Abstract
Recently, a novel verocytotoxin named VT2y was described which belongs to the STx family and is produced by Escherichia coli isolated from domestic poultry with swollen head syndrome (SHS). The VT2y toxin induced apoptosis in Vero, HeLa, CHO, CEF (primary chicken embryo fibroblast) and PCK (primary chicken kidney) cell lines. Morphological evidence (nuclear shrinkage, chromatin condensation and blebbing of the plasma membrane) of apoptosis could be distinguished in 15 min and was maximal at 1 h after treatment with VT2y. This was confirmed by the terminal dUTP nick-end-labeling (TUNEL) method.
Keywords: Verocytotoxin, Apoptosis, Swollen head syndrome, Escherichia coli
1. Introduction
Swollen head syndrome (SHS) in avian species was first described by Morley and Thomson [16] who attributed the disease to a mixed infection with Escherichia coli and coronavirus, paramyxovirus, or pneumovirus in which the initial viral infection caused acute rhinitis that permitted E. coli to invade the subcutaneous facial tissues [19]. This acute respiratory disease observed in domestic poultry is characterized by swelling of the peri- and infra-orbital sinuses, torcicollis, opisthotonus and lack of coordination [16,23]. Since the syndrome was described in South Africa, in 1984, there has been progressive spread to many other countries.
Verocytotoxin-producing E. coli (VTEC) are associated with infant diarrhea, hemorrhagic colitis and hemolytic–uremic syndrome in humans, dysentery in calves, edema disease (ED) of pigs and, more recently, SHS in birds [6,18,20,26]. VTEC strains produce one or more VTs: VT1, and several variants of VT2. The VT2v is produced by E. coli isolated from pigs with ED [11]. Recently Parreira and Yano [20] described a novel cytotoxin called VT2y and sequencing of a 1560-bp DNA fragment of VT2y showed that it contained the STx gene of Shigella dysenteriae, but the region 300 bp upstream of the start of the gene for the STxA subunit was identical to the corresponding sequence for E. coli O111:H-, except for 1 nucleotide, suggesting that VT2y is a member of the STx family [22].
The aim of this study is to illustrate the morphological and intracellular alterations induced in vitro by cytotoxin VT2y in Vero, HeLa, CHO, HEp-2, PCK and CEF cell lines.
2. Materials and methods
2.1. Bacterial strains
E. coli SHS-8 strain isolated from chickens with SHS was used as a source of VT2y [21]. E. coli strain K12C600 was used as a negative control and strain J2 (O157:H-VT2) was used as a source of VT2 (kindly donated by Yoshifumi Takeda, Japan) in these experiments. All E. coli strains were stored at −20°C in Luria–Bertani (LB) [14] broth to which 15% glycerol was added.
2.2. Purification of VT2y
The cytotoxin VT2y was purified [21], and protein content was assayed as described by Bradford [2].
2.3. Cytotoxicity assays
The cytopathic effect (CPE) of purified VT2y was examined in Vero (African green monkey kidney), HeLa (human epitheloid cervical carcinoma), CHO (Chinese hamster ovary), HEp-2 (human larynx epidermoid carcinoma), PCK (primary chicken kidney) cell lines obtained from the Laboratory of Cell Culture, DMI, UNICAMP, Brazil; CEF (chicken embryo fibroblast) cells were generously supplied by Fort Dodge Laboratories, Inc. Campinas, Brazil. All cell lines were maintained as described by MacLeod and Gyles [10]. The cells were grown at 37°C in Eagle's minimal essential medium (EMEM) with Earle's salts (Cultilab, Campinas, SP, Brazil), supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA), penicillin (1000 U ml−1) and streptomycin (250 μg ml−1). To determine cytotoxic activity [18], 100 μl of medium and 2×105 cells per well were placed in 96-well plastic plates (Costar, Cambridge, MA, USA). After 24 h, culture medium was aspirated and 150 μl of fresh EMEM without FBS, and 50 μl (9 ng ml−1) of purified VT2y were added to each well. The plates were incubated at 37°C in a 5% CO2 atmosphere for 24 h. Morphological changes were observed over the 24-h period and the test was scored as positive if more than 50% of the cells exhibited cytotoxic effects. Control E. coli strains were J2 (O157:H-VT2) and K12C600.
2.4. Cellular viability assay
The cellular viability was determined using a neutral red (2-amino-3-methyl-7-dimethyl-amino-phenazoniumchloride) assay [1].
The neutral red assay was performed in Vero, HeLa, CEF, PCK and CHO cells. Purified VT2y cytotoxin was applied to the cells and after 1 h, the medium was substituted with 0.2 ml of neutral red solution (50 μg ml−1) per well and the plate incubated at 37°C in 5% CO2 for 3 h, to allow for dye incorporation. Afterwards, the dye medium was removed and each well was rapidly washed with formol–calcium solution (10% formaldehyde and 10% calcium chloride) to remove unincorporated neutral red; 0.2 ml of solution containing 1 ml acetic acid with ethanol 50% was added to each well and the plate kept for 15 min at room temperature to extract the dye from the cells.
Controls consisted of toxin-treated and untreated cells but without addition of neutral red, (blank). Normal cells, without addition of cytotoxin, but with addition of neutral red, were utilized as controls of 100% with viable cells.
The color reaction was measured with an ELISA plate reader (Multiskan Bichromatic) at 540 nm.
The CD50, was defined as the minimum amount of VT2y required to kill 50% of the cells (1 CD50), and was determined from the toxin concentration resulting in 50% neutral red absorbance compared to control cells as 100%.
2.5. Determination of lactate dehydrogenase (LDH)
Leakage of the cytosolic enzyme LDH, was assessed [15] to investigate whether VT2y affected the integrity of the plasma membrane of Vero cells. Medium from Vero cells treated with 3 CD50 of VT2y or without VT2y was collected after 15, 30, 60, 90 min and immediately chilled on ice until measurement of LDH activity. A fraction of each sample was added to a cuvette containing sodium pyruvate in 1 ml freshly prepared potassium phosphate buffer (0.1 M, pH 7.4) and the mixture was equilibrated for 30 min at 37°C. The enzyme activity was determined spectrophotometrically and the rate of enzyme leakage min−1 was calculated and expressed as percentage of total LDH activity as compared to untreated control cells.
2.6. Morphological characterization of apoptosis induced by VT2y in Vero, HeLa, CEF, PCK and CHO cells
The Vero, HeLa, CEF, PCK and CHO cells were grown on coverslips in 24-well plates using 104 cells ml−1 per well in a volume of 1 ml. The plates were incubated at 37°C in 5% CO2. After 24 h, culture medium was aspirated and replaced with 1 ml of fresh medium. Then, 3 CD50 VT2y was added to each well, and incubated for the same time intervals as in the LDH assay. After incubation, the coverslips were washed with PBS, fixed in 10% formaldehyde solution in PBS for 1 h, then washed with distilled water and stained with 0.025% toluidine blue in McIlvaine pH 4.0 buffer [13].
The coverslips were washed for 15 min with distilled water, air-dried, cleared in xylene and mounted using Entellan (Merck, Germany).
2.7. DNA fragmentation assay in Vero cells
DNA fragmentation in VT2y-treated and untreated Vero cells was investigated in situ by the terminal dUTP nick-end-labeling (TUNEL) method using an In situ cell death detection POD kit (Roche, Mannheim, Germany). Experiments were performed according to the manufacturer's protocol.
3. Results
3.1. Cytotoxic activity
The VT2y was cytotoxic to Vero, HeLa, CEF, PCK and CHO cells, but not for HEp-2 cells. By light microscopy the cytotoxin-affected cells appeared round and shrivelled, and the cells were dead within 1 h.
3.2. Effect of VT2y on cell viability
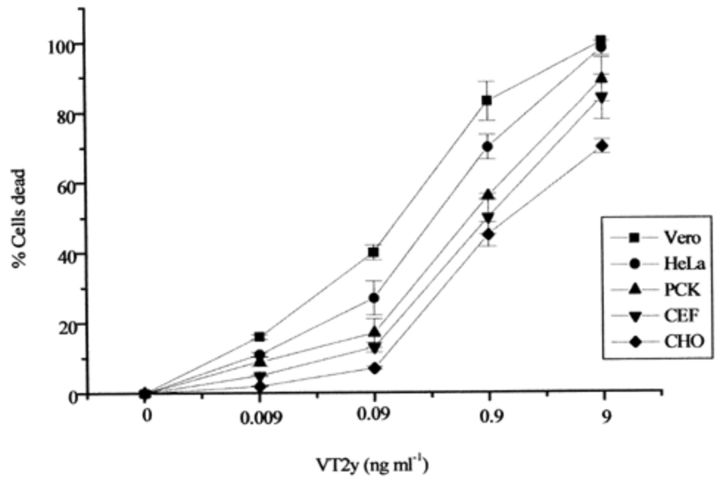
The neutral red assay showed that Vero cells were more sensitive than other cells tested since 50% cell death was observed in the presence of 0.1 ng ml−1 VT2y. A linear increase in the percentage of dead cells was found as protein concentration was increased within the range indicated in Fig. 1.
1.
Effect of treatment for 1 h of various concentrations of VT2y on Vero, HeLa, PCK, CEF and CHO cells as determined by neutral red assay. The experiment was repeated twice and mean values, ±S.D., are shown.
3.3. LDH leakage
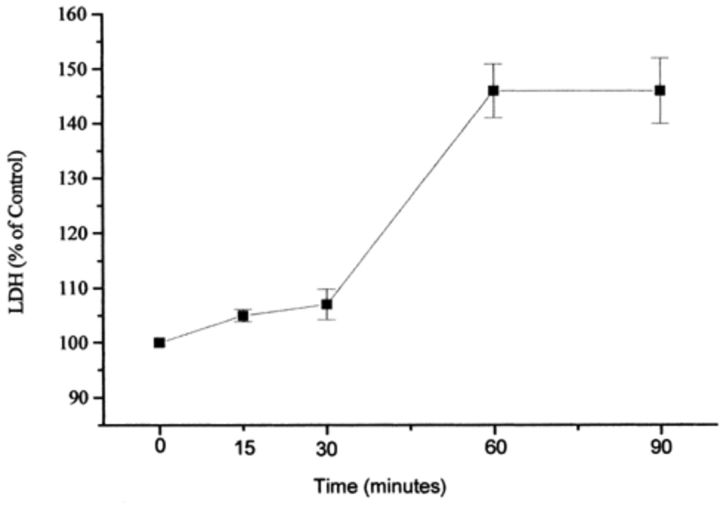
After 15 min minimal (5%) LDH leakage was observed as compared to the control (Fig. 2), but by 60 min an increase to 46% above control LDH leakage had occurred.
2.
LDH leakage after VT2y exposure expressed as a percentage of control values. The experiment was repeated twice and mean values, ±S.D., are shown.
3.4. Kinetic study of the morphological alteration in Vero cells induced by VT2y
The cytotoxin VT2y caused morphological alterations in Vero cells, such as cytoplasm vacuolization, blebbing of the plasma membrane, chromatin condensation within the nucleus, nuclear shrinkage and apoptotic bodies within 15 min. The effects became more pronounced with time to affect the majority of cells. By 1 h, 100% cell death was observed (data not shown).
3.5. Detection of apoptosis in cells after treatment with verotoxin (VT2y)
When cells were cultured in the presence of VT2y, they showed condensed chromatin (Fig. 3B), cleaved nuclei (Fig. 3C) and cytoplasmic blebbing (Fig. 3D), findings typical of apoptosis. We also observed the presence of cytoplasmic vacuoles in conjunction with apoptotic features (Fig. 3C). However, none of these features occurred in control cells (Fig. 3A).
3.
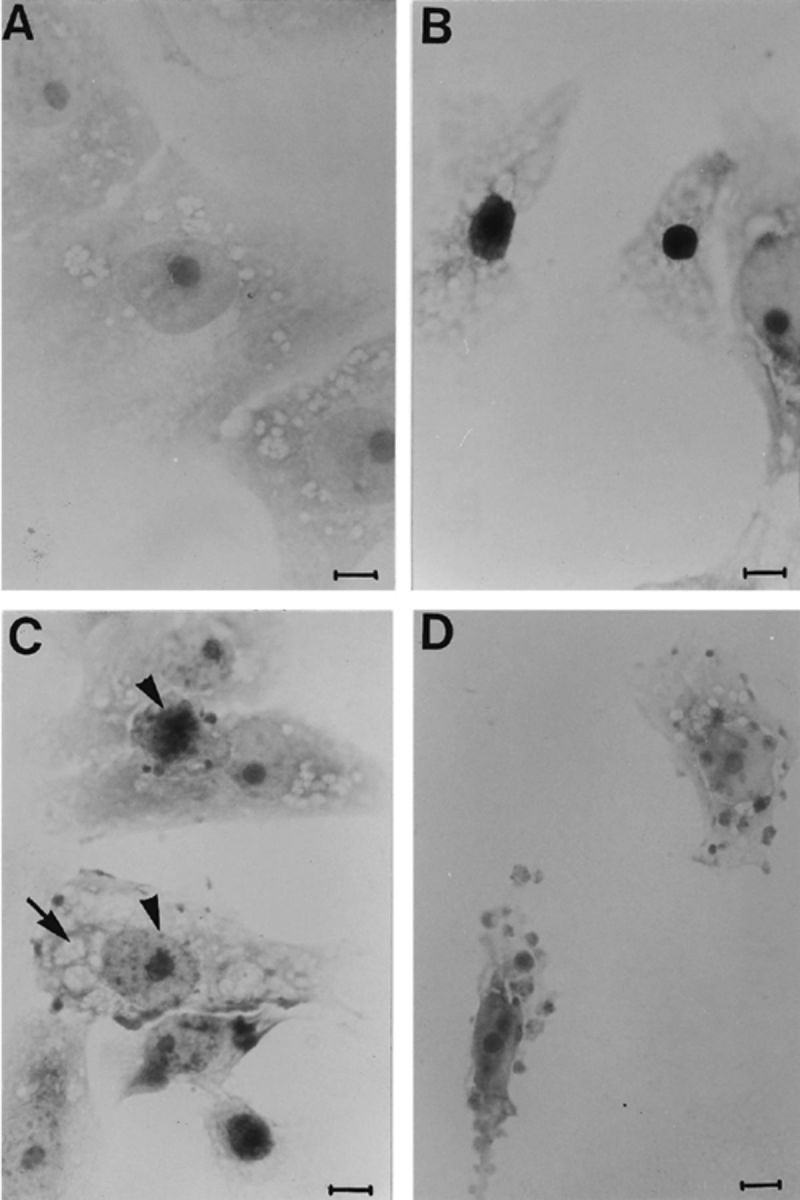
Light Microscopy. Vero cells incubated with culture supernatant from E. coli negative control strain K12C600 (A), and Vero cells 1 h after VT2y treatment (B) showed chromatin condensation, (C) cleaved nuclei (small arrowhead) and cytoplasmic vacuolization (long arrow), (D) formation of cytoplasmic blebbing. Bars, 10 μm. These features are characteristic of apoptosis following treatment with VT2y.
The cleavage of nuclear DNA, a major feature of apoptosis, occurred during VT-induced death of Vero cells, as confirmed by in situ detection by the TUNEL method (Fig. 4B). The same Vero cells clearly incorporated biotinylated nucleotides, indicating the occurrence of DNA fragmentation induced by VT2y, in contrast with control cells (Fig. 4A).
4.
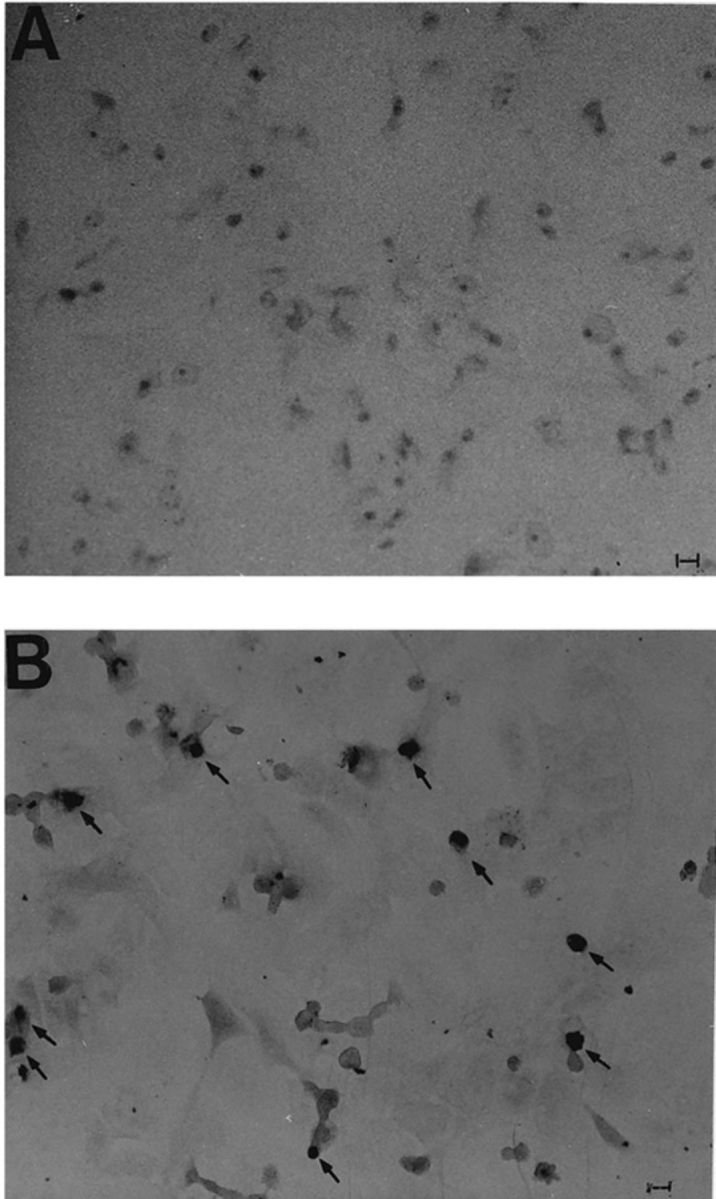
The in situ detection of nuclear DNA cleavage in Vero cells after VT2y treatment. To test the cleavage of nuclear DNA, Vero cells were treated with (B) and without (A) VT2y for 15 min and tested by the TUNEL method. Bars, 100 μm. Typical positive cells are indicated by arrows.
4. Discussion
Programmed cell death, or apoptosis, is defined by the cell's ultrastructural morphology [7] and is characterized by cell shrinkage, membrane blebbing, and condensation of nuclear chromatin. These morphological changes are accompanied by DNA fragmentation, and this form of cell death is a natural process by which an organism removes damaged or unnecessary cells. It may also be triggered by external stimuli [12]. Recently, reports have claimed that VTs induce programmed cell death, apoptosis, in cell lines such as Vero, MDCK [5,25] and others.
In our studies, we showed evidence of morphological alterations characteristic of apoptosis (Fig. 3) in three mammalian cell (Vero, HeLa and CHO) and two avian cell lines (PCK and CEF), but not in HEp-2 cells. The most sensitive line to VT2y was Vero cells (Fig. 1), probably due to receptor specificity of the cells, as it is known that variants of VT2 are more cytotoxic to Vero cells than HeLa cells [4]; the receptor for VT1 and VT2 is globotriosyl ceramide (GB3) [9], whereas the preferred receptor for VT2v is globotetraosyl ceramide (GB4) [24]. It will be interesting to study the receptor for this toxin on the avian cells PCK and CEF, since VT2y is produced by avian E. coli strains. We also observed the presence of multiple vacuoles in the cytoplasm of these cell lines (Fig. 3C), that simultaneously showed apoptotic features induced by VT2y cytotoxin. The microscopic nuclear fragmentation and incorporation of nucleotides shown by the TUNEL method occurred following VT-treatment of Vero cells (Fig. 4). By performing the LDH assay we verified a similar time-response using 3 CD50 VT2y; marked apoptosis effects were also observed when cells were assessed using a toluidine blue assay. Based on these data, we suggest that the VT2y-induced cell death occurs by the apoptotic pathway after plasma membrane damage.
Interestingly morphological evidence of apoptosis was observed within 15 min and was maximal within 1 h of toxin administration to Vero cells, which is considerably more rapid than previously described for apoptosis induced by VT2 in epithelial cells [8,27].
ED in pigs is caused by the toxin variant VT2v produced by E. coli [11]. This disease is characterized by anorexia, edema of the eye-lids and neurological abnormalities, such as lack of coordination and paralysis [3]; these characteristics, are similar in some respects to those shown by chickens with SHS [17]. Here we observed that VT2v induced apoptosis in Vero cells, but in contrast to VT2y the morphological alterations of apoptosis were detected 3 h after VT-treatment (data not shown). From this evidence, a histopathological study of the action of VT2y in vivo is necessary to determine whether apoptosis as a VT-mediated mechanism of cell damage is a major feature in the pathogenesis of SHS.
Acknowledgements
We thank Dr. Hernandes F. Carvalho for the analysis of cytopathic effects, A. Stella Degrossoli for technical assistance and Paulo Yoshio Inoki for photographic facilities. We are grateful to Fort Dodge Laboratories, Inc. for donating the CEF cells. This work was supported by grants from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
References
- [1]. Borenfreund E., Puerner J.A. (1984) A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J. Tissue Cult. Methods 9, 7–9. [Google Scholar]
- [2]. Bradford M.M. (1976) A rapid method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- [3]. Gannon V.P.J., Gyles C.L., Wilcock B.P. (1989) Effects of Escherichia coli Shiga-like toxins (Verotoxins) in pigs. Can. J. Vet. Res. 53, 306–312. [PMC free article] [PubMed] [Google Scholar]
- [4]. Gannon V.P.J., Gyles C.L. (1990) Characteristics of the Shiga-like toxin produced by Escherichia coli associated with porcine edema disease. Vet. Microbiol. 24, 89–100. [DOI] [PubMed] [Google Scholar]
- [5]. Inward C.D., Williams J., Chant I., Crocker J., Milford D.V., Rose P.E., Taylor C.M. (1995) Verocytotoxin-1 induces apoptosis in Vero cells. J. Infect. 30, 213–218. [DOI] [PubMed] [Google Scholar]
- [6]. Karmali M.A., Petric M., Lim C., Fleming P.C., Arbus G.S., Lior H. (1985) The association between hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151, 775–782. [DOI] [PubMed] [Google Scholar]
- [7]. Kerr J.F.R., Wyllie A.H., Currie A.R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Kodama T., Nagayama K., Yamada K., Ohba Y., Akeda Y., Honda T. (1999) Induction of apoptosis in human renal proximal tubular epithelial cells by Escherichia coli verocytotoxin 1 in vitro. Med. Microbiol. Immunol. 188, 73–78. [DOI] [PubMed] [Google Scholar]
- [9]. Lingwood C.A., Law H., Richardson S., Petric M., Brunton J.L., De Grandis S., Karmali M. (1987) Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J. Biol. Chem. 262, 8834–8839. [PubMed] [Google Scholar]
- [10]. MacLeod D.L., Gyles C.L. (1990) Purification and characterization of an Escherichia coli Shiga-like toxin II variant. Infect. Immun. 58, 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Marques L.R.M., Peiris J.S.M., Cryz S.J., O'Brien A.D. (1987) Escherichia coli strains isolated from pigs with edema disease produce a variant of Shiga-like toxin II. FEMS Microbiol. Lett. 44, 33–38. [Google Scholar]
- [12]. McConkey D.J., Nicotera P., Orrenius S. (1994) Signaling and chromatin fragmentation in thymocyte apoptosis. Immunol. Rev. 142, 343–363. [DOI] [PubMed] [Google Scholar]
- [13]. Mello M.L., Vidal B. (1980) Práticas em Biologia Celular (Edgard Blucher R.J., Ed.), p. 71.
- [14]. Miller J.H. (1972) Experiments in Molecular Biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- [15]. Mitchell D.B., Santone K.S., Acosta D. (1980) Evaluation of cytotoxicity in cultured cells by enzyme leakage. J. Tissue Cult. Methods 6, 113–116. [Google Scholar]
- [16]. Morley A.J., Thomson D.K. (1984) Swollen head syndrome in broiler chicken. Avian Dis. 28, 238–243. [PubMed] [Google Scholar]
- [17]. Nakamura K., Mase M., Tanimura N., Yamaguchi S., Yuasa N. (1998) Attempts to reproduce swollen head syndrome in specific pathogen-free chickens by inoculating with Escherichia coli and/or turkey rhinotracheits. Avian Pathol. 27, 21–27. [DOI] [PubMed] [Google Scholar]
- [18]. O'Brien A.D., Holmes R.K. (1987) Shiga and Shiga-like toxins. Microbiol. Rev. 51, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Pages Mante A., Costa Quintana L. (1987) Síndrome de cabeza hinchada (SH). Etiología y profilaxis. Med. Vet. 4, 53–57. [Google Scholar]
- [20]. Parreira V.R., Yano T. (1998) Cytotoxin produced by Escherichia coli isolated from chickens with swollen head syndrome (SHS). Vet. Microbiol. 62, 111–119. [DOI] [PubMed] [Google Scholar]
- [21]. Parreira V.R.P. (1998) Caracterização de uma citotoxina produzida por amostras de Escherichia coli de origem aviária. Tese de Doutorado, pp. 27–29. ICB, Universidade de São Paulo, São Paulo. [Google Scholar]
- [22]. Parreira V.R., Yano T., Gyles C. (2000) Shiga toxin gene in E. coli from swollen head syndrome in chickens. 4th International Symposium and Workshop on ‘Shiga toxin (verocytotoxin)-producing Escherichia coli infections’, 149 pp. Kyoto.
- [23]. Pattison M., Chettle N., Randall C.J., Wyeth P.J. (1989) Observations on swollen head syndrome in broiler and broiler breeder chickens. Vet. Rec. 125, 229–231. [DOI] [PubMed] [Google Scholar]
- [24]. Samuel J.E., Perera L.P., Ward S., O'Brien A.D., Ginsburg V., Krivan H.C. (1990) Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect. Immun. 58, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Sandvig K., van Deurs B. (1992) Toxin-induced cell lysis: protection by 3-methyladenine and cycloheximide. Exp. Cell Res. 200, 253–262. [DOI] [PubMed] [Google Scholar]
- [26]. Smith H.W., Green P., Parsell Z. (1983) Vero cell toxins in Escherichia coli and related bacteria: Transfer by phage and conjugation and toxic action in laboratory animals, chickens and pigs. J. Gen. Microbiol. 129, 3121–3137. [DOI] [PubMed] [Google Scholar]
- [27]. Taguchi T., Uchida H., Kiyokawa N., Mori T., Sato N., Horie H., Takeda T., Fujimoto J. (1998) Verotoxins induce apoptosis in human renal tubular epithelium derived cells. Kidney Int. 53, 1681–1688. [DOI] [PubMed] [Google Scholar]