Abstract
Treatment with first-generation antihistamines reduces sneezing, rhinorrhea, nasal mucus weight, and, in some instances, cough in subjects with experimental or natural colds; however, treatment with second-generation antihistamines has not been effective for these complaints in trials in subjects with natural colds. This article reports the negative results of a clinical trial with loratadine, a second-generation antihistamine, in adults in the rhinovirus challenge model. This finding in the highly controlled setting of the challenge model confirms the earlier negative studies with second-generation antihistamines in natural colds. First-generation antihistamines block both histaminic and muscarinic receptors as well as passing the blood-brain barrier. Second-generation antihistamines mainly block histaminic receptors and do not pass the blood-brain barrier. The effectiveness of first-generation antihistamines in blocking sneezing in colds may be due primarily to neuropharmacological manipulation of histaminic and muscarinic receptors in the medulla.
The individual symptoms of a common cold are due to multiple and somewhat specific pathways of inflammation [1]. Sneezing has been generally thought to result from the release of histamine from nasal mast cells and basophils, which are activated by a cold virus infection. Supporting this belief is the finding that intranasal challenge with histamine in normal volunteers elicited sneezing whereas intranasal exposure to other mediators did not [2]. Also, treatment with first-generation antihistamines is highly effective in reducing sneezing in subjects with experimental and natural colds [3–5]. It is, therefore, surprising that, unlike with allergic rhinitis, histamine levels are not elevated in nasal secretions of patients with colds [6–9], although nasal mucosal sensitivity to histamine has been reported to be increased [10–13].
Both first- and second-generation antihistamines are competitive antagonists to histamine at the H1-receptor site [14]. An additional pharmacological activity of first-generation, but not second-generation antihistamines is the competitive antagonism of acetylcholine at neuronal and neuromuscular muscarinic receptors. First-generation antihistamines also pass the blood-brain barrier and thus have a potential for activity in the brain; second-generation antihistamines do not. In limited testing, and despite their H1-blocking activity, second-generation antihistamines have been ineffective in suppressing sneezing in patients with natural colds [15–18].
These findings raise an interesting question about the mechanism of action of first-generation antihistamines in reducing sneezing in patients with colds and about the ineffectiveness of second-generation antihistamines in this setting. Also, natural cold studies have certain technical problems, such as difficulty in enrolling patients in the early stages of a cold, when treatment effects are most accurately measured [19]. Therefore, it would be desirable to confirm the results of the natural cold studies by testing a second-generation antihistamine in the rhinovirus challenge model, which provides more precision in the measurement of sneezing. This article reports a clinical trial using a second-generation antihistamine, loratadine, in adults with experimental rhinovirus colds and reviews the possible sites of action of first-generation and second-generation antihistamines. Although the study was originally designed to determine whether loratadine by down-regulating expression of intercellular adhesion molecule-1 (ICAM-1) on nasal epithelial cells reduces rhinovirus infection rates, it provides heretofore-missing information on the results of testing a second-generation antihistamine in the virus challenge mode.
Materials and Methods
Subjects. A total of 66 adult volunteers from the Charlottesville, Virginia, area with neutralizing antibody titers of ⩽2 to rhinovirus type 16 were enrolled in the study. Subjects were required to have been free of cold symptoms and fever (>37.8°C) for 1 week prior to entering the trial and to have no history of hypersensitivity to antihistamines. In addition, subjects were excluded if they had (or had a history of) allergic rhinitis, bronchial asthma, or other lower respiratory tract diseases such as chronic obstructive lung disease or emphysema. Subjects with a history of alcohol and drug abuse were excluded, as were volunteers who had used investigational drugs within 30 days, antihistamines and/or cold preparations within 14 days, monoamine oxidase inhibitors within 7 days, astemizole within 90 days, or any other medication thought to interfere with the study drug. Other exclusion criteria included pregnancy or lactation, glaucoma, and renal, hepatic, endocrine, digestive, genitourinary, neurologic, or psychologic disease. The protocol was reviewed by the Human Investigation Committee of the University of Virginia.
Study medication. Loratadine was administered in 10-mg tablets. The placebo tablets were identical to the loratadine tablets but contained pharmacologically inert ingredients.
Virus challenge. Intranasal challenge with rhinovirus type 16 was performed by coarse drops by use of 0.5 mL (0.25 mL per nostril) an inoculum pool containing 100 tissue culture infection dose 50/mL (TCID50/mL) of virus. The challenge was performed twice with a 20-min interval between challenges. The inoculum pool was safety tested for extraneous agents [20].
Measures of infection. Nasal washings were collected 8 days prior to and immediately before the inoculation of the challenge virus to determine whether subjects were infected with a wild-type virus. After virus challenge, nasal washings were collected each morning before administration of medication. Washings were cultured for rhinovirus for 5 days after challenge in human embryonic lung cells (WI-38). Isolates were identified as rhinovirus type 16 by neutralization with type-specific antibody. Venous blood was obtained 7 days prior to treatment and 14–21 days after intranasal inoculation for measurement of homotypic neutralizing antibody [21].
Measures of illness. The presence and severity of symptoms were determined daily beginning on the first day of treatment, 7 days prior to viral challenge, for a total of 13 days. Data on symptoms were collected immediately before administration of loratadine or placebo by a nurse who recorded the subject's assessment of the severity of symptoms over the prior 24 h on a 5-point scale (0, “none”; 1, “mild”; 2, “moderate”; 3, “severe”; 4, “very severe”) [22]. The symptoms assessed were sneezing, runny nose, nasal obstruction, sore throat, cough, headache, malaise, and chilliness. The total symptom score was determined by adding severity scores for the symptoms over the 5-day period after viral challenge. The score for each symptom present immediately before challenge was subtracted from each of the daily scores for that symptom.
Evaluation of illness severity also included daily measurements of nasal secretion weights [23]. Each subject kept a daily log of the number of coughs and sneezes. Nasal secretion measurements and cough and sneeze counts were started after the subjects were cloistered in a hotel after challenge had occurred. Before leaving the hotel on the last day, subjects were asked whether, in their opinion, they experienced a cold. Information on the occurrence and severity of any adverse effects was collected daily, graded as “none,” “mild,” “moderate,” or “severe.”
Experimental design. The trial was a 13-day, single-center, randomized double-blind, placebo-controlled, parallel group study in healthy volunteers 18–40 years of age, of either sex. Loratadine or placebo was administered each morning between 6 A.M. and 8 A.M., on days 1–13 of the study, with viral challenge given on day 8. The subjects were randomly assigned to receive either treatment or placebo and were blinded as to their treatment status, as were the observers recording clinical information.
Data analysis. For comparing proportions, Fisher's exact test was used. The t test was used for comparing ordinal and interval data. The results of probability testing were 2-tailed.
Results
Subjects. Of the 66 subjects enrolled, 34 received loratadine and 32 received placebo. Four subjects in the loratadine group and 1 in the placebo group were infected with a wild-strain rhinovirus at the time of entry into the study. They were excluded from evaluation, as was 1 subject on placebo who discontinued medication after developing a migraine headache on day 10, leaving 60 evaluable subjects for analysis.
Infection rates. Twenty-eight (93%) of 30 subjects on loratadine and 24 (80%) of 30 subjects on placebo shed the challenge virus in nasal fluid on ⩾1 days (P = .25; table 1). The mean number of days on which virus was shed was similar for all challenged volunteers and for all infected volunteers in the 2 groups. Homotypic antibody responses occurred in 12 (40%) of 30 subjects treated with loratadine and 11 (37%) of 30 subjects who received placebo. The infection rate (viral shedding and/or antibody rise) was 29 (97%) of 30 in the loratadine group and 24 (80%) of 30 in the placebo group (P = .1).
Table 1.

Infection and illness rates in adults with experimental rhinovirus colds given loratadine or placebo.
Viral titers. Geometric mean viral titers peaked on the second day after viral challenge, as expected (figure 2A). Viral titers were similar in the 2 groups, although there was trend for lower titers in the placebo group on day 2.
Figure 2.
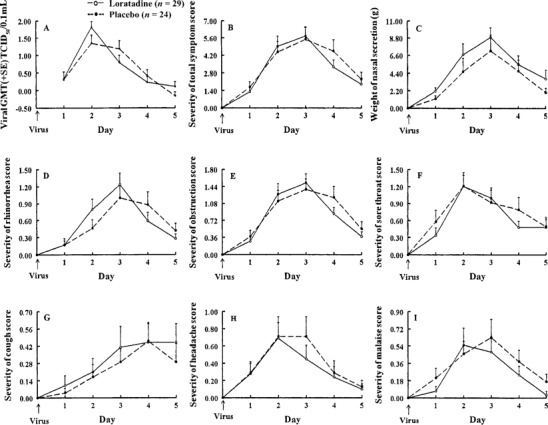
Geometric mean (±SE) viral titers, mean (±SE) nasal mucus weights, and mean (±SE) symptom scores in adults with experimental rhinovirus colds given loratadine or placebo.
Occurrence of illness. Twenty-three (79%) of 29 infected subjects on loratadine and 14 (58%) of 24 infected subjects on placebo met the modified Jackson criteria for illness (P = .2).
Symptom scores. Mean (±SE) sneezing severity scores were similar for the first 3 days and tended to be lower in the placebo group on the fourth day (figure 1). Mean (±SE) total symptom scores were similar in the 2 groups (figure 2B). Rhinorrhea scores tended to be lower in the placebo group on days 2 and 3, but the reverse was seen on days 4 and 5 (figure 2D). Mean (±SE) nasal obstruction, sore throat, and cough scores were similar in the 2 groups (figure 2C, figure 2F, figure 2G). Mean (±SE) headache and malaise scores tended to be lower in the loratadine group for the latter days of illness (figure 2H, figure 2I).
Figure 1.
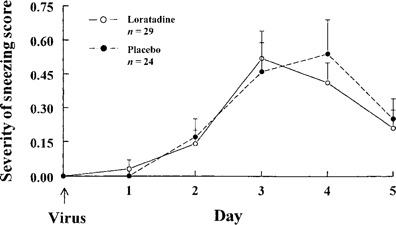
Mean (±SE) sneezing severity scores in adults with experimental rhinovirus colds given loratadine or placebo.
Nasal mucus weights. There was a consistent trend for nasal secretion weights to be lower in the placebo group (figure 2C). Total mean ± SE nasal mucus weights for 5 days were 27.1 ± 4.0 g for the loratadine group and 19.7 ± 5.2 g for the placebo group (P = .3).
ICAM-1 levels. Mean (±SE) nasal fluid ICAM-1 levels rose from the baseline on day 2 and peaked on day 3. The levels were similar in both groups (figure 3).
Figure 3.
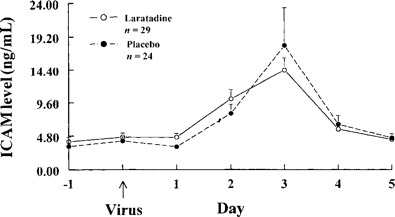
Mean (±SE) nasal fluid ICAM-1 levels in adults with experimental rhinovirus colds given loratadine or placebo.
Adverse events. One subject in the placebo group had a migraine headache and vomiting and another had vomiting. Otherwise, no adverse events were reported.
Discussion
In regard to the original purpose of the study, no differences were observed between the groups receiving loratadine and groups receiving placebo for viral shedding rates, viral titers, overall infection rates, illness rates, or symptom scores. ICAM-1 levels in nasal secretions in the 2 groups were also similar.
The results also showed no therapeutic effect of loratadine on sneezing. This supports earlier work in patients with natural colds in whom second-generation antihistamines were ineffective in reducing sneezing [15–18]. Why first-generation antihistamines are effective in reducing sneezing in colds [3, 5] and second-generation antihistamines are not is of interest. First-generation antihistamines, beside their ability to block H1-receptors, also block muscarinic receptors and pass the blood-brain barrier [14]. Second-generation antihistamines are specific H1-receptor blockers without other recognized pharmacological properties and do not pass the blood-brain barrier.
Information on the neurologic pathways of the sneeze reflex comes mainly from work in animals [24, 25]. The sneeze reflex travels along peripheral nerves and through the medulla oblongata. The neuropharmacology of the sneeze involves H1, muscarinic, and nicotinic receptors. With colds, the sneeze reflex begins in the nose with the infection of nasal cells by a cold virus (figure 4). There is no evidence that activation of basophils and mast cells with release of histamine occurs in colds; however, there is presumed stimulation of free nerve endings of the ethmoidal branch of the trigeminal nerve by inflammatory mediators known to be present, such as bradykinin [1]. The nerve impulse then travels along the afferent nerve fibers to the sensory trigeminal nucleus in the medulla oblongata and arrives at the adjacent sneeze center [26–28]. Via another synapse, the impulse then arrives at the parasympathetic superior salivary nucleus of the facial nerve. Here, it crosses a synapse and travels via the preganglionic fibers of the greater petrosal nerve to the sphenopalatine ganglion. Supporting this route of transmission in sneezing is the finding that injection of alcohol into the sphenopalatine ganglion blocks the sneeze reflex [29]. The impulse then travels across another synapse (mainly nicotinic, some muscarinic) and proceeds via postganglionic fibers to the synapses (muscarinic) of mucus glands and blood vessels, which are then stimulated. The resultant glandular secretion and vascular transudation restimulate the free nerve endings of the trigeminal nerve by which pathway the impulse is redirected to the trigeminal nucleus and, subsequently, to the sneeze center in the medulla. When the restimulation of the sneeze center is sufficient, the impulse is then directed by intramedullary fibers to the synapses of multiple respiratory centers in the reticular formation, and from there to the synapses of respiratory neurons of the vagus, phrenic, and intercostal nerves. Then, via nicotinic synapses, the nerve impulse stimulates the muscular contractions responsible for a sneeze.
Figure 4.
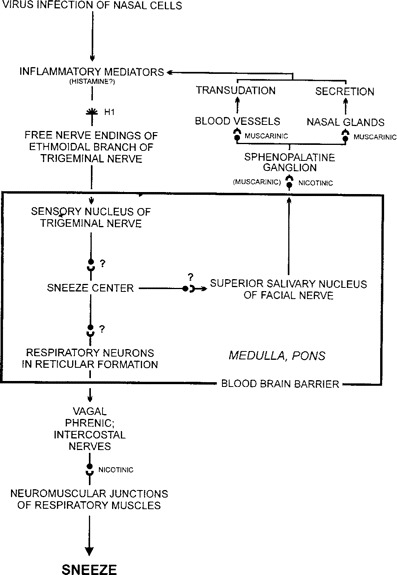
Pathway of the sneeze reflex
The nose is the first site at which histamine may be involved in the pathogenesis of sneezing and where an antihistamine might inhibit the sneeze reflex. H1-receptors are present on the free nerve endings of the trigeminal nerve [30]. Against the possibility that the nasal mucosa is the primary site of action of first-generation antihistamines is the finding that histamine levels have not been found to be elevated in nasal secretions during colds [6–9]. Also, in earlier natural cold studies and in the current study, second-generation antihistamines, despite reaching the nasal mucosa and despite having H1-blocking activity, have been ineffective in sneeze reduction [15–18]; however, because the nasal mucosa appears to have enhanced sensitivity to histamine during colds [10,1–13], the role of the nasal sites cannot be entirely excluded.
The next possible site for a first-generation antihistamine to block sneezing is in the medulla oblongata, where synaptic junctions are present at several locations. Both H1 and muscarinic receptors have been identified in certain areas of the brain [14, 31]. Such receptors would be potential targets for the action of first-generation, but not second-generation antihistamines; however, the synaptic mediators that are involved in the sneeze reflex in the medulla have not been characterized. The observed failure of the second-generation antihistamines to reduce sneezing in colds supports the possibility of these sites being important.
Muscarinic activity is exclusively responsible for parasympathetic stimulation of the glandular secretion and vascular dilatation with transudation that occurs next. The anticholinergic activity of first-generation antihistamines would be expected to operate at these sites. This is supported by the well-documented effect of first-generation antihistamines in reducing the volume of nasal fluid production during colds [3, 4]. The second afferent impulse to the medulla is again initiated by stimulation of the free nerve endings of the trigeminal nerve. Vascular transudation leads to release of kininogen with resultant generation of kinin [14]. This event provides a means for direct stimulation of free nerve endings by kinins as well as by histamine released from mast cells by kinin stimulation. This provides another possible target for the action of an antihistamine. Also, when the nerve impulse is redirected to the medulla, H1 and muscarinic synaptic sites may be blocked by first-generation antihistamines as described above. From that point on, when the motor neurons become involved, nerve transmission depends on nicotinergic receptors and thus would not be susceptible to the action of an antihistamine.
This analysis suggests that an important site for the therapeutic effect of first-generation antihistamines on sneezing is in the medulla oblongata, where both H1 and muscarinic receptors may be involved. H1 receptors are known to be present in high concentrations in the hypothalamus, where histamine acts as a neurotransmitter to help regulate the level of wakefulness [14]. This accounts for the drowsiness associated with the use of first-generation antihistamines. Because of the dense concentration of H1 receptors in the hypothalamus, and because parasympathetic nerve fibers arise from and are activated by the hypothalamus, it is probable that histamine plays a role in signal transmission in this region.
First-generation antihistamines are also known to be helpful in reducing the nausea associated with motion sickness [31]. Scopolamine, an anticholinergic drug, which passes the blood-brain barrier, is an effective treatment for motion sickness, whereas atropine, which does not pass the blood-brain barrier as readily, is not effective. The antinausea effect of scopolamine depends partially on blocking of muscarinic receptors of the vestibular nuclei and the area postrema of the brain. Other nuclei in the brainstem may use the muscarinic receptor system as well, which supports the idea of muscarinic receptors in the CNS being part of the sneeze reflex. At present, information is not complete on the medullary synapses and neurotransmitters involved in the sneeze reflex, and further work is needed in this area; however, available information suggests that to be effective, a treatment for the sneezing of colds requires compounds that pass the blood-brain barrier and possess both H1 and muscarinic-blocking activity.
Footnotes
Written, informed consent was obtained from each subject, and human experimentation guidelines of the United States Department of Health and Human services and those of the University of Virginia Human Investigation Committee were followed in the conduct of clinical research.
Financial support: Schering-Plough.
References
- 1.Gwaltney JM, Jr, Rueckert RR. Rhinovirus. In: Richman DD, Whitley RG, Hayden FG, editors. Clinical virology. New York: Churchill Livingstone; 1997. pp. 1025–47. [Google Scholar]
- 2.Doyle WJ, Boehm S, Skoner DP. Physiologic responses to intranasal dose-response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J Allergy Clin Immunol. 1990;86:924–35. doi: 10.1016/s0091-6749(05)80156-3. [DOI] [PubMed] [Google Scholar]
- 3.Gwaltney JM, Jr, Druce HM. Efficacy of brompheniramine maleate treatment for rhinovirus colds. Clin Infect Dis. 1997;25:1188–94. doi: 10.1086/516105. [DOI] [PubMed] [Google Scholar]
- 4.Gwaltney JM, Jr, Park J, Paul RA, Edelman DA, O'Connor RR, Turner RB. Randomized controlled trial of clemastine fumarate for treatment of experimental rhinovirus colds. Clin Infect Dis. 1996;22:656–62. doi: 10.1093/clinids/22.4.656. [DOI] [PubMed] [Google Scholar]
- 5.Turner RB, Sperber SJ, Sorrentino JV, et al. Effectiveness of clemastine fumarate for treatment of rhinorrhea and sneezing associated with the common cold. Clin Infect Dis. 1997;25:824–30. doi: 10.1086/515546. [DOI] [PubMed] [Google Scholar]
- 6.Eggleston PA, Hendley JO, Gwaltney JM, Jr, Eggleston AW, Leavell BS., Jr Histamine in nasal secretions. Int Arch Allergy Appl Immunol. 1978;57:193–200. doi: 10.1159/000232103. [DOI] [PubMed] [Google Scholar]
- 7.Naclerio RM, Proud D, Kagey-Sobotka A, Lichtenstein LM, Hendley JO, Gwaltney JM., Jr Is histamine responsible for the symptoms of rhinovirus colds: a look at the inflammatory mediators following infection. Pediatr Infect Dis J. 1988;7:218–22. doi: 10.1097/00006454-198803000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993;92:722–31. doi: 10.1016/0091-6749(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 9.Smith TF, Remigio LK. Histamine in nasal secretions and serum may be elevated during viral respiratory tract infection. Int Arch Allergy Appl Immunol. 1982;67:380–3. doi: 10.1159/000233051. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun WJ, Swenson CA, Dick EC, et al. Experimental rhinovirus 16 infection potentiates histamine release after antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1991;144:1267–73. doi: 10.1164/ajrccm/144.6.1267. [DOI] [PubMed] [Google Scholar]
- 11.Greiff L, Andersson M, Akerlund A, et al. Mircovascular exudative hyperresponsiveness in human coronavirus-induced common cold. Thorax. 1994;49:121–7. doi: 10.1136/thx.49.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle WJ, Skoner DP, Seroky JT, Fireman P, Gwaltney JM., Jr Effect of experimental rhinovirus 39 infection on the nasal response to histamine and cold air challenges in allergic and nonallergic subjects. J Allergy Clin Immunol. 1994;93:534–42. doi: 10.1016/0091-6749(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 13.Busse WW. Viral infections and allergic disease. Clin Exper Allergy. 1991;1((S21)):68–71. doi: 10.1111/j.1365-2222.1991.tb01708.x. [DOI] [PubMed] [Google Scholar]
- 14.Bube KS, Jr, Serafin WE. Histamine, bradykinin and their antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill; 1996. pp. 581–600. [Google Scholar]
- 15.Gaffey MJ, Kaiser DL, Hayden FG. Ineffectiveness of oral terfenadine in natural colds: evidence against histamine as a mediator of common cold symptoms. Pediatr Infect Dis J. 1988;7:215–42. doi: 10.1097/00006454-198803000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Berkowitz RB, Tinkelman DG. Evaluation of oral terfenadine for treatment of the common cold. Ann Allergy. 1991;67:593–7. [PubMed] [Google Scholar]
- 17.Henauer SA, Glück U. Efficacy of terfenadine in the treatment of common cold: a double-blind comparison with placebo. Eur J Pharmacol. 1988;34:35–40. doi: 10.1007/BF01061414. [DOI] [PubMed] [Google Scholar]
- 18.Janssens MM, Howarth PH. The antihistamines of the nineties. Clin Rev Allergy. 1993;11:111–53. doi: 10.1007/BF02802296. [DOI] [PubMed] [Google Scholar]
- 19.Gwaltney JM, Jr, Buier RM, Rogers JL. The influence of signal variation, bias, noise, and effect size on statistical significance in treatment studies of the common cold. Antiviral Res. 1996;29:287–95. doi: 10.1016/0166-3542(95)00935-3. [DOI] [PubMed] [Google Scholar]
- 20.Gwaltney JM, Jr, Hendley O, Hayden FG, et al. Updated recommendations for safety-testing of viral inocula used in volunteer experiments on rhinovirus colds. In: Melnick JL, editor. Progress in medical virology. Vol 39. Basel: Karger; 1992. pp. 256–63. [PubMed] [Google Scholar]
- 21.Gwaltney JM, Jr, Colonno RJ, Hamparian VV, Turner RB. Rhinovirus. In: Schmidt NJ, Emmonds RW, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 6th ed. Vol. 142. Washington, DC: American Public Health Association; 1989. pp. 811–5. [Google Scholar]
- 22.Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. Arch Intern Med. 1958;101:267–78. doi: 10.1001/archinte.1958.00260140099015. [DOI] [PubMed] [Google Scholar]
- 23.Doyle WJ, McBride TP, Skoner DP, Maddren BR, Gwaltney JM, Jr, Uhrin M. A double-blind, placebo-controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear and eustachian tube to provocative rhinovirus challenge. Pediatr Infect Dis J. 1988;7:229–38. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 24.Batsel HL, Lines AJ. Neural mechanisms of sneeze. Am J Physiol. 1975;229:770–6. doi: 10.1152/ajplegacy.1975.229.3.770. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka S, Unno T, Ohta Y, Mori S. Sneeze-evoking region within the brainstem. Brain Research. 1990;511:265–70. doi: 10.1016/0006-8993(90)90171-7. [DOI] [PubMed] [Google Scholar]
- 26.Martin RA, Stanley FH, Aldama AE. Inability to sneeze as a manifestation of medullary neoplasm. Neurology. 1991;41:1675–6. doi: 10.1212/wnl.41.10.1675. [DOI] [PubMed] [Google Scholar]
- 27.Korpas J, Tomori Z. Progress in Respiratory Research. Vol. 23. Basel, Switzerland: Karger; 1979. Cough and other respiratory reflexes; pp. 218–23. [Google Scholar]
- 28.Whitman BW, Packer RJ. The photic sneeze reflex: literature review and discussion. Neurology. 1993;43:868–71. doi: 10.1212/wnl.43.5.868. [DOI] [PubMed] [Google Scholar]
- 29.Stromberg BV. Sneezing: its physiology and management. Eye Ear Nose Throat Mon. 1975;54:449–53. [PubMed] [Google Scholar]
- 30.Gwaltney JM, Jr, Hayden FG. The nose and infection. In: Proctor DF, Andersen IB, editors. The nose: upper airway physiology and the atmospheric environment. Amsterdam: Elsevier Biomedical; 1982. pp. 399–422. [Google Scholar]
- 31.Brown JH, Taylor P. Muscarinic receptor agonists and antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill; 1996. pp. 141–60. [Google Scholar]


