Abstract
Background. Avian H5N1 influenza A virus is an emerging pathogen with the potential to cause substantial human morbidity and mortality. We evaluated the ability of currently licensed seasonal influenza vaccine to confer cross-protection against highly pathogenic H5N1 influenza virus in mice.
Methods. BALB/c mice were inoculated 3 times, either intranasally or subcutaneously, with the trivalent inactivated influenza vaccine licensed in Japan for the 2005–2006 season. The vaccine included A/NewCaledonia/20/99 (H1N1), A/NewYork/55/2004 (H3N2), and B/Shanghai/361/2002 viral strains and was administered together with poly(I):poly(C12U) (Ampligen) as an adjuvant. At 14 days after the final inoculation, the inoculated mice were challenged with either the A/HongKong/483/97, the A/Vietnam/1194/04, or the A/Indonesia/6/05 strain of H5N1 influenza virus.
Results. Compared with noninoculated mice, those inoculated intranasally manifested cross-reactivity of mucosal IgA and serum IgG with H5N1 virus, as well as both a reduced H5N1 virus titer in nasal-wash samples and increased survival, after challenge with H5N1 virus. Subcutaneous inoculation did not induce a cross-reactive IgA response and did not afford protection against H5N1 viral infection.
Conclusions. Intranasal inoculation with annual influenza vaccine plus the Toll-like receptor—3 agonist, poly(I): poly(C12U), may overcome the problem of a limited supply of H5N1 virus vaccine by providing cross-protective mucosal immunity against H5N1 viruses with pandemic potential.
In 1997, people in the Hong Kong area became infected with a highly pathogenic avian influenza A virus, H5N1, apparently before that virus adapted to a mammalian species [1–3]. Of the 18 patients who developed respiratory disease, 3 died. The World Health Organization has reported 168 deaths for 278 cases of laboratory-confirmed infection with H5N1 avian influenza, in Southeast Asia, Europe, and Africa, between January 2003 and March 2007. In some instances, human-to-human transmission of the H5N1 virus appears to have occurred [4], suggesting that this virus has the potential to cause an influenza pandemic [5]. Furthermore, an H5N1 virus (A/Hanoi/30408/2005) resistant to oseltamivir was isolated from a Vietnamese girl [6], and H5N1 viruses isolated from individuals in Hong Kong in 1997 were found to be resistant to interferon and tumor-necrosis factor—α [7]. The development of anti-H5N1 vaccines is thus a priority in efforts to prevent a human pandemic of H5N1 influenza.
We recently have shown that the combination of poly(I:C), a synthetic double-stranded RNA, and intranasal vaccine (split-product virus vaccine of either strain A/PuertoRico/8/34 or strain A/HongKong/156/97) protects mice against infection with avirulent A/PuertoRico/8 or highly pathogenic H5N1 (A/HongKong/483/97) influenza virus [8, 9]. poly(I:C), however, has a poor safety profile. poly(I): poly(C12U) (Ampligen) is structurally similar to double-stranded RNA and has exhibited a safe profile in double-blind placebo-controlled phase 2/3 clinical trials [10–12], in which it has been administered, in >75,000 intravenous doses (average dose, 400 mg), to humans. Our preliminary observations indicated that, as an adjuvant, Ampligen also has a protective effect against A/PuertoRico/8 and H5N1 influenza viruses. Furthermore, intranasal inoculation with either a formalin-inactivated H5N1 vaccine or an adenovirus vector—based influenza vaccine protected mice against lethal and heterologous H5N1 virus [13–15]. In 2003, Takada et al. [16] reported that intranasal inoculation with a formalin-inactivated virus vaccine (strain H1N1, H1N2, H3N1, H3N2, H5N4, or H9N2) at high doses protected mice against infection with heterologous A/HongKong/483/97 (H5N1) virus. These findings led us to examine whether intranasal inoculation with both Ampligen and a trivalent inactivated influenza vaccine—A/NewCaledonia/20/99 (H1N1), A/NewYork/55/2004 (H3N2), and B/Shanghai/361/2002—prepared for the 2005–2006 season protected mice against challenge with lethal and heterologous H5N1 virus.
In the present report, we demonstrate that intranasal inoculation with the current trivalent inactivated influenza vaccine combined with Ampligen as a mucosal adjuvant elicited protective immunity against both an H5N1 strain (A/HongKong/483/97) isolated in 1997 and more-recent H5N1 isolates (A/Vietnam/1194/04 and A/Indonesia/6/05) and that it significantly improved the survival rate after challenge with H5N1 virus. The results of our study suggest that the cross-protective immunity induced by such vaccination is mediated by a mucosal immune response, most likely by secretory IgA antibodies specific for influenza-virus proteins.
Materials and Methods
Mice. Female BALB/c mice 6–8 weeks old were purchased from Japan SLC and were kept under specific-pathogen—free conditions.
Viruses. The wild-type strains A/HongKong/483/97(H5N1), A/Vietnam/1194/04 (H5N1), and A/Indonesia/6/2005 (H5N1) were used in the present study. The A/HongKong/483/97 virus [17], isolated from patient with fatal influenza, was prepared in Mardin-Darby canine kidney (MDCK) cells without any special step for adaptation to mice. The Vietnam/1194/04 virus and the Indonesia/6/05 virus were propagated in 10-day—old embryonated chicken eggs, for 2 days at 37°C. These viruses were stored at -80°C, and virus titers were quantified by plaque assay using MDCK cells.
Vaccine and adjuvant. The trivalent inactivated influenza vaccine (split-product virus vaccines, hemagglutinin [HA] vaccine) prepared for the 2005–2006 season and including A/NewCaledonia/20/99 (H1N1), A/NewYork/55/2004 (H3N2), and B/Shanghai/361/2002 was purchased from Kitasato Institute (Saitama, Japan). poly(I):poly(C12U) (Ampligen) was provided by Hemispherx Biopharma.
Inoculation and viral challenge. Mice were anesthetized with diethyl ether and were inoculated 3 times (at consecutive intervals of 3 and 2 weeks), either intranasally or subcutaneously, with 1 μ of the trivalent vaccine (0.33 μ of each vaccine strain) with or without 10 μ of Ampligen. According to a modification of a procedure described elsewhere [18–20], each mouse (5–10 mice/group) was anesthetized and then subjected to infection by inoculation with 4 μL of H5N1 virus suspension (1000 pfu) in PBS into both nostrils (2 μL/nostril) at 14 days after the final inoculation (1000 pfu corresponds to ∼63 times the 50% mouse lethal dose for the A/Vietnam H5N1 strain in the 10-μL infection model). All animal experiments were performed in accordance with the Guides for Animal Experiments Performed at the National Institute of Infectious Diseases (NIID) and were approved by the Animal Care and Use Committee of NIID; infection with H5N1 virus was performed under Biosafety Level 3 containment and was approved by NIID.
Measurement of virus titers and anti-vaccine antibodies. From mice euthanized while anesthetized with chloroform, nasal-wash and serum samples were collected for measuremen tof virus titers and vaccine-specific antibodies. ELISA for determination of the levels of specific IgA and IgG antibodies was performed as described elsewhere [19], with plates coated either with the trivalent vaccine (split-product virus vaccines, HA vaccine) used for vaccination (table 1) or with a formalin-inactivated H5N1 virus vaccine (NIBRG14) [21] derived from a recombinant avirulent avian virus that contains modified HA and neuraminidase (NA) from the highly pathogenic avian influenza strain A/Vietnam/1194/04 and other viral proteins from influenza virus A/PuertoRico/8/34 (H1N1) (table 2). Before the hemagglutination inhibition (HI) tests were performed, receptor-destroying enzyme II (Denka Seiken) was added to the red blood cell—treated serum samples overnight at 37°C, to inactivate nonspecific hemagglutination inhibitors, followed by incubation for 1 h at 56°C, to inactivate receptor-destroying enzyme. HI tests were performed according to the microtiter method of Sever [22]. Virus titers were measured by a plaque assay using MDCK cells, as described elsewhere [23, 24].
Table 1.
Titers of antibodies specific for the trivalent vaccine.
Table 2.
Titers of IgA and IgG antibodies cross-reactive with A/Vietnam/1194/2004 (H5N1), and virus titer after challenge, in immunized mice.
Antigen-specific T cell response. Antigen-specific T cell responses were measured as described elsewhere [25]. The spleen was removed from mice 10 days after the third vaccination. T cells were purified from a single-cell suspension by depletion of CD11b+, CD45R+, DX5+, and Ter-119+ cells, by use of a magnetic cell-sorting system (Miltenyi Biotec). For preparation of antigen-presenting cells, splenocytes from normal BALB/c mice were depleted of CD90 (Thy1.2)—positive cells and were irradiated at 2000 cGy. Purified splenic T cells (1×105 cells/ well) were cultured for 4 days with irradiated antigen-presenting cells (5×105 cells/well) in the absence or presence of viral antigens (at concentrations of 0.1 μ/mL and 1 μ/mL). The concentration of interferon (IFN)—γ in culture supernatants was then measured by use of ELISA for the mouse cytokine (Biosource International). T cell proliferation was monitored by the addition of [3H]thymidine (18.5 kBq/well) (ICN Biomedicals) 8 h before the cells were harvested. The cells were harvested onto a 96-well microplate bonded with a GF/B filter (Packard Instruments). Incorporated radioactivity was calculated by a microplate scintillation counter (Packard Instruments). Data are means ± SDs of values from 2 independent experiments, each performed with T cells from 5 mice/group.
Statistics. All data are presented as means ±SEs or means ± SDs, and experimental groups were compared by use of Student's t test for paired observations. P < .05 was considered to be statistically significant.
Results
Ampligen's effects as a mucosal adjuvant for seasonal influenza vaccine. To evaluate the efficacy of Ampligen as a mucosal adjuvant for the trivalent inactivated influenza vaccine, we examined the IgA and IgG responses to each of the 3 component strains—A/NewCaledonia (H1N1), A/NewYork(H3N2), and B/Shanghai—in mice inoculated intranasally or subcutaneously with 1 μ of the vaccine with or without 10 μ of Ampligen. A high concentration of IgA antibodies to HA was apparent in the nasal-wash samples from mice inoculated intranasally with both the trivalent vaccine and Ampligen (table 1); in contrast, a mucosal IgA response was not observed either in mice inoculated intranasally with the trivalent vaccine without Ampligen or in mice inoculated subcutaneously with the trivalent vaccine with or without Ampligen. Compared with that in noninoculated mice, the concentration of IgG antibodies to HA in serum was significantly increased in mice inoculated intranasally with both the trivalent vaccine and Ampligen and in mice inoculated subcutaneously with the trivalent vaccine with or without Ampligen (table 1). A serum IgG response was not induced either in mice inoculated intranasally with vaccine without Ampligen or in mice inoculated, via either route, with Ampligen alone.
HI titers with regard to the A/NewCaledonia (H1N1), A/NewYork (H3N2), and B/Shanghai virus strains were examined in vitro, by use of serum samples from the same group of mice in which antibodies were examined. The serum samples from mice inoculated subcutaneously with vaccine with or without Ampligen showed high HI activity, with titers of 160–320, whereas serum samples from mice inoculated intranasally with Ampligen without the trivalent vaccine showed little response. No HI titer was detected in serum samples from noninoculated mice, from those inoculated intranasally with vaccine without Ampligen, or from those inoculated, via either route, with Ampligen alone.
Induction of H5N1-reactive antibody by intranasal inoculation with both seasonal influenza vaccine and Ampligen. We next characterized the cross-reactive antibody response to A/Vietnam/1194/04 (H5N1) in the various groups of inoculated mice. Compared with that in the noninoculated mice, the concentration of IgA antibodies to A/Vietnam/1194/04 in nasalwash samples was significantly increased in mice inoculated intranasally with both the trivalent vaccine and Ampligen (table 2). The concentration of IgG antibodies to A/Vietnam/1194/04 in serum was also significantly increased in mice inoculated intranasally with both the trivalent vaccine and Ampligen and in mice inoculated subcutaneously with the trivalent vaccine with or without Ampligen (table 2).
HI titers with regard to heterologous A/Vietnam/1194/2004 (H5N1), A/HongKong (H5N1), and A/Indonesia/6/2005 (H5N1) virus were examined in vitro using serum samples from the same group of mice in which antibodies were examined. However, these antibodies did not show any appreciable crossneutralizing activity against H5N1 virus strains (table 2). We did not detect HI activity against A/NewCaledonia (H1N1), A/NewYork (H3N2), B/Shanghai, A/Vietnam/1194/2004 (H5N1), A/HongKong (H5N1), and A/Indonesia/6/2005 (H5N1) virus strains in the nasal-wash samples from inoculated mice (data not shown). The lack of such activity was likely due to the dilution of antibodies intrinsic to collection of nasal-wash samples.
We also examined the cross-protective effect that Ampligen combined with the trivalent vaccine has in mice challenged with 1000 pfu of A/Vietnam/1194/04 (H5N1) virus. In noninoculated mice, the virus titer was 103.2 pfu/mL in nasal-wash samples obtained 3 days after challenge. Compared with those from noninoculated mice, nasal-wash samples from mice inoculated intranasally with either the trivalent vaccine alone or Ampligen alone and from mice inoculated subcutaneously with both the trivalent vaccine and Ampligen showed a (nonsignificant) 1-log-unit reduction in virus titer; in contrast, nasalwash samples from mice inoculated intranasally with both the trivalent vaccine and Ampligen did not contain detectable virus (table 2). The nasal-wash and serum samples from the same groups of mice were tested for the ability to inhibit hemagglutination induced by the A/Vietnam/1194/04, A/HongKong/483/97, or A/Indonesia/6/05 viruses; no HI activity was detected in the nasal-wash or serum samples from the different inoculated groups (data not shown).
Cross-protection against different H5N1 influenza virus strains by intranasal inoculation with seasonal influenza vaccine. We next examined whether the combination of the trivalent vaccine and Ampligen conferred cross-protection against heterologous H5N1 influenza viruses, including the A/Vietnam/1194/04, A/HongKong/483/97, and A/Indonesia/6/05 strains (figure 1). In challenges with 1000 pfu of A/Vietnam/1194/04 virus, mice that had been inoculated with both the trivalent virus and Ampligen showed a significant reduction in virus titer, compared with noninoculated mice (figure 1A). Furthermore, at 14 days after challenge with A/Vietnam/1194/04virus, 50% of the intranasally inoculated mice (n=10) were still alive, whereas all of the subcutaneously inoculated mice (n=10) and all of the noninoculatedmice (n=10) were dead. The subcutaneously inoculated mice showed the typical ruffled fur and neurological symptoms, such as tremor and spinning, evidenced by moribund mice; in contrast, the mice not challenged with A/Vietnam/1194/04 virus showed no ruffled fur and appeared to be healthy. In challenges with 1000 pfu of A/HongKong/483/97 virus, mice that had been inoculated with both the trivalent vaccine and Ampligen showed a 25% reduction in virus titer, compared with noninoculated mice (figure 1B). At 14 days after challenge with A/HongKong/483/97, all of the intranasally inoculated mice were still alive, whereas 30% of the subcutaneously inoculated mice (n=10) and 40% of the noninoculated mice (n=10) had died. Finally, in challenges with 1000 pfu of A/Indonesia/6/05 virus, mice that had been inoculated with both the trivalent vaccine and Ampligen showed a significant reduction in virus titer, compared with noninoculated mice (figure 1C). At 14 days after challenge with A/Indonesia/6/05 virus, all of the intranasally inoculated mice were still alive, whereas 80% of the subcutaneously inoculated mice (n=5) and 80% of the noninoculated mice (n=5) had died. Taken together, these results indicate that intranasal inoculation with both the trivalent vaccine and Ampligen was more effective against challenge with heterologous H5N1 influenza virus than was subcutaneous inoculation.
Figure 1.
Cross-protective effect of inoculation with trivalent inactivated influenza vaccine and Ampligen against highly pathogenic H5N1 influenza viruses. Mice were inoculated, intranasally (in) or subcutaneously (sc), with trivalent inactivated vaccine and Ampligen, as described in table 1. At 14 days after the final inoculation, the mice were challenged by in administration of 1000 pfu of A/Vietnam/1194/04 virus (A), A/HongKong/483/97 virus (B), or A/Indonesia/6/05 virus (C). At 3 days after challenge, nasal-wash samples were collected, and the titer of each virus was determined (left panels); data are means ± SEs 5 mice/group. White circles indicate values for individual mice. Survival rates of the mice in each group (n=5 or n=10) also were monitored, for 14 days after challenge with H5N1 (right panels). *P < .05 vs. noninoculated (Naive) mice.
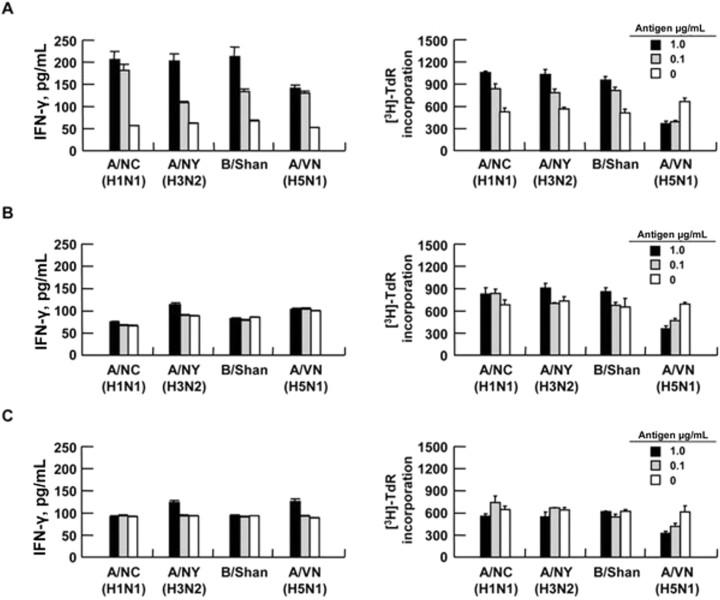
T cell responses induced by intranasal inoculation with both the trivalent vaccine and Ampligen. We next examined whether intranasal inoculation with the trivalent vaccine with or without Ampligen induced a T cell response against either homologous virus strains or heterologous H5N1 viruses. At 10 days after the final inoculation, T cells were isolated from the spleens of vaccinated mice and were cultured with irradiated antigen-presenting cells, in the absence or presence of the trivalent-vaccine components (at concentrations of 0.1 μ/mL and 1.0 μ/mL). T cells from mice inoculated with both the trivalent vaccine and Ampligen were proliferated and produced IFN-γ in a manner dependent on the concentration of the trivalentvaccine antigens (figure 2A); however, these antigens had no effect on the proliferation of and IFN-γ production by T cells isolated either from mice inoculated with the trivalent vaccine alone (figure 2B) or from noninoculated mice (figure 2C), and mice intranasally inoculated with both the trivalent vaccine and Ampligen did not show a cross-reactive T cell response to A/Vietnam/1194/04 (H5N1) whole-virus antigen (figure 2A). These results suggest that intranasal inoculation with both the trivalent vaccine and Ampligen induces a systemic T cell response to homologous vaccine strains but not to heterologous H5N1 virus strains.
Figure 2.
Interferon (IFN)—γ (left panels) and [3H]thymidine incorporation (right panels) by T cells from mice inoculated intranasally (in) with the trivalent vaccine and Ampligen (A), mice inoculated with the trivalent vaccine alone (B), and noninoculated mice (C), as described in table 1. Splenic T cells were isolated from the mice 10 days after the final inoculation and were cultured with irradiated antigen-presenting cells in the absence or presence of A/NewCaledonia (A/NC), A/NewYork (A/NY), B/Shanghai (B/Shan), or A/Vietnam/1194/04 (A/VN) antigens, at concentrations of 0.1 μ/mL and μ/mL. After 4 days of culture, the concentration of IFN-γ in culture supernatants was measured by ELISA; the minimum detectable dose of mouse IFN-γ is <1 pg/mL. Data are means ± SDs for 2 independent experiments, each performed with T cells from 5 mice/group.
Discussion
We have evaluated both the immunogenicity of the trivalent inactivated influenza vaccine (HA vaccine) prepared for the 2005–2006 season and its cross-protective efficacy against different H5N1 influenza viruses when it is administered either alone or with Ampligen. Compared with noninoculated mice, those inoculated, intranasally or subcutaneously, with both the trivalent vaccine and Ampligen manifested a lower virus titer in nasal-wash samples at 3 days after challenge with H5N1 influenza virus. However, whereas intranasal inoculation effectively improved the survival rate of mice infected with antigenically distinct H5N1 virus strains, subcutaneous inoculation did not. We speculate that this cross-protection may be mediated by the mucosal immune response, probably via secretory IgA antibodies to viral proteins. Elsewhere, we have shown that polymeric immunoglobulin receptor—knockout mice do not secrete IgA and show less cross-protective efficacy against variant influenza virus infection, when challenged with either influenza A virus or influenza B virus [26, 27]. However, anti-HA or anti-NA antibodies that normally act as neutralizing antibodies do not seem to contribute to such cross-protection, because none of the nasal-wash and serum samples collected from inoculated mice in the present study exhibited crossneutralizing activity in vitro (table 2); these antibodies neutralized the HI activity of the viruses whose subtypes are the same as those of the respective vaccine strains (table 1). These findings may be interpreted as suggesting that IgA antibodies specific for viral internal proteins are important for heterosubtypic protection, because some secretory IgA antibodies may neutralize virus infectivity during transcytosis in the infected epithelial cells [28–30].
In the present study, intranasal inoculation with both the trivalent vaccine and Ampligen induced a systemic T cell response to homologous virus strains but not to the heterologous H5N1 virus strain. These results further support the notion that the heterologous protection achieved by such inoculation is largely attributable to cross-reactive secretory IgA. The cross-protective immunity seems to correlate with virus replication in noninoculated mice. Although noninoculated and intranasally inoculated mice were challenged with the same titer of the A/Vietnam (H5N1) virus, the virus titer in the noninoculated mice was different in every experiment, a finding that explains the observed difference in cross-protective effects (table 2 and figure 1).
Our other recent studies have presented a strong case for the use of double-stranded RNA (dsRNA) as an adjuvant for intranasal vaccines [8, 9]. dsRNA is a powerful Toll-like receptor -3 agonist in the induction of innate immune responses; particularly significant is its apparent ability, as adjuvant, to broaden the range of viral mutant strains against which the wild-type vaccine provides full or partial protection, a property that will become increasingly important as new strains of influenza virus appear with ever increasing frequency and exhibit the potential to evolve into a global pandemic. The limitation to this approach has been that traditional dsRNAs are experimental agents, which have not been approved for human use and/or have exhibited significant toxicity (i.e., in the case of polyI:polyC) in human clinical trials. The one exception is the dsRNA product, Ampligen (polyI:polyC12U). Ampligen has been studied in 1700 patients with a cumulative systemic drug exposure of 76,000 doses; Ampligen has completed pivotal phase II/III trials for chronic fatigue syndrome and has an excellent safety record in humans.
In summary, intranasal inoculation with both the trivalent inactivated influenza vaccine and Ampligen induced cross-protective mucosal immunity to heterologous H5N1 influenza virus in mice. Intranasal inoculation with both the annual influenza vaccine and Ampligen may thus represent a strategy that, in humans, can generate protective mucosal immunity against newly emerging and highly pathogenic avian influenza viruses.
Acknowledgments
We thank Dr. Wilina Lim of the Department of Health of the government of Hong Kong, for providing us with influenza virus strain A/Vietnam/1194/04 (H5N1), and Drs. U. Suzuki and Komase, of Kitasato Institute (Saitama, Japan), for providing us with the vaccines.
Footnotes
Potential conflicts of interest: none reported.
Financial support: Japan Society for the Promotion of Science (grant-in-aid 07205 to T.I.); Ministry of Health, Labor and Welfare (grant H19 trans 002 to H.H.); Research on Health Sciences Focusing on Drug Innovation (grant KH51045 to H.H.).
References
- 1.Claas EC, Osterhaus AD, van Beek R, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–7. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 2.de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL. A pandemic warning? Nature. 1997;389:554–554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbarao K, Klimov A, Katz J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 4.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 5..Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11:1515–21. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108–1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 7.Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat Med. 2002;8:950–4. doi: 10.1038/nm757. [DOI] [PubMed] [Google Scholar]
- 8.Ichinohe T, Watanabe I, Ito S, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005;79:2910–9. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahi-Ozaki Y, Itamura S, Ichinohe T, et al. Intranasal administration of adjuvant-combined recombinant influenza virus HA vaccine protects mice from the lethal H5N1 virus infection. Microbes Infect. 2006;8:2706–14. doi: 10.1016/j.micinf.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Suhadolnik RJ, Reichenbach NL, Hitzges P, et al. Changes in the 2–5A synthetase/RNase L antiviral pathway in a controlled clinical trial with poly(I)-poly(C12U) in chronic fatigue syndrome. In Vivo. 1994;8:599–604. [PubMed] [Google Scholar]
- 11.Suhadolnik RJ, Reichenbach NL, Hitzges P, et al. Upregulation of the 2–5A synthetase/RNase L antiviral pathway associated with chronic fatigue syndrome. Clin Infect Dis. 1994;18(Suppl 1):S96–104. doi: 10.1093/clinids/18.supplement_1.s96. [DOI] [PubMed] [Google Scholar]
- 12.Thompson KA, Strayer DR, Salvato PD, et al. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:polyC12U in the treatment of HIV infection. Eur J Clin Microbiol Infect Dis. 1996;15:580–7. doi: 10.1007/BF01709367. [DOI] [PubMed] [Google Scholar]
- 13.Hoelscher MA, Garg S, Bangari DS, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–81. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takada A, Kuboki N, Okazaki K, et al. Avirulent Avian influenza virus as a vaccine strain against a potential human pandemic. J Virol. 1999;73:8303–7. doi: 10.1128/jvi.73.10.8303-8307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75:5141–50. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003;21:3212–8. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 17.Gao P, Watanabe S, Ito T, et al. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–9. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura S, Iwasaki T, Thompson AH, et al. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J Gen Virol. 1998;79(Pt 2):291–9. doi: 10.1099/0022-1317-79-2-291. [DOI] [PubMed] [Google Scholar]
- 19.Tamura S, Miyata K, Matsuo K, et al. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–900. [PubMed] [Google Scholar]
- 20.Yetter RA, Lehrer S, Ramphal R, Small PA., Jr Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980;29:654–62. doi: 10.1128/iai.29.2.654-662.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolson C, Major D, Wood JM, Robertson JS. Generation of influenza vaccine viruses on Vero cells by reverse genetics: an H5N1 candidate vaccine strain produced under a quality system. Vaccine. 2005;23:2943–52. doi: 10.1016/j.vaccine.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 22.Sever JL. Application of a microtechnique to viral serological investigations. J Immunol. 1962;88:320–9. [PubMed] [Google Scholar]
- 23.Tobita K. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med Microbiol Immunol (Berl) 1975;162:23–7. doi: 10.1007/BF02123574. [DOI] [PubMed] [Google Scholar]
- 24.Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol (Berl) 1975;162:9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- 25.Takasuka N, Fujii H, Takahashi Y, et al. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int Immunol. 2004;16:1423–30. doi: 10.1093/intimm/dxh143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asahi Y, Yoshikawa T, Watanabe I, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168:2930–8. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 27.Asahi-Ozaki Y, Yoshikawa T, Iwakura Y, et al. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol. 2004;74:328–35. doi: 10.1002/jmv.20173. [DOI] [PubMed] [Google Scholar]
- 28.Bomsel M, Heyman M, Hocini H, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–87. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 29.Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–5. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–43. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]