Abstract
In 1997, avian influenza virus H5N1 was transmitted directly from chicken to human and resulted in a severe disease that had a higher mortality rate in adults than in children. The characteristic mononuclear leukocyte infiltration in the lung and the high inflammatory response in H5N1 infection prompted us to compare the chemokine responses between influenza virus–infected adult and neonatal monocyte-derived macrophages (MDMs). The effects of avian influenza virus A/Hong Kong/483/97 (H5N1) (H5N1/97), its precursor A/Quail/Hong Kong/G1/97 (H9N2) (H9N2/G1), and human influenza virus A/Hong Kong/54/98 (H1N1) (H1N1/98) were compared. Significantly higher expression of CCL2, CCL3, CCL5, and CXCL10 was induced by avian influenza viruses than by human influenza virus. Moreover, the increase in CCL3 expression in H5N1/97-infected adult MDMs was significantly higher than that in neonatal MDMs. Enhanced expression of CCR1 and CCR5 was found in avian virus–infected adult MDMs. The strong induction of chemokines and their receptors by avian influenza viruses, particularly in adult MDMs, may account for the severity of H5N1 disease
Chemokines are a large family of small secreted proteins that can chemoattract leukocyte subpopulations from the blood to sites of inflammation. They are classified according to the cysteine motif into 4 subgroups—namely, CC, CXC, C, and CX3C chemokines [1, 2]. The biological effects of chemokines are exerted by their binding to specific G protein–coupled receptors expressed on the surface of leukocytes [3]. CC chemokine receptors are expressed on a wide variety of cells, including monocytes/macrophages, lymphocytes, NK cells, eosinophils, and basophils. CXC receptors predominate on neutrophils [4, 5]
After the initial infection of respiratory epithelial cells by influenza A virus, small numbers of blood-derived neutrophils and large numbers of blood monocytes/macrophages extrude into the infected lung. Viral replication continues in the epithelial cells, and infection spreads to macrophages [6]. A series of inflammatory CC chemokines (CCL2, CCL3, CCL5, and CCL11) and interferon (IFN)–γ–responsive chemokines (CXCL1, CXCL8, CXCL9, and CXCL10) are induced in the infected macrophages [7–10 ]. These chemokines act as important mediators for immune cell activation and recruitment; however, they may be detrimental to the infected host, resulting in inflammation and organ pathology [1, 2]. CCL3, CCL5, and CXCL10 potentially contribute to lung pathology by inducing the activation and migration of cytotoxic T lymphocytes (CTLs) to the site of influenza infection [11, 12]
Avian influenza virus H5N1 is novel to the human population and was first identified as transmitting to humans directly in Hong Kong in 1997 [13]. In contrast to infection with common human influenza viruses, infection with this virus is characterized by severe lung damage with infiltration of macrophages, lymphocytes, and reactive fibroblasts, as well as by high inflammatory responses. Reactive hemophagocytosis is one of the notable systemic signs [13–15 ]. Age-related severity is also found in avian influenza. Among the 18 humans infected with H5N1 in 1997, 5 of 9 patients aged >12 years died, but only 1 of 9 patients aged <12 years died [13]. Avian virus H9N2/G1, the precursor of H5N1 virus, shares some similarities with H5N1 virus, such as 6 internal genes, a high proinflammatory induction phenotype in adult macrophages, and mild clinical features in children [15–17 ]
Our previous studies have demonstrated that neonatal immune cells are functionally immature, compared with their adult counterparts [18–22 ]. Hence, we hypothesized that there are differential immune responses to avian influenza viruses during the development of the host’s immune system and that these differences may be related to the clinical severity. In the present study, we compared the expression of inflammatory chemokines CCL2, CCL3, CCL5, and CXCL10, as well as that of the chemokine receptors CCR1 and CCR5, between adult and neonatal monocyte-derived macrophages (MDMs) infected with avian influenza virus H5N1/97 or with its precursor, H9N2/G1
Materials and Methods
Sample collectionHuman umbilical-cord blood was obtained from the placentas of healthy, full-term infants, after the placentas were delivered and separated from the infants, with the written informed consent of their mothers. Adult blood was collected from healthy blood donors (from the Hong Kong Red Cross Blood Transfusion Service). The research protocol was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster
Culture of MDMsHuman MDMs were generated from mononuclear cells, as described elsewhere [15, 23]. The purity of monocytes, as determined by flow cytometry (Coulter Epics Elite; Beckman Coulter) with anti-CD14 monoclonal antibody (PharMingen) was consistently >90%. The monocytes were refed by fresh medium every 2 days and allowed to differentiate for 14 days in vitro
Virus preparation, titration, and infectionIn view of the biosafety issues involved in handling highly pathogenic avian influenza virus A/Hong Kong/483/97 (H5N1/97), we used avian influenza virus A/Quail/Hong Kong/G1/97 (H9N2/G1) for the optimization of experiments. The findings were then confirmed using H5N1/97 virus, and all experiments with H5N1/97 virus were performed in a Biosafety Level 3 facility
As described elsewhere [23], viruses were cultured in Madin-Darby canine kidney (MDCK) cells (ATCC), were purified by adsorption to and elution from turkey red blood cells [24], and were stored at −70°C until use. The titer of virus stock was determined by titration in MDCK cells and by daily observation for cytopathogenic effect and was confirmed by hemagglutination assay [25]. The TCID50 was calculated by the Reed-Muench formula
Day-14 differentiated MDMs were infected by influenza viruses at an MOI of 2. This is taken to be the 0-h point of infection (POI) for the experiments described below. After virus adsorption for 1 h at 37°C, unadsorbed virus was removed by washing with PBS. Mock-treated cells were similarly treated in parallel, except that virus was not added. To determine the infectivity, cells were fixed and analyzed by immunofluorescent staining specific for influenza A virus matrix protein (M) and nucleoprotein (NP) (DAKO Imagen; Dako Diagnostics)
Quantification of mRNA by real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR)Infected MDMs (∼5×105) cultured in macrophage serum–free medium (Invitrogen Life Technologies) were harvested at various time points for total RNA extraction by use of TRIzol Reagent (Invitrogen Life Technologies). As described in our previous report [26], reverse transcription was performed on DNase-treated total RNA. The cDNA was synthesized from mRNA with oligo(dT)12–18 primer and Superscript II reverse transcriptase (Invitrogen Life Technologies). The cDNA samples were diluted (1:10) and used as template. Specific primers and probes ([26, 27] and table 1) were used in a real-time PCR assay, detected by use of an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). The standard curve was generated using serial dilution of plasmids (from ∼10 to 107 copies) containing the respective cloned gene targets. Viral M1 gene copies were quantified on the basis of a SYBR green fluorescence signal after PCR (forward primer, 5′-CTT CTA ACC GAG GTC GAA ACG-3′; reverse primer, 5′-GGC ATT TTG GAC AAA GCG TCT A-3′). Dissociation curve analysis was performed after each assay, to ensure specific target detection. To standardize results for variability in RNA and cDNA quantity, we expressed them as the number of target gene copies per 104 copies of β-actin
Table 1.
Primer and Taqman probe sequences used in real-time polymerase chain reaction assays
ELISA for supernatants from virus-infected MDMsSupernatants from 106 mock- or virus-infected MDMs per well incubated in a volume of 800 μL of RPMI 1640 plus 10% fetal bovine serum were collected after 6, 12, or 24 h and stored at −70°C; thawed; and irradiated at a dosage of 0.2 J/cm2 for 15 min in a UV crosslinker (Spectrolinker) before use. The concentrations of CCL3 and CCL5 in culture supernatants were measured by use of commercially available Duoset ELISA kits purchased from R&D Systems, in accordance with the manufacturer’s instructions. Each sample was assayed in duplicate. Samples were read at 450 nm, using a microplate reader (Bio-Rad), and were analyzed using Microplate Manager software (version 4.0; Bio-Rad)
Statistical analysisData are expressed as mean±SE. Statistical significance was determined by pair or nonpair nonparametric tests, using Instat software (version 3.05; GraphPad). P<.05 was considered to be significant
Results
Detection of similar infectivity and viral replication in adult and neonatal MDMs infected with human and avian influenza virusesDifferences in virulence between avian and human influenza viruses may be dependent on differences in cell tropism and in the kinetics of infection and replication. We first determined the infectivity of human and avian influenza viruses in adult and neonatal MDMs by immunofluorescence staining of viral NP. Similar to our previous findings in adult MDMs [15, 23], >90% of neonatal MDMs were infected at the 12-h POI—that is, within the first round of viral replication (figure 1)
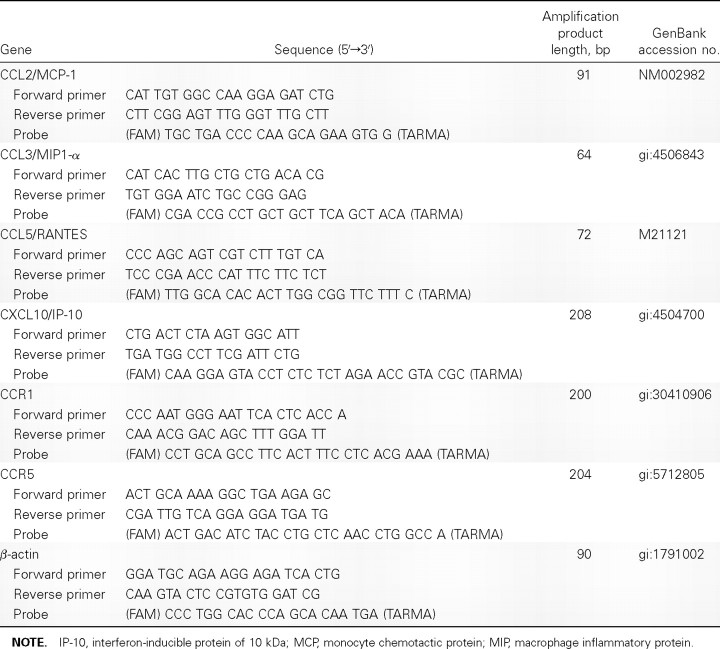
Figure 1.
Immunofluorescence staining of viral nucleoprotein (NP) in influenza virus–infected adult and neonatal monocyte-derived macrophages (MDMs). Day-14 differentiated MDMs were infected by influenza viruses H1N1/98, H9N2/G1, and H5N1/97 at an MOI of 2. At the 12-h point of infection, cells were fixed and analyzed by immunofluorescent staining specific for influenza A virus matrix protein and NP (green) with Evan’s blue (red) as a counterstaining. More than 90% of cells were infected
To quantify viral replication in the infected MDMs, we measured the copies of viral matrix (M1) gene at the 4-h and 7-h POIs. An increase in the number of viral M1 gene copies was observed in adult and neonatal MDMs between the 4-h and 7-h POIs (adult MDMs: H1N1, from 10,949.84±9211.48 to 48,618.35±27,473.55 copies; H9N2, from 8681.06±4066.48 to 49,205.68±11,589.67 copies; neonatal MDMs: H1N1, from 24,446.35±11,045.67 to 46,883.99±13,758.16 copies; H9N2, from 8111.12±3529.33 to 38,131.56±5455.63 copies; n=4). At the 7-h POI, similarly high numbers of M1 gene copies were found in both adult and neonatal MDMs infected with H1N1/98, H9N2/G1, or H5N1/97 (n=4; P>.05)
We then measured the titers of viruses released from H1N1/98- or H9N2/G1-infected adult and neonatal MDMs at the 24-h POI. The human and avian influenza viruses replicated to similar extents in adult and neonatal MDMs, and the virus titers were ∼104 TCID50/mL in the supernatants from MDMs infected with H1N1/98 or H9N2/G1 (n=3; P>.05)
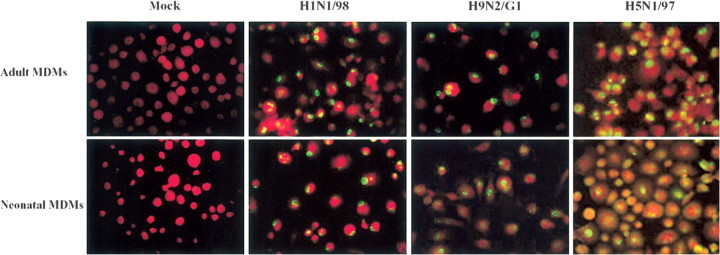
Up-regulation of CCL2, CCL3, CCL5, and CXCL10 mRNA expression in adult and neonatal MDMs infected with avian influenza virus H9N2/G1After viral infection, the expression of CCL2, CCL3, CCL5, and CXCL10 mRNA was induced and progressively increased with time (figure 2). In adult MDMs, avian influenza virus H9N2/G1 induced higher expression of CCL3, CCL5, and CXCL10 mRNA at all time points assessed (4-, 8-, and 12-h POIs) than that induced by human virus H1N1/98 (n=6; P<.01) (figure 2B–2D). However, neonatal MDMs showed chemokine responses to both H1N1/98 and H9N2/G1 that were similar in extent at early time points (4-h or 8-h POI), and only later did the difference in expression of these genes between them become significant (figure 2F–2H)
Figure 2.
Kinetics of CCL2, CCL3, CCL5, and CXCL10 mRNA expression in influenza virus–infected adult and neonatal monocyte-derived macrophages (MDMs). The graphs depict the kinetics of CCL2, CCL3, CCL5, and CXCL10 mRNA expression in mock-infected, H1N1/98-infected, and H9N2/G1-infected adult MDMs (A–D) and neonatal MDMs (E–H). After influenza virus infection, total RNA was harvested at the 4-h, 8-h, and 12-h points of infection (POIs). The target genes were quantified by quantitative reverse-transcription polymerase chain reaction and normalized to 104 copies of β-actin mRNA. The data shown are the mean±SE from 6 sets of independent experiments. In contrast to H1N1/98, avian influenza virus H9N2/G1 induced higher levels of CCL2, CCL3, CCL5, and CXCL10. *P<.05; **P<.01
Only at the 12-h POI was a significant difference in CCL2 expression between H9N2/G1- and H1N1/98-infected MDMs found (P=.0079 for adult MDMs [n=6; figure 2A]; P=.0429 for neonatal MDMs [n=6; figure 2E]). Higher expression of CCL2 and CCL3 was found in adult MDMs than in neonatal MDMs infected with H9N2/G1, although the difference did not reach statistical significance (figure 2A 2B 2E and 2F)
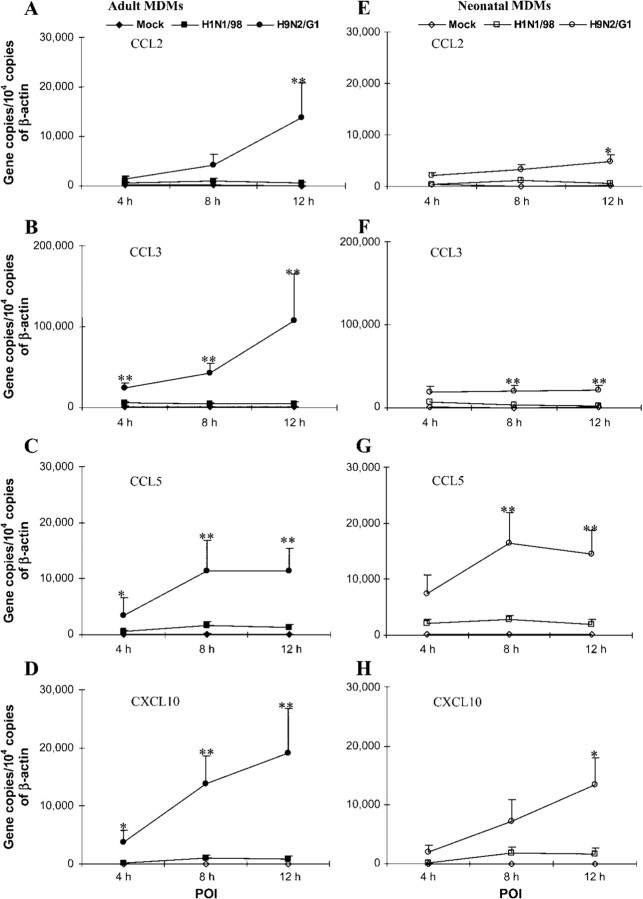
Significantly higher expression of CCL3 mRNA in adult MDMs than in neonatal MDMs infected with H5N1/97We subsequently repeated the above experiments on H5N1/97-infected MDMs in a Biosafety Level 3 laboratory. To compare the adult and neonatal MDM chemokine responses to H5N1/97 with those to human influenza virus H1N1/98, we analyzed our data as fold increases in chemokine mRNA expression, by dividing the number of normalized target-gene copies in H5N1/97-infected MDMs by the number in H1N1/98-infected MDMs. At the 7-h POI, the expression of CCL2, CCL3, CCL5, and CXCL10 mRNA was significantly up-regulated by avian influenza virus H5N1/97 (figure 3). Generally, there were greater fold increases in gene expression in H5N1/97-infected adult MDMs than in neonatal MDMs (adult MDMs: 18.75 ± 15.4, 25.67±11.62, 17.24±4.84, and 19.95±9.75; neonatal MDMs: 14.31±8.02, 5.66±1.30, 12.56±4.41, and 16.75 ± 4.99). Of note, a significantly greater increase in CCL3 expression was found in H5N1/97-infected adult MDMs than in neonatal MDMs (P=.0317; n=6) (figure 3)
Figure 3.
Increase in CCL2, CCL3, CCL5, and CXCL10 expression in H5N1/97-infected adult and neonatal monocyte-derived macrophages (MDMs), compared with that in H1N1/98-infected MDMs. After influenza virus infection, total RNA was harvested at the 7-h point of infection. The target genes were quantified by quantitative reverse-transcription polymerase chain reaction and normalized to 104 copies of β-actin mRNA. The fold increase is the normalized no. of target-gene copies in H5N1/97-infected MDMs divided by the no. in H1N1/98-infected MDMs. Significantly higher fold increases in CCL3 expression were detected in H5N1/97-infected adult MDMs than in neonatal MDMs. The data shown are the mean±SE from 6 sets of independent experiments. *P<.05
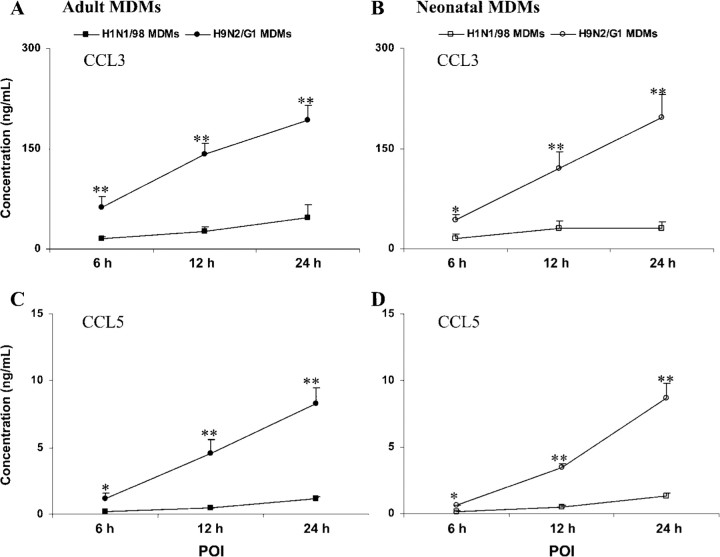
Higher production of CCL3 and CCL5 at an early stage of infection in adult MDMs than in neonatal MDMs infected with H9N2/G1 In the supernatants from mock-treated MDMs after 24 h, CCL3 production was <0.2 ng/mL (0.105±0.028 ng/mL for adult MDMs; 0.088±0.008 ng/mL for neonatal MDMs; n=4), and CCL5 was not detectable. In parallel with the gene expression, significantly higher production of CCL3 and CCL5 was found in both H1N1/98-infected and H9N2/G1-infected MDMs than in mock-infected MDMs (figure 4). Enhanced production of inflammatory chemokines CCL3 and CCL5 was detected early, at the 6-h POI, and the concentrations of these chemokines increased with time. At early time points (6-h and 12-h POIs), much higher concentrations of CCL3 and CCL5 were detected in H9N2/G1-infected adult MDMs than in neonatal MDMs. Compared with H1N1/98-infected MDMs, there was a 5–10-fold increase in CCL3 and CCL5 production in H9N2/G1-infected MDMs at the 24-h POI (CCL3 in adult MDMs, 192.73±21.65 ng/mL vs. 46.41±19.40 ng/mL; CCL3 in neonatal MDMs, 196.90±34.11 ng/mL vs. 30.59±9.21 ng/mL; CCL5 in adult MDMs, 8.31±1.17 ng/mL vs. 1.16±0.13 ng/mL; CCL5 in neonatal MDMs, 8.67±1.12 ng/mL vs. 1.31±0.23 ng/mL; P<.001; n=5)
Figure 4.
CCL3 and CCL5 produced in H1N1/98- and H9N2/G1-infected monocyte-derived macrophages (MDMs). The supernatants from H1N1/98- and H9N2/G1-infected MDMs were collected at the 6-h, 12-h, and 24-h points of infection (POIs) and assayed by ELISA, as described in Materials and Methods. At early time points (6-h and 12-h POIs) after infection with H9N2/G1, much higher concentrations of CCL3 and CCL5 were detected in adult MDMs than in neonatal MDMs. Compared with those from H1N1/98-infected MDMs, there were 5–10-fold increases in CCL5 and CCL3 concentrations in the culture supernatants from H9N2/G1-infected MDMs at the 24-h POI. The data shown are the mean±SE from 5 sets of independent experiments. *P<.05; **P<.01
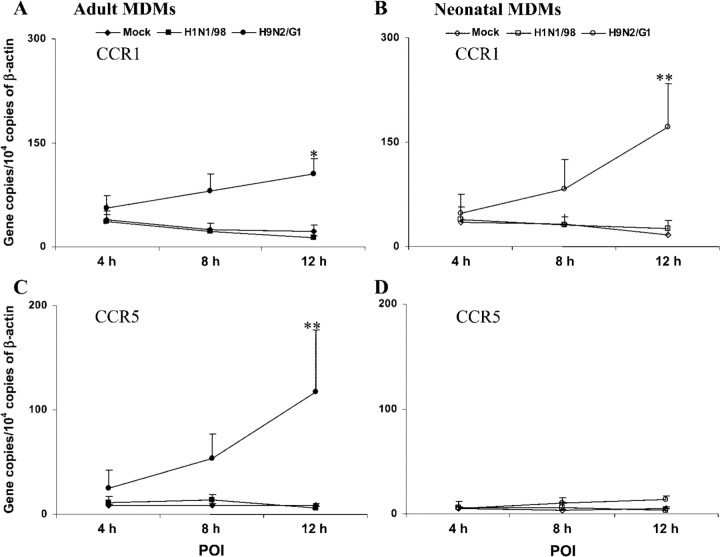
Differential CCR1 and CCR5 expression in adult and neonatal MDMs infected with H9N2/G1It has been reported that influenza A virus abolishes the functional responsiveness of monocytes via down-regulation of CCR2 expression but could induce a slight increase in CCR1 and CCR5 expression [8]. Virus-induced CCL5 and activation of CCR5 are required to prevent apoptosis of influenza virus–infected mouse macrophages in vivo and of human macrophages ex vivo [28]. Therefore, we determined whether the expression of chemokine receptors differed between virus-infected adult and neonatal MDMs. Chemokine receptors could bind several members of the same chemokine subgroup with high affinity. Both CCR1 and CCR5 could bind to CCL3 and CCL5 [1, 2, 4, 11]. As shown in figure 5, little change in CCR1 and CCR5 expression was found in H1N1/98-infected MDMs. At the 12-h POI, significantly higher expression of both CCR1 and CCR5 was detected in H9N2/G1-infected adult MDMs than in H1N1/98-infected adult MDMs (CCR1, P=.0025 [figure 5A]; CCR5, P=.0022 [figure 5C]; n=6). Similar to that in adult MDMs, CCR1 expression in H9N2/G1-infected neonatal MDMs displayed a gradual increase with time and, at the 12-h POI, was significantly higher than that in H1N1/98-infected neonatal MDMs (P=.0087; n=6) (figure 5B). However, there was little change in the expression of CCR5 in H1N1/98- or H9N2/G1-infected neonatal MDMs, and <20 gene copies/104 copies of β-actin were detected (figure 5D)
Figure 5.
CCR1 and CCR5 mRNA expression in influenza virus–infected adult and neonatal monocyte-derived macrophages (MDMs). The graphs depict the kinetics of CCR1 and CCR5 expression in H1N1/98- and H9N2/G1-infected adult MDMs (A and C) and neonatal MDMs (B and D). After influenza virus infection, total RNA was harvested at the 4-h, 8-h, and 12-h points of infection (POIs). The target genes were quantified by quantitative reverse-transcription polymerase chain reaction and normalized to 104 copies of β-actin mRNA. Significantly higher expression of both CCR1 and CCR5 were detected in H9N2/G1-infected adult MDMs than in H1N1/98-infected adult MDMs. Up-regulation of CCR1 expression, but not of CCR5 expression, was detected in H9N2/G1-infected neonatal MDMs. The data shown are the mean±SE from 6 sets of independent experiments. *P<.05; **P<.01
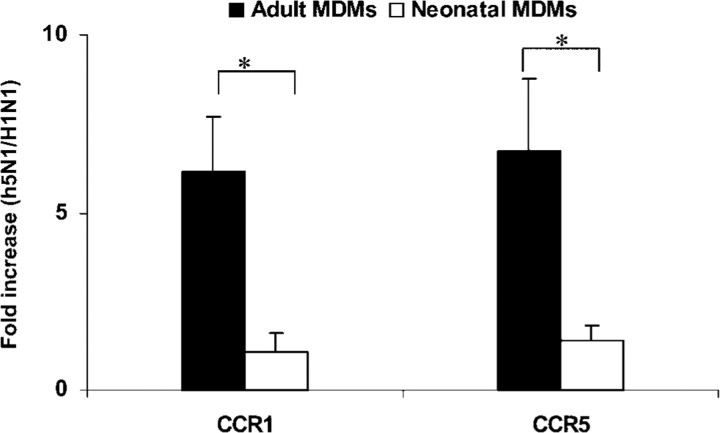
Enhanced CCR1 and CCR5 expression in H5N1/97-infected adult MDMsWe further compared the expression of CCR1 and CCR5 in H5N1/97-infected MDMs with that in H1N1/98-infected MDMs at the 7-h POI. The increase in CCR1 and CCR5 expression in H5N1/97-infected adult MDMs, compared with that in human virus H1N1/98–infected cells, was ∼6–7-fold. However, there was no increase seen in H5N1/97-infected neonatal MDMs, compared with H1N1/98-infected cells. Significantly higher fold increases in CCR1 and CCR5 expression were found in H5N1/97-infected adult MDMs than in neonatal MDMs (CCR1, P=.0223; CCR5, P=.0152; n=6) (figure 6)
Figure 6.
Enhanced CCR1 and CCR5 expression in H5N1/97-infected adult monocyte-derived macrophages (MDMs). After influenza virus infection, total RNA was harvested at the 7-h point of infection. The target genes were quantified by quantitative reverse-transcription polymerase chain reaction and normalized to 104 copies of β-actin mRNA. The fold increase is the normalized no. of target-gene copies in H5N1/97-infected MDMs divided by the no. in H1N1/98-infected MDMs. Higher fold increases in CCR1 and CCR5 expression were detected in H5N1/97-infected adult MDMs than in neonatal MDMs. The data shown are the mean±SE from 6 sets of independent experiments. *P<.05
Discussion
In this study, we have demonstrated that human MDMs have differential responses to human influenza virus H1N1/98 and avian viruses H9N2/G1 and H5N1/97, in spite of their similar infectivity and viral replication. Moreover, stronger chemokine and chemokine-receptor responses to avian influenza viruses were detected in adult MDMs than in neonatal MDMs
Consistent with the findings in other studies of chemokine responses to human influenza viruses [7, 8, 10], inflammatory CC chemokines and IFN-responsive chemokines were induced by avian influenza viruses in our experiments. Furthermore, significantly higher expression of CCL2, CCL3, CCL5, and CXCL10 was found in response to avian influenza viruses, compared with human influenza viruses (figures 2 and 4). Expression of these chemokines is also up-regulated in other viral infections, such as lymphocyte choriomeningitis virus, West Nile virus, respiratory syncytial virus, and hepatitis C virus infections [29–32 ]. The chemokine-dependent trafficking of monocytes/macrophages, dendritic cells, NK cells, and lymphocytes between the site of infection, the circulation, and the draining lymph nodes provides protective immunity against viral infections. Paradoxically, the same chemokines could exacerbate virus-induced inflammation and pathology via exaggerated cell-mediated responses [1, 2, 31, 32]. Therefore, the rapid and strong chemokine response detected in both adult and neonatal MDMs infected with avian influenza viruses may partially explain the greater clinical severity observed
The kinetics of chemokine production in avian influenza virus H9N2/G1–infected MDMs suggested that the CCL3 and CCL5 responses were stronger in adult MDMs than in neonatal MDMs at an early stage (figure 4). Strikingly, the fold increase in CCL3 expression in H5N1/97-infected adult MDMs was significantly higher than that in neonatal MDMs (figure 3). The detrimental effects of CCL3 have been widely studied in viral infections. Higher CCL3, interleukin (IL)–6, IL-8, and IL-1 levels have been found in plasma from patients with fatal H5N1 infection than in plasma from survivors [14]. Elevated CCL3 and IFN-γ levels have been detected in inflammatory tissues from patients with infection-induced hemophagocytic syndrome [33]. Additionally, levels of CCL3, but not of other CC chemokines (CCL2 and CCL5), have been found to be higher in nasopharyngeal secretions from infants with more-severe hypoxic bronchiolitis due to influenza virus [34]. It has been found that less mononuclear cell infiltration and pulmonary edema occurs in gene-deficient CCL3−/− mice than in wild-type mice during respiratory virus infection [35, 36]. The higher CCL3 response in adult MDMs may be one of the important factors responsible for the age-related severity of avian influenza virus infection in 1997
Both CCR1 and CCR5 are able to bind CCL3. The process by which human monocytes mature into tissue macrophages is accompanied by a loss in CCR2 expression, an increase in expression of CCR1 and CCR5, and an enhanced functional response to CCL3 [37, 38]. During influenza virus infection, CCL3 may primarily function through CCR1 or CCR4, and its interaction with CCR5 is limited [39]. Moreover, activation of CCR1 is an early event in the systemic inflammatory response and occurs in various cell types [40, 41]. The trafficking of myeloid cells and neutrophils is disordered in CCR1−/− mice [41]. Improved survival was detected in gene-deleted CCR1−/− mice infected with pneumonia virus, compared with that in wild-type mice [42]. We detected the up-regulation of both CCR1 and CCR5 expression in avian virus H9N2/G1–infected adult MDMs; however, only CCR1 expression was up-regulated in H9N2/G1-infected neonatal MDMs (figure 5). These results suggested that the activation of CCR1 may be the major response in macrophages to avian influenza virus. In contrast to H5N1/97-infected adult MDMs, which had a several-fold increase in CCR1 and CCR5 production, H5N1/97-infected neonatal MDMs had a minimal fold increase of CCR1 and CCR5 production at the 7-h POI (figure 6). The difference may be explained by delayed chemokine-receptor activation of neonatal MDMs in response to H5N1/97 virus, compared with that of adult MDMs, or by an intrinsically weaker response in neonatal MDMs. The latter is supported by the observation that there is an age-related increase of CCR5 expression in monocytes, lymphocytes, and NK cells [43]
Via chemokine receptor CCR5, chemokines such as CCL3, CCL4, CCL5, CCL6, and CCL8 exert their biological functions. Recently, it has been reported that virus-induced CCL5 (but not CCL3 or CCL4) and activation of CCR5 (but not CCR1 or CCR3) are required to prevent apoptosis of influenza virus–infected mouse macrophages in vivo and human macrophages ex vivo [28]. As is shown in our study, significantly higher expression of CCL5 was detected in avian influenza virus–infected human MDMs than in human influenza virus H1N1/98–infected MDMs (figures 2, 3, and 4). In human patients and mouse models, it has been found that the replication of H5N1 virus or H5N1 nonstructural gene reassortant virus is prolonged [14, 44]. We have recently demonstrated that lesser cytopathogenic effects were caused by avian influenza viruses H9N2/G1 and H5N1/97 in Jurkat T cells [23] and other cell types, such as dendritic cells (data not shown), compared with human virus H1N1/98. The lesser cytopathogenic effects or lower percentages of apoptosis in avian influenza virus–infected cells may contribute to the prolonged viral replication, and this process may be a result of the antiapoptotic effects of CCL5 and CCR5
H9N2/G1 and H5N1/97 share 6 internal genes [17]. The fact that enhanced chemokine and chemokine-receptor responses were induced by both avian viruses suggested that these viral genes or their proteins may be involved in the induction of responses. More recently, mice infected with the reconstructed virus bearing hemagglutinin and/or neuraminidase of the 1918 Spanish influenza virus also showed significantly enhanced production of tumor necrosis factor–α, CCL3, CXCL2, and IFN-γ, as well as high mortality [45, 46]. Since the amino acid changes detected in polymerase PA, PB1, and PB2 of the 1918 Spanish influenza virus were also detected in H5N1 and H9N2 viruses [47], further investigation is needed to determine their roles
Altogether, our findings suggest that there is similar infectivity and viral replication but different chemokine and chemokine-receptor responses of human MDMs to human and avian influenza viruses. In general, the chemokine and chemokine-receptor responses of MDMs to avian influenza viruses were much stronger than those to human virus, which may account for the high pathogenicity of avian viruses. There were specific differences in responses—for example, greater CCL3, CCR1, and CCR5 expression—in avian influenza virus H5N1/97–infected adult MDMs, compared with those in neonatal MDMs. These data suggest that host factors may influence the disease process or outcome
Acknowledgments
We thank all of the staff in the Labour Ward of Queen Mary Hospital for their assistance in collecting the cord blood, as well as all of the blood donors and their families
Footnotes
Potential conflicts of interest: none reported
Financial support: Research Grants Council of the Hong Kong Special Administrative Region, China (project HKU 7532/05M); University of Hong Kong (Outstanding Researcher Awards to Y.L.L. and J.S.M.P. and Postgraduate Studentship to J.Z.)
References
- 1.Mahalingam S, Karupiah G. Chemokines and chemokine receptors in infectious diseases. Immunol Cell Biol. 1999;77:469–75. doi: 10.1046/j.1440-1711.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- 2.Maghazachi AA. Chemokines, G proteins and natural killer cells. Arch Immunol Ther Exp (Warsz) 2000;48:65–72. [PubMed] [Google Scholar]
- 3.Glass WG, Rosenberg HF, Murphy PM. Chemokine regulation of inflammation during acute viral infection. Curr Opin Allergy Clin Immunol. 2003;3:467–73. doi: 10.1097/00130832-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Mahalingam S, Friedland JS, Heise MT, Rulli NE, Meanger J, Lidbury BA. Chemokines and viruses: friends or foes? Trends Microbiol. 2003;11:383–91. doi: 10.1016/s0966-842x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Fujisawa H, Tsuru S, Taniguchi M, Zinnaka Y, Nomoto K. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J Gen Virol. 1987;68(Pt 2):425–32. doi: 10.1099/0022-1317-68-2-425. [DOI] [PubMed] [Google Scholar]
- 7.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76:886–95. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 8.Salentin R, Gemsa D, Sprenger H, Kaufmann A. Chemokine receptor expression and chemotactic responsiveness of human monocytes after influenza A virus infection. J Leukoc Biol. 2003;74:252–9. doi: 10.1189/jlb.1102565. [DOI] [PubMed] [Google Scholar]
- 9.Peiris JS, Yu WC, Leung CW, et al. Re‐emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–9. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus‐induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12:171–80. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 11.Luster AD. Chemokines‐chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 12.Hennet T, Ziltener HJ, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–9. [PubMed] [Google Scholar]
- 13.Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 14.Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 15.Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–7. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 16.Peiris M, Yuen KY, Leung CW, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 17.Chin PS, Hoffmann E, Webby R, et al. Molecular evolution of H6 influenza viruses from poultry in southeastern China: prevalence of H6N1 influenza viruses possessing seven A/Hong Kong/156/97 (H5N1)‐like genes in poultry. J Virol. 2002;76:507–16. doi: 10.1128/JVI.76.2.507-516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu E, Law HK, Lau YL. Tolerance associated with cord blood transplantation may depend on the state of host dendritic cells. Br J Haematol. 2004;126:517–26. doi: 10.1111/j.1365-2141.2004.05061.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu EM, Tu W, Law HKW, Lau YL. Decreased yield, phenotypic expression and function of immature monocyte‐derived dendritic cells in cord blood. Br J Haematol. 2001;113:240–6. doi: 10.1046/j.1365-2141.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 20.Tu W, Cheung PT, Lau YL. Insulin‐like growth factor 1 promotes cord blood T cell maturation and inhibits its spontaneous and phytohemagglutinin‐induced apoptosis through different mechanisms. J Immunol. 2000;165:1331–6. doi: 10.4049/jimmunol.165.3.1331. [DOI] [PubMed] [Google Scholar]
- 21.Tu W, Cheung PT, Lau YL. IGF‐I increases interferon‐gamma and IL‐6 mRNA expression and protein production in neonatal mononuclear cells. Pediatr Res. 1999;46:748–54. doi: 10.1203/00006450-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Tu W, Zhang DK, Cheung PT, Tsao SW, Lau YL. Effect of insulin‐like growth factor 1 on PHA‐stimulated cord blood mononuclear cell telomerase activity. Br J Haematol. 1999;104:785–94. doi: 10.1046/j.1365-2141.1999.01272.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Law HKW, Cheung CY, et al. Functional tumor necrosis factor–related apoptosis‐inducing ligand production by avian influenza virus–infected macrophages. J Infect Dis. 2006;193:945–53. doi: 10.1086/500954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirst GK. Adsorption of influenza hemagglutinins and virus by red blood cells. J Exp Med. 1942;76:195–209. doi: 10.1084/jem.76.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payment P, Trudel M. Isolation and identification of viruses. In: Payment P, Trudel M, editors. Methods and techniques in virology. New York: Marcel Dekker; 1993. pp. 19–38. [Google Scholar]
- 26.Law HK, Cheung CY, Ng HY, et al. Chemokine up‐regulation in SARS‐coronavirus‐infected, monocyte‐derived human dendritic cells. Blood. 2005;106:2366–74. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte‐derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol. 2004;173:189–96. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 28.Tyner JW, Uchida O, Kajiwara N, et al. CCL5‐CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med. 2005;11:1180–7. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asensio VC, Campbell IL. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J Virol. 1997;71:7832–40. doi: 10.1128/jvi.71.10.7832-7840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirato K, Kimura T, Mizutani T, Kariwa H, Takashima I. Different chemokine expression in lethal and non‐lethal murine West Nile virus infection. J Med Virol. 2004;74:507–13. doi: 10.1002/jmv.20205. [DOI] [PubMed] [Google Scholar]
- 31.John AE, Berlin AA, Lukacs NW. Respiratory syncytial virus induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur J Immunol. 2003;33:1677–85. doi: 10.1002/eji.200323930. [DOI] [PubMed] [Google Scholar]
- 32.Nischalke HD, Nattermann J, Fischer HP, Sauerbruch T, Spengler U, Dumoulin FL. Semiquantitative analysis of intrahepatic CC‐chemokine mRNAs in chronic hepatitis C. Mediators Inflamm. 2004;13:357–9. doi: 10.1155/S0962935104000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teruya‐Feldstein J, Setsuda J, Yao X, et al. MIP‐1alpha expression in tissues from patients with hemophagocytic syndrome. Lab Invest. 1999;79:1583–90. [PubMed] [Google Scholar]
- 34.Garofalo RP, Hintz KH, Hill V, Patti J, Ogra PL, Welliver RC., Sr A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J Med Virol. 2005;75:282–9. doi: 10.1002/jmv.20268. [DOI] [PubMed] [Google Scholar]
- 35.Cook DN, Beck MA, Coffman TM, et al. Requirement of MIP‐1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–5. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 36.Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. The chemokine macrophage‐inflammatory protein‐1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J Immunol. 2000;165:2677–82. doi: 10.4049/jimmunol.165.5.2677. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann A, Salentin R, Gemsa D, Sprenger H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP‐1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol. 2001;69:248–52. [PubMed] [Google Scholar]
- 38.Zylla D, Li Y, Bergenstal E, et al. CCR5 expression and beta‐chemokine production during placental neonatal monocyte differentiation. Pediatr Res. 2003;53:853–8. doi: 10.1203/01.PDR.0000059749.82140.4A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–9. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su SB, Mukaida N, Wang J, Nomura H, Matsushima K. Preparation of specific polyclonal antibodies to a C‐C chemokine receptor, CCR1, and determination of CCR1 expression on various types of leukocytes. J Leukoc Biol. 1996;60:658–66. doi: 10.1002/jlb.60.5.658. [DOI] [PubMed] [Google Scholar]
- 41.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12:313–35. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 42.Bonville CA, Lau VK, DeLeon JM, et al. Functional antagonism of chemokine receptor CCR1 reduces mortality in acute pneumovirus infection in vivo. J Virol. 2004;78:7984–9. doi: 10.1128/JVI.78.15.7984-7989.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shalekoff S, Gray GE, Tiemessen CT. Age‐related changes in expression of CXCR4 and CCR5 on peripheral blood leukocytes from uninfected infants born to human immunodeficiency virus type 1‐infected mothers. Clin Diagn Lab Immunol. 2004;11:229–34. doi: 10.1128/CDLI.11.1.229-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipatov AS, Andreansky S, Webby RJ, et al. Pathogenesis of Hong Kong H5N1 influenza virus NS gene reassortants in mice: the role of cytokines and B‐ and T‐cell responses. J Gen Virol. 2005;86:1121–30. doi: 10.1099/vir.0.80663-0. [DOI] [PubMed] [Google Scholar]
- 45.Kobasa D, Takada A, Shinya K, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–7. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 46.Tumpey TM, Garcia‐Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:889–93. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]