Abstract
The presence of cytokines and the toxic eosinophil granule product major basic protein (MBP) was investigated in nasal aspirates from children with naturally occurring virus-induced asthma exacerbations and compared with levels in nasal aspirates taken from the same children when asymptomatic. Increased levels of MBP accompanied by increased levels of the chemokines RANTES and macrophage-inhibitory protein 1α were observed in nasal aspirates from children during the virus-induced exacerbations. Granulocyte-macrophage colony-stimulating factor was mostly undetectable in samples obtained during both symptomatic and asymptomatic periods. Interleukin-5 levels were low, but tended to increase in samples from symptomatic children. These data confirm that the eosinophil product MBP and the eosinophil chemoattractant chemokines RANTES and macrophage-inhibitory protein 1α are increased in upper respiratory viral infections associated with asthma exacerbations and suggest an important role for these chemokines in regulating eosinophil influx and activation. These chemokines may represent targets for therapeutic intervention in virus-induced asthma exacerbations.
Respiratory viral infections are the major cause of asthma exacerbations in children and adults [1, 2]. In a community-based study of 9- to 11-year-old children, viral infections were associated with over 80% of asthma exacerbations, and rhino-viruses were responsible for 50% of the episodes [1]. There is no specific treatment for asthma exacerbations, and the mechanisms of such exacerbations are poorly understood. Two recent studies compared responses to experimental rhinovirus infections in asthmatic or allergic rhinitic subjects and normal subjects, and both implicated an increased bronchial eosinophil infiltrate in the pathogenesis of virus-induced asthma exacerbations [3, 4]. Eosinophils can cause tissue damage by a variety of mechanisms, including the generation of toxic oxygen species and the release of toxic cationic molecules contained in their granules such as major basic protein (MBP) and eosinophil cationic protein (ECP).
We recently showed that naturally occurring upper respiratory viral infections in asthmatic children induce the release of interleukin (IL)-8 into nasal secretions and that IL-8 is associated with the presence of neutrophil-derived proteins, suggesting that IL-8 may be important in neutrophil recruitment and activation [5]. However, no studies have investigated the possible role of eosinophils in the pathogenesis of naturally occurring asthma exacerbations associated with respiratory viral infections. Because molecules promoting eosinophil recruitment and activation are likely targets for the development of new therapies for virus-induced asthma, we investigated whether eosinophil chemoattractants, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-5, and the chemokines RANTES and macrophage-inhibitory protein 1α (MIP-1α) [6–8] are implicated in eosinophil recruitment and activation in naturally occurring upper respiratory viral infections in children with acute asthma exacerbations.
Material and Methods
Clinical samples
Diluted nasal aspirate samples were taken from children in the Southampton area during acute naturally occurring colds associated with exacerbations of asthma as part of another study of 108 children aged 9–11 years [1]. For the present study, we obtained 35 nasal aspirate samples during periods of acute asthma exacerbation from 26 children (16 boys, 10 girls; 14 atopic, 12 nonatopic). We obtained another 26 samples when the children were asymptomatic. The “acute exacerbation samples” were selected if the child had a proven viral infection and sufficient nasal aspirate remained in both acute and asymptomatic samples after completion of the previous study for the planned assays. Methods of sample collection have been described [1].
Virus detection methods
Rhinoviruses were detected by poly-merase chain reaction (PCR) as previously reported [1]. We detected other respiratory viruses by cell culture on five cell lines, immuno-fluorescence microscopy, and serology. Differentiation of rhino-viruses from enteroviruses was done by acid lability testing [9]. Full details of the methods have been published [1].
Cytokine and MBP measurements
We measured RANTES, MIP-1α, and IL-5 in nasal aspirates by ELISA according to the manufacturer's protocol (R&D Systems, Abingdon, UK). The lower limit of detection of RANTES, MIP-1α, and IL-5 in nasal aspirates was 10, 15, and 1 pg/mL, respectively. In the GM-CSF ELISA, we used paired antibodies specific for GM-CSF from PharMingen (Cambridge, UK). The ELISA was performed according to the manufacturer's protocol. The lower limit of detection was 8 pg/mL. Eosinophil MBP in nasal aspirates was measured in duplicate using a two-site RIA as previously described [10]. The lower limit of detection for MBP was 8.8 ng/mL.
Statistical analysis
We used the Mann-Whitney U test to examine differences in cytokine levels in nasal aspirate samples obtained when symptomatic and asymptomatic, and to examine RANTES and MIP-1α levels between atopic and nonatopic children in symptomatic samples. Spearman rank correlations were used to examine the relationships between MBP, RANTES, and MIP-1α levels and respiratory symptom scores during an acute asthma exacerbation. Cytokine and MBP levels were reported and analyzed as measured in the diluted nasal aspirate samples.
Results
Description of exacerbations
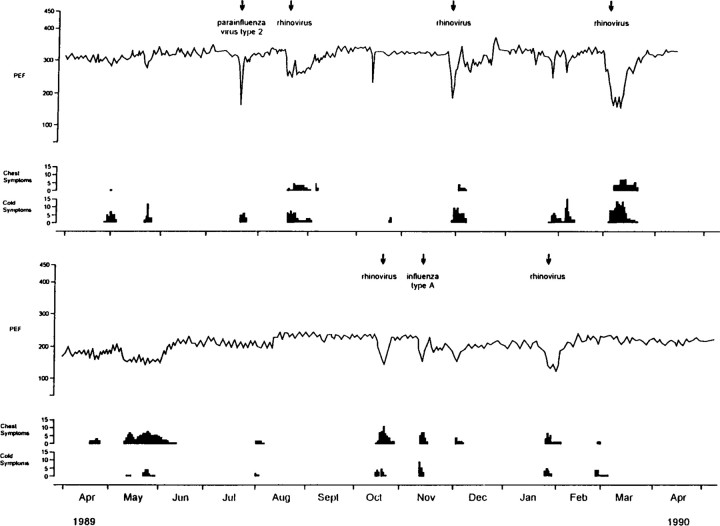
The 26 children had 35 acute asthma exacerbations. Rhinoviruses were detected in 25 (71%), influenza virus type A in 5 (14%), parainfluenza virus type 2 in 3 (9%), adenovirus in 1 (3%), and coronavirus 229E in 1 (3%). Rhinovirus was also detected in 2 asymptomatic samples, although only by very weak PCR signals. Significant episodes of upper respiratory symptoms were reported in 33 (94.2%) of the 35 exacerbations. Lower respiratory episodes characterized by cough, wheeze, or shortness of breath of at least moderate severity were recorded in 25 (71%), and 20 (57%) had a significant drop in peak flow (PEF). All episodes were defined as previously reported [1]. The median maximal percentage fall in PEF from baseline during these episodes was 33% (equivalent to a fall >102 L/min [1]). Figure 1 shows symptoms, PEF, and viruses identified in 2 study children to illustrate the nature of the clinical episodes analyzed in this study.
Figure 1.
Respiratory symptoms (cold and chest), peak flow (PEF) recordings, and viruses identified in 2 children. Samples were obtained at times indicated by arrows.
RANTES and MIP-1α levels in nasal aspirate samples
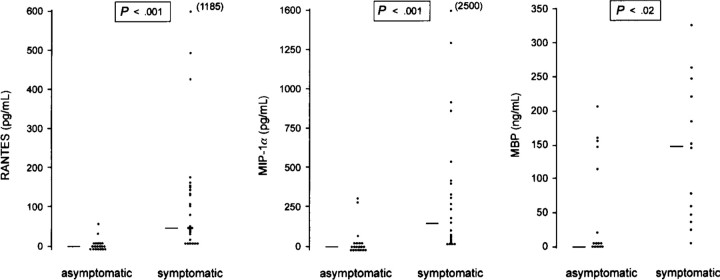
Levels of RANTES and MIP-1α in samples obtained during periods of acute exacerbation were significantly higher (median, 43 pg/mL [range, <10–1185] and 123 pg/mL [range, <15–2500], respectively) than samples obtained when the children were asymptomatic (median <10 pg/mL [range, <10–55] and <15 pg/mL [range, 29.2–280.0], respectively; P <.001; figure 2). When samples from atopic and nonatopic children were compared, concentrations of RANTES and MIP-1α were 3-fold higher in nasal aspirates from atopic children than from nonatopic children during acute exacerbations of asthma (median RANTES levels: 101.3 vs. 33 pg/mL, respectively; median MIP-1α: 123 vs. 39 pg/mL, respectively); however, this difference did not reach statistical significance.
Figure 2.
Levels of RANTES, MIP-1α, and major basic protein (MBP) in nasal aspirates from children with virus-induced asthma exacerbations and when well. RANTES and MIP-1α were measured by ELISA in nasal aspirates obtained during acute asthma exacerbations associated with proven viral infections (symptomatic) and when completely well (asymptomatic). Horizontal lines represent median values. Statistical analysis, Mann-Whitney U test.
GM-CSF, IL-5, and MBP levels in nasal aspirates
Concentrations of GM-CSF (n = 23), MBP (n = 13), and IL-5 (n = 11) were measured in nasal aspirates if sufficient material remained. GM-CSF concentrations were mostly undetectable in samples from both symptomatic and asymptomatic children, and median values were below the limit of detection (8 pg/mL) of the GM-CSF ELISA. IL-5 levels tended to increase in the nasal aspirates of symptomatic when compared with asymptomatic children (median, 1.5 pg/mL [range, <1.0–31.4] vs. <1.0 pg/mL [range, <1.0–12.1]); however, this difference failed to reach statistical difference (P = .1).
MBP levels were significantly higher in samples from acute exacerbation episodes (median, 60 ng/mL; range, <8.8–335) than from asymptomatic periods (median, <8.8 ng/mL; range, <8.8–212; P <.001; figure 2). There were no significant correlations between MBP and RANTES levels (r = .11, P = .7, n = 13) or between MBP and MIP-1α levels (r = .43, P = .14, n = 13) in nasal samples from periods of acute exacerbation.
Correlation of MBP levels with respiratory symptoms
To investigate the possible relationship between eosinophil influx and activation and local respiratory symptoms, we sought correlations between the levels of MBP and severity (as previously defined [1]) of upper respiratory tract symptoms recorded during the episode. There was no significant correlation between the severity of upper respiratory symptoms and MBP levels (r = −.2, P = .4, n = 13). Similarly, no significant correlation was found between the severity (also previously defined [1]) of lower respiratory symptoms and MBP concentrations (r = .24, P = .5, n = 9).
Discussion
In this study, we observed increased levels of the eosinophil chemoattractants RANTES and MIP-1α and of the eosinophil product MBP in nasal aspirates from children during periods of proven virus-induced asthma exacerbations. These data implicate these chemokines in eosinophil recruitment and activation in virus-induced asthma and suggest that they or their receptors may represent promising targets for the development of therapies for virus-induced asthma.
Respiratory syncytial virus induces selective production of the RANTES by upper airway epithelial cells in vitro [11]. In the present study, we showed that viral infection of the upper respiratory tract induces release of the chemokines RANTES and MIP-1α and release of the eosinophil product MBP in vivo. This is of interest, as both of these chemokines are eosinophil chemoattractants. While MIP-1α is less potent than RANTES as an eosinophil chemoattractant, it is an important mediator in the inflammatory response to viral infection [12]. Furthermore, in a mouse model, absence of CCR1, which binds MIP-1α most strongly, resulted in reduced host defense to infection and a tilt in favor of a Th2-type response to infection [13]. Given the role of Th2 cells in asthma, these properties suggest that both MIP-1α and RANTES may be important mediators of virus-induced asthma exacerbations. This observation is supported by the finding that levels of these cytokines in atopic children were 3-fold greater than in nonatopic children.
We have observed increases in chemokine and mediator levels in the upper airway in children with simultaneous virus-related colds and asthma exacerbations. The known properties of these chemokines strongly suggest that they are likely to be important in eosinophil recruitment and activation in the upper airway, and therefore by implication, are likely to be important in the eosinophil recruitment and activation known to be important in the pathogenesis of virus-induced asthma exacerbation. These data suggest that studies investigating the importance of these chemokines in the lower airway should be done to test this hypothesis.
The lack of correlation between chemokine levels and either eosinophil mediator levels or respiratory symptoms raises some doubts about the relevance of these chemokines to eosinophil recruitment and activation in the airway. However, such correlations are difficult to find in clinical studies where many variables may affect the strength of a relationship between two biologic measurements. For example, other chemokines such as eotaxin or IL-8 may also be involved, perhaps in concert with RANTES and MIP-1α. Furthermore, the finding of a correlation is still not proof of a causal relationship between two variables. In our view, the known biologic functions of these chemokines and the presence of increased eosinophil mediators is sufficient to support our hypothesis that these chemokines are important in eosinophil recruitment and activation in vivo. Further studies with chemokine antagonists will be required to test the strength of this relationship.
The cytokines and the eosinophil product MBP detected in the nasal aspirates could be derived from local production or from serum via vascular leakage. We believe that the high levels detected (especially considering that mucus was diluted 20–40 times before storage and analysis) suggest that local production predominates.
GM-CSF and IL-5 are two other cytokines that play an important role in leukocyte trafficking. GM-CSF is chemotactic for both neutrophils and eosinophils, while IL-5 is important in eosinophil hematopoiesis, priming, and chemoattraction [6, 7]. Concentrations of GM-CSF in nasal secretions were mostly undetectable, suggesting that this cytokine plays a minor role in the pathogenesis of naturally occurring virus-induced asthma exacerbations. This finding is consistent with previous reports showing that GM-CSF plays a minor role in upper respiratory viral infections [14,15]. In a small group of children from whom enough clinical material remained, we also investigated the presence of IL-5. Although levels of IL-5 tended to be increased in the nasal aspirates from symptomatic children, there were no significant differences between symptomatic and asymptomatic children. The role of IL-5 in viral exacerbations of asthma must be further studied in greater numbers of children.
In summary, we found increased levels of the chemokines RANTES and MIP-1α in nasal secretions during exacerbations of asthma associated with upper respiratory viral infection in children compared with asymptomatic samples from the same children. GM-CSF was not detectable in most nasal aspirates, but IL-5 concentrations tended to increase in samples from symptomatic children. This study also found increased levels of the eosinophil granule protein MBP during virus-induced exacerbations of asthma. These findings suggest that the development of new drugs to neutralize the effects of RANTES and MIP-1α on eosinophils may affect virus-induced asthma exacerbations.
Acknowledgments
We thank Philip Pattemore, Gwen Sanderson, and Sandy Smith for help with sample collection and virus detection.
Footnotes
Presented in part: American Academy of Allergy and Immunology meeting, New York, 1995.
Informed consent was obtained from all study subjects. The study was approved by the Southampton Hospitals joint ethical subcommittee.
Grant support: Medical Research Council (UK) Travelling Research Fellowship (S.L.J.), University of Ferrara (A.P.), NIH (AI-34577, AI-07047, and AI-09728).
References
- 1.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of virus infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–8. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–86. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–8. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teran LM, Johnston SL, Schröder J, Church MK, Holgate ST. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am J Respir Crit Care Med. 1997;155:1362–6. doi: 10.1164/ajrccm.155.4.9105080. [DOI] [PubMed] [Google Scholar]
- 6.Teran LM, Montefort S, Douglass J, Holgate ST. Neutrophil and eosinophil chemotaxins in asthma. Q J Med. 1993;86:761–9. [PubMed] [Google Scholar]
- 7.Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Mantovani A. Recombinant human interleukin-5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989;19:701–5. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- 8.Teran LM, Davies DE. The chemokines: their potential role in allergic inflammation. Clin Exp Allergy. 1996;26:1005–19. [PubMed] [Google Scholar]
- 9.Johnston SL, Tyrrell DAJ. Lennette EH, Schmidt NJ. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington, DC: American Public Health Association; 1995. Rhinoviruses; pp. 553–63. [Google Scholar]
- 10.Wagner JM, Bartemes K, Vernof KK, et al. Analysis of pregnancy-associated major basic protein levels throughout gestation. Placenta. 1993;14:671–81. doi: 10.1016/s0143-4004(05)80384-4. [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Deskin RW, Casola A, et al. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 12.Cook DN, Beck MA, Coffman TM, et al. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–5. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 13.Gao JL, Wynn TA, Chang Y, et al. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–68. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linden M, Greiff L, Anderson M, et al. Nasal cytokines in common cold and allergic rhinitis. Clin Exp Allergy. 1995;25:166–72. doi: 10.1111/j.1365-2222.1995.tb01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noah TL, Henderson FW, Wortman IA, et al. Nasal cytokine production in viral acute upper respiratory infection of children. J Infect Dis. 1995;171:584–92. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]