Abstract
Many studies suggest that probiotic, prebiotic and symbiotic foods may be beneficial in the prevention and management of nutrition and health, the objective of this work was to develop a symbiotic drink based on coconut water. Fermentation was performed using lyophilized Lactobacillus rhamnosus SP1 and inulin as a source of soluble fiber. Different formulations were developed, determining the concentrations of fiber and probiotics. The growth of the probiotic in MRS broth was evaluated, using the plate counting technique in different periods of time. The fermentation time of the drink was 8 h and the shelf life in refrigeration was 14 days evaluated by pH and hedonic scale. The pH of the final drink was 3.48 and the probiotic content was 82 × 10 8 CFU/ml. It is concluded that coconut water can be processed by adding probiotic and prebiotic characteristics with sensory acceptance and adequate preservation characteristics.
Keywords: Food science, Food technology, Symbiotic drink, Functional food, L. rhamnosus, Coconut water
Food science, Food technology, Symbiotic drink, Functional food, L. rhamnosus, Coconut water
1. Introduction
Coconut is the fruit of coconut palm (Cocus nucifera), also known as coconut, coconut palm, adiavan or Indian palm, and belongs to the Arecaceae family. It is a palm tree native to the eastern tropical regions, grown in Asia, America and Africa (Ramkhelawan and Paul, 2016).
In recent years the consumption of coconut products such as oil, dry coconut snacks, cosmetic products, bottled coconut water, coconut cream and milk has shown a much faster growth than the production of this tropical fruit (Granados and López, 2002; Prades et al., 2012). Coconut water is the liquid found in a young green coconut and should not be confused with coconut milk, since it refers to liquid products obtained by grating the solid endosperm, with or without the addition of water (Yong et al., 2009).
Coconut water has been used successfully in various parts of the world for oral rehydration, treatment of childhood diarrhea, gastroenteritis and cholera (Balit et al., 2018; Mujahid et al., 2019) and contains organic and inorganic compounds that play a vital role in helping the antioxidant system of the human body (Evans and Halliwell, 2001).
As it easily deteriorates once exposed to the air, most of the coconut water is consumed in its natural form in the areas where it is produced. Coconut water is comercially processed using ultra high temperature technology. However, it loses its delicate fresh flavor and some of its nutrients during heating (Awua et al., 2011; Kailaku et al., 2017), so would be desirable a non-thermal process to protect the fresh flavor and nutrient content of coconut water (Gautam et al., 2017). Until now, there is few studies in which alternative forms of conservation or transformation are proposed.
Consumers demand foods that, in addition to satisfying the taste, are healthy or contribute to their health, this demand can be covered by the so-called functional foods, hence the interest in the development of products through fermentation since these foods and Its components have many possible health benefits (Melini et al., 2019). Fermented foods can be produced with simple economic ingredients and techniques, and can contribute significantly to the human diet, especially in rural homes and communities in villages around the world, so this research aims to develop a symbiotic functional drink using Coconut water as a base, Lactobacillus rhamnosus SP1 as probiotic and inulin as source of soluble fiber in quantity that allows to classify the product as functional and evaluate its probiotic characteristics through techniques that allow its standardization easily.
2. Materials and methods
A lyophilized strain of Lactobacillus rhamnosus SP1 (Clerici-Sacco, Italy), also known as Lactobacillus rhamnosus GG, was selected as it is one of the best studied and documented probiotic strains, with more than 300 clinical studies that have proven its efficacy and safety, is stable in acid and bile, has good adhesion to the mucous cells of the human intestine, and produces lactic acid. In experimental animals it has been shown to improve insulin sensitivity and reduce lipogenesis (Kim et al., 2013; Kun-Young et al., 2015), while in humans it has a positive immunomodulatory effect that could help reduce repeated rotavirus diarrhea episodes (Sindhu et al., 2014).
2.1. Preparation of the starter culture
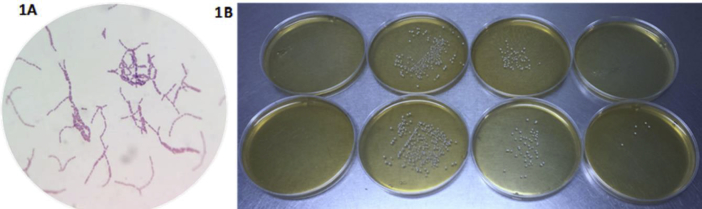
To prepare the starter culture, the contents of the lyophilized strain sachet (1011UFC) were poured into 1 L of sterile distilled water, homogenizing and subsequently cooling to 1.5 °C, the purity was verified via a Gram staining under a microscope, and short chains of gram positive bacilli were observed (Figure 1A). The strain was subcultured twice to obtain an active culture for the preparation of an inoculum by inoculation in De Man Rogosa Sharpe broth (MRS, Merck, Germany) and incubated at 35 °C for 24 h. The culture was then stored at 1.5 °C and maintained on MRS broth until further use. In accordance with NOM-092-SSA1-1994, the CFU/ml plate count of the strain was performed to verify its growth (Figure 1B).
Figure 1.
Purity and viability check of L. rhamnosus SP1 strain. 1A. Microscopic observation of the strain stained by Gram staining, Gram positive short bacilli are observed. 1 B. Plate count according to NOM-092-SSA1-1994 of CFU/ml of the different dilutions of the stock culture of the strain, on the left the negative control.
2.2. Development of the formulation and fermentation of the beverage
Once the viability of the strain was verified, a literature review was conducted to determine fermentable carbohydrates by L. rhamnosus, selecting sucrose and lactose (to improve palatability), and it was decided to use a commercial coconut water, since the different treatments to which the fresh coconut water was subjected for pasteurization caused loss of aroma and development of slightly amber colors. The criteria for water coconut brand selection were that the only ingredient was coconut water (without additives or preservatives) and that would have pasteurized or sterilized by a UHT treatment. As a source of soluble fiber it was used agave inulin (Nupromic ®), because it is known that the intake of inulin and FOS (fructooligosaccharides) cause an increase in short chain fatty acids in the colon, reduction of the pH of the colon and a reduction in serum triglyceride levels, stimulating the growth of some bacteria of the intestinal flora, improves constipation, improves mineral absorption and causes a general stimulation of the immune system (Lomax and Calder, 2008; Tarini and Wolever, 2010), and it has been discovered that consuming from 8 to 15 g of chicory inulin, functional effects occur such as constipation reduction and better quality of life (Marteau et al., 2011). For the fermentation inoculum, 1 ml of the starter culture was inoculated in 99 ml of sterile MRS broth, and then incubation was performed at 35 °C for 24 h. For the development of the beverage formulations, a randomized block design was used and included three treatments with three different concentrations of lactose, sucrose and inulin, for a total of 15 formulations (Table 1), 990ml of each of these formulations was inoculated with 10 ml of the fermentation inoculum, then incubated at 35 °C and the pH was monitored until it decreased at least to pH < 5.0.
Table 1.
Random blocks diagram of the formulas worked.
| Block | Lactose (%) | Sucrose (%) | Inulin (%) |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| A | 0 | 0 | 10 |
| B | 0 | 0 | 15 |
| C | 5 | 0 | 0 |
| D | 5 | 0 | 10 |
| E | 5 | 0 | 15 |
| F | 10 | 0 | 0 |
| G | 10 | 0 | 10 |
| H | 10 | 0 | 15 |
| I | 0 | 5 | 0 |
| J | 0 | 5 | 10 |
| K | 0 | 5 | 15 |
| L | 0 | 10 | 0 |
| M | 0 | 10 | 10 |
| N | 0 | 10 | 15 |
CFU colony forming units.
2.3. Sensory evaluation
A panel of 30 untrained homogenize (30 university students between 20 and 25 years of age) evaluated the sensory attributes of coconut water drinks for color, smell, taste and texture. The test was accomplished based on 5-point hedonic scale by panelists and scaled as 1 = dislike extremely, 2 = dislike moderately, 3 = neither like nor dislike, 4 = like moderately and 5 = like extremely. The samples, each of which received three digit code, served in plastic containers under normal light. The panelists received the random samples. They were asked to rinse their mouth with water between each simple tests.
2.4. Analytical determinations
Once the appropriate formulation was selected based on the sensory evaluation, the following analytical determinations were carried out, both for the fermented beverage and for the coconut water (control).
pH: pH readings were made from the beginning of the incubation every hour to a pH < 5 and then every 3 days with a potentiometer (SM25CW Science Med, Finland), taking 20 ml aliquots of the drink (NMX-F-317-NORMEX-2013), until a minimum of pH 3 is reached, since at this pH all beverages were sensorially unacceptable.
Humidity: by thermobalance (BL-MB45 Ohaus, New Jersey, USA). At a temperature of 70 °C.
Ashes: by the calcination method, bringing the samples at a temperature of 500 °C for 8 h in a muffle (FB1300 Barnstead/Thermolyne, Iowa, USA).
Proteins: by the method of Kjeldahl (KC24800 Labconco, Kansas, USA).
Additionally, the dietary fiber to the fermented beverage was determined by the gravimetric-enzymatic method (AOAC 993.19).
2.5. Statistic analysis
The data obtained were analyzed using a unidirectional analysis of variance, and the Tukey test was used to evaluate the differences between treatments. All statistical analyzes were performed with the statistical package SPSS version 21 for Windows (IBM, Corp., Armonk, NY, USA). Significant differences were considered if p < 0.05, and the results were expressed as mean values ± standard deviations.
2.6. Ethical aspects
The present study was presented to the Biochemistry Departmental Review Board, who after having made a series of questions concerning the safety of the study, informed participation and confidentiality of the information, decided to approve the study. All the tasters signed the consent form before performing sensory analyses.
3. Results and discussion
Currently, symbiotic foods (with mixtures of probiotics and prebiotics) are often used to take advantage of their synergistic effects in the application to food products (Al-Sheraji et al., 2013), and represent the new challenge for functional beverages, since prebiotics can improve the viability of probiotic bacteria and actively stimulate the beneficial microbiota in the human gastrointestinal tract (Corbo et al., 2014).
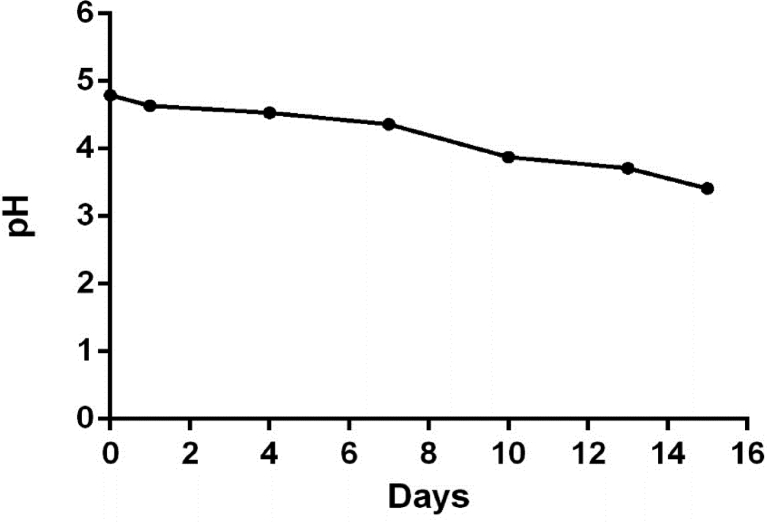
Once the viability and purity of the strain was verified, the different formulations shown in Table 1 were developed. The carbohydrates selected to add as carbon source were lactose and sucrose, both fermentable by Lactobacillus sp. (Camargo-Prado et al., 2015), however, in the case of lactose, it would have the disadvantage of not being suitable for the consumption of intolerant people. With regard to the prebiotic to be added, in addition to the multiple benefits reported in the literature for inulin, an important aspect is that the addition of 5% inulin increases the survival capacity of Lactobacillus spp. (Önal Darilmaz et al., 2019) with the additional advantage of further improving its activity against E. coli, so the initial percentage of inulin addition was 5% and it was decided to increase it to 15%, looking for the developed beverage to be prebiotic to show other possible functional effects in addition to those due to the probiotic (symbiotic). Maintaining in all formulations an inoculum of 1% starter culture, the incubation was carried out at 35 °C and pH readings were made every 3 days until reaching a pH of 3, since from this value the sensory characteristics of the beverage was no longer properly preserved (Figure 2), which determines that the shelf life of the beverage in refrigeration once processed, is approximately 15 days.
Figure 2.
pH variation during storage of symbiotic drink.
In order to evaluate the repeatability of the formula and establish the time and temperature of incubation, 5 formulations in 200 ml bottles were prepared for one week, and allowed to incubate in the oven at 35 °C until the desired pH was reached and after that they were refrigerated to 1.5 °C, obtaining in each case a very similar drink in each processing batch (Figure 3) and establishing that the initial inoculum must be 1% of the starter culture and the incubation conditions 35 °C for 8 h.
Figure 3.
Batch of replicas of the symbiotic drink developed. It was verified that the final formulation was reproducible; batches of the beverage made on five consecutive days, after 8 h of incubation are shown.
It was decided to employ untrained panelists because the objective was to determine the reaction of the potential consumer to the beverage and, according to Watts et al. (1989), with untrained panelists it is possible to obtain information about likes and dislikes, preferences and acceptability requirements. The results of the sensory evaluation are shown in Table 2. The color was statistically the same in all the formulations, however, in relation to other characteristics (smell, taste and texture), the lactose-containing formulations obtained the lowest scores, this finding was very favorable since it allowed to discard lactose as an ingredient and develop a suitable drink for lactose intolerant people. Of the formulations with lactose, the only one that was not rejected was the 10% formulation but without inulin which would lead to a probiotic but not symbiotic drink. Significantly, the formulations K and M, both with sucrose, were the most accepted in terms of taste and general acceptance, without differences between them, so the formulation K was finally selected because it contained less sucrose and more fiber so it could be considered symbiotic.
Table 2.
Comparison of sensory analysis in different formulations.
| Coconut water | A | B | C | D | E | F | G | H | I | J | K | L | M | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smell | 2.8 ± 0.84 | 3 ± 0.91 | 3.2 ± 0.95 | 3.13 ± 0.86 | 2.97 ± 0.93 | 3.23 ± 0.89l | 3.4 ± 1l | 2.83 ± 0.79 | 2.7 ± 1.1 | 3.17 ± 0.91 | 3.15 ± 1.1 | 3.43 ± 0.85l | 2.37 ± 0.92n | 3.2 ± 1.03 | 3.27 ± 1.28 |
| Color | 3.43 ± 0.67 | 3.53 ± 0.73 | 3.63 ± 0.61 | 3.57 ± 0.72 | 3.4 ± 0.62 | 3.73 ± 0.64 | 3.5 ± 0.71 | 3.27 ± 0.64 | 3.4 ± 0.62 | 3.63 ± 0.66 | 3.3 ± 0.91 | 3.63 ± 0.71 | 3.27 ± 0.7 | 3.87 ± 0.81 | 3.63 ± 0.93 |
| Taste | 2.03 ± 0.89f,g,h,i,k,m | 2.1 ± 0.92 f,g,i,k,m |
2.57 ± 1.35k,m | 2.57 ± 1.19k,m | 2.4 ± 1.22 f,k,m |
2.57 ± 1.07k,m | 3.47 ± 0.93j | 3.07 ± 1.1j | 3 ± 1.31j,k | 3.27 ± 0.86j | 1.67 ± 0.62 k,l,m |
3.97 ± 0.72l,n | 2.67 ± 1.4 | 3.53 ± 1.04 | 2.63 ± 1.27 |
| Texture | 3.2 ± 0.88 | 3.23 ± 0.94 | 3.4 ± 0.89 | 3.13 ± 1i,k | 3.23 ± 1 | 3.2 ± 0.88 | 3.63 ± 0.72 | 3.7 ± 0.92 | 3.57 ± 1 | 4 ± 0.69j | 2.85 ± 1k,m | 4 ± 0.72l | 3.17 ± 1.23 | 3.77 ± 0.97 | 3.43 ± 1.16 |
| General acceptance | 2.9 ± 0.5 f,i,k,m |
2.9 ± 0.6k,m | 3.1 ± 0.6 | 3.2 ± 0.6k | 2.9 ± 0.7k | 3.2 ± 0.7 | 3.5 ± 0.7j,l | 3.2 ± 0.6 | 3.2 ± 0.8k | 3.4 ± 0.5j,l | 2.7 ± 0.6k,m | 3.7 ± 0.4l | 2.8 ± 0.7m | 3.6 ± 0.7 | 3.2 ± 0.9 |
Data are expressed as the mean ± standard deviation.
a: Significantly different respect formulation A, b: Significantly different respect formulation B, c: Significantly different respect formulation C, d: Significantly different respect formulation D, e: Significantly different respect formulation E, f: Significantly different respect formulation F, g: Significantly different respect formulation G, h: Significantly different respect formulation H, i: Significantly different respect formulation I, j: Significantly different respect formulation J, k: Significantly different respect formulation K, l: Significantly different respect formulation L, m: Significantly different respect formulation M, n: Significantly different respect formulation N; via repeated one-way ANOVA followed by Tukey's test.
One of the factors that most influence eating behavior, along with cost, safety and accessibility is the sensory aspect (Włodarska et al., 2019), so the main criterion for the choice of the final formula was the sensory evaluation, also because the sensory effect derived from the inclusion of probiotics in products of plant origin is critical in the development of functional drinks (Ozcan et al., 2016).
This parameter has been explored by some authors, for example, salty, sour and scented flavors have been reported in fruit drinks with the addition of probiotics (Perricone et al., 2015). Luckow and Delahunty (2004) concluded that orange juices with probiotics (Lactobacillus rhamnosus GG, Lactobacillus casei imunitass®, Lactobacillus paracasei NFBC 43338) presented unpleasant sensory profiles described as medicinal flavors and without dairy touches. However, it is suggested that exposure and familiarity with probiotic drinks helps improve consumer acceptance and taste for the sensory characteristics of fruit drinks with probiotics (Luckow et al., 2005).
Once the formulation was selected, the corresponding physicochemical analysis was performed. Table 3 shows the composition of the developed beverage. It can be seen that the proximal composition in the developed beverage is very similar to that reported for coconut water, however, according to different studies, it can be very variable, so it is difficult to establish comparisons (Prades et al., 2012; Yong et al., 2009).
Table 3.
Percentage composition of the symbiotic drink developed and coconut water used compared to values reported in the literature.
| Symbiotic drink | Coconut water used | Prades et al. (2012) | Yong et al. (2009) | |
|---|---|---|---|---|
| Dry material | 25.07 ± 0.02 | 6.0 ± 0.08 | 4.5 ± 0.7 | 5.01 |
| Humidity | 74.93 ± 0.02 | 94 ± 0.08 | NR | 94.99 |
| Ashes | 0.40 ± 0.01 | 0.45 ± 0.01 | 0.43 ± 0.04 | 0.39 |
| Protein | 0.04 ± 0.002 | 0.19 ± 0.002 | 0.25 ± 0.26 | 0.72 |
| Fat | ND | ND | 0.51 ± 0.33 | 0.20 |
| Dietary fiber | 12.43 ± 0.19 | ND | NR | 1.1 |
| Nitrogen Free Extract | ND | 5.2 | NR | 3.71 |
ND not detected, NR not reported.
Few processes have been reported for the transformation of coconut water, some of the works developed so far include a patent granted to Soccol (2009) and subsequently published (Camargo-Prado et al., 2015) of a fermented beverage with Lactobacillus plantarum using coconut water added with yeast extract, sugar and soy protein hydrolyzate; the cobiotication of coconut water with Bacillus clausii and Saccharomyces boulardii (Rachana, 2017); the development of a hydration drink without fermentation based on serum and up to 30% coconut water (Murillo-Calderón, 2015); a drink fermented with Lactobacillus plantarum DW12 but of mature coconut water, which has different characteristics to those of immature coconut water, added with glutamate and fermented sugar cane or honey up to 20% (Kantachote et al., 2017), and a drink based on coconut water but fermented with Bacillus coagulans and oriented mainly to determine the probiotic potential of this bacterium (Gangwar et al., 2018), so these results allow us to point out that it is possible to develop a symbiotic drink with proximal characteristics quite similar to those of coconut water with the additional contribution of the reported beneficial effects on the health of L. rhamnosus and the content of dietary fiber that makes it possible to consider the developed beverage as symbiotic.
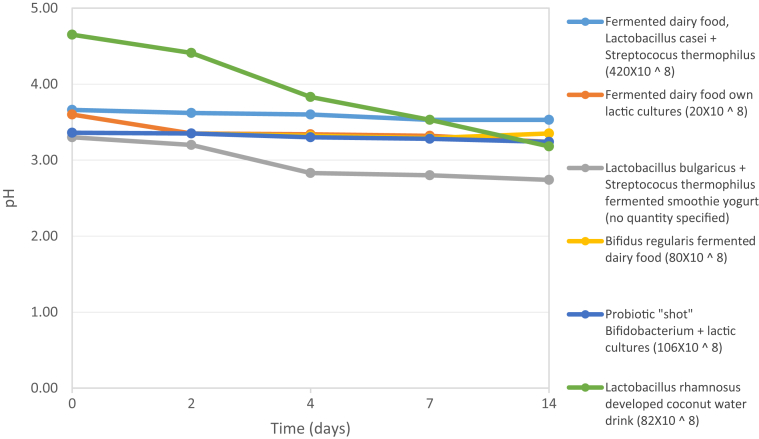
As a quick method to evaluate the growth of Lactobacillus rhamnosus, the use of the MacFarland scale and spectrophotometric readings were evaluated, however, due to the characteristics of the beverage, this method was not viable and the variation in pH with respect to the time compared to some commercial probiotic products was used as reference (Figure 4). Based on the pH, it could be concluded that the developed beverage is stable with respect to the pH compared to similar commercial products, the slow but continuous decrease of the pH in the developed beverage is probably due to the inulin content, since according to several researchers, It can be used by Lactobacillus spp. as carbon source (Önal Darilmaz et al., 2019; Balthazar et al., 2018; De Souza et al., 2012).
Figure 4.
pH of commercial probiotic drinks vs developed beverage.
4. Conclusions
It was possible to develop a fermented coconut water formulation with L. rhamnosus stable in relation to physical and chemical characteristics, further, the viability of L. rhamnosus was above the minimum required to be considered probiotic.
The fermented beverage developed has a good sensory acceptance and meets the criteria of functional feeding, since it has an amount of 82 × 10 8 CFU/ml of L. rhamnosus and 15 g of inulin. The shelf life of the product developed in refrigeration was 15 days.
The critical characteristic for the shelf life of coconut water proved to be of a sensory nature, related to the appearance of flavors, odors and undesirable appearance, probably caused by residual enzymatic activity of the product. The critical parameter would be the decrease in consumer satisfaction, after day 15 of storage at 1.5 °C.
Declarations
Author contribution statement
Orietta Segura-Badilla, Martín Lazcano-Hernández, Obdulia Vera-López, Joaquín Ramírez-Calixto: Performed the experiments; Wrote the paper.
Ashuin Kammar-García, Patricia Aguilar-Alonso: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Addí Rhode Navarro-Cruz: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Al-Sheraji S.H., Ismail A., Manan M.Y., Mustafa S., Yusof R.M., Hassan F.A. Prebiotics as functional foods: a review. J. Funct. Foods. 2013;5(4):1542–1553. [Google Scholar]
- Awua A.K., Doe E.D., Agyare R. Exploring the influence of sterilisation and storage on some physicochemical properties of coconut (Cocos nucifera L.) water. BMC Res. Notes. 2011;4 doi: 10.1186/1756-0500-4-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balit T., Asae A., Boonyoung P., Chanchula K., Hiranphan P., Panityakul T., Radenahmad N. Optimal doses and neuroprotective effects of prolonged treatment with young coconut juice in orchidectomized rats. A preliminary study. Songklanakarin J. Sci. Technol. 2018;40(2):475–483. [Google Scholar]
- Balthazar C.F., Silva H.L.A., Esmerino E.A., Rocha R.S., Moraes J., Carmo M.A.V. The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chem. 2018;246:464–472. doi: 10.1016/j.foodchem.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Camargo-Prado F., De Dea-Lindner J., Inaba J., Thomaz-Soccol V., Kaur-Brar S., Soccol C.R. Development and evaluation of a fermented coconut water beverage with potential health benefits. J. Funct. Foods. 2015;12:489–497. [Google Scholar]
- Corbo M.R., Bevilacqua A., Petruzzi L., Casanova F.P., Sinigaglia M. Functional beverages: the emerging side of functional foods. Compr. Rev. Food Sci. Food Saf. 2014;13(6):1192–1206. [Google Scholar]
- De Souza R.P., Perego P., de Oliveira M.N., Converti A. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT - Food Sci. Technol. 2012;47(2):358–363. [Google Scholar]
- Evans P., Halliwell B. Micronutrients: oxidant/antioxidant status. Br. J. Nutr. 2001;85:67–74. [PubMed] [Google Scholar]
- Gangwar A.S., Bhardwaj A., Sharma V. Fermentation of tender coconut water by probiotic bacteria Bacillus coagulans. Int. J. Food Stud. 2018;7(1):100–110. [Google Scholar]
- Gautam D., Umagiliyage A.L., Dhital R., Joshi P., Watson D.G., Fisher D.J., Choudhary R. Nonthermal pasteurization of tender coconut water using a continuous flow coiled UV reactor. LWT - Food Sci. Technol. 2017;83:127–131. [Google Scholar]
- Granados D., López G.F. Manejo de la palma de coco (Cocos nucifera L.) en México. Revista Chapingo. Serie Ciencias Forestales y del Ambiente. 2002;8:39–48. [Google Scholar]
- Kailaku S., Setiawan B., Sulaeman A. The shelf life estimation of cold sterilized coconut water. Planta Tropika J. Agro Sci. 2017;5(1):62–69. [Google Scholar]
- Kantachote D., Ratanaburee A., Hayisama-ae W., Sukhoom A., Nunkaew T. The use of potential probiotic Lactobacillus plantarum DW12 for producing a novel functional beverage from mature coconut water. J Funct Foods. 2017;32:401–408. [Google Scholar]
- Kim S.W., Park K.Y., Kim B., Kim E., Hyun C.K. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem. Biophys. Res. Commun. 2013;431(2):258–263. doi: 10.1016/j.bbrc.2012.12.121. [DOI] [PubMed] [Google Scholar]
- Kun-Young P., Bobae K., Chang-Kee H. Lactobacillus rhamnosus GG reverses insulin resistance but does not block its onset in diet-induced obese mice. J. Microbiol. Biotechnol. 2015;25(5):753–757. doi: 10.4014/jmb.1409.09016. [DOI] [PubMed] [Google Scholar]
- Lomax A., Calder P. Prebiotics, immune function, infection and inflammation: a review of the evidence. Br. J. Nutr. 2008;101(5):633–658. doi: 10.1017/S0007114508055608. [DOI] [PubMed] [Google Scholar]
- Luckow T., Delahunty C. Which juice is “healthier”? A consumer study of probiotic non-dairy juice drinks. Food Qual. Prefer. 2004;15:751–759. [Google Scholar]
- Luckow T., Sheehan V., Delahunty C., Fitzgerald G. Determining the odor and flavor characteristics of probiotic, healthpromoting ingredients and the effects of ripéate esposaré on consumer acceptance. J. Food Sci. 2005;70(1):S53–59. [Google Scholar]
- Marteau P., Jacobs H., Cazaubiel M., Signoret C., Prevel J.M., Housez B. Effects of chicory inulin in constipated elderly people: a double-blind controlled trial. Int. J. Food Sci. Nutr. 2011;62(2):164–170. doi: 10.3109/09637486.2010.527323. [DOI] [PubMed] [Google Scholar]
- Melini F., Melini V., Luziatelli F., Ficca A.G., Ruzzi M. Health-promoting components in fermented foods: an up-to-date systematic review. Nutrients. 2019;11(5):1–24. doi: 10.3390/nu11051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid I., Mulyanto A., Khasanah T.U. The effectiveness of coconut water in inhibiting shigella sp. bacteria from diarrhea. Medisains. 2019;17(1):8. [Google Scholar]
- Murillo-Calderón L.A. 2015. Desarrollo de una Bebida Hidratante Elaborada a Base de Agua de Coco y Suero de Leche Siguiendo la Normativa Para Bebidas Isotónicas.https://www.dspace.espol.edu.ec/retrieve/89003/D-88108 Retrieved from. [Google Scholar]
- NMX-F-317-NORMEX . Normas Mexicanas. Dirección General de Normas; 2013. Foods-determination of pH in foods and beverages-potenciometric method-test method. [Google Scholar]
- NOM-092-SSA1-1994, bienes y servicios. Método para la cuenta de bacterias aerobias en placa, 1994.
- Önal Darilmaz D., Sönmez Ş., Beyatli Y. The effects of inulin as a prebiotic supplement and the synbiotic interactions of probiotics to improve oxalate degrading activity. Int. J. Food Sci. Technol. 2019;54(1):121–131. [Google Scholar]
- Ozcan O., Ozcan T., Yilmaz-ersan L., Akpinar-bayizit A., Delikanli B. 2016. The Use of Prebiotics of Plant Origin in Functional Milk Products the Use of Prebiotics of Plant Origin in Functional Milk Products; pp. 14–22. (April) [Google Scholar]
- Perricone M., Bevilacqua A., Altieri C., Sinigaglia M., Corbo M.R. Challenges for the production of probiotic fruit juices. Beverages. 2015;1:95–103. [Google Scholar]
- Prades A., Dornier M., Diop N., Pain J.P. Coconut water uses, composition and properties: a review. Fruits. 2012;67:87–107. [Google Scholar]
- Rachana P. Studies on development of probioticated coconut water. Online Int. Interdiscipl. Res. J. 2017;7:9–17. [Google Scholar]
- Ramkhelawan E., Paul C. International Trade Centre; Geneva, Switzerland: 2016. Coconut Production Technology. [Google Scholar]
- Sindhu K.N., Sowmyanarayanan T.V., Paul A., Babji S., Ajjampur S.S., Priyadarshini S., Sarkar R., Balasubramanian K.A., Wanke C.A., Ward H.D., Kang G. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2014;58(8):1107–1115. doi: 10.1093/cid/ciu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccol, C. R., 2009. Processo tecnológico para a produção de uma bebida fermentada a base de água de coco com propriedades probióticas. Brazilian Patent No. PI0703244-7 A2.
- Tarini J., Wolever T. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl. Physiol. Nutr. Metabol. 2010;35(1):9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- Watts B.M., Ylimaki G.L., Jeffery L.E., Elias L.G. International Development Research Center; Ottawa: 1989. Basic Sensory Methods for Food Evaluation; pp. 60–63. [Google Scholar]
- Włodarska K., Pawlak-Lemánsk a K., Górecki T., Sikorska E. Factors influencing consumers’ perceptions of food: a study of apple juice using sensory and visual attention methods. Foods. 2019;8:545. doi: 10.3390/foods8110545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J.W.H., Ge L., Ng Y.F., Tan S.N. The chemical composition and biological properties of coconut (Cocos Nucifera L.) water. Molecules. 2009;14:5144–5164. doi: 10.3390/molecules14125144. [DOI] [PMC free article] [PubMed] [Google Scholar]