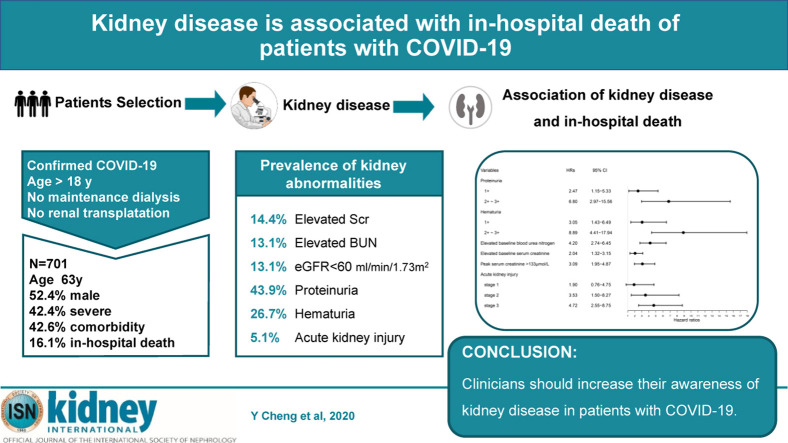
Abstract
In December 2019, a coronavirus 2019 (COVID-19) disease outbreak occurred in Wuhan, Hubei Province, China, and rapidly spread to other areas worldwide. Although diffuse alveolar damage and acute respiratory failure were the main features, the involvement of other organs needs to be explored. Since information on kidney disease in patients with COVID-19 is limited, we determined the prevalence of acute kidney injury (AKI) in patients with COVID-19. Further, we evaluated the association between markers of abnormal kidney function and death in patients with COVID-19. This was a prospective cohort study of 701 patients with COVID-19 admitted in a tertiary teaching hospital that also encompassed three affiliates following this major outbreak in Wuhan in 2020 of whom 113 (16.1%) died in hospital. Median age of the patients was 63 years (interquartile range, 50-71), including 367 men and 334 women. On admission, 43.9% of patients had proteinuria and 26.7% had hematuria. The prevalence of elevated serum creatinine, elevated blood urea nitrogen and estimated glomerular filtration under 60 ml/min/1.73m2 were 14.4, 13.1 and 13.1%, respectively. During the study period, AKI occurred in 5.1% patients. Kaplan-Meier analysis demonstrated that patients with kidney disease had a significantly higher risk for in-hospital death. Cox proportional hazard regression confirmed that elevated baseline serum creatinine (hazard ratio: 2.10, 95% confidence interval: 1.36-3.26), elevated baseline blood urea nitrogen (3.97, 2.57-6.14), AKI stage 1 (1.90, 0.76-4.76), stage 2 (3.51, 1.49-8.26), stage 3 (4.38, 2.31-8.31), proteinuria 1+ (1.80, 0.81-4.00), 2+∼3+ (4.84, 2.00-11.70), and hematuria 1+ (2.99, 1.39-6.42), 2+∼3+ (5.56,2.58- 12.01) were independent risk factors for in-hospital death after adjusting for age, sex, disease severity, comorbidity and leukocyte count. Thus, our findings show the prevalence of kidney disease on admission and the development of AKI during hospitalization in patients with COVID-19 is high and is associated with in-hospital mortality. Hence, clinicians should increase their awareness of kidney disease in patients with severe COVID-19.
Keywords: acute kidney injury, COVID-19, in-hospital death, kidney disease, pneumonia
Graphical abstract
Editor’s Note.
Coronavirus disease 2019 (COVID-19) is now a considered a pandemic by the World Health Organization. This article, published in this issue of Kidney International, is the first study that reported an association between kidney disease (or abnormal kidney function) and mortality in hospitalized patients with COVID-19. In this cohort, approximately 13% of patients had underlying kidney disease. More than 40% had evidence of abnormal kidney function and 5.1% had acute kidney injury (AKI) during their hospital stay. There was a dose-dependent relationship between AKI stages and death, with an excess risk of mortality by at least 4 times among those with stage 3 AKI. Kidney disease is a major complication of COVID-19 and a significant risk factor of death. However, data of this study was originated from a single center, therefore, generalizability of the cohort characteristics could not be confirmed, and only short-term, catastrophic outcome (death) was discussed. Nonetheless, the study findings suggest that early identification of those at risk, interventions to provide appropriate support, and avoidance of nephrotoxins may help to improve the prognosis of patients with COVID-19.
In December 2019, a series of unknown origin cases of acute respiratory illness occurred in Wuhan, Hubei Province, China.1 , 2 High-throughput sequencing showed that the disease was caused by named “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2).3 On February 11, 2020, the World Health Organization officially changed the name of the disease caused by SARS-CoV-2 to coronavirus disease 2019 (COVID-19). The disease rapidly spread from Wuhan to other areas worldwide. As of February 29, 2020, Chinese health authorities announced that 79,389 confirmed cases of novel coronavirus infection and 2,838 death cases had been reported in 31 provincial-level regions. Of note, in Wuhan, 48,557 COVID-19 cases with 2,169 deaths were confirmed on that same day, suggesting a much higher proportion of severe cases and mortality rate in Wuhan than in other provinces of China. However, all clinical characteristics of the patients suffering from COVID-19 cases were defined only progressively. Identifying and eliminating factors predicting a negative outcome is a key to improving survival from COVID-19.
Although diffuse alveolar damage and acute respiratory failure were the main features of COVID-19,4 the involvement of other organs needed to be explored. After lung infection, the virus may enter the blood, accumulate in kidney, and cause damage to resident renal cells. Indeed, COVID-19 RNA was found in the plasma of 15% of patients by real-time polymerase chain reaction.4 Of note, it is reported showed that 6.7% of patients with SARS developed acute kidney injury (AKI, and the mortality of those with AKI was 91.7%.5 Thus, understanding how the kidney is affected by SARS-CoV-2 is urgently warranted.
In this large prospective cohort study of adult patients with COVID-19 in a tertiary teaching hospital with 3 branches and more than 4000 beds, which was designated for critical COVID-19 cases by local government, we aimed to determine the prevalence of AKI in patients with COVID-19 and to define the association between markers of kidney disease and death in patients infected with COVID-19.
Results
Baseline characteristics
A total of 701 patients were included in our study. Table 1 shows the clinical features of patients with COVID-19. Median age was 63 years, and 52.4% of patient were male. Median duration from illness onset to admission was 10 days. Of the total patients, 42.6% were reported as having ≥1 comorbidity: 2.0%, 1.9%, 33.4%, 14.3%, and 4.6% reported having, respectively, chronic kidney disease, chronic obstructive pulmonary disease, hypertension, diabetes, and tumor. Mean lymphocyte count was 0.9 ± 0.5 × 109/l below the lower limit of normal. Most patients had elevated levels of high-sensitivity C-reactive protein (83.0%) and erythrocyte sedimentation rate (81.6%), but elevated levels of procalcitonin were rare (9.8%). Coagulopathies were common in patients with COVID-19. In addition, mean serum lactose dehydrogenase (377 ± 195 U/l) was increased, especially in those with high baseline serum creatinine levels (Table 2 ).
Table 1.
Characteristics and outcomes of patients with COVID-2019
| Variables | All patients | Normal baseline serum creatinine | Elevated baseline serum creatinine | P value |
|---|---|---|---|---|
| Number | 701 | 600 | 101 | |
| Age, yr | 63 (50–71) | 61 (49–69) | 73 (62–79) | <0.001 |
| Male patients | 367 of 701 (52.4) | 294 of 600 (49.0) | 73 of 101 (72.3) | <0.001 |
| Days from illness onset to admission, d | 10 (7–13) | 10 (7–13) | 9 (7–12) | 0.381 |
| Fever on admission | 213 of 655 (32.5) | 187 of 560 (33.4) | 26 of 95 (27.4) | 0.246 |
| Systolic blood pressure, mm Hg | 128 (117–143) | 128 (118–142) | 128 (114–144) | 0.942 |
| Diastolic blood pressure, mm Hg | 79 (72–87) | 79 (73–87) | 77 (70–86) | 0.282 |
| Severe disease | 297 of 701 (42.4) | 244 of 600 (40.7) | 53 of 101 (52.5) | 0.026 |
| Any comorbidity | 297 of 698 (42.6) | 237 of 598 (39.6) | 60 of 100 (60.0) | <0.001 |
| Chronic kidney disease | 14 of 698 (2.0) | 5 of 598 (0.8) | 9 of 100 (9.0) | <0.001 |
| Chronic obstructive pulmonary disease | 13 of 698 (1.9) | 9 of 598 (1.5) | 4 of 100 (4.0) | 0.191 |
| Hypertension | 233 of 698 (33.4) | 185 of 598 (30.9) | 48 of 100 (48.0) | 0.001 |
| Diabetes | 100 of 698 (14.3) | 84 of 598 (14.0) | 16 of 100 (16.0) | 0.606 |
| Tumor | 32 of 698 (4.6) | 28 of 598 (4.7) | 4 of 100 (4.0) | 0.965 |
| Admission to intensive care unit | 73 of 701 (10.4) | 60 of 600 (10.0) | 13 of 101 (12.8) | 0.382 |
| Administration of mechanical ventilation | 97 of 701 (13.4) | 75 of 600 (12.5) | 22 of 101 (21.8) | 0.012 |
| Acute kidney injury | 36 of 701 (5.1) | 24 of 600 (4.0) | 12 of 101 (11.9) | 0.001 |
| Stage 1 | 13 of 701 (1.9) | 10 of 600 (1.7) | 3 of 101 (3.0) | 0.356 |
| Stage 2 | 9 of 701 (1.3) | 4 of 600 (0.7) | 5 of 101 (5.0) | |
| Stage 3 | 14 of 701 (2) | 10 of 600 (1.7) | 4 of 101 (4.0) | |
| In-hospital death | 113 of 701 (16.1) | 79 of 600 (13.2) | 34 of 101 (33.7) | <0.001 |
COVID-19, coronavirus disease 2019.
Data are presented as number/total (percentage) or median (interquartile range). The severity was staged based on the guidelines for diagnosis and treatment of COVID-19 (trial fifth edition) published by the Chinese National Health Commission on February 4, 2020.
Table 2.
Laboratory data of patients with COVID-19 on admission
| Variables | All patients | Normal baseline serum creatinine | Elevated baseline serum creatinine | P value |
|---|---|---|---|---|
| Leukocyte count, × 10⁹/l | 7.5 ± 7.5 | 7.2 ± 7.4 | 9.5 ± 8.0 | 0.005 |
| Lymphocyte count, × 10⁹/l | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.8 ± 0.5 | 0.015 |
| Hemoglobin, g/l | 128 ± 17 | 127 ± 17 | 131 ± 20 | 0.110 |
| Platelet count, × 10⁹/l | 213 ± 94 | 216 ± 94 | 191 ± 94 | 0.014 |
| Prothrombin time > 14.5 s | 260 of 670 (38.8) | 215 of 573 (37.5) | 45 of 97 (46.4) | 0.097 |
| Activated partial thromboplastin time > 42 s | 210 of 495 (42.4) | 171 of 423 (40.4) | 39 of 72 (54.2) | 0.029 |
| D-dimer > 0.5 mg/l | 512 of 661 (77.5) | 424 of 563 (75.3) | 88 of 98 (89.8) | 0.002 |
| Procalcitonin ≥ 0.5 ng/ml | 61 of 620 (9.8) | 37 of 538 (6.9) | 24 of 82 (29.3) | <0.001 |
| High-sensitivity C-reactive protein ≥ 10 mg/l | 560 of 675 (83.0) | 478 of 581 (82.3) | 82 of 94 (87.2) | 0.235 |
| Erythrocyte sedimentation rate > 15 mm/h | 541 of 663 (81.6) | 463 of 568 (81.5) | 78 of 95 (82.1) | 0.891 |
| Alanine aminotransferase, U/l | 35 ± 38 | 36 ± 40 | 32 ± 29 | 0.206 |
| Aspartate aminotransferase, U/l | 42 ± 42 | 41 ± 43 | 47 ± 33 | 0.142 |
| Total bilirubin, mmol/l | 12 ± 23 | 11 ± 7 | 21 ± 57 | 0.061 |
| Lactose dehydrogenase, U/l | 377 ± 195 | 364 ± 180 | 458 ± 254 | 0.001 |
| Creatinine kinase, U/l | 164 ± 233 | 149 ± 185 | 257 ± 410 | 0.108 |
| Sodium, mmol/l | 139 ± 5 | 138 ± 5 | 139 ± 7 | 0.373 |
| Potassium, mmol/l | 4.2 ± 0.7 | 4.2 ± 0.7 | 4.5 ± 0 .7 | <0.001 |
| Proteinuria | ||||
| Negative | 248 of 442 (56.1) | 232 of 389 (59.6) | 16 of 53 (30.2) | <0.001 |
| 1+ | 149 of 442 (33.7) | 128 of 389 (32.9) | 21 of 53 (39.6) | |
| 2+∼3+ | 45 of 442 (10.2) | 29 of 389 (7.5) | 16 of 53 (30.2) | |
| Hematuria | ||||
| Negative | 324 of 442 (73.3) | 299 of 389 (76.9) | 25 of 53 (47.2) | <0.001 |
| 1+ | 68 of 442 (15.4) | 52 of 389 (13.4) | 16 of 53 (133.3) | |
| 2+∼3+ | 50 of 442 (11.3) | 38 of 389 (9.8) | 12 of 53 (22.6) | |
| Blood urea nitrogen, mmol/l | 5.7 ± 3.9 | 4.8 ± 2.3 | 11 ± 7 | <0.001 |
| Serum creatinine, μmol/l | 77 ± 31 | 68 ± 16 | 132 ± 39 | <0.001 |
| eGFR, ml/min per 1.73 m2 | 87 ± 23 | 94 ± 17 | 48 ± 13 | <0.001 |
| Peak serum creatinine, μmol/l | 91 ± 67 | 79 ± 48 | 163 ± 109 | <0.001 |
COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate.
Data are presented as number/total (percentage) or mean ± SD.
Kidney abnormalities
On admission, serum creatinine and blood urea nitrogen (BUN) were elevated in 14.4% and 13.1% of the patients, respectively. Estimated glomerular filtration rate < 60 ml/min per 1.73 m2 was reported in 13.1% of patients. During hospitalization, peak serum creatinine was 91 ± 67 μmol/l; 43.9% of patients had proteinuria, and relatively fewer patients (26.7%) had hematuria (Table 2). On admission, urethral catheterization was done in only 9.8% of patients, some of whom were due to the application of invasive mechanical ventilation. This indicated that the impact of catheterization on abnormal urinary analysis on admission was small.
Compared with patients with normal serum creatinine, those who entered the hospital with an elevated serum creatinine were predominantly male and older and were more severely ill (Table 1). Moreover, patients with elevated baseline serum creatinine demonstrated a higher leukocyte count and lower lymphocyte and platelet counts. Coagulation pathway abnormalities, including prolonged activated partial thromboplastin time and higher D-dimer, were more common in patients with elevated baseline serum creatinine. The percentage of patients with increased procalcitonin, and the levels of aspartate aminotransferase and lactose dehydrogenase were also higher in patients with elevated baseline serum creatinine. Of note, the gap between peak and baseline serum creatinine was also much greater in patients with elevated baseline serum creatinine (Table 2).
Incidence of AKI and in-hospital death
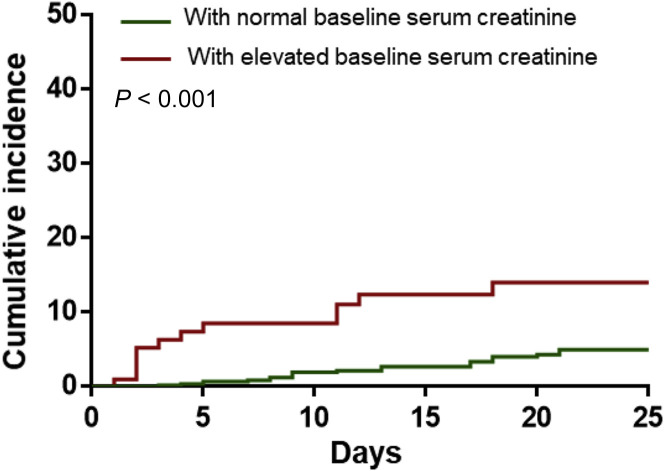
During hospitalization, AKI occurred in 5.1% of patients. The incidence of AKI was significantly higher in patients with elevated baseline serum creatinine (11.9%) than in patients with normal baseline values (4.0%) (Table 1, Figure 1 ).
Figure 1.
Cumulative incidence of acute kidney injury of patients with coronavirus disease 2019 subgrouped by baseline serum creatinine.
In-hospital death occurred in 16.1% of patients. The median time to death was 6 days (interquartile range, 3–12 days). The incidence of in-hospital death in the patients with elevated baseline serum creatinine was 33.7%, which was significantly higher than in those with normal baseline serum creatinine (13.2%) (Table 1).
Association of kidney disease indicators with in-hospital death
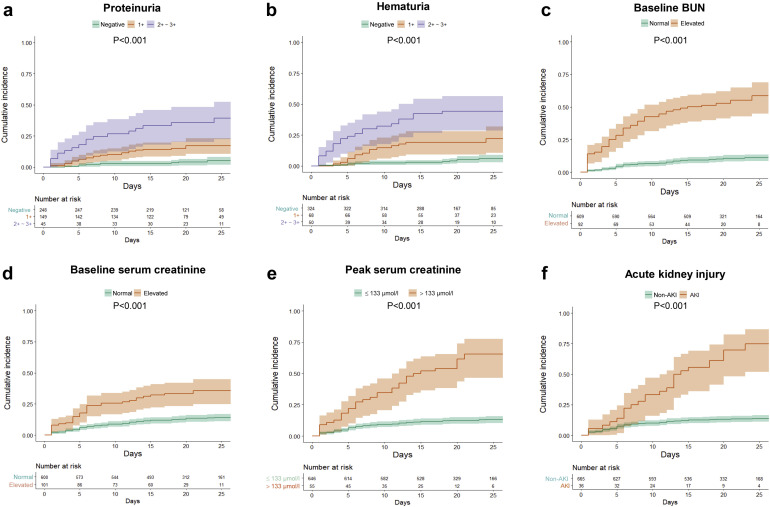
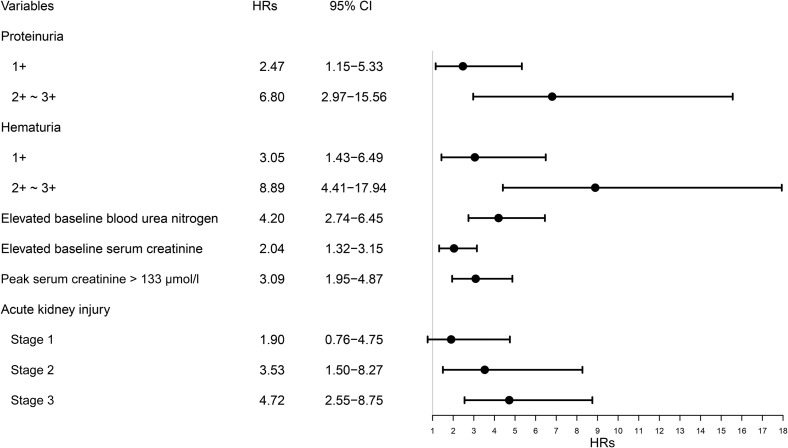
Kaplan-Meier analysis revealed a significantly higher in-hospital death rate for patients with kidney abnormalities, including elevated baseline serum creatinine, elevated baseline BUN, proteinuria, hematuria, and AKI (P < 0.001) (Figure 2 ). Univariate Cox regression analysis showed that age above 65 years, male sex, and severe COVID-19 disease were associated with in-hospital death. In addition, the kidney disease indicators mentioned above were also associated with in-hospital death (Table 3 ). After adjusting for age, sex, disease severity, comorbidities, and lymphocyte count, the following were all associated with in-hospital death: proteinuria of any degree, hematuria of any degree, elevated baseline BUN, serum creatinine, peak serum creatinine > 133 μmol/l, and AKI over stage 2 (Figure 3 ).
Figure 2.
Cumulative incidence for in-hospital death of patients with coronavirus disease 2019 subgrouped by kidney disease indicators. Shadows indicate the 95% confidence intervals of the corresponding estimates: (a) proteinuria, (b) hematuria, (c) baseline blood urea nitrogen (BUN), (d) baseline serum creatinine, (e) peak serum creatinine, and (f) acute kidney injury.
Table 3.
Univariate Cox regression analysis of association between kidney disease and in-hospital death in patients with coronavirus disease 2019
| Variables | Hazard ratios | 95% Confidence interval | P value |
|---|---|---|---|
| Age > 65 yr | 2.43 | 1.66–3.56 | <0.001 |
| Sex, male | 2.15 | 1.45–3.21 | <0.001 |
| Severe disease | 6.10 | 3.86–9.64 | <0.001 |
| Any comorbidity | 1.06 | 0.73–1.54 | 0.771 |
| Leukocyte count > 10× 10⁹/l | 1.06 | 0.73–1.54 | 0.771 |
| Lymphocyte count < 1.5 × 10⁹/l | 1.02 | 0.70–1.48 | 0.931 |
| Proteinuria | |||
| Negative | Reference | Reference | |
| 1+ | 4.12 | 1.97–8.62 | <0.001 |
| 2+∼3+ | 10.92 | 5.00–23.86 | <0.001 |
| Hematuria | |||
| Negative | Reference | Reference | |
| 1+ | 4.64 | 2.24–9.62 | <0.001 |
| 2+∼3+ | 12.20 | 6.32–23.53 | <0.001 |
| Elevated baseline blood urea nitrogen | 7.15 | 4.92–10.39 | <0.001 |
| Elevated baseline serum creatinine | 2.99 | 2.00–4.47 | <0.001 |
| Peak serum creatinine >133 μmol/l | 5.88 | 3.90–8.87 | <0.001 |
| Acute kidney injury | |||
| Stage 1 | 3.51 | 1.53–8.02 | 0.003 |
| Stage 2 | 6.24 | 2.73–14.27 | <0.001 |
| Stage 3 | 9.81 | 5.46–17.65 | <0.001 |
The severity was staged based on the guidelines for diagnosis and treatment of coronavirus disease 2019 (trial fifth edition) published by the Chinese National Health Commission on February 4, 2020. Comorbidities include chronic kidney disease, chronic obstructive pulmonary disease, hypertension, diabetes, and tumor.
Figure 3.
Association of kidney disease with in-hospital death in patients with coronavirus disease 2019 (COVID-19). Hazard ratios (HRs) of each variable were obtained using separate proportional hazard Cox models after adjustment for age, sex, disease severity, any comorbidity, and lymphocyte count. The severity was staged based on the guidelines for diagnosis and treatment of COVID-19 (trial fifth edition) published by the Chinese National Health Commission on February 4, 2020. Comorbidities include chronic kidney disease, chronic obstructive pulmonary disease, hypertension, diabetes, and tumor. 95% CI, 95% confidence interval.
Medications
The medications before hospitalization were not available, thus we only summarized the medications during hospitalization in Table 4 . On the first day of admission, antivirus (73.0%), antibiotics (71.0%), and glucocorticoids (36.9%) were the 3 most common medications among patients with COVID-19, and the percentage of treatment with antivirus (P = 0.041) and glucocorticoid (P = 0.006) medications was significant higher in patients with AKI. However, when it comes to specific antivirus medications, including umifenovir, ganciclovir, interferon, lopinavir with ritonavir, oseltamivir, and ribavirin, there is no significant difference between patients with AKI and those without. Throughout the hospitalization, the percentage of medications increased, especially for antivirals, diuretics, and glucocorticoids. In addition, the percentages of diuretics during hospitalization were significantly higher in patients with AKI than in those without (72.2% vs. 6.2%, P < 0.001).
Table 4.
Medications used on admission and during hospitalization
| Drugs | Medications on admission |
Medications during hospitalization |
||||||
|---|---|---|---|---|---|---|---|---|
| All patients | AKI | Non-AKI | P value | All patients | AKI | Non-AKI | P value | |
| RAAS inhibitors | 33 of 701 (4.7) | 0 of 36 (0.0) | 33 of 665 (5.0) | 0.334 | 41 of 701 (5.8) | 0 of 36 (0.0) | 41 of 665 (6.2) | 0.242 |
| Antibiotics | 498 of 701 (71.0) | 27 of 36 (75.0) | 471 of 665 (70.8) | 0.591 | 600 of 701 (85.6) | 35 of 36 (97.2) | 565 of 665 (85.0) | 0.041 |
| Antivirals | 512 of 701 (73.0) | 21 of 36 (58.3) | 491 of 665 (73.8) | 0.041 | 658 of 701 (93.9) | 32 of 36 (88.9) | 626 of 665 (94.1) | 0.357 |
| Umifenovir | 343 of 701 (48.9) | 14 of 36 (38.9) | 329 of 665 (49.5) | 0.216 | 475 of 701 (67.8) | 17 of 36 (47.2) | 458 of 665 (68.9) | 0.007 |
| Ganciclovir | 24 of 701 (3.4) | 0 of 36 (0.0) | 24 of 665 (3.6) | 0.491 | 27 of 701 (3.9) | 0 of 36 (0.0) | 27 of 665 (4.1) | 0.430 |
| Interferon | 129 of 701 (18.4) | 6 of 36 (16.7) | 123 of 665 (18.5) | 0.783 | 169 of 701 (24.1) | 9 of 36 (25.0) | 160 of 665 (24.1) | 0.898 |
| Lopinavir and ritonavir | 74 of 701 (10.6) | 2 of 36 (5.6) | 72 of 665 (10.8) | 0.469 | 196 of 701 (28.0) | 4 of 36 (11.1) | 192 of 665 (28.9) | 0.021 |
| Oseltamivir | 39 of 701 (5.6) | 1 of 36 (2.8) | 38 of 665 (5.7) | 0.707 | 79 of 701 (11.3) | 1 of 36 (2.8) | 78 of 665 (11.7) | 0.166 |
| Ribavirin | 8 of 701 (1.1) | 0 of 36 (0.0) | 8 of 665 (1.2) | 1.000 | 33 of 701 (4.7) | 0 of 36 (0.0) | 33 of 665 (5.0) | 0.334 |
| Antidiabetic | 68 of 701 (9.7) | 3 of 36 (8.3) | 65 of 665 (9.8) | 1.000 | 119 of 701 (17.0) | 4 of 36 (11.1) | 115 of 665 (17.3) | 0.336 |
| Diuretics | 10 of 701 (1.4) | 1 of 36 (2.8) | 9 of 665 (1.4) | 0.412 | 67 of 701 (9.6) | 26 of 36 (72.2) | 41 of 665 (6.2) | <0.001 |
| Glucocorticoids | 259 of 701 (36.9) | 21 of 36 (58.3) | 238 of 665 (35.8) | 0.006 | 387 of 701 (55.2) | 22 of 36 (61.1) | 365 of 665 (54.9) | 0.465 |
AKI, acute kidney injury; RAAS, renin-angiotensin-aldosterone system.
RAAS inhibitors include angiotensin-converting-enzyme inhibitor and angiotensin receptor blocker.
Discussion
In this large prospective cohort study conducted in a tertiary teaching hospital with 3 branches in Wuhan, China, we observed a high prevalence of kidney disease in hospitalized patients with COVID-19. More than 40% of them had evidence of kidney disease, with elevated serum creatinine and BUN values in over 13% of them. Strikingly, the presence of kidney disease was associated with greater in-hospital mortality.
Multiple organ involvement including the liver, gastrointestinal tract, and kidney have been reported during the course of SARS in 20036 and very recently in patients with COVID-19.7 One possible explanation of the high prevalence of kidney involvement at hospital admission is that some the patients with COVID-19 had a past history of chronic kidney disease. Such patients have a proinflammatory state with functional defects in innate and adaptive immune cell populations8 and are known to have a higher risk for upper respiratory tract infection9 and pneumonia.10 Of note, the median time period between the first symptoms and signs of COVID-19 and hospital admission was slightly more than a week in our study. An alternative explanation is that many patients with COVID-19 could not be admitted in the very early stage of disease outbreak because of the acutely increasing, large number of patients and limited availability of hospital beds in Wuhan. Earlier admission to hospital might have helped to prevent disease spread and deterioration.
This is the first study showing an association between kidney involvement and poor outcome in patients with COVID-19. We found that patients with elevated baseline serum creatinine were more likely to be admitted to the intensive care unit and to undergo mechanical ventilation, suggesting that kidney disease on admission represented a higher risk of deterioration. It has been reported previously that kidney injury was associated with an increased risk of death in patients with influenza A virus subtype H1N1 and SARS.5 , 11 In our study, indicators of kidney involvement at admission were associated with a higher risk of in-hospital death even after adjustment for potential confounders. This observation indicated poor prognosis regardless of initial COVID-19 severity and general physical condition. Monitoring kidney function must therefore be emphasized even in patients with mild respiratory symptoms, and altered kidney function should be given particular attention after admission in clinical practice. Early detection and treatment of renal abnormalities, including adequate hemodynamic support and avoidance of nephrotoxic drugs, may help to improve the vital prognosis of COVID-19.
AKI results from an abrupt loss of kidney function and is strongly associated with increased mortality and morbidity.12 We found that patients with elevated serum creatinine were more likely to develop AKI during hospitalization, which is consistent with study in SARS.5 It is therefore important to increase the awareness of AKI in those who entered the hospital with an elevated serum creatinine. In our cohort, the detection rate of AKI in patients with COVID-19 was 5.1%, which is in keeping with recent reports of small sample size1 , 4 , 7 , 13 and much higher than the 0.5% of a large observational study.14 This may be explained by an extremely high proportion of severely sick patients in a previous case series and only 15.7% in the large observational study. In our cohort study, 42.7% of patients were severely ill, and this may explain the higher detection rate of AKI in clinic practice in Wuhan. Importantly, the present method of detecting AKI is mainly based on acute changes in serum creatinine and the frequency of serum creatinine tests has a substantial impact on detection rate.15 In a nationwide cross-sectional survey of hospitalize adult patients in China, the detection rate of AKI was only 0.99% by Kidney Disease: Improving Global Outcomes (KDIGO) criteria.16 After adjusting for the frequency of serum creatinine, determinations of the incidence of AKI in Chinese hospitalized adults rose to 11.6%.17 Thus, to improve early detection of kidney injury, more frequent serum creatinine measurements should be performed in patients with COVID-19.
The etiology of kidney disease involvement in patients with COVID-19 is likely to be multifactorial. First, the novel coronavirus may exert direct cytopathic effects on kidney tissue. This is supported by the detection of polymerase chain reaction fragments of coronavirus in blood and urine in both the patients with the 2003 SARS virus18 and those with COVID-19.4 Recently, it has been shown that the novel coronavirus uses angiotensin converting enzyme 2 (ACE2) as a cell entry receptor, which is identical to that of the SARS-CoV as reported in 2003.19 Recent human tissue RNA-sequencing data demonstrated thatACE2 expression in urinary organs (kidney) was nearly 100-fold higher than in respiratory organs (lung).20 Therefore, the kidney disease may be caused by coronavirus entering kidney cells through an ACE2-dependent pathway. Second, deposition of immune complexes of viral antigen or virus-induced specific immunological effector mechanisms (specific T-cell lymphocyte or antibody) may damage the kidney. However, kidney microscopy specimens from patients with SARS were reported to show a normal glomerular aspect and absence of electron-dense deposits.5 This is not in support of an active immune-mediated glomerulonephritis. Clearly, potential pathological kidney changes in patients with COVID-19 require further study. Third, virus-induced cytokines or mediators might exert indirect effects on renal tissue, such as hypoxia, shock, and rhabdomyolysis. In fact, some of the patients with the 2009 H1N1 virus had mild to moderate elevations of serum creatine kinase.21 In keeping with this observation, 138 patients with COVID-19, who were admitted to an intensive care unit, showed a tendency toward increased creatine kinase levels,13 and the patients with kidney involvement in our study tended to have increased creatine kinase levels as well.
We compared the medications on the first day of admission and during the hospitalization in patients with AKI and non-AKI. We found that patients with AKI were more likely to have higher proportion of glucocorticoid and lower proportion of antiviral drugs and renin-angiotensin-aldosterone system inhibitors treatment on admission. The difference in the use of glucocorticoids may be explained by the condition of patients with AKI that was more severe, thus physicians tended to use glucocorticoids in the most critically ill patients, even if there is a controversy on the use of glucocorticoids in patients with COVID-19.22 , 23 However, the oral antiviral drugs, including umifenovir, oseltamivir, and lopinavir with ritonavir, were preferred in moderate patients on admission. Given that ACE2 is a functional receptor for SARS-CoV-2, the safety and potential effects of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19 should be carefully considered.24 These potentially harmful effects may account for the low proportion of renin-angiotensin-aldosterone system inhibitors used by physicians in our cohort, especially in patients with AKI who had higher level of creatinine on admission. Due to the small number of patients with AKI and the bias in different therapy of patients with COVID-19, causal relationship between drug and AKI in patients with COVID-19 remains undetermined.
Even though this study included a large number of patients from a tertiary teaching hospital in Wuhan, it has several limitations. First, an accurate baseline serum creatinine was not available, which may have led to an underestimation of AKI or erroneous associations. Second, although we attempted to adjust for many confounders, other unmeasured or unknown confounders might have played a role. Third, clinical data of patients after discharge were lacking, so we could not assess COVID-19 effects on long-term outcomes. The precise impact of COVID-19 on kidney structure and function and the incidence of chronic kidney disease in these patients require further investigation.
In conclusion, the prevalence of kidney disease in patients with COVID-19 hospitalized in Wuhan, China, was high. After adjustment for confounders, kidney disease on admission and AKI during hospitalization were associated with an increased risk of in-hospital death. Clinicians should increase their awareness of kidney disease in hospitalized patients with COVID-19. Early detection and effective intervention of kidney involvement may help to reduce deaths of patients with COVID-19.
Methods
Participants
All consecutive patients with COVID-19 admitted to Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, from January 28 to February 11, 2020, were enrolled. Tongji Hospital, located in Wuhan, Hubei Province, the major endemic area of COVID-19, is one of the main tertiary teaching hospitals. Tongji Hospital was assigned responsibility for the treatments of patients with severe COVID-19 by the Wuhan government on January 31. All patients who were enrolled in this study were diagnosed COVID-19–positive according to the guidance provided by the Chinese National Health Commission. The diagnosis criteria were as follow: clinical diagnosis criteria of (i) fever or respiratory symptoms and (ii) leukopenia or lymphopenia; and (iii) the computerized tomography scan showed radiographic abnormalities in lung. Those with ≥2 clinical diagnosis criteria and a positive result to high-throughput sequencing or real-time polymerase chain reaction assay were diagnosed COVID-19–positive.
Pediatric patients are excluded. Patients with a history of maintenance dialysis or renal transplantation were also excluded from the study. Clinical outcomes were monitored up to February 29, 2020, the final date of follow-up.
Data sources
The demographic characteristics, clinical symptoms, laboratory data, and medications were extracted from electronic medical records. Laboratory data consisted of complete blood count; liver and renal function tests; examination of hemostasis parameters; measurement of high-sensitivity C-reactive protein, procalcitonin, lactate dehydrogenase, and creatine kinase serum levels; and erythrocyte sedimentation rate. The thresholds of these measures were given by our laboratory. The upper limits of normal serum creatinine in men and women were 104 μmol/l and 84 μmol/l, respectively. The upper limits of normal BUN in men with age <60, 60 to 80, >80 years were 8.0, 9.5, and 8.3 mmol/l, respectively. The upper limits of normal BUN in women with age <60, 60 to 80, >80 years were 7.5, 8.8, and 8.3 mmol/l, respectively. The data were reviewed by a trained team of physicians. Estimated glomerular filtration rate was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.25 The date of disease onset was defined as the day when the first symptom was noticed. The endpoint was the in-hospital death.
Definition
Severity of the disease was staged according to the guidelines for diagnosis and treatment of COVID-19 (trial fifth edition) published by Chinese National Health Commission on February 4, 2020. Severe cases were defined as (i) respiratory rate > 30 breaths/min, (ii) oxygen saturation ≤ 93%, or (iii) PaO2/FiO2 ratio ≤ 300 mm Hg. Critical severe cases were defined as including ≥1 of the following criteria: shock; respiratory failure requiring mechanical ventilation; combination with other organ failures; and admission to intensive care unit.
AKI was defined as an increase in serum creatinine by 0.3 mg/dl within 48 hours or a 50% increase in serum creatinine from baseline within 7 days according to the KDIGO criteria.26 Baseline serum creatinine was defined as the serum creatinine value on admission. The date of AKI onset was defined as the earliest day of a serum creatinine change meeting KDIGO criteria. The stage of AKI was determined using the peak serum creatinine level after AKI detection, with increases of 1.5 to 1.9, 2.0 to 2.9, and ≥3 times baseline being defined as AKI stage 1, 2, and 3, respectively.
Covariates
Based on review of the literature,14 , 27 , 28 several covariates were selected for analysis as potential confounding variables in our regression analyses. These included age, sex, comorbidities, disease severity, and lymphocyte count.
Statistical analysis
Categorical variables were summarized as percentages, and continuous variables were expressed as the mean ± SD or median with interquartile range. Two-sample t test or Wilcoxon rank-sum test were used for continuous variables and chi-square test or Fisher’s exact test for categorical variables as appropriate. Cumulative rates of in-hospital death were determined using the Kaplan-Meier method. The associations between kidney disease indicators and in-hospital death were examined using Cox proportional hazard regression analysis. Cox proportional hazards assumptions were tested with the Schoenfeld residuals. No violations of the Cox proportional hazards assumptions were detected. In full multivariable models, we assessed interactions of kidney disease indicators with age, sex, comorbidities, disease severity, and lymphocyte count (cutoff P < 0.01). None were eligible for retention. Patients with missing value in proteinuria and hematuria (n = 259) were excluded when assessed the association of those 2 indicators and in-hospital death in Cox proportional hazard model. Statistical analyses were performed using R software (version 3.6.1; R Foundation, Vienna, Austria), with statistical significance set at 2-sided P < 0.05.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors greatly appreciate all the hospital staff for their efforts in recruiting and treating patients and thank all patients involved in this study.
The study protocol and waived written informed consent were approved by the Medical Ethics Committee of Tongji Hospital (No. TJ-C20200132).
This work was financially supported by the International (Regional) Cooperation and Exchange Projects (NSFC-DFG Grant No. 81761138041); National Natural Science Foundation of China (Grant Nos. 81570667, 81470948, 81670633); Major Research Plan of the National Natural Science Foundation of China (Grant No. 91742204); The National Key R&D Program of China (Grant Nos. 2018YFC1314003-1, 2015BAI12B07); and National Key Research and Development Program (Grant No. 2016YFC0906103).
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
GX and SG designed the study. YC, RL, KW, MZ, ZW, LD, JL, and YY collected the data and prepared the figures and tables. YC and SG contributed analytical tools. YC and SG wrote the paper. SG and GX conceived the project and supervised and coordinated all the work.
Contributor Information
Shuwang Ge, Email: geshuwang@tjh.tjmu.edu.cn.
Gang Xu, Email: xugang@tjh.tjmu.edu.cn.
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. [DOI] [PMC free article] [PubMed]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:556–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsang K.W., Ho P.L., Ooi G.C. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;48:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betjes M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Hagai K., Rozenberg I., Korzets Z. Upper respiratory tract infection among dialysis patients. Isr Med Assoc J. 2016;18:557–560. [PubMed] [Google Scholar]
- 10.Sibbel S., Sato R., Hunt A. The clinical and economic burden of pneumonia in patients enrolled in Medicare receiving dialysis: a retrospective, observational cohort study. BMC Nephrol. 2016;17:199. doi: 10.1186/s12882-016-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung J.Y., Park B.H., Hong S.B. Acute kidney injury in critically ill patients with pandemic influenza A pneumonia 2009 in Korea: a multicenter study. J Crit Care. 2011;26:577–585. doi: 10.1016/j.jcrc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Vanmassenhove J., Kielstein J., Jorres A., Biesen W.V. Management of patients at risk of acute kidney injury. Lancet. 2017;389:2139–2151. doi: 10.1016/S0140-6736(17)31329-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China [e-pub ahead of print]. N Engl J Med. https://doi.org/10.1056/NEJMoa2002032. Accessed March 14, 2020. [DOI] [PMC free article] [PubMed]
- 15.Ge S., Nie S., Liu Z. Epidemiology and outcomes of acute kidney injury in elderly Chinese patients: a subgroup analysis from the EACH study. BMC Nephrol. 2016;17:136. doi: 10.1186/s12882-016-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L., Xing G., Wang L. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465–1471. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 17.Xu X., Nie S., Liu Z. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10:1510–1518. doi: 10.2215/CJN.02140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peiris J.S.M., Chu C.M., Cheng V.C.C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z., Wu M., Guo J. Caution on kidney dysfunctions of 2019-nCoV patients 2020. MedRxiv preprint. Available at: Accessed March 14, 2020. [DOI]
- 21.Kumar A., Zarychanski R., Pinto R. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 22.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang L., Zhao J., Hu Y. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system [e-pub ahead of print]. Nat Rev Cardiol.https://doi.org/10.1038/s41569-020-0360-5. Accessed March 14, 2020. [DOI] [PMC free article] [PubMed]
- 25.Levey A.S., Stevens L.A., Schmid C.H. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): a new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 27.Guan W.-J., Liang W.-H., Zhao Y. Comorbidity and its impact on 1,590 patients with COVID-19 in China: a nationwide analysis. MedRxiv preprint. February 27, 2020. Available at: Accessed March 14, 2020. [DOI] [PMC free article] [PubMed]
- 28.Tan L., Wang Q., Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. MedRxiv preprint. March 3, 2020. Available at: Accessed March 14, 2020. [DOI] [PMC free article] [PubMed]