Abstract
Severe acute respiratory syndrome (SARS) is caused by a novel coronavirus (SARS-CoV) strain. Analyses of T cell repertoires in health care workers who survived SARS-CoV infection during the 2003 outbreak revealed that their effector memory Vγ9Vδ2 T cell populations were selectively expanded ∼3 months after the onset of disease. No such expansion of their αβ T cell pools was detected. The expansion of the Vγ9Vδ2 T cell population was associated with higher anti–SARS-CoV immunoglobulin G titers. In addition, in vitro experiments demonstrated that stimulated Vγ9Vδ2 T cells display an interferon-γ–dependent anti–SARS-CoV activity and are able to directly kill SARS-CoV–infected target cells. These findings are compatible with the possibility that Vγ9Vδ2 T cells play a protective role during SARS
Severe acute respiratory syndrome (SARS) is caused by a novel coronavirus (SARS-CoV) strain and is clinically similar to many other acute respiratory infections [1, 2]. Its symptoms include high fever, chills, rigors, dyspnea, nonproductive cough, myalgias, lymphopenia, and chest infiltrates, which begin after an incubation period of 2–10 days. SARS initially appeared in southern China at the end of 2002 and was first recognized as a new disease entity by World Health Organization infectious-diseases expert Carlo Urbani. By the beginning of 2003, SARS had spread into 29 countries. The 2003 epidemic lasted >16 weeks and affected 8098 people, 774 of whom died. Nosocomial transmission of SARS-CoV was a striking feature of the epidemic. If another SARS epidemic were to occur, surveillance efforts in containing the secondary transmission of SARS-CoV would ideally be combined with better prophylactic and therapeutic measures. Despite the substantial number of cases and the intensive studies that have been conducted, the mechanism of the protective immune response against SARS-CoV remains unknown
Understanding of the magnitude, specificity, and quality of anti–SARS-CoV immune responses is important to pursue, particularly because some infected patients do not develop severe, life-threatening disease. It is conceivable that, in patients with mild disease, various adaptive and innate immune responses cooperate to control and eventually eradicate SARS-CoV infection in vivo. The cell-mediated antiviral mechanisms against CoVs involve T cells that express αβ or γδ T cell receptors (TCRs) [3–5]. Thus, we analyzed, ∼3 months after the onset of disease, αβ and γδ T cell profiles in health care workers (HCWs) who had had SARS and had convalesced (SC-HCWs). In contrast to there being no measurable changes in the population of peripheral-blood αβ T cells, the effector memory Vγ9Vδ2 T cell population was selectively expanded in the peripheral blood of SC-HCWs. In addition, Vγ9Vδ2 T cells were able to inhibit SARS-CoV replication in Vero cells in vitro and to kill SARS-CoV–infected target cells, suggesting a potential role for Vγ9Vδ2 T cells in SARS immunosurveillance
Patients, materials, and methodsChanges in effector memory peripheral-blood T cell subsets were analyzed in 15 SC-HCWs (mean ± SD age, 29.4 ± 7.9 years; 10 men and 5 women). Peripheral-blood mononuclear cells (PBMCs) were collected ∼3 months after the onset of disease (their fevers had lasted for a mean ± SD of 4.9 ± 2.4 days), and the mean ± SD anti–SARS-CoV–specific IgG titer, as measured by ELISA, was 485.3 ± 360.9. Eleven putatively healthy volunteers (mean ± SD age, 32.3 ± 6.1 years; 5 men and 6 women) who had not been infected with SARS-CoV served as control subjects. All of the SC-HCWs and control subjects were recruited at the Prince of Wales Hospital in Hong Kong. The present study was approved by the institutional ethics committee
The Vβ and Vδ T cell repertoires in the 15 SC-HCWs and the 11 control subjects were studied by flow cytometry with 22 TCR chain–specific monoclonal antibodies (MAbs) (Beckman Coulter). Isotype-matched control MAbs from BD Biosciences were used to measure background staining. PBMCs were isolated by LeucoSep centrifugation (Arnica), in accordance with the manufacturer’s instructions; incubated with individual MAbs for 15 min at 4°C; washed in PBS containing 1% bovine serum albumin and 0.1% sodium azide; fixed in 4% paraformaldehyde; and immediately analyzed using a FACScalibur flow cytometer (BD Biosciences). Expression of CD45RA and CD27 molecules on Vβ and Vδ T cells was analyzed using the MAbs from BD Biosciences. Because the blood samples were obtained from convalescent healthy persons with normal lymphocyte counts, the percentage changes are also likely to reflect quantitative differences in T cell subset distributions
γδ T cells from the control subjects were purified using magnetic microbeads (Miltenyi Biotech), stimulated with isopentenyl pyrophosphate (IPP; 10 μmol/L; Sigma) plus interleukin (IL)–2 (100 U/mL; Boehringer Mannheim), and cocultured with Vero cells. To evaluate the noncytolytic antiviral activity of cell-released soluble factors, 1×106 γδ T cells were plated on 1 × 104 Vero cells/mL of culture medium. The Vero cell cultures were maintained in flat-bottom 24-well plates and separated (from the stimulated γδ T cells) by a semipermeable polycarbonate membrane with a 0.4-μm pore size (Transwell; BD Labware). After 24 h, the Vero cell cultures were infected with SARS-CoV at an MOI of 0.01. The supernatants were collected 4 days afterward, and the TCID50 per milliliter was determined as described elsewhere [6]. RNA was extracted using NucliSens isolation reagents (NASBA Diagnostics, Organon Teknica), and the number of SARS-CoV RNA copies was assessed by real-time polymerase chain reaction (Artus). Moreover, Vδ2 T cell lines (expression of Vδ2 by >80% of cells) were obtained by stimulating the PBMCs from 2 control subjects with IPP (10 μmol/L) and IL-2 (100 U/mL) for 12 days. The Vδ2 T cell lines were then stimulated with IPP for another 24 h, and the supernatants that contained factors released by activated γδ T cells (termed “γδ factors” [GDFs]) from these cultures were diluted 1:2 and added to the Vero cell cultures (1×105 cells/mL) for an additional 24 h. The cultures were then infected with SARS-CoV at an MOI of 0.01. Twenty-four hours after infection, the Vero cell culture supernatants were collected, and the TCID50 per milliliter was determined. A cytokine neutralization assay was performed using a polyclonal rabbit anti–interferon (IFN)–γ (original titer, 50,000 neutralization units/mL). The concentration of antibody against IFN-γ was 10,000 U/mL, that is, 10–100-fold higher than that required to neutralize the IFN-γ present in the supernatants of cultures in which γδ T cells are stimulated with 10 μmol/L IPP
Because the functional activation of Vγ9Vδ2 T cells requires species-specific interaction with target cells [7], Vero monkey kidney cells could not be used in the human Vγ9Vδ2 T cell stimulation experiments. Therefore, we used THP-1 cells, a human monocytic cell line that is infectable with SARS-CoV in vitro [8]. Specifically, Vγ9Vδ2 T cell lines were cocultured with uninfected- and SARS-CoV–infected THP-1 cells (MOI, 10) at 1:1 ratio. After 48 h, the culture supernatants from Vγ9Vδ2 T cell/THP-1 cocultures were analyzed for the presence of IFN-γ by ELISA (TEMA Ricerca), and the percentage of dead THP-1 cells was determined by propidium iodide labeling of hypodiploid nuclei after 24 h of coculture
ResultsThe relative frequencies of different Vβ and Vδ T cell subsets in the peripheral blood of the SC-HCWs and control subjects are shown in figure 1A. Although the Vβ T cell profiles (figure 1A) and the effector memory αβ T cell profiles (figure 1B) were not significantly altered in the SC-HCWs, compared with those in the control subjects, a substantial expansion of the Vδ2 T cell population was observed in the SC-HCWs (figure 1A). These results are compatible with the hypothesis that the Vγ9Vδ2 T cell population (an absolute majority of Vδ2 T cells coexpress Vγ9) is stimulated during infection with SARS-CoV. A representative analysis of the Vδ2 T cell subsets (the percentages of Vδ2 T cells expressing or not expressing the CD45RA and CD27 markers—i.e., CD45RA+CD27+ cells [naive], CD45RA−CD27+ cells [central memory], CD45RA−CD27− cells [effector memory], and CD45RA+CD27− cells [effector]) in one of the SC-HCWs is shown in figure 1A(inset). The representation of naive and fully differentiated effector Vδ2 T cells was not significantly different (P⩾.05, Mann-Whitney U test) between the 2 groups. In contrast, the central and effector memory subsets were significantly (P<.05, Mann-Whitney U test) expanded in the SC-HCWs, compared with those in the control subjects (figure 1A, inset). Interestingly, the higher number of circulating Vδ2 T cells was associated with higher anti–SARS-CoV–specific IgG titers (titer, >1:500; P=.036, Mann-Whitney U test). Therefore, it is conceivable that SARS-CoV infection induces a Vδ2 T cell response that may be involved in the anti–SARS-CoV immunosurveillance in vivo
Figure 1.
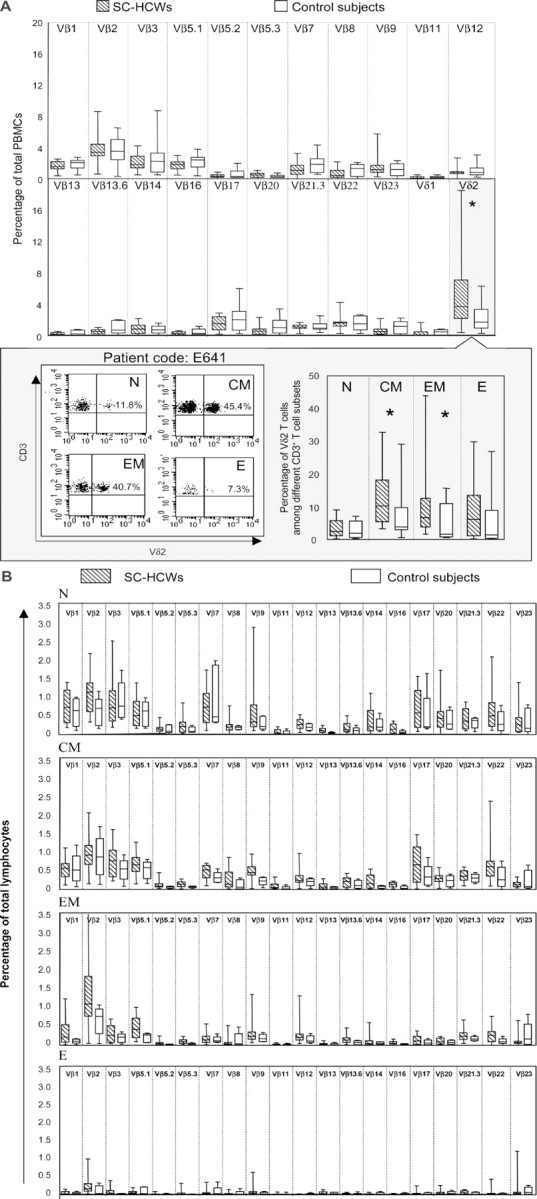
Induction of distinct changes in T cell subsets by severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) infection. A Vβ and Vδ T cell repertoires in health care workers (HCWs) who had had SARS and had convalesced (SC-HCWs; n=15; hatched bars) and in healthy control subjects (n=11; white bars), as assessed by flow cytometry with 22 T cell receptor chain–specific monoclonal antibodies. A representative experiment using peripheral-blood mononuclear cells (PBMCs) from one of the SC-HCWs is shown in the inset, which illustrates the frequencies of Vδ2 cells among naive (N), central memory (CM), effector memory (EM), and effector (E) CD3+ T cells. B Vβ T cell repertoire among the different effector memory αβ T cell subsets. In both panel A and panel B, the bars indicate the interquartile ranges of the individual measurements, the horizontal lines within the boxes indicate the median values, and the vertical lines indicate the ranges of the lowest and highest measurements. Statistical analysis was performed using the nonparametric Mann-Whitney U test. *Statistically significant (P<.05)
Subsequent experiments were performed to assess the noncytolytic antiviral activity of soluble factors released by Vγ9Vδ2 T cells, by use of an in vitro system of Vero cells infected with SARS-CoV (figure 2A). Both freshly purified γδ T cells and γδ T cell lines were used. Initially, γδ T cells from the control subjects were purified by magnetic microbead selection and stimulated with IPP. Then, the γδ T cells were cocultured with SARS-CoV–infected Vero cells separated from the γδ T cells by a semipermeable membrane. In addition, Vγ9Vδ2 T cell lines were obtained by stimulating PBMCs from the control subjects with IPP and IL-2, and the resulting γδ T cell lines were restimulated with IPP. The supernatants from these cultures that contained soluble molecules released by IPP-activated cell lines were tested for their anti–SARS-CoV activity. These experiments demonstrated that stimulated Vγ9Vδ2 T cells release noncytolytic antiviral GDFs. The presence of GDFs in the SARS-CoV–infected Vero cell cultures substantially reduced the quantity of SARS-CoV infectious units (measured as TCID50) produced in the system, compared with that in control cultures (figure 2A). Also, the total viral load (measured as the number of SARS-CoV RNA copies) was considerably decreased in the presence of GDFs. Interestingly, the anti–SARS-CoV activity of the GDFs was completely abolished by the addition of an antibody against IFN-γ (figure 2A). These results strongly suggest that IFN-γ is at least partially responsible for the observed antiviral action of the GDFs. Additional experiments demonstrated that the GDFs complemented IFN-α in blocking SARS-CoV replication (data not shown), confirming a synergistic SARS-CoV–inhibitory activity of human type I and type II IFNs [9]. Finally, the Vγ9Vδ2 T cell/THP-1 cocultures were used to assess cytotoxic function and cytokine production. Our results demonstrated that production of IFN-γ is augmented in Vγ9Vδ2 T cells stimulated by SARS-CoV in vitro (769.8 pg/mL in uninfected cocultures vs. 979.0 pg/mL in SARS-CoV–infected cocultures) (figure 2B). In addition, analysis of hypodiploid nuclei demonstrated a substantial increase in the cytotoxicity of Vγ9Vδ2 T cells against SARS-CoV–infected THP-1 target cells (87.5%), compared with that against uninfected target cells (21.9%). Thus, it appears that SARS-CoV infection of THP-1 cells promotes the cytotoxic effector function of Vγ9Vδ2 T cells
Figure 2.
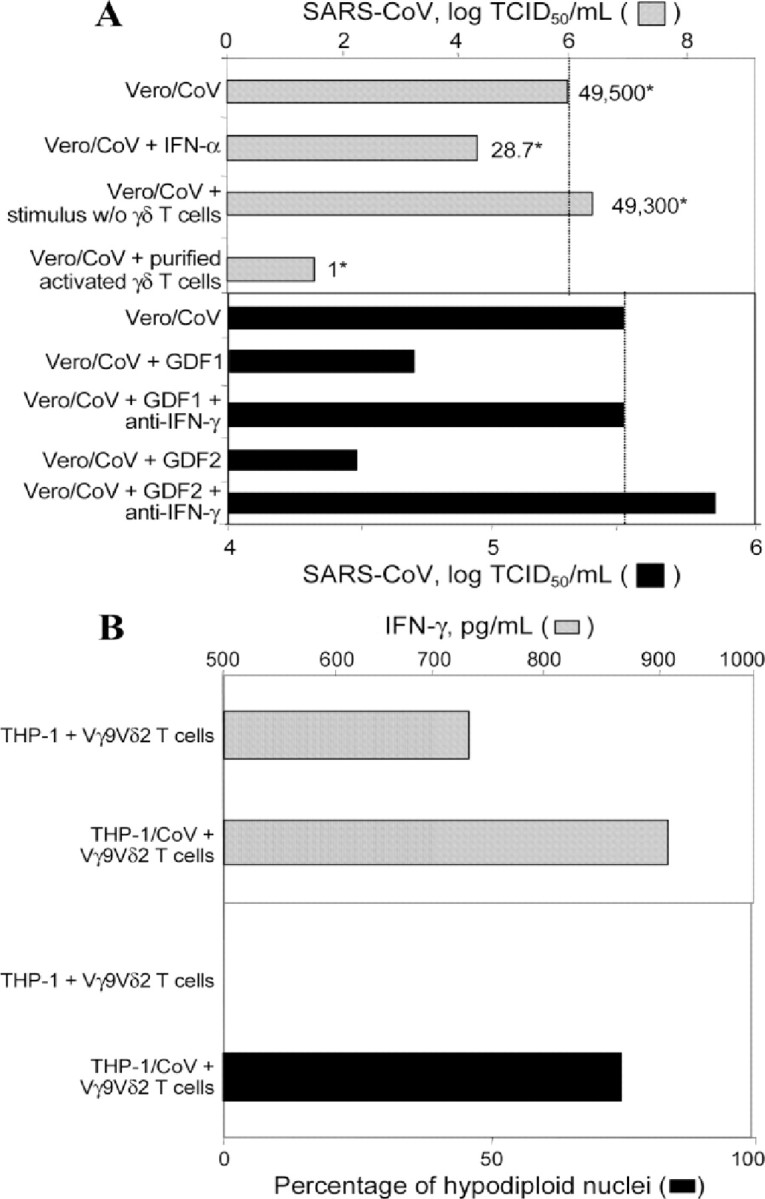
Noncytolytic antiviral activity and cytokine production. A Inhibition of severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) replication by interferon (IFN)–γ–like factors produced by activated Vγ9Vδ2 T cells. The gray and black bars show the log TCID50 per milliliter. One representative experiment (of 3 independent experiments) is shown. The relative numbers of SARS-CoV RNA copies (compared with those in control cultures) are indicated by asterisks and reflect the average of measurements in 3 separate wells. GDF, γδ factor released by Vδ2 T cell lines during the 24-h isopentenyl pyrophosphate–stimulation period; Vero/CoV, Vero cells infected with SARS-CoV. B Ability of SARS-CoV–infected THP-1 cells (THP-1/CoV) to induce IFN-γ production by Vγ9Vδ2 T cells, as determined by ELISA. The amount of IFN-γ in supernatants from 48-h cultures (gray bars) was analyzed in the presence and the absence of Vγ9Vδ2 T cell lines. The spontaneous (background) release of IFN-γ was <7% of the stimulation-induced release and was subtracted from the values measured in the experimental cocultures. In the same experiments, the percentages of hypodiploid target cells were calculated using propidium iodide labeling (black bars) after 24 h of coculture. The relative frequencies of dead THP-1 cells in control cultures without Vγ9Vδ2 T cells never exceeded 15% and were subtracted from the values measured in the experimental cocultures
DiscussionThe role played by T-cell–mediated immunity during SARS-CoV infection is not well understood. One of the traits of SARS is the rapid development of lymphopenia, with CD4+ T cells being more severely reduced than CD8+ T cells during acute infection [10, 11]. In recovering patients, peripheral αβ T cell subsets are rapidly restored, suggesting that tissue sequestration may contribute to the decrease in lymphocyte counts during acute SARS. In addition, CD8+ αβ T cell responses to SARS-CoV spike protein epitopes have been observed in patients who had recovered from SARS [12]. Indeed, certain HLA class I molecules may direct the CD8+ T cell response, conferring either protection or increased susceptibility [13]. No Vβ-specific changes were measured in our SC-HCWs ∼3 months after the onset of disease, indicating that any oligoclonal αβ T cell response that might have occurred did not persist in the peripheral blood in a form that was detectable by our MAb analysis
SARS-CoV proteins contain superantigen (SAg) domains [14] that may play crucial roles in targeting host cells and interfering with the immune system. SAgs are proteins produced by certain bacteria, mycoplasma, and viruses that stimulate a large number of T cells that express specific variable regions of the TCR β chains. In the present study, we analyzed the TCR Vβ repertoire in the SC-HCWs, and no differences were observed in the frequencies of the different Vβ chains or in the effector memory Vβ T cell subsets in the peripheral blood of the SC-HCWs and the control subjects. Typically, any SAg response is expected to induce a significant perturbation of the Vβ T cell repertoire, resulting in specific deletions or expansions of selected Vβ T cell subsets. Therefore, in light of our data, the involvement of a Vβ-specific SAg in the immunopathogenesis of SARS appears to be unlikely
However, the effector memory Vγ9Vδ2 T cell population was selectively expanded in the peripheral blood of the SC-HCWs. These cells can display a broad antiviral activity against different viruses, including retroviruses, flaviviruses, paramyxoviruses, orthomyxoviruses, picornaviruses, CoVs, rhabdoviruses, arenaviruses, herpesviruses, hepadnaviruses, and orthopoxviruses [15]. The antiviral action of γδ T cells may play an important defensive role, especially given their relatively large numbers (e.g., ∼1 of 30 adult human PBMCs is a Vγ9Vδ2 T cell) and their ability to respond very quickly (typically, no antigen processing is required for the SAg-like activation of Vγ9Vδ2 T cells). The molecules recognized by γδ T cells during viral infections are probably of cellular rather than viral origin and may be metabolites of altered cellular pathways [15]. Moreover, virus-exposed γδ T cells can be rapidly activated by type I IFNs (IFN-α and IFN-β), a phenomenon that is likely to contribute to an effective antiviral response. Thus, the potential involvement of a possible γδ-specific SAg in SARS-associated immune responses cannot be excluded
The antiviral role played by γδ T cells has been intensively studied in mice and was found to be correlated with the production of IFN-γ by distinct γδ T cell subsets [15]. In a rodent model of CoV infection (infection with mouse hepatitis virus [MHV]), T cells expressing the γδ TCR appeared to be the major T cell effectors and were found predominantly in areas of virus replication [3]. In MHV-infected mice, γδ T cells may function by both lysing infected target cells and secreting proinflammatory cytokines and could be important for anti-MHV responses in vivo. In our experiments, we observed that Vγ9Vδ2 T cells are able to exert a potent cytolytic activity against SARS-CoV–infected target cells. The remarkable similarities of the NKG2D receptor between rodents and humans may support a role for NKG2D ligands in the recognition and killing of CoV-infected cells. In the present study, Vγ9Vδ2 T cells appeared to be able to inhibit SARS-CoV replication in vitro through an IFN-γ–dependent process. Recently, Dandekar et al. have shown that γδ T cells mediate demyelination in mice infected with MHV (strain JHM), with IFN-γ and NKG2D as the critical players in this process [4]. NKG2D is an activating, C-type lectin NK cell receptor that is recognized as a potent costimulator of the cytotoxic functions of human Vγ9Vδ2 T cells [16]. Our data do not exclude the possibility that NKG2D ligands may be involved in the recognition and killing of SARS-CoV–infected cells. Furthermore, our results are compatible with the idea that Vγ9Vδ2 T cells contribute to anti-SARS innate immune responses by employing both cytotoxic and noncytolytic antiviral mechanisms
Activated human Vγ9Vδ2 T cells may promote antigen processing and presentation and so provide costimulatory signals to dendritic and αβ T cells [17–19]. Therefore, they may also participate in the induction of adaptive immune responses against SARS-CoV. It is noteworthy that the in vitro and in vivo activities of Vγ9Vδ2 T cells can be stimulated by many nonpeptidic molecules, including nitrogen-containing bisphosphonates (which are frequently used in the treatment of bone-demineralization disorders) and pyrophosphomonoester drugs (which are currently being tested in phase 1 cancer trials) [20]. The relatively low in vivo toxicity of many of these drugs may facilitate novel approaches to the treatment of SARS-CoV infection, including ones that could be based on type II IFN released by activated Vγ9Vδ2 T cells in combination with type I IFN chemotherapy
Footnotes
Potential conflicts of interest: none reported
Financial support: Italian Ministry of Health (grant 03.118UO04 to Istituto di Ricovero e Cura a Carattere Scientifico); National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R01AI48401)
References
- 1.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 2.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 3.Dandekar AA, Perlman S. Virus-induced demyelination in nude mice is mediated by gamma delta T cells. Am J Pathol. 2002;161:1255–63. doi: 10.1016/s0002-9440(10)64402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dandekar AA, O’Malley K, Perlman S. Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T-cell receptor beta-deficient mice infected with a coronavirus. J Virol. 2005;79:9388–96. doi: 10.1128/JVI.79.15.9388-9396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gioia C, Horejsh D, Agrati C, et al. T-cell response profiling to biological threat agents including the SARS coronavirus. Int J Immunopathol Pharmacol. 2005;18:525–30. doi: 10.1177/039463200501800312. [DOI] [PubMed] [Google Scholar]
- 6.Stroher U, DiCaro A, Li Y, et al. Severe acute respiratory syndrome–related coronavirus is inhibited by interferon-α. J Infect Dis. 2004;189:1164–7. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato Y, Tanaka Y, Tanaka H, Yamashita S, Minato N. Requirement of species-specific interactions for the activation of human gamma delta T cells by pamidronate. J Immunol. 2003;170:3608–13. doi: 10.4049/jimmunol.170.7.3608. [DOI] [PubMed] [Google Scholar]
- 8.Ng LF, Hibberd ML, Ooi EE, et al. A human in vitro model system for investigating genome-wide host responses to SARS coronavirus infection. BMC Infect Dis. 2004;4:34. doi: 10.1186/1471-2334-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castilletti C, Bordi L, Lalle L, et al. Coordinate induction of IFN-alpha and -gamma by SARS-CoV also in the absence of virus replication. Virology. 2005;341:163–9. doi: 10.1016/j.virol.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004;189:648–51. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui W, Fan Y, Wu W, Zhang F, Wang JY. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin Infect Dis. 2003;37:857–9. doi: 10.1086/378587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YD, Sin WY, Xu GB, et al. T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T-cell immune response in patients who recover from SARS. J Virol. 2004;78:5612–8. doi: 10.1128/JVI.78.11.5612-5618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin M, Tseng HK, Trejaut JA, et al. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:9. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Luo C, Li W, et al. Structure-based preliminary analysis of immunity and virulence of SARS coronavirus. Viral Immunol. 2004;17:528–34. doi: 10.1089/vim.2004.17.528. [DOI] [PubMed] [Google Scholar]
- 15.Poccia F, Agrati C, Martini F, Capobianchi MR, Wallace M. Antiviral reactivities of gammadelta T cells. Microbes Infect. 2005;7:518–28. doi: 10.1016/j.micinf.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das H, Groh V, Kuijl C, et al. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 17.Martino A, Casetti R, D’Alessandri A. Alessandri A, Sacchi A, Poccia F. Complementary function of gamma delta T-lymphocytes and dendritic cells in the response to isopentenyl-pyrophosphate and lipopolysaccharide antigens. J Clin Immunol. 2005;25:230–7. doi: 10.1007/s10875-005-4080-8. [DOI] [PubMed] [Google Scholar]
- 18.Martino A, Poccia F. Close encounters of different kinds: dendritic cells and gammadelta T cells heighten therapeutic applications. Immunol Lett. 2005;101:115. doi: 10.1016/j.imlet.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 20.Casetti R, Perretta G, Taglioni A, et al. Drug-induced expansion and differentiation of Vγ9Vδ2 T cells in vivo: the role of exogenous IL-2. J Immunol. 2005;175:1593–8. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]


