Abstract
This review examines the question of whether exercise can be used as an experimental model to further our understanding of innate antimicrobial peptides and proteins (AMPs) and their role in susceptibility to infection at mucosal surfaces. There is strong evidence to suggest that AMPs, in combination with cellular and physical factors, play an important role in preventing infection. Although AMPs act directly on microorganisms, there is increasing recognition that they also exert their protective effect via immunomodulatory mechanisms, especially in noninflammatory conditions. Further studies that manipulate physiologically relevant concentrations of AMPs are required to shed light on the role they play in reducing susceptibility to infection. Evidence shows that in various form prolonged and/or exhaustive exercise is a potent modulator of the immune system, which can either sharpen or blunt the immune response to pathogens. The intensity and duration of exercise can be readily controlled in experimental settings to manipulate the degree of physical stress. This would allow for an investigation into a potential dose–response effect between exercise and AMPs. In addition, the use of controlled exercise could provide an experimental model by which to examine whether changes in the concentration of AMPs alters susceptibility to illness.
Keywords: antimicrobial peptides and proteins, lactoferrin, lysozyme, innate immunity, exercise, upper respiratory tract infection
Introduction
There is a higher risk of infection at epithelial surfaces of the body, such as the respiratory, gastrointestinal or urogenital tract, and the skin, that interface with, and separate the host from, the external environment. These epithelial surfaces are protected from invading microorganisms by the innate mucosal/epithelial defence system, which will be referred to as innate mucosal immunity. Although the mucosal immune system does not function independently of the systemic immune system, it is regarded as a distinct entity because it has localized defence factors and is autonomously regulated (Toy & Mayer, 1996). In addition to its defence mechanisms, the mucosal immune system also suppresses potentially damaging inflammatory activity. This suppression plays an important role in the prevention of chronic inflammation at mucosal surfaces by preventing infection without the initiation of an immune response. Under inflammatory conditions the suppression of inflammatory activity acts as a measure of control to bring the inflammatory process to a conclusion. Dysfunction in mucosal immunity is associated with increased illness and morbidity (Daele & Zicot, 2000), suggesting that immune competence at mucosal surfaces is an important factor for the maintenance of health and well‐being.
Although effective protection of mucosal surfaces requires both innate and adaptive immune components, this review will address the innate mucosal immune system and in particular antimicrobial peptides and proteins (AMPs) with focus on lactoferrin and lysozyme. Innate mechanisms are primarily responsible for preventing pathogens from entering the body and initiating a rapid response should infection occur. The prophylactic role of the innate immune system has, in recent years, received increased attention as the search continues for ways to reduce the burden of infectious illness worldwide. There is a diverse range of innate physical (cilia, epithelia and mucus), cellular (neutrophils and macrophages) and humoral factors (AMPs) that function as a barrier to infectious agents. Although the role of physical and cellular factors has been well characterized, humoral factors, such as AMPs, have only recently been acknowledged as important components at mucosal surfaces. AMPs are constituent and inducible factors of secretions at mucosal surfaces that display activity against a broad range of pathogens. Their presence in secretions without the need for prior exposure to infectious agents is indicative of their integral role in the innate mucosal immune system.
Although there is extensive evidence from in vitro and animal studies to suggest that AMPs have a role in innate mucosal defence, their activity in humans needs to be confirmed by in vivo experiments. Host immune status is recognized as an important factor in susceptibility to infection. Studies that employ experimental models of physical stress to manipulate local immune factors, such as AMPs, may shed further insight into the relationship between immunity, stress and infection. We propose that exercise could be a useful experimental model to study changes in the concentration of AMPs and improve knowledge of their role in reducing susceptibility to illness. Heavy and/or prolonged exercise in humans is known to cause transient perturbations in many cellular and humoral immune factors (1999a, 1999b). Investigations have shown that the serum concentration of lactoferrin increases after moderate‐ and high‐intensity running (Inoue et al., 2004). To date there have been no published investigations examining the relationship between the concentration of AMPs located in respiratory secretions and exercise. Using exercise as an experimental model to study the relationship between physiologically relevant changes in AMPs and susceptibility to infection may shed further light on the role of AMPs in mucosal immunity.
Antimicrobial peptides and proteins
Since Alexander Fleming's discovery in the 1920s that lysozyme kills bacteria, there has been a steady interest in the role of AMPs at mucosal surfaces. The term antimicrobial peptide traditionally refers to small (<100 amino acids) cationic peptides that have antimicrobial activity. The discovery in recent years of a wide range of biological factors, such as cytokines, that display antimicrobial activity has broadened the number of innate antimicrobial factors. Throughout this review we will use the generic abbreviation AMP to refer to both small cationic peptides and polypeptides and proteins, such as lactoferrin and lysozyme. An extensive number of AMPs have been identified in plants and animals. Each mucosal location has a unique profile of AMPs (Tjabringa et al., 2005). This site‐specific difference is the result of a number of factors, including the effect of commensal microbial communities and the presence or absence of microbial challenge. AMPs are classified into groups based on structural features, including size, amino acid structure, physical structure and charge (Reddy et al., 2004). In addition to their antimicrobial properties, AMPs exert substantial immunomodulatory influence locally by inducing the secretion of cytokines and recruiting immune cells to sites of infection, and participating in the remodelling of injured epithelia (Bowdish et al., 2005). AMPs contribute to the health and well‐being of mucosal surfaces by engaging in a diverse range of activities.
The diverse activities attributed to AMPs relate to the fact that they contain multiple functional domains. The antimicrobial properties of lactoferrin are related to the N‐terminal fragment of lactoferrin, known as lactoferricin (Wakabayashi et al., 2003). The ability of lactoferrin to act as a microbistatic agent through its iron‐binding capability, however, relates to the two homologous lobes at either end of the peptide. Kanyshkova et al. (2003) noted that other enzymatic activities displayed by lactoferrin relate to different subfractions of the peptide. Similar observations have been made with other AMPs. Investigations into the human cathelicidin LL‐37, an AMP secreted from leukocytes and epithelial cells, have identified several isoforms each of which has a different function (Murakami et al., 2002). Many AMPs require enzymatic processing after secretion for synthesis into an active form for their antimicrobial activity. For example, lactoferricin is derived by pepsin digestion of lactoferrin postsecretion (Wakabayashi et al., 2003) and pepsin processing of lysozyme is responsible for generating the antimicrobial potency of lysozyme (Ibrahim et al., 2005). Postsecretory processing of the mature cathelicidin occurs once it has been secreted on to the skin surface to generate multiple AMPs that display antimicrobial activity. Many AMPs lose their ability to undertake other functions once processed from their parent form. The mechanisms that regulate postsecretory processing are uncertain, but it is reasonable to surmise that the processing enzymes are regulated by the mucosal milieu and may be a mechanism that allows the host to adapt to altered environmental circumstances. Other AMPs are secreted in their processed form. Neutrophil defensins are stored as processed peptides in the azurophilic granules of neutrophils. The secretion of peptides in a processed form gives the body an immediate antimicrobial platform by which to attack pathogens, while the ability to process peptides into different forms with various capabilities provides the body with a broad spectrum of agents to protect host tissues.
As part of the innate immune system, AMPs do not show antigen specificity. They do, however, discriminate between prokaryotic and mammalian cells. This preferential selectivity is related to fundamental differences between the membranes of the two types of cells, specifically membrane charge – microorganism cell membranes have a net anionic charge while host cells are zwitterionic – and membrane lipid composition (Matsuzaki, 1999). Traditionally, the interaction between AMPs and microorganisms was thought to be as a result of electrostatic interaction caused by this difference in cell charges. More recent investigations with bomimetic structures indicate, however, that membrane lipid composition is more important a determinant than the overall net charge between the membranes in the ability of AMPs to select preferentially, and then interact with, microorganism cells over host cells (Arnt et al., 2006). Microorganism cell membranes contain phosphatidylglycerol (PG), cardiolipin and phosphatidylethanolamine (PE), which AMPs show high affinity toward. By contrast, mammalian cells are composed of phosphatidylcholine (PC) and cholesterol, which reduce the sensitivity of the membrane to the activity of AMPs. Furthermore, the lipids with negatively charged headgroups are in the inner leaflet of the membrane in mammalian cells, facing the cytoplasm. Targeting fundamentally common features of microorganism cell membranes provides AMPs with their nonspecific, broad capability, and contributes to the continuing effectiveness of AMPs against infectious agents. Changing the charge density on the membrane has been identified as one of the primary mechanisms by which bacteria evade AMPs (Devine & Hancock, 2002). Targeting fundamentally different features between prokaryotic and eukaryotic cells also protects eukaryotic cells from antimicrobial activity.
The focus on AMPs has traditionally been on their antimicrobial properties. AMPs act against a broad spectrum of infectious pathogens in vitro, including Gram‐positive and Gram‐negative bacteria, viruses and fungi. The antibacterial activity of AMPs has been measured against a range of bacteria, including Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli (Travis et al., 1999). Although there are conflicting reports, it appears that AMPs are effective antiviral agents. Lactoferrin inhibits the growth of respiratory syncytial virus, a common respiratory virus, at a concentration 10‐fold lower than that found in human milk (Grover et al., 1997). Lactoferrin also acts against HIV and human cytomegalovirus (hCMV) in vitro (Harmsen et al., 1995). Both lactoferrin (Arnold et al., 2002) and the cathelicidin LL‐37 (Gordon et al., 2005) prevent adenovirus, a respiratory virus, from replicating in vitro. Whereas AMPs act against a broad spectrum of pathogens, they display different selective activity for various microorganisms. Lactoferrin's activity against adenovirus can be contrasted with its action against another common respiratory virus, rhinovirus, which it did not inhibit the growth of (Clarke & May, 2000). AMPs have been shown to have fungicidal and fungistatic effects, with processed forms of cathelicidin displaying activity against Candida albicans at mucosal surfaces (Lopez‐Garcia et al., 2005). Several AMPs are induced in vitro by microorganisms (Duits et al., 2003). The evidence from in vitro studies suggests that AMPs display selective activity against a range of common infectious pathogens. No published information appears available on the effect of common stressors, such as exercise, on AMP functionality. Given the effect of exercise on cellular activation, further investigation is warranted to determine if AMP functionality is diminished, and susceptibility to infection altered, as a result of intensive exercise training.
Mechanisms of antimicrobial activity
The mechanisms by which AMPs exert their antimicrobial activity are illustrated in Fig. 1. Whether AMPs are capable of attacking multiple targets simultaneously or are target specific is a matter of debate. Direct attack can be lethal or have an inhibitory effect on the growth and activity of microorganisms, with the concentration of the peptide the determining factor. In order to exert antimicrobial activity, AMPs must reach pathogen‐specific minimum concentrations. A key step in their antimicrobial activity is disruption of the microorganism cell membranes. This occurs as a two‐step process during the initial interaction between AMPs and bacteria. The first step involves electrostatic interaction that results in a depolarization of the microorganism cell membrane. The loss of charge between the inside and outside of the cell membrane allows polar substances, which are usually tightly regulated under normal conditions, greater freedom to traverse into the cellular environment. The loss of charge also allows physical interaction between the peptide and the microorganism. Following contact with pathogen membranes, AMPs form amphiphilic structures that have a polar hydrophilic and a nonpolar hydrophobic section at opposite ends. This conformational change allows the peptide to insert into the membrane, further destabilizing its barrier function.
Figure 1.
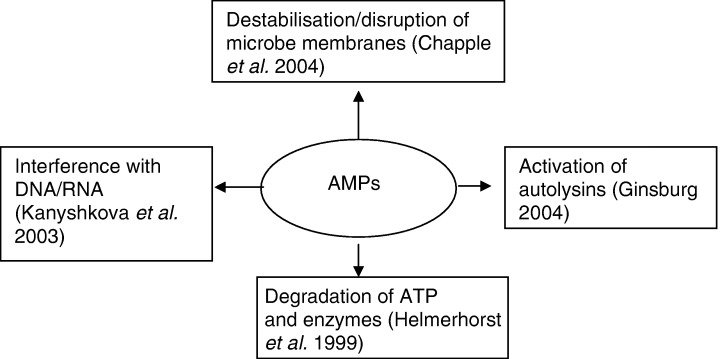
Mechanisms by which AMPs exert their antimicrobial activity.
Destabilization of the microorganism cell membranes has traditionally been thought to be the mechanism by which AMPs eliminate microorganisms. A number of models have been proposed to explain the process by which this may occur (Hancock, 2001). The first is the carpet model, in which a layer of AMPs carpets the membranes, resulting in the membranes collapsing and eventually disintegrating as the concentration of AMPs reaches a critical threshold. The AMPs then gain direct access to the intracellular environment (Matsuzaki, 1999). The second mechanism of destabilization is the barrel‐stave model, which involves the formation of ion channels. These channels form after insertion of the hydrophobic section of the AMP into the membrane. The lipid membrane then separates, with the nonpolar section of the AMP facing the membrane and a hydrophilic barrel forming that spans the membrane and exposes the cell to the external environment. The final model is the aggregate model, where clusters of AMPs penetrate the membrane causing transient pores to form and the cell contents to leak out. Although there is debate about the exact mechanism(s) of destablization, there is agreement that disruption of the cell membrane would cause cell lysis if the permeability of the membrane overwhelms the ability of other mechanisms to maintain homeostasis within the cell. It has been suggested more recently that membrane permeabilization is not the mechanism by which AMPs eliminate microorganisms but an activity that facilitates AMP access to internal targets, such as DNA/RNA (Kanyshkova et al., 2003), protein synthesis (Helmerhorst et al., 1999) and autolytic cell wall enzymes (Ginsburg, 2004). The exact mechanism by which AMPs act is determined by a number of factors, including the strain of microorganism, characteristics of the peptide and the way that AMPs interact with other immune factors, including other peptides, within the mucosal milieu (Matsuzaki, 1999). Membrane permeabilization is recognized, however, as a central feature to the antimicrobial properties of AMPs.
The potency of AMPs against microorganisms is increased by their ability to act synergistically, whereby AMPs interact to have a combined effect, and additively, in which case the increased potency is the result of a number of AMPs working independently on a pathogen simultaneously (Singh et al., 2000). This synergistic and additive activity has a number of important effects. First, it reduces the minimum concentration of AMPs required to eliminate microorganisms. A cocktail of AMPs working in combination at mucosal surfaces lowers the concentration required to eliminate microorganisms. Secondly, synergistic and additive interactions increase the spectrum of infectious agents against which AMPs can act. Investigations have shown that lactoferrin enhances the effectiveness of lysozyme to eliminate Gram‐positive bacteria (Leitch & Willcox, 1999). The cell membrane of Gram‐positive bacteria is protected from lysozyme by lipotechoic acid. Lactoferrin neutralizes the lipotechoic acid, thereby rendering bacterial membranes of Gram‐positive bacteria more susceptible to the activity of lysozyme (Leitch & Willcox, 1999). Thirdly, synergistic and additive interactions increase the speed at which AMPs eliminate infectious pathogens. Combining lactoferrin, lysozyme and serum leukoprotease inhibitor resulted in a faster rate of elimination of Escherichia coli than any one protein used individually (Singh et al., 2000). Finally, synergistic and additive interactions provide an element of redundancy to mucosal surfaces, lowering the likelihood that deficiencies will result in increased clinical susceptibility to infection.
AMPs can also inhibit the growth and activity of microorganisms indirectly. One mechanism by which this is done is by making the mucosal environment unsuitable for colonization. In order to multiply and colonize epithelia, microorganisms require the presence of nutrients on mucosal surfaces. One well‐characterized antimicrobial agent, lactoferrin, binds free iron, a nutrient essential to the growth and multiplication of microorganisms, at mucosal surfaces to restrict its use by bacteria (Legrand et al., 2004). Making mucosal surfaces inhospitable reduces the ability of microorganisms to colonize epithelia and slows their ability to multiply, thus giving the host tissues increased time to marshal other defensive modalities. However, microorganisms have evolved and developed mechanisms to overcome the ability of AMPs to reduce the availability of nutrients. In the case of iron, some bacteria have evolved strategies to sequester it from lactoferrin. This is one mechanism by which commensal microbial communities are able to survive at mucosal surfaces.
Recent studies have reported inconsistencies in the direct antimicrobial properties of AMPs between in vitro and in vivo conditions. In vitro studies examine AMPs as a standalone factor, which is not easily translated to their action in vivo, where agonistic and antagonistic factors in the local environment can exert considerable influence on AMP activity. For example, AMPs lose their antimicrobial activity in the presence of physiological levels of NaCl or serum (Travis et al., 1999). Furthermore, there are discrepancies between the concentrations of AMPs used in in vitro studies compared with the concentrations in the physiological milieu present in vivo (McPhee & Hancock, 2005). The concentrations at which AMPs display their antimicrobial activity in vitro is in the micro‐ to milligram per millilitre range, far higher than that found in many locations in the body, especially in noninflammatory conditions, where the concentration of AMPs is below the minimum inhibitory concentration used in vitro. This suggests that the direct antimicrobial properties demonstrated by AMPs may not be their sole, or even primary, role in host defence in vivo. Instead, it may be an activity that occurs only under inflammatory conditions, where the substantially higher concentrations of AMPs overcome the inhibitory effect of physiological salt concentrations.
Immunodulatory activity of the AMP family
There is evidence that AMPs have strong immunomodulatory influence (Box 1). These activities have fuelled debate about the mechanism by which AMPs exert their protective effect at mucosal surfaces because they have been conducted under relevant in vivo conditions (Bowdish et al., 2005). AMPs exert their anti‐inflammatory activity by preventing interaction between microorganisms and host cells and stimulating the secretion of cytokines. AMPs prevent interaction between microorganisms and host cells by interfering with cell receptors that recognize microorganisms and by neutralizing microorganism‐specific immunoactivating structures. The ability of AMPs to block binding between host cells and microorganisms has been demonstrated against a variety of pathogens. Human β‐defensin (hBD‐2) prevents interaction between host cells and HIV by down‐regulating receptors on host cells involved in viral transmission (Quinones‐Mateu et al., 2003) while lactoferrin binds to host cell receptors and blocks their interaction with viral pathogens, such as adenovirus (Arnold et al., 2002). AMPs bind with CD14 and lipopolysaccharide binding protein (LBP) to impair the binding of inflammatory components on bacterial membranes to host cells (Kirkland et al., 1993). CD14 is a receptor found on monocyte, macrophage and neutrophil membranes (mCD14) and in serum (sCD14) and is the primary mechanism of immune activation to small concentrations of lipopolysaccharide (Le Roy et al., 2001). mCD14 facilitates binding of immune cells and lipopolysaccharide, while sCD14 mediates binding between lipopolysaccharide and cells involved in immune activation that do not have a membrane‐bound CD14 receptor, such as epithelial cells (Arditi et al., 1993). Each of the major structural classes of AMPs block binding between lipopolysaccharide and LBP (Scott et al., 2000). This blocking prevents the transfer of lipopolysaccharide to CD14 that would otherwise initiate an lipopolysaccharide‐induced inflammatory response. The ability of lipopolysaccharide to induce an inflammatory response is further impaired by the ability of AMPs, such as lactoferrin, also to bind with high affinity to CD14 to prevent an LBP–lipopolysaccharide complex from binding to it (Baveye et al., 2000). Preventing interaction between a potential host cell and microorganisms in this way prevents the initiation of an immune response.
Table Box 1.
Immunomodulatory functions of AMPs
| Neutralizing immunoactivating structures |
| Acting directly on cellular cytokine expression |
| Regulating receptor expression |
| Binding host cell proteins |
| Chemoattractant for immune cells |
The anti‐inflammatory influence of AMPs is also mediated by the inactivation of microorganism‐specific proinflammatory motifs (Elsbach, 2003). The innate immune system is alerted to the presence of an infectious pathogen by structural features that are microorganism‐specific. These motifs are recognized by a variety of receptors, such as the toll‐like receptor, which then induce the release of inflammatory mediators. Altering the characteristics of these microorganism‐specific proinflammatory features may have a substantial impact on the ability of a microorganism to induce an immune response (Brandenburg et al., 2001). With regard to lipopolysaccharide, the lipid A moiety is the proinflammatory motif that induces an inflammatory response. Lipid A is characterized by its shape and charge, having either a concave or a conical shape and two or more negative charges (Seydel et al., 2003). The depolarization and insertion of the AMP into the cell membrane during the initial interaction between microorganisms and AMPs cancels the negative charge and changes the shape of the lipid A section to render it inactive (Brandenburg et al., 2001). AMPs can bind to other inflammatory‐inducing factors, such as the unmethylated CpG dinucleotides in bacterial DNA (Britigan et al., 2001), that are also responsible for inducing an array of proinflammatory activities. Blocking the ability of these immunoactivating motifs from inducing an inflammatory response is thought to explain the antiendotoxin activity (Bennett‐Guerrero et al., 2001) of serum. It is reasonable to postulate that the presence of AMPs in mucosal secretions would have the same effect.
Several AMPs also affect inflammatory activity by influencing the secretion of cytokines from host cells. Lactoferrin inhibits the secretion of tumour necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6 and IL‐8 from monocytes whether added before or after an inflammatory‐inducing agent (Haversen et al., 2002), and inhibits lipopolysaccharide from inducing the classical complement pathway (Samuelsen et al., 2004). By contrast, the induction of AMPs during inflammation facilitates local up‐regulation of the immune response. The secretion of AMPs increases significantly during inflammation. Although this increase may mediate antimicrobial activity, it also serves to attract, recruit and activate other components of the immune system central to an effective immune response. The increase in AMP concentration establishes a chemical gradient, which attracts cells to the site of infection. LL‐37 is a chemoattractant for neutrophils, monocytes and T cells to sites of infection (De et al., 2000). AMPs also induce the secretion of proinflammatory cytokines, such as IL‐8 secretion from neutrophils and TNF‐α from macrophages (Shinoda et al., 1996), that in turn recruit cells to the source of the cytokine secretion. These studies suggest AMPs are integral to the process of inflammation, although their influence, either in promoting or in resolving it, will depend on the interplay between a variety of other factors, including cytokines and cellular activation.
The secretion of AMPs during inflammation acts as a link between the innate and adaptive components of the immune system (Yang et al., 2001). Activation of the adaptive immune system is mediated by the uptake of antigen at sites of infection and presentation of antigen to T and B cells in lymph nodes. Dendritic cells have been identified as key cells in the respiratory tract that take up and present antigen to activate the adaptive immune system. There is a broad spectrum of stimuli that induce the trafficking of dendritic cells to sites of infection. Both α and β defensins recruit immature dendritic cells to sites of infection through chemoattraction and induce their maturation by binding with CCR6 receptors on the dendritic cell surface (Yang et al., 1999). The activation of dendritic cells via the CCR6 receptor also induces the secretion of IL‐8, which serves further to promote an inflammatory response. AMPs clearly have an important role in activating the adaptive immune response and recruiting adaptive immune effector cells toward sites of infection.
The immunomodulatory action of AMPs has been shown to be independent of their antimicrobial activity, suggesting that this may be the mechanism by which AMPs exert their protective effect. The ability of AMPs to neutralize immunoactivating structures on microorganisms and block microorganisms from binding to host cells prevents infectious agents from inducing an inflammatory response. This activity may play a part in preventing unintended or constant inflammation at mucosal surfaces. The mucosal surfaces of the body are constantly exposed to a high antigenic load and a balance must be maintained between active and passive immunity, so these sites are not in a permanent state of inflammation. This activity may be particularly relevant to AMPs expressed constitutively at mucosal surfaces. Given their immediate induction during an innate immune response, the ability of AMPs to modulate inflammation may contribute to an appropriate immune response at sites of infection. The resolution of inflammation is a crucial aspect of an immune response, and through their anti‐inflammatory influence, AMPs may act as a counter‐regulatory mechanism that dampens the immune response (Bowdish et al., 2005).
Although much of the evidence for AMPs having a protective role in host defence is inferred from in vitro studies, in vivo studies have confirmed that AMPs play a prophylactic role at mucosal surfaces. In an in vivo animal study, selective inhibition of cathelicidin in mutant mice, via deletion of the relevant gene, resulted in severe necrotic infection following inoculation with group A Streptococcus, which did not occur in wild‐type mice (Nizet et al., 2001). Dysfunctional AMP secretion is also associated with greater susceptibility to infection. Overproduction of AMPs in vivo, as occurs with psoriasis, reduces the risk of secondary infection, which contrasts with the decreased expression of AMPs in patients suffering atopic dermatitis, who experience increased susceptibility to secondary infection (Ong et al., 2002). Reversing conditions that inhibit the antimicrobial activity of AMPs reduces susceptibility to common infectious pathogens in disease states. A link has been proposed between AMPs and an increased susceptibility to infection in cystic fibrosis (CF). Cystic fibrosis patients have high salt concentration in respiratory secretions that are thought to inactivate the antimicrobial activity of AMPs, thus leading to a heightened susceptibility to infection. Reducing the salt concentration enabled CF secretions being able to kill common pathogens (Travis et al., 1999). Collectively, these studies indicate that AMPs play an important role in susceptibility to infection in vivo.
Beyond their protective capability there is an accumulating body of evidence indicating that AMPs are involved in wound healing. This activity is mediated through a variety of mechanisms, including promotion of angiogenesis/arteriogenesis, the proliferation and migration of epithelial cells and, indirectly, by attracting immune cells that secrete factors promoting wound closure to sites of infection (Zanetti, 2004). Vascularization is an essential component in restoring tissue integrity after injury by allowing, among other things, the trafficking and migration of cells and molecules to sites of injury from the bloodstream. In vivo and ex vivo studies have shown that the cathelicidin LL‐37 induces vascularization by binding to a formyl peptide receptor like 1 (FPRL1) on epithelial cells (Koczulla et al., 2003). In addition, human epithelial cell lines treated with synthetic biologically active LL‐37 peptide showed a significant increase in cell proliferation, while reduced expression of this AMP delays healing. Similar findings have been made with regard to defensins. Human neutrophil defensins induce airway epithelial cell proliferation and cell migration. The recruitment of immune cells to sites of infection may form an indirect mechanism by which AMPs enhance wound healing. The recruitment of immune cells improves the body's ability to mount an immune response and up‐regulates inflammation by the release of chemokines. Chemokines also affect wound healing, by acting as growth factors and angiogenic agents (Sorensen et al., 2003). The finding that AMPs play a role in wound closure supports an expanded functional role beyond that of host defence.
AMPs and the upper respiratory tract
The upper respiratory tract is a key entry point for viral pathogens. Upper respiratory tract infections (URTIs) are associated with a high morbidity burden that may have sequelae that lead to death, especially in infants and in those aged over 70 years (Hashem & Hall, 2003), and form the most common presentation to general medical practice. Essentially there are five main causes of respiratory illness: viral infection, bacterial infection, allergic responses, exercise‐induced asthma and noninfectious inflammation (Pyne & Gleeson, 1998). Most infectious causes of illness are viral, with the most common being caused by rhinovirus, coronavirus, respiratory syncitial virus, parainfluenza virus or adenovirus. Many of these viruses are enveloped viruses that are susceptible to the antimicrobial activity of AMPs. URTI of an infective nature is accompanied by a variety of symptoms, including sore throat, cough, runny nose, congested sinuses, headache, myalgia and fibralgia (Barrett et al., 2002).
URTIs are associated with substantial burden and are a primary reason for absence from work and school, and, when associated with healthcare costs, driven largely by physician visits and over‐the‐counter products to remedy associated symptoms, URTI is a significant economic burden (Hashem & Hall, 2003). An economic analysis in the United States estimated the direct cost of respiratory tract infections was $9 billion, not including lost work days (Dixon, 1985). The human cost of URTIs is less clear but no less significant. In many cases, these illnesses necessitate reduced social interaction and rest, reduced feelings of health and well‐being and reduced quality of life (Hashem & Hall, 2003). For various population groups, such as athletes, URTIs may have increased significance. Although evidence is thus far inconclusive, there are data suggesting that athletes remaining free of URTIs in the lead up to and during competition perform marginally better than athletes reporting illness (Pyne et al., 2001).
The innate mucosal immune system is a key element in the maintenance of an infection‐free state in the upper respiratory tract. One mechanism by which the role of AMPs could be assessed in the upper respiratory tract in human subjects is through experimental manipulation of physical stress. A laboratory or field‐based model of physical stress that elicits substantial variations in the concentration and/or function of AMPs is required. This approach could be useful in studying the relationships between AMPs and clinical consequences in terms of the incidence, severity or duration of infection. This method of investigation is warranted because substantial perturbations in immunity would be expected to alter susceptibility to illness, and there is evidence that the aetiology of some infectious episodes after exercise is from pathogens susceptible to AMPs (Spence et al., 2004). Although there are established links between immunodeficiency and infection, the literature is less clear about the way in which normal perturbations in immunity affect risk of infection. There is a large variation in susceptibility to URTIs among healthy individuals, with incidence lower in some individuals than others (Gwaltney, 2002). Further work is required to characterize better the way in which clinically normal variations in immunity in healthy people, including changes in the concentration of AMPs, affect susceptibility to common illnesses and infections.
Exercise as a model by which to study AMPs
The effect of stress on the immune system has been well documented. By affecting the secretion of various neuropeptides, or stress hormones, stress has a direct effect on the immune system by causing changes to the trafficking and activity of effector cells (neutrophils, lymphocytes, macrophages), the secretion of cytokines and the induction of endogenous factors that regulate immune activity (heat shock proteins). Many of the acute changes that occur in response to stress enhance immunity. However, the effects of stress hormones on immune function may suppress immunity if elevated too acutely, for long periods of time, or too frequently. Psychological stress can impact negatively on adaptive immune parameters, resulting in the reactivation of three latent herpesviruses, Epstein–Barr virus (EBV), herpes simplex virus type‐1 (HSV‐1) and human herpesvirus 6 (HHV‐6) (Glaser et al., 1999). Similar findings are reported on the effect of stress on factors of innate immunity.
Exercise, particularly prolonged intense exercise, is known to cause a transient perturbation in cellular and humoral aspects of immunity, which is consistent with our understanding about the effects of stress on the body. The extent of the disturbance to immunity is determined by the intensity, duration and frequency of the exercise workload (Gleeson et al., 2003). The acute immune response to prolonged intense exercise in blood is characterized by a biphasic increase in leucocyte numbers, particularly neutrophils, macrophages and natural killer (NK) cells, during and immediately after exercise (Pyne, 1994). Lymphocyte numbers then decrease in the period after exercise to concentrations below resting values (Nieman et al., 1995). The secondary increase in leucocyte cell numbers over the following hours is largely attributable to the mobilization of neutrophils. Prolonged intense exercise is associated with substantial changes in cell functional activity. Neutrophil respiratory burst activity and degranulation increase during and immediately after exercise, before decreasing to levels below the pre‐exercise period. High‐intensity exercise is also associated with a reduction in the expression of neutrophil cell‐surface receptors immediately and for an hour postexercise (Peake et al., 2004). These studies indicate that discrete aspects of neutrophil function can be negatively affected by prolonged intense exercise. Variations have also been found in NK cell activity (NKCA). However, these perturbations in activity appear dependent on the training history of the subjects, with healthy and well‐conditioned subjects experiencing a fall below pre‐exercise values in NKCA, while prolonged intense exercise had no effect on NKCA in highly trained athletes. The changes associated with prolonged intense exercise, while only transient, reflect a period of immunosuppression.
Prolonged intense exercise has a negative effect on mucosal immunology. The effect of exercise on mucosal immunology has been assessed by quantifying changes in salivary immunoglobulin A (SIgA) between the pre‐ and postexercise period (Tomasi et al., 1982). SIgA is secreted by B cells and constitutes a humoral component of the adaptive immune system that provides antigen‐specific immunity at mucosal surfaces. There is an acute and chronic decrease in SIgA following a session of prolonged intense exercise or over a heavy training period (Gleeson et al., 1999). Considerable change has also been observed in innate mucosal defences following prolonged intense exercise, including impaired cilia beat frequency and mucocilliary transit time (Muns et al., 1995), an influx of polymorphonuclear leukocytes (PMNs) to the respiratory tract for several days and reduced phagocytic activity for up to 24 h in the URT (Muns, 1994). Collectively, these data suggest that prolonged intense exercise has a suppressive effect on mucosal immunity.
There are a diverse range of AMPs and proteins in saliva, including lactoferrin, lysozyme, secretory leukocyte protease inhibitor (SLPI), defensins, LL‐37 and histatins. Similar to other mucosal surfaces, many of these AMPs form a constitutive barrier to foreign objects entering the oral, nosocomial and URT (Singh et al., 2000). These factors are secreted from surface epithelial cells and salivary glands (Dubin et al., 2004). Following infection, the concentration of these AMPs, and other nonconstitutive peptides, increases as they are induced from epithelial and immune cells. To date there are no studies that have systematically examined the acute (min to h) and chronic (days to weeks) changes in AMP concentration in saliva after exercise or training.
There are a diverse number of mechanisms by which exercise could alter the concentration of AMPs in the respiratory tract (Box 2). Prolonged intense exercise is associated with hyperventilation, which, during exercise, would dry the respiratory tract, potentially reducing the protective shield provided by AMPs. However, in the postexercise period, hyperventilation may increase the secretion of AMPs by inducing an inflammatory response. Airway epithelial cells lining the respiratory tract may experience mechanical trauma as large amounts of air are forcefully inspired. The greater volume of ventilation will increase exposure to environmental irritants and microorganisms. Epithelial cells increase their expression of AMPs following physical damage (Dorschner et al., 2001) and contact with microorganisms (Duits et al., 2003). The recruitment and activation of neutrophils during exercise could increase the concentration of AMPs at mucosal surfaces during and immediately after exercise, as neutrophils secrete soluble proteins, including AMPs, when activated. Inhaled particles may indirectly stimulate the expression of AMPs by inducing proinflammatory cytokines. The expression of AMPs is increased in the presence of proinflammatory chemokines, especially interferon‐γ, IL‐1β and IL‐8. This suggests that in the immediate period after exercise (min to h), local mechanisms may increase the concentration of AMPs in the respiratory tract.
Table Box 2.
Mechanisms by which acute exercise may affect the concentration of AMPs
| Increased secretion of neuropeptides |
| Induction of AMP secretion by proinflammatory cytokines |
| Damaged epithelial cells releasing AMPs |
| Neutrophil secretion of AMPs |
In addition to exerting local influence on the expression of AMPs, intense exercise may exert indirect effects by increasing the secretion of neuropeptides. Exercise stimulates the hypothalamic–pituitary–adrenal axis to secrete stress hormones, thus increasing the body's ability to meet the physical and metabolic demands of exercise. Prolonged intense exercise is associated with substantially increased secretion of human growth hormone, β‐endorphin, catecholamines and glucocorticoids. These factors have a strong influence on the immune system by activating specific receptors on host cells. Exercise‐induced changes in plasma concentrations of stress hormones have been associated with changes in circulating leucocyte distribution and activity. The effect of stress hormones on AMPs, however, is somewhat less certain. Catecholamines could induce the expression of AMPs by activating the transcription factor NF‐κ B, which is a pathway involved in up‐regulating the secretion of peptides such as defensins. Neuropeptides can induce expression of AMPs from glands in animal models; however, they have little impact on AMP secretion from epithelial cells (Dubin et al., 2004). Although further studies confirming the effects of neuropeptides on AMP secretion are required, it is plausible to suggest that their secretion during exercise may alter the concentration of AMPs in the postexercise period.
The clinical significance of these changes would suggest that, except during the period when exercise is undertaken and the antimicrobial shield may be diminished by drying, there would be a reduced susceptibility to infection in the period (1–3 h) postexercise. However, exercise‐induced secretion of AMPs may result in a refractory period, where the ability of host cells to secrete AMPs in the immediate postexercise period is reduced. Neutrophils have a transient reduction in the ability to undertake further activity once activated. Given that neutrophils are a substantial source of AMPs, this may have implications for the ability of the URT to respond to pathogenic challenge should it occur in the postexercise period. This may be similar for epithelial cells. The increased secretion of lysozyme from glands is a function of prior accumulation of the protein over time (Dubin et al., 2004). This suggests the existence of a refractory period where innate defences are suppressed until recovery and restoration is achieved. The significance of a postexercise refractory period in AMP protection may be negligible after one bout of exercise but become more significant over a training period of several weeks to months. Elite athletes participating in sports such as rowing, swimming, cycling and running undertake a multitude of high‐intensity training sessions on a weekly basis. Indeed, chronic stress is associated with a reduced secretion of salivary lysozyme (Koh et al., 2002). Hence, it could be postulated that prolonged intense exercise may have a negative effect on the concentration of AMPs in the URT.
In addition to the suppressive effects of exercise on mucosal immunity there are other reasons justifying its use as an experimental model. Exercise can easily be controlled and reproduced in animal and human settings. This means that the exercise load (physical stress) applied can be prescribed relative to an individual's capability. Although prolonged intense exercise causes transient perturbations in immunity, there are individual differences in the relative load or intensity of exercise required to achieve such an effect. The magnitude of this between‐subject variation is influenced by physical capacity, training history and fitness. These factors need to be considered when determining the load (intensity and duration) to be applied in experimental settings. Failure to apply the relevant exercise load might confound study results.
The use of exercise as a model to study the role of AMPs forms part of the discipline of exercise immunology, which has extensively studied the effect of prolonged intense exercise on the immune system in athletic populations to determine why this subgroup of athletes appears to be at greater risk of illness. Studies examining the incidence of URTIs in elite athletes engaging in prolonged intense exercise have had variable outcomes, with some studies reporting a heightened incidence (Spence et al., 2004) and others reporting no change (Pyne et al., 2001) in comparison with sedentary control groups. There is general consensus that athletes may experience higher rates of illness during critical training periods and competition. This relationship has been characterized as a theoretical J‐shaped curve. The J curve relates the incidence of illness to exercise load. According to this model, individuals engaging in moderate exercise have a reduced risk of illness compared with sedentary individuals or athletes undertaking a high exercise load. Whether this model also applies to AMPs is unclear and worthy of investigation to shed insight on underlying mechanisms and clinical outcomes for the altered susceptibility to illness. A pilot investigation conducted at the Australian Institute of Sport has demonstrated that the concentration of salivary lactoferrin, one of the most abundant AMPs, decreased during a season of training in highly trained elite rowers (our unpublished data).
Future directions
Further in vivo studies of AMPs are required to elucidate their role with regard to susceptibility to infection at mucosal surfaces. Given that changes in the concentration of AMPs have substantial implications for their interaction with microorganisms, there is a need to employ investigative models that physiologically suppress their presence at mucosal surfaces. Exercise may be an appropriate mechanism to further our understanding of the role of AMPs in the control of URT immune status. Further studies are required to examine acute and long‐term changes in AMPs in recreational subjects and highly trained athletes undertaking intense, prolonged training. These studies need to address the relationship between intense prolonged exercise on AMP concentrations and determine whether acute or long‐term alterations in the concentration and/or function of AMPs is associated with increased incidence of infection. A methodological approach that takes into account confounding variables of exercise such as frequency, intensity and duration is required.
Collection of saliva to study mucosal immunity is well established. Saliva collection is noninvasive and straightforward and can be more easily standardized in relation to other secretions of the mucosal immune system (Gleeson, 2000). Ease of collection and validity as a marker of the mucosal immune system, particularly with respect to IgA, makes saliva collection the preferred method of antimicrobial assessment in athletes, especially in comparison with the collection of other mucosal secretions. Nasal secretions have been used to study AMPs in respiratory secretions. However, most collection techniques for nasal secretions are complex and invasive, such as nasal lavage and suction, because spontaneously secreted fluid is not released in a large enough volume at a constant rate in healthy individuals. The detection of AMPs in nasal lavage is also markedly diminished by dilution of airway secretions (Cole et al., 1999). The collection of tear fluid may also be an effective method for studying changes in mucosal immunity. However, changes in the concentration of tear fluid may not accurately reflect changes occurring in respiratory tract AMPs. Given their role in protection of the URT and the ease with which their status can be collected, salivary AMPs offer promise as useful parameters in monitoring the status of the mucosal immune system.
Conclusion
AMPs play a diverse role in the innate mucosal immune system. As a constituent product at mucosal surfaces, AMPs participate in the barrier function that prevents microorganisms from causing infection. This activity is mediated by acting directly on microorganisms, which can be lethal or inhibit their growth and activity, or by preventing them from initiating an inflammatory response. There should also be recognition that AMPs act more broadly to participate in an immune response by recruiting cells, inducing cytokines and aiding in tissue repair. Although there is a growing body of evidence that AMPs play a role in mucosal immunity, further research is required to quantify their role with regard to susceptibility to infection. One mechanism by which this can be explored is through prolonged intense exercise, which causes a transient suppression of immunity. Individuals undertaking heavy prolonged exercise appear to suffer an increased incidence of URTIs. As yet, however, no link has been found between exercise‐induced immunosuppression and increased incidence of illness. Further prospective, well‐designed and controlled studies are required to clarify the relationships between exercise‐induced perturbations in AMPs and incidence of illness. This line of investigation should enhance our understanding of the role of AMPs in mucosal immunity.
Editor: Willem van Leeuwen
References
- Arditi M, Zhou J, Dorio R, Rong GW, Goyert SM & Kim KS (1993) Endotoxin‐mediated endothelial cell injury and activation: role of soluble CD14. Infect Immun 61: 3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D, Di Biase AM, Marchetti M, Pietrantoni A, Valenti P, Seganti L & Superti F (2002) Antiadenovirus activity of milk proteins: lactoferrin prevents viral infection. Antiviral Res 53: 153–158. [DOI] [PubMed] [Google Scholar]
- Arnt L, Rennie JR, Linser S, Willumeit R & Tew GN (2006) Membrane activity of biomimetic facially amphiphilic antibiotics. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys 110: 3527–3532. [DOI] [PubMed] [Google Scholar]
- Barrett B, Locken K, Maberry R, Schwamman J, Brown R, Bobula J & Stauffacher EA (2002) The Wisconsin Upper Respiratory Symptom Survey (WURSS): a new research instrument for assessing the common cold. J Fam Pract 51: 265–273. [PubMed] [Google Scholar]
- Baveye S, Elass E, Fernig DG, Blanquart C, Mazurier J & Legrand D (2000) Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E‐selectin and ICAM‐1, induced by the CD14‐lipopolysaccharide complex. Infect Immun 68: 6519–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett‐Guerrero E, Barclay GR, Weng PL, Bodian CA, Feierman DE, Vela‐Cantos F & Mythen MG (2001) Endotoxin‐neutralizing capacity of serum from cardiac surgical patients. J Cardiothorac Vasc Anesth 15: 451–454. [DOI] [PubMed] [Google Scholar]
- Bowdish DM, Davidson DJ & Hancock RE (2005) A re‐evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 6: 35–51. [DOI] [PubMed] [Google Scholar]
- Brandenburg K, Jurgens G, Muller M, Fukuoka S & Koch MH (2001) Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol Chem 382: 1215–1225. [DOI] [PubMed] [Google Scholar]
- Britigan BE, Lewis TS, Waldschmidt M, McCormick ML & Krieg AM (2001) Lactoferrin binds CpG‐containing oligonucleotides and inhibits their immunostimulatory effects on human B cells. J Immunol 167: 2921–2928. [DOI] [PubMed] [Google Scholar]
- Clarke NM & May JT (2000) Effect of antimicrobial factors in human milk on rhinoviruses and milk‐borne cytomegalovirus in vitro . J Med Microbiol 49: 719–723. [DOI] [PubMed] [Google Scholar]
- Cole AM, Dewan P & Ganz T (1999) Innate antimicrobial activity of nasal secretions. Infect Immun 67: 3267–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daele J & Zicot AF (2000) Humoral immunodeficiency in recurrent upper respiratory tract infections. Some basic, clinical and therapeutic features. Acta Oto-Rhino-Laryngol Belg 54: 373–390. [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt AP et al (2000) LL‐37, the neutrophil granule‐ and epithelial cell‐derived cathelicidin, utilizes formyl peptide receptor‐like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 192: 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DA & Hancock RE (2002) Cationic peptides: distribution and mechanisms of resistance. Curr Pharm Des 8: 703–714. [DOI] [PubMed] [Google Scholar]
- Dixon RE (1985) Economic costs of respiratory tract infections in the United States. Am J Med 78: 45–51. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, Pestonjamasp VK, Tamakuwala S et al (2001) Cutaneous injury induces the release of cathelicidin anti‐microbial peptides active against group A Streptococcus . J Invest Dermatol 117: 91–97. [DOI] [PubMed] [Google Scholar]
- Dubin RF, Robinson SK & Widdicombe JH (2004) Secretion of lactoferrin and lysozyme by cultures of human airway epithelium. Am J Physiol Lung Cell Mol Physiol 286: L750–L755. [DOI] [PubMed] [Google Scholar]
- Duits LA, Nibbering PH, Van Strijen E, Vos JB, Mannesse‐Lazeroms SP, Van Sterkenburg MA & Hiemstra PS (2003) Rhinovirus increases human beta‐defensin‐2 and ‐3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol 38: 59–64. [DOI] [PubMed] [Google Scholar]
- Elsbach P (2003) What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J Clin Invest 111: 1643–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I (2004) Bactericidal cationic peptides can also function as bacteriolysis‐inducing agents mimicking beta‐lactam antibiotics?; it is enigmatic why this concept is consistently disregarded. Med Hypotheses 62: 367–374. [DOI] [PubMed] [Google Scholar]
- Glaser R, Friedman SB, Smyth J et al (1999) The differential impact of training stress and final examination stress on herpesvirus latency at the United States military academy at west point. Brain Behav Immun 13: 240–251. [DOI] [PubMed] [Google Scholar]
- Gleeson M (2000) Mucosal immune responses and risk of respiratory illness in elite athletes. Exerc Immunol Rev 6: 5–42. [PubMed] [Google Scholar]
- Gleeson M, Hall ST, McDonald WA, Flanagan AJ & Clancy RL (1999a) Salivary IgA subclasses and infection risk in elite swimmers. Immunol Cell Biol 77: 351–355. [DOI] [PubMed] [Google Scholar]
- Gleeson M, McDonald WA, Pyne DB, Cripps AW, Francis JL, Fricker PA & Clancy RL (1999b) Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc 31: 67–73. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Pyne DB & Callister R (2003) Exercise effects on mucosal immunity and risk of upper respiratory illness. Int J Sports Med 4: 1–14. [Google Scholar]
- Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ & McDermott AM (2005) Human cathelicidin (LL‐37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res 30: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover M, Giouzeppos O, Schnagl RD & May JT (1997) Effect of human milk prostaglandins and lactoferrin on respiratory syncytial virus and rotavirus. Acta Paediatr 86: 315–316. [DOI] [PubMed] [Google Scholar]
- Gwaltney JM (2002) Clinical significance and pathogenesis of viral respiratory infections. Am J Med 112: 13S–18S. [DOI] [PubMed] [Google Scholar]
- Hancock RE (2001) Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1: 156–164. [DOI] [PubMed] [Google Scholar]
- Harmsen MC, Swart PJ, De Bethune MP, Pauwels R, De Clercq E, The TH & Meijer DK (1995) Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis 172: 380–388. [DOI] [PubMed] [Google Scholar]
- Hashem M & Hall CB (2003) Respiratory syncytial virus in healthy adults: the cost of a cold. J Clin Virol 27: 14–21. [DOI] [PubMed] [Google Scholar]
- Haversen L, Ohlsson BG, Hahn‐Zoric M, Hanson LA & Mattsby‐Baltzer I (2002) Lactoferrin down‐regulates the LPS‐induced cytokine production in monocytic cells via NF‐kappa B. Cell Immunol 220: 83–95. [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Breeuwer P, Van't Hof W et al (1999) The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem 274: 7286–7291. [DOI] [PubMed] [Google Scholar]
- Ibrahim HR, Inazaki D, Abdou A, Aoki T & Kim M (2005) Processing of lysozyme at distinct loops by pepsin: a novel action for generating multiple antimicrobial peptide motifs in the newborn stomach. Biochim Biophys Acta 1726: 102–114. [DOI] [PubMed] [Google Scholar]
- Inoue H, Sakai M, Kaida Y & Kaibara K (2004) Blood lactoferrin release induced by running exercise in normal volunteers: antibacterial activity. Clin Chim Acta 341: 165–172. [DOI] [PubMed] [Google Scholar]
- Kanyshkova TG, Babina SE, Semenov DV et al (2003) Multiple enzymic activities of human milk lactoferrin. Eur J Biochem 270: 3353–3361. [DOI] [PubMed] [Google Scholar]
- Kirkland TN, Finley F, Leturcq D, Moriarty A, Lee JD, Ulevitch RJ & Tobias PS (1993) Analysis of lipopolysaccharide binding by CD14. J Biol Chem 268: 24818–24823. [PubMed] [Google Scholar]
- Koczulla R, Von Degenfeld G, Kupatt C et al (2003) An angiogenic role for the human peptide antibiotic LL‐37/hCAP‐18. J Clin Invest 111: 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D, Yong Y, Ng V & Chia SE (2002) Stress, mucosal immunity, upper respiratory tract infections, and sickness absence. J Occup Environ Med 44: 987–988. [DOI] [PubMed] [Google Scholar]
- Le Roy D, Di Padova F, Adachi Y, Glauser MP, Calandra T & Heumann D (2001) Critical role of lipopolysaccharide‐binding protein and CD14 in immune responses against gram‐negative bacteria. J Immunol 167: 2759–2765. [DOI] [PubMed] [Google Scholar]
- Legrand D, Elass E, Pierce A & Mazurier J (2004) Lactoferrin and host defence: an overview of its immuno-modulating and anti-inflammatory properties. Biometals 17: 225–229. [DOI] [PubMed] [Google Scholar]
- Leitch EC & Willcox MD (1999) Elucidation of the antistaphylococcal action of lactoferrin and lysozyme. J Med Microbiol 48: 867–871. [DOI] [PubMed] [Google Scholar]
- Lopez‐Garcia B, Lee PH, Yamasaki K & Gallo RL (2005) Anti‐fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol 125: 108–115. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K (1999) Why and how are peptide‐lipid interactions utilized for self‐defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta 1462: 1–10. [DOI] [PubMed] [Google Scholar]
- McPhee JB & Hancock RE (2005) Function and therapeutic potential of host defence peptides. J Pept Sci 11: 677–687. [DOI] [PubMed] [Google Scholar]
- Muns G (1994) Effect of long‐distance running on polymorphonuclear neutrophil phagocytic function of the upper airways. Int J Sports Med 15: 96–99. [DOI] [PubMed] [Google Scholar]
- Muns G, Singer P, Wolf F & Rubinstein I (1995) Impaired nasal mucociliary clearance in long‐distance runners. Int J Sports Med 16: 209–213. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ohtake T, Dorschner RA & Gallo RL (2002) Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res 81: 845–850. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Sampson CS et al (1995) The acute immune response to exhaustive resistance exercise. Int J Sports Med 16: 322–328. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X et al (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414: 454–457. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C et al (2002) Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347: 1151–1160. [DOI] [PubMed] [Google Scholar]
- Peake J, Wilson G, Hordern M et al (2004) Changes in neutrophil surface receptor expression, degranulation, and respiratory burst activity after moderate‐ and high‐intensity exercise. J Appl Physiol 97: 612–618. [DOI] [PubMed] [Google Scholar]
- Pyne DB (1994) Regulation of neutrophil function during exercise. Sports Med 17: 245–258. [DOI] [PubMed] [Google Scholar]
- Pyne DB & Gleeson M (1998) Effects of intensive exercise training on immunity in athletes. Int J Sports Med S183–S191; discussion S191–S194. [DOI] [PubMed] [Google Scholar]
- Pyne DB, McDonald WA, Gleeson M, Flanagan A, Clancy RL & Fricker PA (2001) Mucosal immunity, respiratory illness, and competitive performance in elite swimmers. Med Sci Sports Exerc 33: 348–353. [DOI] [PubMed] [Google Scholar]
- Quinones‐Mateu ME, Lederman MM, Feng Z et al (2003) Human epithelial beta‐defensins 2 and 3 inhibit HIV‐1 replication. Aids 17: F39–F48. [DOI] [PubMed] [Google Scholar]
- Reddy KV, Yedery RD & Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24: 536–547. [DOI] [PubMed] [Google Scholar]
- Samuelsen O, Haukland HH, Ulvatne H & Vorland LH (2004) Anti‐complement effects of lactoferrin‐derived peptides. FEMS Immunol Med Microbiol 41: 141–148. [DOI] [PubMed] [Google Scholar]
- Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE & Gold MR (2000) Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol 164: 549–553. [DOI] [PubMed] [Google Scholar]
- Seydel U, Hawkins L, Schromm AB, Heine H, Scheel O, Koch MH & Brandenburg K (2003) The generalized endotoxic principle. Eur J Immunol 33: 1586–1592. [DOI] [PubMed] [Google Scholar]
- Shinoda I, Takase M, Fukuwatari Y, Shimamura S, Koller M & Konig W (1996) Effects of lactoferrin and lactoferricin on the release of interleukin 8 from human polymorphonuclear leukocytes. Biosci Biotechnol Biochem 60: 521–523. [DOI] [PubMed] [Google Scholar]
- Singh PK, Tack BF, McCray PB Jr & Welsh MJ (2000) Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol 279: L799–L805. [DOI] [PubMed] [Google Scholar]
- Sorensen OE, Cowland JB, Theilgaard‐Monch K, Liu L, Ganz T & Borregaard N (2003) Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol 170: 5583–5589. [DOI] [PubMed] [Google Scholar]
- Spence L, Nissen M, Sloots T et al (2004) Identifying the aetiology of upper respiratory illness in elite and recreational athletes. Proceedings of the Australian Conference of Science and Medicine in Sport, 6–9 October 2004, Alice Springs, Australia, Abstract 80.
- Tjabringa GS, Vos JB, Olthuis D et al (2005) Host defense effector molecules in mucosal secretions. FEMS Immunol Med Microbiol 45: 151–158. [DOI] [PubMed] [Google Scholar]
- Tomasi TB, Trudeau FB, Czerwinski D & Erredge S (1982) Immune parameters in athletes before and after strenuous exercise. J Clin Immunol 2: 173–178. [DOI] [PubMed] [Google Scholar]
- Toy LS & Mayer L (1996) Basic and clinical overview of the mucosal immune system. Semin Gastrointest Dis 7: 2–11. [PubMed] [Google Scholar]
- Travis SM, Conway BA, Zabner J et al (1999) Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol 20: 872–879. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H, Takase M & Tomita M (2003) Lactoferricin derived from milk protein lactoferrin. Curr Pharm Des 9: 1277–1287. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN et al (1999) Beta‐defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286: 525–528. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O & Oppenheim JJ (2001) The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci 58: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M (2004) Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol 75: 39–48. [DOI] [PubMed] [Google Scholar]


