Abstract
In 1972, a 27-nm virus-like particle was discovered by use of immune electron microscopy (IEM) in an infectious stool filtrate derived from an outbreak of gastroenteritis in an elementary school in Norwalk, Ohio. IEM enabled the direct visualization of antigen-antibody interaction, as the particles were aggregated and coated by specific antibodies. This allowed the recognition and identification of a 27-nm virus-like particle that did not have a distinctive morphology, was low-titered, and was among the smallest viruses known. Serum antibody responses to the 27-nm particle were demonstrated in key individuals infected under natural or experimental conditions; this and other evidence suggested that this virus-like particle was the etiologic agent of the Norwalk gastroenteritis outbreak. The fastidious 27-nm Norwalk virus is now considered to be the prototype strain of a group of noncultivatable viruses that are important etiologic agents of epidemic gastroenteritis in adults and older children.
I was invited to present my reflections on the discovery of the Norwalk virus. This is a personal odyssey that chronicles the steps in making the arduous leap from the discovery of a 27-nm virus-like particle to its etiologic association with epidemic gastroenteritis. I will describe how, by necessity, we bypassed the classical tissue-culture virology approach, which relies on the ability of a virus to infect and produce a change in cells or to infect an animal model. Rather, we used a novel approach—“direct virology” or “particle virology”—in which the virus particle itself is studied directly as the “center of attention” without the benefit of an in vitro or animal model system.
Rationale for the Search for a Cause of Viral Gastroenteritis
The goals of the Laboratory of Infectious Diseases (LID) at the National Institutes of Health (NIH) traditionally have focused on the definition of the natural history and epidemiologic characteristics of a disease, the elucidation of its etiologic agent, and the development of a vaccine for its prevention. With the termination in 1969 of the longitudinal Junior Village study of infants and young children, which for 15 years had encompassed each of these goals, the emphasis of the Epidemiology Section of the LID turned to the study of the etiology of acute nonbacterial (viral) gastroenteritis. The Junior Village study generated seminal information on the epidemiology of respiratory and enteric infections and led to the discovery of many respiratory and enteric viruses [1]. Although nonbacterial diarrheal illnesses had occurred frequently in this study and many enteric viruses were readily recovered in tissue culture, none emerged as etiologic agents of diarrhea. The search for a viral etiologic agent for acute gastroenteritis began in the late 1960s and was intensified in the early 1970s. The search for a viral agent was based on the rationale that (1) the etiology of most episodes of infectious gastroenteritis among pediatric and adult populations was unknown [2, 3]; (2) it was assumed that viruses were important in these outbreaks because bacteria were associated etiologically only infrequently [2, 3]; (3) bacteria-free stool filtrates induced gastroenteritis in adult volunteer studies [4–12]; and (4) new techniques, such as organ culture, that might enable the cultivation of a fastidious etiologic agent had become available.
Early Transmission Studies in Volunteers
Bacteria-free filtrates derived from naturally occurring outbreaks of gastroenteritis in the United States and Japan were successfully used to transmit infection to adult volunteers, providing particularly strong evidence of a viral etiology for gastroenteritis. A brief survey of volunteer studies done in the 1940s and 1950s demonstrates the intensive efforts to detect an etiologic agent. Reimann et al. [4] induced gastroenteritis by administering aerosolized bacteria-free throat washings or fecal suspensions from persons in a gastroenteritis outbreak. Gordon et al. [5] induced an afebrile diarrheal illness following oral administration of pooled bacteria-free fecal filtrates or throat washings from patients in an outbreak at Marcy State Hospital (located near Utica, NY). This agent, the Marcy strain, was passaged serially seven additional times in volunteers [5–8]. Short-term (several weeks) and longer-term (9–15 months) immunity was described in rechallenge studies [5–8].
Kojima et al. [9] induced gastroenteritis following oral administration of bacteria-free fecal filtrates derived from ill individuals in Niigata Prefecture and other Japanese prefectures [9]. Serial passage in volunteers was successful, and short-term immunity was shown with a single strain. Yamamoto et al. [10] induced gastroenteritis following oral administration of bacteria-free filtrates from gastroenteritis patients in the Gumma Prefecture outbreak. Jordan et al. [11] induced gastroenteritis following oral administration of a bacteria-free filtrate from a gastroenteritis patient in the Cleveland Family Study (FS strain). It was serially passaged in volunteers, and cross-challenge studies with the Marcy and FS strains indicated that these agents were antigenically distinct. Fukumi et al. [12] induced gastroenteritis following intraduodenal administration of the Marcy strain. Challenge of these volunteers 2 months later with the Niigata strain by the same route did not induce illness, suggesting that the 2 strains were antigenically related.
Attempts to Detect a Virus Associated with Gastroenteritis by Tissue-Culture Techniques
Although known infectious filtrates were available from these studies, all attempts to identify an etiologic agent using newly available tissue-culture techniques were unsuccessful. Similarly, studies of numerous outbreaks of naturally occurring gastroenteritis consistently failed to reveal an etiologic agent even during the “golden age” of virology in the 1950s and 1960s, when the use of tissue culture led to the discovery of scores of new cultivatable viruses, such as the ECHO and coxsackie-viruses, many of which grew to high titer in the enteric tract [2–3].
Attempts to Detect a Virus Associated with Gastroenteritis by Novel Organ Culture Techniques
During the late 1960s, new techniques were developed for the detection of fastidious viruses. These included the use of human embryonic nasal or tracheal organ culture, which preserved cells in their normal state of differentiation and architecture. Organ culture was used successfully for the discovery of several new respiratory coronaviruses that did not grow in conventional tissue cultures [13, 14]. The success in growing fastidious coronaviruses in organ culture stimulated renewed efforts to cultivate the elusive agents of “viral” gastroenteritis; human fetal intestinal organ cultures were established in an effort to find a method to support the growth of a heretofore noncultivatable gastroenteritis virus [15, 16]. However, the organ culture technique (as well as standard tissue-culture techniques) also failed to yield an etiologic agent [17]. Although the study specimens that were tested were derived from individuals with nonbacterial gastroenteritis, there was no practical way of knowing whether they contained infectious material that was capable of producing disease. It was possible that the inability to detect a virus resulted from the absence of an infectious agent in the test specimen. Fecal specimens of known infectivity from early volunteer studies described above could not be accessed because they were either not available at the time or had been exhausted.
Later Transmission Studies of Gastroenteritis in Volunteers
In the early 1970s, a second generation of volunteer studies was initiated, using newer techniques, in the United States and the United Kingdom in hopes of identifying known disease-producing infectious fecal suspensions [18, 19]. In 1970, fecal filtrates from four separate gastroenteritis outbreaks were studied in groups of 3–4 volunteers [18]. One of the four outbreaks took place in an elementary school in Norwalk, Ohio, in October 1968, and was investigated by the Centers for Disease Control [20]. During a 2-day period, 50% of the students and teachers (116/232) developed a gastrointestinal illness, and there was a secondary attack rate of 32% among contacts of primary cases. The incubation period was ∼48 h, and the illness, which lasted ∼24 h, was described as “winter vomiting disease” because it was reminiscent of the syndrome first described by Zahorsky in 1929 [21]. Indeed, the prominent clinical manifestations in the Norwalk outbreak were vomiting and nausea, although some of the patients developed diarrhea. Laboratory studies did not reveal an etiologic agent.
A rectal swab specimen from a secondary case in the Norwalk outbreak was prepared as a 2% filtrate and administered orally to 3 volunteers, 2 of whom developed gastroenteritis [18]. The agent was passaged serially to other volunteers, and the biological characteristics relating to size (66 nm) and its acid, ether, and relative heat stability were determined using the ability to induce illness in volunteers as the indicator system [22]. However, attempts to identify an etiologic agent in cell or organ culture were again unsuccessful, and studies in various animals, including monkeys, failed to yield a virus.
In the volunteer studies in the United Kingdom, material from three gastroenteritis outbreaks was administered to volunteers, and one (the “W” agent) was studied extensively because it produced gastroenteritis [19]. The agent was determined to be ether stable and <50 nm in size, as judged from volunteer studies; however, attempts to cultivate a virus in tissue culture and organ culture were unsuccessful.
The Rationale for Using Immune Electron Microscopy (IEM) for Detection of Fastidious Viruses
An unanticipated course of events influenced the ultimate progress of the gastroenteritis program at NIH. In 1970, I spent 6 months in Anthony Waterson's Department of Virology at the Royal Postgraduate Medical School of the University of London to learn electron microscopy under the tutelage of June Almeida, an outstanding electron microscopist and a pioneer in the application of IEM to virology. This technique, a method defined as the direct observation of antigen-antibody interaction [23], was not new—it had been described in 1941 in studies with tobacco mosaic virus [24, 25]. Even with the development of electron microscopes with increased resolving power and the introduction of negative-staining techniques, which greatly enhanced contrast, thus facilitating the recognition of viruses [26], the application of IEM was underutilized. We examined human coronaviruses by IEM to visualize the formation of complement holes in the envelope following incubation with uninactivated serum, as previously described for an avian coronavirus [23]. We also examined rhinovirus preparations by IEM in an attempt to visualize these 27-nm viruses clearly because they did not grow to high titer, were of rather small size (even for a virus), and did not have a distinctive morphologic appearance [27].
The power of this technique was shown clearly in these rhinovirus studies, in which a relatively low-titered tissue-culture suspension of rhinovirus 1A was reacted with a specific goat serum or a control (PBS). The mixture was then centrifuged, and the pellet was reconstituted with distilled water and stained with phosphotungstic acid [27]. Examination of the control preparation revealed scattered, randomly distributed, 27-nm particles, some of which could not be identified conclusively as a virus (figure 1). However, in the virus-serum preparation, the 27-nm rhinovirus particles were no longer randomly distributed but appeared in the form of large and small aggregates coated with antibody and standing out clearly from the background, leaving no doubt that they were virus particles (figure 2). These observations had a major impact on the course of my future research as I realized that IEM might enable the detection of fastidious viruses that do not grow in tissue culture. Key to this concept was the realization that although hyperimmune serum would not be available for enabling the detection of a noncultivatable unknown agent, convalescent sera could be used for screening as it would provide the specificity needed to detect a putative viral agent.
Figure 1.

Single particle of rhinovirus 1A from control preparation in which rhinovirus 1A suspension was incubated with PBS prior to further preparation for electron microscopy. Particles were randomly distributed, and it was difficult to determine whether certain objects were virus particles. Bar = 100 nm. (From Kapikian et al. [27], bar added.)
Figure 2.

Micrograph illustrating large, distinctive aggregate of rhinovirus particles in rhinovirus 1A suspension, which was incubated with 1 : 180 dilution of rhinovirus 1A goat antiserum prior to further preparation for electron microscopy. Bar = 100 nm. (From Kapikian et al. [27], bar added.)
Discovery of the Norwalk Virus
Shortly after my return to NIH, I began to search for respiratory coronaviruses in harvests of human embryonic tracheal organ cultures inoculated with nasal wash specimens from an NIH common-cold study [28]. I used convalescent sera from the patient as the source of antibody in order to facilitate or enable the visualization of a low-titered coronavirus preparation [29]. In this way, we identified a new coronavirus strain (no. 692) that was distinct by IEM from the 2 most completely characterized human coronavirus strains, OC 43 and 229E.
Because of the failure to cultivate virus from infectious stool material from ill volunteers from the Norwalk outbreak, I extended the IEM studies [30] and examined the Norwalk agent stool filtrates, using a volunteer's convalescent serum as the source of antibody [31]. In June 1972, almost 20 months after beginning such studies, I examined a Norwalk agent stool filtrate (designated 8FIIa) derived from a volunteer who became ill after oral administration of the Norwalk agent [31]. This filtrate was known to contain an infectious agent because it had induced a diarrheal illness in 6 of 10 volunteers. Following incubation of the stool filtrate with a volunteer's convalescent serum and further preparation for electron microscopy, glistening aggregates of nonenveloped, antibody-coated 27-nm, virus-like particles, which resembled rhinoviruses, were visualized. A characteristic aggregate observed in early experiments is shown in figure 3A. The visualization of virus-like particles was very promising, but it was clear that further studies were needed to determine the significance of this finding. Had the incubation of the Norwalk agent stool filtrate with convalescent serum merely facilitated the detection by IEM of an adventitious virus that had no relationship to the Norwalk outbreak?
Figure 3.

A, An aggregate observed after incubation of 0.8 mL of Norwalk (8FIIa) stool filtrate with 0.2 mL of 1 : 5 dilution of prechallenge serum of volunteer A and further preparation for electron microscopy. Quantity of antibody on these glistening particles was rated as 1+. B, Aggregate observed after incubation of 0.8 mL of Norwalk (8FIIa) stool filtrate with 1 : 5 dilution of postchallenge serum from volunteer B and further preparation for electron microscopy. Particles were very heavily coated with antibody. Heavily coated particles were usually found in small aggregates, whereas those with less antibody were usually in larger aggregates. Quantity of antibody on these particles was rated as 4+. Bar = 100 nm and applies to both A and B. (From Kapikian et al. [31], bar added.)
It was essential to determine if the volunteers who became ill following challenge with the Norwalk agent had developed a serologic response to the 27-nm particle visualized by IEM. How could this be done with a particle that could not be cultivated? An antibody-rating system was developed, in which a low dilution of pre- or postchallenge serum (1 : 5) was reacted with the Norwalk agent (8FIIa) stool filtrate for examination by IEM: The amount of antibody coating the particles was rated on a 0–4+ scale, with 4+ being a very heavy coating of antibody, which almost obscured the particle, and 3+, 2+, or 1+ indicating lesser amounts of antibody. A 1+ difference in antibody rating was a significant seroresponse. An example of a 1+ rating is shown in figure 3A and a 4+ rating in figure 3B. Crucial to this rating system was the requirement that the ratings be made under code to assure objectivity. An example of a significant seroresponse using a volunteer's pre- or post-challenge serum from a later study with the Norwalk virus is shown in figure 4.
Figure 4.

Aggregate observed after incubation of 0.8 mL of Norwalk stool filtrate with 0.2 mL of 1 : 5 dilution of volunteer's prechallenge serum and further preparation for electron microscopy. Volunteer developed gastroenteritis after challenge with 2d-passage Norwalk filtrate, which had been heated for 30 min at 60°C [22]. Quantity of antibody on particles in this aggregate was rated 1-2-2+, and prechallenge serum was given overall rating of 1–2+. Bar = 100 nm and applies to entire figure. B, Single particle. C, 3 single particles observed after incubating 0.8 mL of Norwalk stool filtrate with 0.2 mL of 1 : 5 dilution of same volunteer's convalescent serum and further preparation for electron microscopy. These particles are heavily coated with antibody. Quantity of antibody on these particles was rated 4+, and serum was given overall rating of 4+ also. Difference in quantity of antibody coating particles in prechallenge and postchallenge sera is clearly evident. (From Kapikian et al. [30], bar added.)
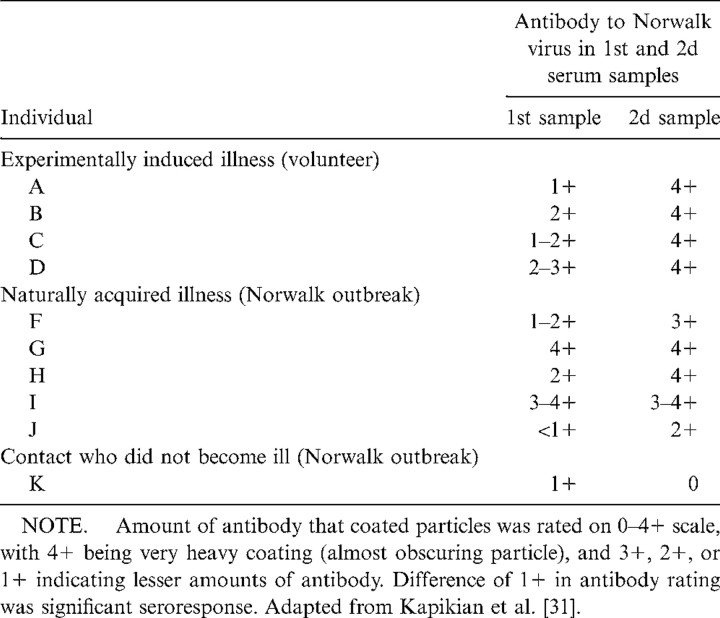
Each of the 4 volunteers who became ill after challenge with the second human-passaged Norwalk virus stool filtrate (8FII) developed a seroresponse to the newly discovered 27-nm Norwalk particle (table 1). A fifth volunteer, who did not become ill, did not develop a significant seroresponse (data not shown). Although these findings moved us closer to an etiologic association, another step in establishing causation was necessary. Could the 27-nm virus-like particle be an adventitious virus that was present in the Norwalk stool filtrate and, thus, had merely infected each volunteer nonspecifically and was unrelated to the illness? The adventitious virus could have been a contaminant in the original rectal swab specimen (along with the real Norwalk virus) or could have been picked up during passage in volunteers.
Table 1.
Antibody responses to the 27-nm Norwalk virus, as determined by immune electron microscopy.
We examined this possibility by testing paired acute and convalescent sera from patients who developed naturally occurring illness during the outbreak in Norwalk, Ohio [31]. Four of the paired sera were obtained from primary cases, 1 from a secondary case (“J”) and 1 from a contact who did not become ill (table 1). This was a crucial experiment because it would be highly unlikely that the newly discovered 27-nm particle was an adventitious agent if the naturally occurring cases developed an antibody response to it. We found that 3 of the 5 individuals who had become ill developed a significant antibody response to the 27-nm Norwalk particle. The 2 who did not develop such a response had a high level of antibody to the 27-nm particle in both acute and convalescent sera, most likely a reflection of the time of acquisition of the acute phase sera, probably several days after the onset of disease. In addition, the contact who did not become ill did not develop a seroresponse. The demonstration of an antibody response in 3 of 5 individuals who developed illness under natural conditions, independently of the responses observed in the challenged volunteers, was a major link in establishing the chain of causation. One of the 3 (“J”) who developed a seroresponse was the donor of the original rectal swab specimen from which the 27-nm particle was derived. In addition, 1 of the 2 volunteers who became ill after challenge with the original rectal swab specimen developed a seroresponse and the other did not (data not shown) [31].
Although the evidence for an etiologic association was becoming quite convincing, there was an additional obstacle to consider. Was it possible that gastroenteritis induced a nonspecific antibody response, thereby negating the presumed importance of demonstrating a seroresponse to the 27-nm particle under experimental and natural conditions? We examined this possibility by examining serum specimens from volunteers who had become ill after challenge with stool filtrates derived from other outbreaks of gastroenteritis not related to the Norwalk outbreak—one in Hawaii and the other in Maryland (Montgomery County). We tested sequential sera from 2 volunteers who underwent three successive challenges. The first volunteer was challenged three times—the first and second times with the Hawaii agent and a third time with the Norwalk agent. This volunteer became ill after the first and third challenges, suggesting that the Hawaii and Norwalk agents were unrelated. Examination of 5 sequential serum samples by IEM revealed that this volunteer did not develop a seroresponse to the Norwalk virus after the first or second challenge with the Hawaii agent but did develop a seroresponse to the Norwalk virus after the third challenge [31].
The second volunteer also underwent three sequential challenges—the first and second with the Maryland agent and then a third with the Norwalk agent. This volunteer became ill after the first and third challenges also. Five sequential sera were tested by IEM as above. The volunteer developed a significant antibody response to the Norwalk virus after the first challenge but not after the second and developed a further increase in antibody to the Norwalk virus after the third challenge. The Norwalk virus and the Maryland agent appeared to be anti-genically related. Thus, it appeared from these sequential challenges that antibody responses detected to the 27-nm Norwalk particle after experimentally or naturally induced infection were specific [31].
Because of the evidence outlined above, we were satisfied that the major obstacles to establishing an etiologic association between the Norwalk virus particle and the Norwalk outbreak had been addressed. Thus, we stated in our abstract of the original report that “A 27 nm particle was observed by immune electron microscopy in an infectious stool filtrate derived from an outbreak in Norwalk, Ohio, of acute infectious nonbacterial gastroenteritis. Both experimentally and naturally infected individuals developed serological evidence of infection; this along with other evidence suggested that the particle was the etiological agent of Norwalk gastroenteritis” [31].
Further Role of IEM in Direct or Particle Virology
After the discovery of the Norwalk virus, the IEM technique proved to be important in its characterization. For example, the density of the virus in CsCl [32] and the pattern of virus shedding during illness [33] was determined. In addition, IEM was used for the discovery of other 27-nm gastroenteritis viruses, such as the Hawaii virus in 1977 [34] and the Snow Mountain virus in 1982 [35]. Moreover, in 1973, with a similar type of analysis, IEM was key for the discovery of the 27-nm hepatitis A virus [36]. Later, shortly after the discovery of rotavirus in Australia [37], IEM was used to detect antibody responses to the 70-nm rotaviruses in infants hospitalized with diarrhea [38]. The technique of IEM, which has been so important in the study of gastroenteritis and hepatitis viruses, holds promise in the future to help elucidate the cause of diseases of unknown etiology including a substantial portion of diarrheal episodes [39].
Footnotes
All volunteers gave informed consent. Human experimentation guidelines of the US Department of Health and Human Services were followed in the conduct of the clinical research.
References
- 1.Bell JA, Huebner RJ, Rosen L, et al. Illness and microbial experiences of nursery children at Junior Village. Am J Hyg. 1961;74:267–92. [Google Scholar]
- 2.Connor JD, Barrett-Connor E. Infectious diarrheas. Pediatr Clin North Am. 1967;14:197–221. doi: 10.1016/s0031-3955(16)31951-4. [DOI] [PubMed] [Google Scholar]
- 3.Yow MD, Melnick JL, Blattner RJ, Stephenson NB, Robinson NM, Burkhardt MA. The association of viruses and bacteria with infantile diarrhea. Am J Epidemiol. 1970;92:33–9. doi: 10.1093/oxfordjournals.aje.a121177. [DOI] [PubMed] [Google Scholar]
- 4.Reimann HA, Prince AH, Hodges JH. The cause of epidemic diarrhea, nausea and vomiting (viral dysentery?) Proc Soc Exp Biol Med. 1945;59:8–9. [Google Scholar]
- 5.Gordon I, Ingraham HS, Korns RF. Transmission of epidemic gastroenteritis to human volunteers by oral administration of fecal filtrates. J Exp Med. 1947;86:409–22. doi: 10.1084/jem.86.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon I, Meneely JK, Jr, Currie GD, Chicine A. Clinical laboratory studies in experimentally induced epidemic nonbacterial gastroenteritis. J Lab Clin Med. 1953;41:133–41. [PubMed] [Google Scholar]
- 7.Gordon I, Ingraham HS, Korns RF, Trussel RE. Gastroenteritis in man due to a filtrable agent. NY State J Med. 1949;49:1918–20. [PubMed] [Google Scholar]
- 8.Gordon I, Patterson PR, Whitney E. Immunity in volunteers recovered from nonbacterial gastroenteritis. J Clin Invest. 1956;35:200–5. doi: 10.1172/JCI103264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima S, Fukumi H, Kusama H, et al. Studies on the causative agent of the infectious diarrhea. Records of the experiments on human volunteers. Jpn Med J. 1948;1:467–76. [Google Scholar]
- 10.Yamamoto A, Zennyoji H, Yanagita K, Kato S. Research into the causative agent of epidemic gastroenteritis which prevailed in Japan in 1948. Jpn Med J. 1948;1:379–84. [Google Scholar]
- 11.Jordan WS, Jr, Gordon I, Dorrance WR. A study of illness in a group of Cleveland families. VII. Transmission of acute nonbacterial gastroenteritis to volunteers: evidence for two different etiologic agents. J Exp Med. 1953;98:461–75. doi: 10.1084/jem.98.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumi H, Nakaya R, Hatta S, et al. An indication as to identity between the infectious diarrhea in Japan and the afebrile infectious nonbacterial gastroenteritis by human volunteer experiments. Jpn J Med Sci Biol. 1957;10:1–17. doi: 10.7883/yoken1952.10.1. [DOI] [PubMed] [Google Scholar]
- 13.Tyrrell DAJ, Bynoe ML, Hoorn B. Cultivation of “difficult” viruses from patients with common colds. Br Med J. 1968;1:606–10. doi: 10.1136/bmj.1.5592.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57:933–40. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubenstein D, Tyrrell DAJ. Growth of viruses in organ cultures of intestine. Br J Exp Pathol. 1970;51:210–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Dolin R, Blacklow NR, Malmgren RA, Chanock RM. Establishment of human fetal intestinal organ cultures for growth of viruses. J Infect Dis. 1970;122:227–31. doi: 10.1093/infdis/122.3.227. [DOI] [PubMed] [Google Scholar]
- 17.Blacklow NR, Dolin R, Fedson DS, et al. Acute infectious nonbacterial gastroenteritis: etiology and pathogenesis. A combined clinical staff conference at the Clinical Center of the National Institutes of Health. Ann Intern Med. 1972;76:993–1008. doi: 10.7326/0003-4819-76-6-993. [DOI] [PubMed] [Google Scholar]
- 18.Dolin R, Blacklow NR, DuPont H, et al. Transmission of acute infectious nonbacterial gastroenteritis to volunteers by oral administration of stool filtrates. J Infect Dis. 1971;123:307–12. doi: 10.1093/infdis/123.3.307. [DOI] [PubMed] [Google Scholar]
- 19.Clarke SKR, Cook GT, Egglestone SI, et al. A virus from epidemic vomiting disease. Br Med J. 1972;3:86–9. doi: 10.1136/bmj.3.5818.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler I, Zickl R. Winter vomiting disease. J Infect Dis. 1969;119:668–73. doi: 10.1093/infdis/119.6.668. [DOI] [PubMed] [Google Scholar]
- 21.Zahorsky J. Hyperemesis hiemis or the winter vomiting disease. Arch Pediatr. 1929;46:391–5. [Google Scholar]
- 22.Dolin R, Blacklow NR, DuPont H, et al. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc Soc Exp Biol Med. 1972;140:578–83. doi: 10.3181/00379727-140-36508. [DOI] [PubMed] [Google Scholar]
- 23.Almeida JD, Waterson AP. The morphology of virus antibody interaction. Adv Viral Res. 1969;15:307–38. doi: 10.1016/S0065-3527(08)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson TF, Stanley WM. A study by means of the electron microscope of the reaction between tobacco mosaic virus and its antiserum. J Biol Chem. 1941;139:339–45. [Google Scholar]
- 25.Ardenne M.v., Friedrich-Freska H, Schramm G. Electronenmikroskopische Untersuchung der Präcipitinreaktion von Tabakmosaikvirus mit Kaninchenantiserum. Arch Ges Virusforsch. 1941;2:80–86. [Google Scholar]
- 26.Brenner S, Horne RW. A negative staining method for high resolution electronmicroscopy of viruses. Biochim Biophys Acta. 1959;34:103–10. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- 27.Kapikian AZ, Almeida JD, Stott EH. Immune electron microscopy of rhinoviruses. J Virol. 1972;10:142–6. doi: 10.1128/jvi.10.1.142-146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapikian AZ, James HD, Jr, Kelly SJ, et al. Isolation from man of “avian infectious bronchitis virus-like” viruses (coronaviruses) similar to 229E virus, with some epidemiological observations. J Infect Dis. 1969;119:282–90. doi: 10.1093/infdis/119.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapikian AZ, James HD, Jr, Kelly SJ, Vaughn AL. Detection of coronavirus strain 692 by immune electron microscopy. Infect Immun. 1973;7:111–6. doi: 10.1128/iai.7.1.111-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapikian AZ, Feinstone SM, Purcell RH, et al. Detection and identification by immune electron microscopy of fastidious agents associated with respiratory illness, acute nonbacterial gastroenteritis, and hepatitis A. In: Lennette Ett., editor. Perspectives in Virology. Vol. 9. New York: Academic Press; 1975. pp. 9–47. (Pollard M, ed. Antiviral Mechanisms; vol. 9) [Google Scholar]
- 31.Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27 nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10:1075–81. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapikian AZ, Gerin JL, Wyatt RG, Thornhill TS, Chanock RM. Density in cesium chloride of the 27 nm “8FIIa” particle associated with acute infectious nonbacterial gastroenteritis: determination by ultra-centrifugation and immune electron microscopy. Proc Soc Exp Biol Med. 1973;142:874–7. doi: 10.3181/00379727-142-37135. [DOI] [PubMed] [Google Scholar]
- 33.Thornhill TS, Kalica AR, Wyatt RG, Kapikian AZ, Chanock RM. Pattern of shedding of the Norwalk particle in stools during experimentally induced gastroenteritis in volunteers as determined by immune electron microscopy. J Infect Dis. 1975;132:28–34. doi: 10.1093/infdis/132.1.28. [DOI] [PubMed] [Google Scholar]
- 34.Thornhill TS, Wyatt RG, Kalica AR, Dolin R, Chanock RM, Kapikian AZ. Detection by immune electron microscopy of 26–27 nm virus-like particles associated with two family outbreaks of gastroenteritis. J Infect Dis. 1977;135:20–7. doi: 10.1093/infdis/135.1.20. [DOI] [PubMed] [Google Scholar]
- 35.Dolin R, Reichman RC, Roessner KD, et al. Detection by immune electron microscopy of the Snow Mountain agent of acute viral gastroenteritis. J Infect Dis. 1982;146:184–9. doi: 10.1093/infdis/146.2.184. [DOI] [PubMed] [Google Scholar]
- 36.Feinstone SM, Kapikian AZ, Purcell RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973;182:1026–8. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- 37.Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with viral gastroenteritis. Lancet. 1973;2:1281–3. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- 38.Kapikian AZ, Kim HW, Wyatt RG, et al. Reovirus-like agent in stools: association with infantile diarrhea and development of serologic tests. Science. 1974;185:1049–53. doi: 10.1126/science.185.4156.1049. [DOI] [PubMed] [Google Scholar]
- 39.Kapikian AZ. Viral gastroenteritis. JAMA. 1993;269:627–30. [PubMed] [Google Scholar]