Abstract
A green fluorescent protein (gfp) gene was ligated to the Lactobacillus reuteri‐specific nisin‐inducible expression‐secretion vector pNIES, generating a pNIES‐GFP vector capable of secreting the cloned gene as a GFP‐fusion protein with fluorescent activity. To develop this system as a live vehicle carrying the heat‐stable enterotoxin (ST) and heat‐labile enterotoxin B (LTB) of the enterotoxigenic Escherichia coli (ETEC), a recombinant 5′‐ST‐LT B‐3′ DNA fragment was cloned into pNIES‐GFP. The resulting L. reuteri/pNIES‐GFP:STLTB system was found to possess the capability of adhering to the mice gut, secreting GFP:STLTB product at 0.14 and 0.026 pgcell−1 under induced and noninduced conditions, respectively. Further analysis of the GFP:STLTB product confirmed its ganglioside‐binding ability, LTB antigenicity and relative freedom from the ST‐associated toxicity, making it suitable for use as an oral vaccine in mice. Oral inoculation of the L. reuteri/pNIES‐GFP:STLTB culture in mice elicited significant (P<0.01) serum IgG and mucosal IgA antibodies against the STLTB antigen. These immunized mice were subsequently challenged with ETEC and showed full protection against the fluid influx response in the gut. This is the first report of using L. reuteri as a vaccine carrier to induce complete immunologic protection against ETEC.
Keywords: Escherichia coli enterotoxin, Lactobacillus reuteri, oral vaccine, green fluorescent protein
Introduction
Lactobacillus reuteri, frequently found in the gastrointestinal tracts of humans and animals, has been discovered to have advantages that make it suitable for use as a probiotic (1989, 2002, 2005). Further development of such a microbe with probiotic effects as a live vehicle carrying immunological molecules would provide the gastrointestinal tract with extra medical benefits. To implement this goal, L. reuteri, along with many other Lactobacillus species already applied in the probiotic field, has been intensively explored for its possible role as a vaccine carrier during the last decade.
However, to our knowledge, only four L. reuteri species –Lactobacillus acidophilus (Chu et al., 2005), L. casei (2005, 2000, 1999), L. johnsonii (Scheppler et al., 2002) and L. plantarum (2005, 2002, 2002, 2000) – have produced foreign antigens (i.e. urease B, proteinase B, tetanus toxin fragment C and Coronavirus glycoprotein S) and elicited a specific immune response in the mucosal membrane system. One reason for this small number may be the limited sources of expression‐secretion vectors (ESV), which, because they are frequently species‐specific, were difficult to adapt to other Lactobacillus reuteri species (Wu et al., 2006).
Therefore, constructing a new ESV is nearly inevitable, as a Lactobacillus reuteri species of interest is chosen for live vehicle development. Given the considerable phylogenic distances existing among the more than 60 Lactobacillus reuteri species (Reveneau et al., 2002), which usually vary greatly in efficacy of transcription, translation and secretion signals (2002, 2002, 2002), a broad host‐spectrum ESV functional in many L. reuteri species seems unlikely to be developed in the near feature. Accordingly, an L. reuteri‐specific nisin‐inducible ESV, the pNIES, was constructed and found to possess advantages, including a high secretion efficiency, a high constitutive ES activity and also a satisfactory inducible capacity, appropriate for use in L. reuteri as a vaccine vector (Wu et al., 2006).
Enterotoxigenic Escherichia coli (ETEC) had been responsible for a sizable fraction of the morbidity and mortality burden from diarrheal diseases suffered by young children in developing countries (Guzman‐Verduzco & Kupersztoch, 1987). The virulence of ETEC is directly related to the production of heat‐labile (LT) and/or heat‐stable (ST) toxins, which alter the hydrosaline balance of the intestinal mucosa. To our knowledge, no licensed vaccine is available for ETEC thus far. Apparently, more efforts are still required before this important goal can be realized.
Fusion of ST and LT enterotoxin B (LTB) together can successfully evoke high titers of neutralization antibodies efficiently against both ST and LTB (Clements, 1990). In addition, a recombinant 5′‐ST‐LT B‐3′ DNA template from the pGSK51 (Guzman‐Verduzco & Kupersztoch, 1987) is immediately available. Bearing these in mind, a STLT B gene encoding the 53‐amino acid ST polypeptide (including the 19‐amino acids of active ST enterotoxin) and 105‐amino acid LTB polypeptide, devoid of their original leader peptides, was PCR‐amplified and tested for its potential role as a subunit vaccine through our newly constructed L. reuteri/pNIES system.
In this study, the pNIES is upgraded to a pNIES‐GFP, which is able to express and secrete the cloned STLT B gene as a GFP:STLTB fusion protein with fluorescent activity. This L. reuteri/pNIES‐GFP system and its secreted GFP:STLTB protein were characterized to evaluate their suitability for use in the gastrointestinal tract of mice. Further mice immunization, antibody titers (IgG and IgA) detection, and protection tests are also included in this report.
Materials and methods
Bacterial strains and growth conditions
The bacterial strains and recombinant plasmids used in this study are listed in Table 1. All experiments were performed with strains L. reuteri DSM20016 (L. reuteri 20016) and WT3‐3 (L. reuteri 3‐3). Lactobacillus reuteri 20016 is the prototype that originated from human intestine (Kandler et al., 1980), whereas L. reuteri 3‐3 is an isolate from mice cecum.
Table 1.
Bacterial strains and plasmids used in this study
| Strains/plasmids | Characteristics | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Escherichia coli TG1 | K‐12Δ(lac‐pro) supE thi hsd5 F’ traD36 proA + B + lacI q lacZΔM15; Ems | New England Biolab |
| Escherichia coli ATCC35401 | Enterotoxigenic E. coli (O78:H11), heat‐stable enterotoxin and labile enterotoxin producing strain; isolated from human feces | ATCC * |
| Lactobacillus reuteri DSM20016 | Type strain; plasmid free; Ems | Kandler et al. (1980) |
| Lactobacillus reuteri WT3‐3 | Wild type strain isolated from cecum of mouse; reuterin producing strain; plasmid free; Ems | This study |
| Plasmids | ||
| pNIES | Nisin‐controlled expression‐secretion vector containing ColE1 ori, DSO‐rep32‐SSO ori, PnisA, nisRK gene, and SPamyL; Emr; 8.2 kb | Wu et al. (2006) |
| pNIES‐GFP | pNIES::gfp; 9 kb | This study |
| pNIES‐GFP:STLTB | pNIES::gfp::hST‐LT B; 9.6 kb | This study |
| pET‐STLTB | pETc:: hST‐LT B; Ampr; 6.4 kb | This study |
| pGSK‐51 | Cloning vector containing the genetic fusion gene of Escherichia coli enterotoxins, hST‐LT B; Ampr; 3.6 kb | ATCC67278; Guzman‐Verduzco & Kupersztoch (1987) |
| pQBI25‐GFP | Eukaryotic expression vector containing gfp gene; Ampr; 6.2 kb | Qbiogene |
ATCC: American Type Culture Collection.
All cloning steps were done with E. coli TG1 strain (New England Biolabs) grown in LB medium (Difco) at 37°C under aeration. Lactobacillus reuteri strains were cultured in buffered MRS broth (Difco) containing 0.2 M potassium phosphate (pH 7) at 37°C without shaking. When required, erythromycin was added to the culture medium at final concentrations of 10 and 150 μg mL−1 for recombinant L. reuteri (rL. reuteri) and E. coli strains, respectively.
Plasmid construction and DNA manipulation
Plasmids from E. coli were extracted using an alkaline lysis method and L. reuteri plasmids were isolated as described by Chassy & Giuffrida (1980). Restriction endonucleases, T4 DNA ligase, and Taq polymerase were purchased from New England Biolabs and used according to the manufacturer's recommendations. Electroporations of L. reuteri and E. coli were performed as described by Lin & Chung (1999).
The construction scheme for pNIES‐GFP:STLTB is shown in Fig. 1. The expression‐secretion vector pNIES was constructed previously (Wu et al., 2006) and the gfp gene (795 bp) was PCR‐amplified from pQBI25‐GFP (Qbiogene) using oligonucleotides 5′‐TGACTAGTGCTAGCAAAGGAGA and 5′‐GTCAGAATTCGCGAAGCTTGCT (the restriction sites are underlined) as primers. The PCR product of gfp gene, after treatment with SpeI/EcoRI, was ligated to the SpeI/EcoRI‐digested pNIES, creating the plasmid pNIES‐GFP (Fig. 1a). Subsequently, an STLT B gene (510 bp), produced by PCR‐amplification from the pGSK51 (Guzman‐Verduzco & Kupersztoch, 1987) using primer sets of ST‐F (5‐GCAGGATCC ATATGAAAAAATCA‐3′) and LTB‐R (5′‐CTAGAATTCTTTTAAG AAAC‐3′), was digested with BamHI/EcoRI and inserted into the corresponding sites in pNIES‐GFP to generate the pNIES‐GFP:STLTB (Fig. 1b). Standard PCR was carried out with an automated thermal cycler (PerkinElmer). Amplification was performed with 1 ng plasmid DNA under the following conditions: 2 min at 94°C followed by 30 cycles consisting of 30 s at 94°C, 30 s at 55°C, and 45 s at 72°C and a final step of 7 min at 72°C. To produce large amounts of STLTB protein in E. coli, the BamHI/EcoRI STLT B fragment was cloned into the expression vector pET32c (Novagen) to create the pET‐STLTB. The integrity of DNA sequencing in every step of the cloning process as mentioned above was confirmed by sequence analysis.
Figure 1.
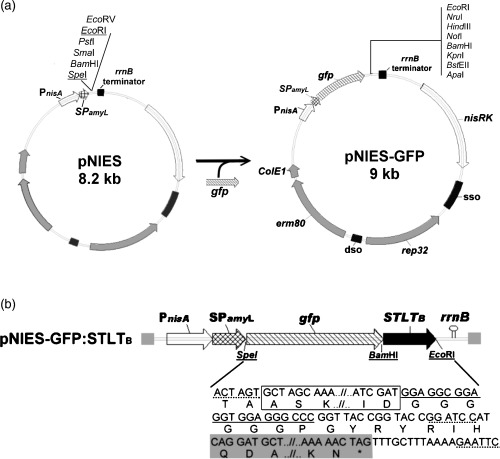
Design of the cassette for the GFP:STLTB fusion protein to be expressed and secreted by Lactobacillus reuteri. (a) Construction and the restriction map of pNIES‐GFP. The pNIES‐GFP was constructed by ligating a gfp reporter gene with multiple cloning sites to the pNIES. The transcription directions are indicated by the arrows. Construction details are given in the text. (b) The GFP:STLTB cassette is expressed under the control of PnisA promoter. The cassette sequence is given below the schematic diagram, in which the restriction endonuclease cleavage sites are underlined with a dashed line. The gfp and STLT B gene sequences are represented by a box and gray background, respectively. The flexible linker (underlined) is between the gfp and STLT B genes.
Epifluorescence microscopy
Fluorescent activity of the L. reuteri/pNIES‐GFP:STLTB (recombinant L. reuteri, rL. reuteri) cells was directly observed by eye using an epifluorescence microscope (Olympus model BX60) fitted with an FITC filter set. Activation of the expression of GFP fusion protein was performed as described in Wu & Chung (2006).
Preparation of cell lysate and culture supernatant for protein analysis
Overnight rL. reuteri cultures were used to inoculate fresh MRS media at 1 : 50 dilution. After incubation for 1 h, the cultures were added (induced culture), or not (noninduced culture), with nisin A (Sigma) at a final concentration of 50 ng mL−1 and allowed to grow for a further 3 h before being adjusted with additional MRS broth to an OD at 600 nm of 0.25–0.27 (c. 1 × 108 CFU mL−1). Then, 10 mL of the OD‐adjusted culture was centrifuged and the supernatant concentrated to 1/100 volume with an Amicon PM‐30 concentrator (Millipore), which was used latterly as the supernatant fraction in the protein analysis. The cell pellet was washed twice, resuspended in 100 μL lysis buffer (1 mL of 10 mMTris‐HCl buffer containing 100 μg lysozyme and mutanolysin), and incubated at 37°C for 3 h to produce the protoplast. Sonication of the protoplast was performed with an ultrasonic homogenizer (Labsonic 2000; Sartorius BBI Systems) from which the clear lysate was obtained by centrifugation and used as the intracellular fraction for protein analysis.
Protein analysis
To visualize the proteins in the lysate and supernatant, the protein concentrations of the samples were analyzed using a Bio‐Rad protein assay kit, mixed well with an equal amount of sample buffer (2 ×), and then subjected to Western blot analysis (Martín et al., 2004), in which polyclonal rabbit antiserum against GFP (Invitrogen) and alkaline phosphatase‐conjugated goat anti‐rabbit IgG antibody (Sigma) were used for the immunoblotting target protein detection. Quantification of the protein in gel was performed by comparing the signal scanned from the target protein with that from the purified GFP:STLTB (as a standard), using an Alphaimager image‐analyzing system (Alpha Innotech) and the computer program alphaimager™ 2200 version 5.5 as described in Wu et al. (2006).
GM1‐ELISA
The ganglioside binding activity of the GFP:STLTB protein was examined following procedures described by Slos et al. (1998). Briefly, microtiter plates, precoated per well with 200 ng of the mixed gangliosides (Sigma) in carbonate‐bicarbonate coating buffer (pH 9.6), were loaded with 100 μl of the concentrated supernatant sample, followed by the addition of rabbit anti‐LTB antiserum (Immunology Consultants Laboratory) and p‐nitrophenyl phosphate (Sigma). Absorbance was measured at 405 nm.
Toxicity assay
The suckling mouse assay, as descried by Giannella (1976), with slight modifications, was performed to determine the ST toxicity of GFP:STLTB protein. In brief, solutions containing 0.1, 0.15 and 0.5 μg mL−1 of ST were prepared by dissolving and diluting the purified ST (100 000 U mg−1, Sigma) with phosphate‐buffered saline (PBS) (containing 1% bovine serum albumin and 0.2% Trypan blue dye). The same process was also performed in preparing the 10 and 100 μg mL−1 GFP:STLTB solutions from a concentrated rL. reuteri culture supernatant, of which the GFP:STLTB concentration was estimated in advance using an alphaimager image‐analyzing system as mentioned above. To assay the ST toxicity in mice, five groups of infant SPF BALB/c mice (3 days old, five mice per group), purchased from the National Laboratory Animal Center (NLAC, Taipei, Taiwan) and housed in cages with free access to water and feed as approved by the local veterinary office, were intragastrically inoculated by group with 100 μL of one of the aforementioned concentrations of ST or GFP: STLTB solutions per mouse. After 3 h, the animals were sacrificed and dissected. Data were collected only from those showing blue coloration in the stomach, which is evidence of a successful inoculation of the solution into the stomach. The entire intestine and remaining carcass were weighed separately, and the weight ratio between the gut and remaining carcass (G/C ratio) calculated.
Survival of recombinant strains in feces
Two groups of mice (five mice per group) were, respectively, administered orally with rL. reuteri 3‐3, or rL. reuteri 20016, at a dose of 1 × 109 CFU per mouse. Fresh feces from five mice in each group were collected 2 days before and every day after the administration for 5 days, and crushed together in sterile PBS to obtain a pooled sample of 100 mg mL−1 of fecal suspension from each group, from which a serial dilution was made. Persistence was estimated by triplicate plating 100 mL of each appropriate dilution onto the Lactobacillus reuteri‐selective Rogosa agar (Merck) containing 10 μg mL−1 erythromycin and counting the visible colonies after 48 h of anaerobic incubation at 37°C. Ten randomly selected colonies per plate were further picked and examined for the fluorescent activity (Flu+) with an epifluorescence microscope as described above, from which the percentage of Flu+ colonies was obtained and used as an index to adjust the fecal CFU value (CFU 100 mg−1 feces) calculated from the Emr colonies.
Immunization procedures
The SPF BALB/c mice (aged 8 weeks), five mice per group, were housed in the same cage for 7 days prior to the commencement of experiment. To prepare the rL. reuteri for mice immunization, large volumes of MRS media were inoculated with overnight cultures at a concentration of 1 : 50. Nisin A was added to the cultures after incubation for 1 h. The cultures were then incubated for another 2 h and harvested by centrifugation (at 8000 g for 10 min at 4°C) to obtain pellets, which were immediately resuspended in an administration buffer (0.2 M sodium bicarbonate, 5% casein hydrolysate, 0.5% glucose) (Robinson et al., 1997) and adjusted by an OD600 nm reading to reach a concentration of 5 × 109 CFU mL−1. Mice were intragastrically inoculated with the freshly prepared cell suspensions daily at a dose of 0.2 mL per mouse for 3 days. This was repeated three times (one prime and two boosts) with a 2‐week interval between each repeat. Ten days after each administration, individual sera and fresh fecal pellets were collected and treated as described by Robinson et al. (1997). These vaccination experiments were carried out on at least two separate occasions and the results demonstrated to be reproducible.
Antibody determinations
STLTB protein was extracted from the IPTG‐induced E. coli BL21 (DE3) (Novagen) transformed with pET‐STLTB, and purified by Ni‐affinity chromatography (Novagen) as recommended by the manufacturer. The titers of the sera (IgG) and fecal (IgA) anti‐STLTB antibodies were determined by enzyme‐linked immunosorbant assay (ELISA) as described by Grangette et al. (2002). Briefly, polystyrene microtiter plates (Nunc), coated overnight at 4°C with 1 μg of the purified STLTB, were added to the twofold serially diluted serum or fecal samples. Bound antibodies were detected using alkaline phosphatase conjugated goat anti‐mouse IgG or IgA (Sigma), followed by color development using p‐nitrophenyl phosphate as substrate. Absorbance was measured at 405 nm. End‐point titers were defined as the highest dilution that gave an absorbance three times higher than background.
Challenge procedures: patent mouse gut (PMG) assay
In vivo enterotoxin neutralization was determined using a PMG assay as described by Guidry et al. (1997) with modifications. Briefly, all immunized mice were given sterile water containing 5 mg streptomycin per milliliter ad libitum for 3 days, and then fasted for 24 h, followed by sterile water only for an additional 6 h prior to challenge. When challenged, mice were inoculated intragastrically with 0.5 mL NaHCO3 10% solution alone or in conjunction with 3 × 108, 1.5 × 109, or 3 × 109 CFU of live ETEC organisms. The mice remained in their cages without food but with water ad libitum for 3 h, and were then sacrificed by CO2 inhalation. The entire intestine and carcass were weighed separately and a G/C ratio was calculated for each animal.
Statistical analysis
The results are expressed as the mean±SE of the mean (SEM). Statistical significance was evaluated by Student's t‐test. Statistical significance was considered to be P<0.05 or 0.01.
Results
Construction of the pNIES‐GFP vector
As shown in Fig. 1a, a promoterless gfp gene was cloned into the L. reuteri‐specific expression‐secretion vector pNIES, generating a chimerical plasmid pNIES‐GFP with eight unique cloning sites immediately downstream of the gfp gene. Lactobacillus reuteri transformed with pNIES‐GFP exhibited intracellular and extracellular green fluorescent activity (Fig. 2a), indicating the gfp gene was successfully cloned and regulated under the expression and secretion elements in pNIES.
Figure 2.
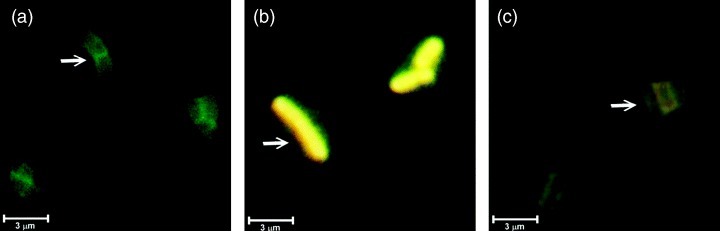
Microscopic photographs of the fluorescent recombinant Lactobacillus reuteri expressing GFP:STLTB in the supernatant (c), and cytoplasm (b). A positive control, the Lactobacillus reuteri/pNIES‐GFP culture, was included (a). Scale bar=3 μm.
To test the function of pNIES‐GFP in expressing and secreting the cloned gene of interest as a GFP‐fusion product, the STLT B gene was ligated into its BamHI/EcoRI sites to translationally (in‐frame) fuse with the upstream gfp gene. The resulting 9.6‐kb plasmid pNIES‐GFP:STLTB (Fig. 1b), which was shown to contain the gfp:STLT B DNA fragment by restriction and PCR‐amplification analysis (Supplementary Fig. S1), was transformed into L. reuteri 20016 and L. reuteri 3‐3. The L. reuteri transforments carrying pNIES‐GFP:STLTB were found to exhibit fluorescent activities (Flu+) in their cytoplasm and culture supernatant, though with a yellow color (Fig. 2). Further Western blot analysis of the culture supernatant detected the presence of a 48‐kDa protein, corresponding to the molecular mass of the mature GFP:STLTB protein predicted from the DNA sequence (Fig. 3a). This confirmed the capability of the pNIES‐GFP to express and secrete GFP‐fusion protein with fluorescent activity.
Figure 3.
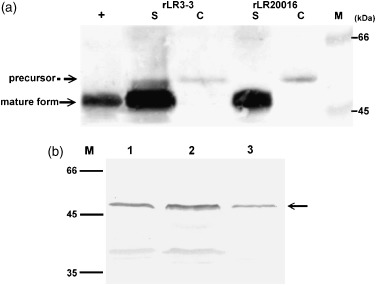
Western blot analysis and quantification of GFP:STLTB fusion protein produced by the recombinant Lactobacillus reuteri cultures which were induced (a) or not (b) with nisin. (a) Target fusion proteins in 20 μL of culture supernatant (S) and cell lysate (C) of recombinant Lactobacillus reuteri 20016 and recombinant Lactobacillus reuteri 3‐3 were detected by rabbit anti‐GFP antibody. Affinity‐purified GFP:STLTB protein 2 μg was used as a standard (+). The size (in kDa) of the molecular mass marker is shown in the left margin. By scanning and comparing signals with the purified protein, concentrations of the secreted GFP:STLTB in recombinant Lactobacillus reuteri 3‐3 and recombinant Lactobacillus reuteri 20016 cultures can be estimated to be 14.3 and 13.6 mg L−1, respectively. (b) Noninducing recombinant L. reuteri cultures. Lanes 1 and 2 were 10 μL of concentrated supernatants of recombinant Lactobacillus reuteri 20016 and Lactobacillus reuteri 3‐3; lane 3 is 0.1 μg of affinity‐purified GFP:STLTB protein (indicated with arrow). The concentration of the secreted GFP:STLTB in noninducing recombinant Lactobacillus reuteri 20016 and recombinant Lactobacillus reuteri 3‐3 cultures can be estimated as 2.1 and 2.6 mg L−1, respectively.
Characterization of the GFP:STLTB fusion protein secreted by the L. reuteri/pNIES‐GFP:STLTB (rL. reuteri)
Quantification of the secreted GFP:STLTB fusion protein using a gel‐protein scan is shown in Fig. 3. Four hours after the inoculation, the yield of fusion protein in the supernatants of the nisin‐induced rL. Reuteri 3‐3 and rL. reuteri 20016 was estimated to be 14.3 and 13.6 mg L−1 (Fig. 3a), respectively, whereas the yield in the supernatants of the noninduced cultures was 2.6 and 2.1 mg L−1, respectively (Fig. 3b). Because the rL. reuteri concentration used in each analysis was about 1 × 108 CFU mL−1, the amounts of GFP:STLTB (pg) secreted per cell in the aforementioned groups could thus be estimated to be 0.14, 0.13, 0.026, and 0.021, respectively.
To estimate the ganglioside‐binding ability of the GFP:STLTB protein, a GM1 ELISA was performed, in which significantly (P<0.05) higher binding indexes (i.e. optical absorbance readings), compared with those in the negative controls, were detected in the culture supernatants of both rL. reuteri strains (Fig. 4), demonstrating the capability of GFP:STLTB to bind to the gangliosides purified from intestinal epithelial cells. Moreover, as anti‐LTB was used in the ELISA test, this positive result also confirmed the existence of LTB antigenicity in the GFP:STLTB protein.
Figure 4.
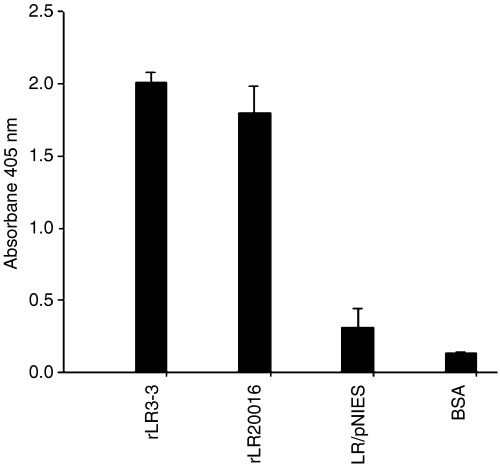
GM1 ELISA evaluation of ability of GFP:STLTB protein to bind the ganglioside. Equal volumes of recombinant Lactobacillus reuteri 20016, recombinant Lactobacillus reuteri 3‐3 concentrated supernatants, and the Lactobacillus reuteri/pNIES and BSA negative controls were loaded onto GM1‐coated wells, followed with detection by rabbit anti‐LTB antiserum. Bars represent the mean absorbance of 405 nm±SEM in each group.
To detect the possible ST toxicity remained in the fusion protein, the GFP:STLTB was orally administered to mice at doses as high as 1000 and 10 000 ng, respectively. The gastrointestinal fluid influx responses, measured as a G/C ratio, with both doses were less than the positive threshold of ST toxicity (i.e. 0.09) determined in this study (Table 2). This, on the contrary, was found to be accomplished by only a mere 15 ng of the purified ST. Thus, as shown in Table 2, the no observed adverse effect level (NOAEL) of GFP:STLTB (>208 pmol) was calculated to be >27 times (208/7.5) greater than that of the purified ST (<7.5 pmol), indicating the relative freedom from the ST‐associated toxicity in the GFP:STLTB protein.
Table 2.
ST toxicity of the GFP:STLTB protein secreted by the recombinant Lactobacillus reuteri in suckling mouse assay
| Protein (MW) | Dose administered | Mean G/C ratio |
|---|---|---|
| PBS/BSA control | Not applicable | 0.08 ± 0.002 |
| ST (2000) | 10 ng * (5 pmol) | 0.075 ± 0.009 |
| ST | 15 ng (7.5 pmol) | 0.106 ± 0.001 |
| ST | 50 ng (25 pmol) | 0.131 ± 0.005 |
| GFP:STLTB (48 000) | 1000 ng † (21 pmol) | 0.07 ± 0.003 |
| GFP:STLTB | 10 000 ng (208 pmol) | 0.082 ± 0.005 |
ST 10 ng (100 000 U mg−1, Sigma) is the amount of toxin capable of producing a G/C ratio of ≥0.083 in Swiss albino mice 3 days old as described in the Sigma product manual. However, by comparing the fluid influx observed in our experiment using BALB/c mice (3 days old), the G/C ratio of ≥0.09 is determined to indicate a positive ST response in this study.
The predicted amount of GFP:STLTB protein was calculated by an alphaimager image‐analyzing system as described by Wu et al. (2006).
MW, molecular weight of protein.
Stability of pNIES‐GFP:STLTB in rL. reuteri and transit times of rL. reuteri in gastrointestinal tract
To analyze the stability of pNIES‐GFP:STLTB in rL. reuteri, rL. reuteri 20016 or rL. reuteri 3‐3 was grown in MRS without erythromycin and analyzed for the percentage of colonies that still maintained the plasmid‐encoded Emr marker at each 12‐generation interval (c. 9.5 h). As shown in Fig. 5, more than 90% of the colonies from both strains, after growing for 72 generations (c. 57 h), were still able to grow on the erythromycin‐containing media, from which the stability index at this stage could thus be interpreted as 90%. However, at the 108th generations (c. 86 h), the stability indexes for both strains declined significantly, reaching only 73.5±3.5% and 48.2±1.8% for the rL. reuteri 3‐3 and rL. reuteri 20016, respectively, indicating that the pNIES‐GFP:STLTB had a better segregational stability in L. reuteri 3‐3 (P<0.01).
Figure 5.
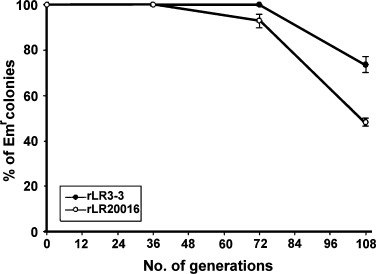
The segregational stability of the plasmid pNIES‐GFP:STLTB in recombinant Lactobacillus reuteri 3‐3 (•) and recombinant Lactobacillus reuteri 20016 (○). The percentage of colonies able to maintain their Emr marker after growing in medium without erythromycin‐selective pressure was used as an index to estimate the stability of this plasmid in its host strain at each generation stage indicated.
To investigate the persistence of rL. reuteri in gastrointestinal tract, mice were inoculated orally with a dose of c. 1 × 109 CFU of the rL. reuteri 20016 or rL. reuteri 3‐3. Large amounts of rL. reuteri began to appear in the feces 24 h after the administration (Fig. 6). Although the numbers of fecal CFU in each time point of both groups were significantly different (P<0.05) from each other, an identical fluctuation pattern of CFU along the time course was observed in both groups. This fluctuation pattern included two significant declines of CFU on the 2nd and 4th days (P<0.05, as compared with the CFU on their last days, respectively) with a substantial presence of rL. reuteri (Emr, Flu+) even 5 days after the administration. The fecal persistence time, in excess of 24 h, which is identical to that of the gastrointestinal‐adhesive lactic acid bacteria (Grangette et al., 2002), strongly indicates the capability of rL. reuteri to colonize in the gastrointestinal tract.
Figure 6.
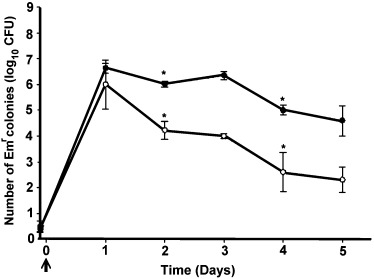
Persistence of recombinant Lactobacillus reuteri reuteri 20016 (○) and recombinant Lactobacillus reuteri 3‐3(•) in the feces of mice. Fecal samples were collected each day after intragastric administration and bacterial recovery was estimated by plating on appropriate selective medium as described in the text. Data were obtained from a triplicate plating of each appropriate dilution. *Indicates significant decline of CFU on that day as compared with the previous day.
To estimate whether our CFU counting had included any comparable colonies such as Emr lactic acid bacteria, which were sometimes found in the gastrointestinal tracts of pigs, chickens and human (2005, 2005, 2005) but still not definitely in mice, a total of 100 randomly picked colonies from 10 plates, each representing a group of rL. reuteri at each day of the experiment, were subjected to fluorescent activity (Flu+) analysis. The result confirmed that the counted CFU all originated from our administered rL. reuteri (Emr, Flu+).
Induction of anti‐STLTB immune responses by the intragastric route
We then examined whether the rL. reuteri system, after intragastric administration to mice, could induce a local and/or systemic immune response against STLTB. Serum IgG analysis (Fig. 7a) revealed that both rL. reuteri 20016 and rL. reuteri 3‐3 strains, after the final boost, elicited significant anti‐STLTB IgG levels (end‐point titers) of 1560 and 4160, respectively (P<0.01, as compared with those in the negative control groups of the L. reuteri 3‐3 and PBS). Notably, the immune response in the rL. reuteri 3‐3 group was so rapid and strong that its IgG end‐point titers of 1480, 2080 and 4160, detected at the three immunization stages (i.e. after priming, first boost, and second boost; Fig. 7a), were significantly (P<0.05) higher than their corresponding titers of 260, 720, and 1560, respectively, in the rL. reuteri 20016 group.
Figure 7.
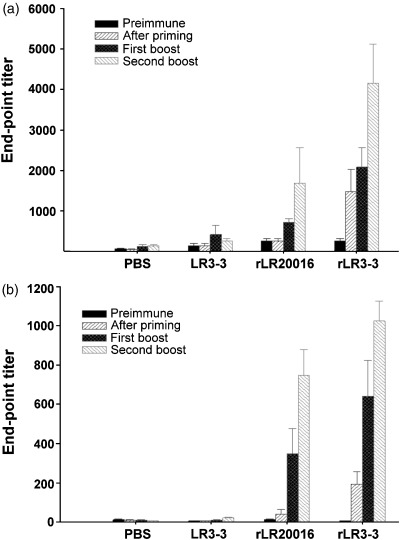
Determination of specific anti‐STLTB sera IgG (a) and fecal IgA (b) titers induced in mice. Individual sera and feces were collected prior to immunization and 10 days after the priming, the first and the second boost from groups of five mice immunized with buffer alone, control strain Lactobacillus reuteri 3‐3, recombinant Lactobacillus reuteri 20016 or recombinant Lactobacillus reuteri 3‐3. Bars represent the mean titers of ELISA antibodies±SEM in each group.
As to the mucosal immune response, analysis of the fecal STLTB‐specific IgA titer revealed that, although evidence of immune activity in both control groups was not detectable, significant STLTB‐specific IgA titers of 746 and 1024, after the final boost, were literally recorded in the rL. reuteri 20016 and rL. reuteri 3‐3 groups, respectively. Again, the rL. reuteri 3‐3 demonstrated a rapid and significantly (P<0.05) higher IgA titer than that of the rL. reuteri 20016, indicating its suitability for studying the immune response of mice to the oral live vaccine.
Challenge of immunized mice with ETEC
As successful induction of mucosal STLTB‐specific IgA was observed, the patent mouse gut assay (Guidry et al., 1997) was then performed to explore whether the onset of immune responses in our rL. reuteri groups could provide substantial protection against ETEC challenge. In our preliminary PMG test, a dose response between the G/C ratio and CFU of ETEC, ranging from 3 × 108 to 3 × 109, was observed (Fig. S2). Moreover, a dose of 3 × 109 CFU of live ETEC was able to induce a serious gastrointestinal fluid influx, building up the gut weight and leading to a high G/C ratio of 0.185. Remarkable contrast was seen in the negative control (NaHCO3 solution) group, which revealed a normal gastrointestinal tract with a G/C ratio of 0.085 only. The gross lesion of fluid accumulation in bowel, especially in cecum, was consistent with the previous observation by Richardson et al. (1984) (Fig. S3). This preliminary PMG test suggested a G/C ratio of ≥0.10 to be the positive threshold for judging the toxic effect of ETEC in this study.
Successful protection against the ETEC challenge was seen in mice immunized with either rL. reuteri 3‐3 or rL. reuteri 20016, because neither visual fluid response nor G/C ratios of ≥0.10 (i.e. 0.090 and 0.095 respectively, Fig. 8) were recorded. In contrast, the negative control groups administered with PBS or L. reuteri 3‐3 strain displayed massive fluid accumulations with G/C ratios of 0.189 and 0.140 significantly (P<0.05) higher than the positive threshold of 0.10. Furthermore, a group of nonimmunized mice, gavaged with NaHCO3 solution instead of the ETEC (shown as a control group in Fig. 8), was found to have normal gastrointestinal tracts with a G/C ratio of only 0.089. This indicated that none of the materials used in this test, except the ETEC strain, was able to cause the fluid accumulation seen in the positive ETEC responses.
Figure 8.
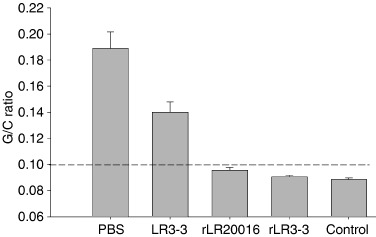
Neutralization of enterotoxin in patent mouse model in vivo by active immunization. At 14 days after the final boost, all immunized groups were challenged with live ETEC (3 × 109 CFU in 0.5 mL NaHCO3 10% solution) intragastrically. Bars represent the mean gut/carcass weight (G/C) ratios for the groups at 3 h after challenge. The control group represents normal mice being challenged with buffer only. G/C ratios of ≥0.10 are considered positive (dashed line).
Discussion
Green fluorescent protein from Aequorea victoria, which has the advantages of being easily and stably detectable by epifluorescence microscopy without the need of adding substrate (or cofactor), has been demonstrated to be a superior reporter to the traditional enzymatic markers in tagging lactic acid bacteria (2000, 2001). In our study, the gfp gene was used in the construction of an L. reuteri/pNIES‐GFP:STLTB (rL. reuteri; Emr, Flu+) system, which expressed the cloned STLT B gene as a GFP:STLTB fusion protein exhibiting fluorescent activity (Flu+) and providing a 2nd selection marker for our rL. reuteri.
More than 400 species of bacteria and a few eukaryotic fungi and protists naturally reside in an individual's digestive tract, and the CFU mL−1 of colon content usually reaches up to 1014 (Lionetti et al., 2006). The Lactobacillus reuteri‐selective Rogosa agar containing 10 μg mL−1 of erythromycin, used in many similar fecal studies, was found to grow some unexpected colonies with morphology comparable to our rL. reuteri during our preliminary test of our rL. reuteri in pigs. The possible microorganisms that appeared on it were suspected to be some yeast and Emr lactic acid bacteria, of which the latter have been reported to emerge frequently in the feces of pigs, chickens, and humans (2005, 2005, 2005) but not definitively in mice. Under such circumstances, developing an rL. reuteri with a double selection marker would certainly provide better accuracy for the fecal CFU estimation.
In the fecal persistence analysis, a total of 100 colonies were examined for their double selection markers in two separate analyses, which demonstrated the freedom from Emr lactic acid bacteria in our experimental mice and also confirmed the accuracy of the CFU data. Noteworthy was that the GFP has demonstrated itself to be a reliable marker, expressing Flu+ consistently in all tested colonies and being easily observed using an epifluorescence microscope. This may indicate the possibility of relying on the gfp gene solely as a selection marker in our rL. reuteri, which otherwise, with an antibiotic‐resistant gene, is not allowed to be used in human and animals as a vaccine carrier.
As the GFP:STLTB product was the substance eventually presented by the rL. reuteri to the mucosal immune system, its characteristics relative to the suitability for use in animal study needs to be evaluated. First, its size (48 kDa), detected in the supernatant of the rL. reuteri culture (Fig. 3), when probed with the specific anti‐GFP or anti‐LTB antibodies, corresponded to the expected molecular mass of the mature GFP:STLTB protein. This indicated the fusion protein was not only expressed and secreted successfully, but also preserved its important GFP and LTB antigenic properties. Second, the appearance of fluorescence, though in yellow in color, signified a successful expression and folding of GFP in the two totally different environments of cell supernatant and cytoplasm. This kind of color change has been described in the Qbiogene manual as a normal result of the excitation‐spectrum shift, caused by the interaction of two moieties in a fusion protein. Third, the receptor (gangliosides GM1 of the intestinal epithelial cell) binding ability, a property naturally possessed by the LTB and correlated with the mucosal immunogenicity and adjuvanticity of LTB when coadministered with other antigens (Guidry et al., 1997), was significantly detectable in our GFP:STLTB protein. This finding strongly qualified the fusion product as an excellent vaccine candidate. Finally, the fusion product, being >27 times greater in NOAEL (Table 2) than the purified ST, indicating that it was free from ST‐associated toxicity. The exact reason for the lost of toxicity in our GFP:STLTB fusion is not clear. However, Clements (Clements, 1990) fused an LTB upstream to the ST, resulting in a fusion protein (i.e. LTB‐ST) free of toxicity, and suggested the toxic determinant of ST had been masked by the upstream LTB moiety. Accordingly, the N‐terminal GFP in our GFP:STLTB product may have the same masking effect on its downstream ST. Studies are required to address this suggestion more stringently.
To induce a successful mucosal immune response, both the amount and duration of an antigen presented to the mucosa are crucial (2002, 2002). The amounts of GFP:STLTB protein secreted by our rL. reuteri are predicted, from the in vitro estimation, to be 0.13–0.14 pg cell−1 during a 3‐h period after the rL. reuteri have been induced by nisin and orally inoculated in mice. After that, only a basal (constitutive) amount of 0.021–0.026 pg cell−1 will be produced if they can manage to grow in the gastrointestinal tract. As there is no method available for detection of antigen levels, delivered by a vaccine carrier in gastrointestinal tract, development of a tissue section technique to calculating the GFP:STLTB amount from the Flu+ intensity is underway.
Should this tissue section technique be developed, it would directly confirm the colonization capability of our rL. reuteri in the gastrointestinal tract, which, in this study, was only indirectly proved by the fecal persistence time that showed a typical pattern of the adhesive microorganisms (Grangette et al., 2002). Generally, the fecal persistence time for a nonadhesive lactic acid bacteria was <24 h after an oral inoculation of 1 × 109 CFU (Oozeer et al., 2002). The extra 4 fecal persistence days of our rL. reuteri strongly indicated their capability of colonizing in the gastrointestinal tract. Accordingly, the gradational and extended secretion of rL. reuteri in feces (Fig. 6) might be attributed to the periodical sloughing of the adhered rL. reuteri from the mucosal membrane.
As for the significant difference (P<0.05) of fecal CFU between the mice strain (rL. reuteri 3‐3) and human strain (rL. reuteri 20016) at each time point (Fig. 6), we suspect it was caused by the possible less adhesive capability of the rL. reuteri 20016, causing most of its inoculated CFU to be quickly excreted within the first 8–14 h, as would occur during normal ingestion in mice (Cunliffe‐Beamer, 1998); the ones remaining on the mucosal membrane, though with the same pattern of behavior as that of the rL. reuteri 3‐3, naturally would have lower CFU counts at each time point.
For many pathogens, the initial infection occurs at the mucosa of the lungs and intestines. It is therefore important to develop vaccines that can induce a mucosal immune response, mediated predominantly by the secretory IgA (sIgA), to neutralize the pathogens at the point where their initial infection and replication takes place. Furthermore, as many infections that have gained entrance through the mucosal surfaces will become systemic, the vaccines should also be able to elicit specific immunity in the systemic lymphoid tissues. In general, foreign antigens, delivered by the oral live vaccine carrier system, were able to elicit both specific sIgA and systemic serum IgG (2002, 2005, 2002, 2000). Failures to induce antibody response, especially when using lactobacilli as a carrier, were mainly attributable to the low concentration of antigen expressed (2002, 2000), although other factors, including bacteria strain, cellular location of antigen, immunization scheme and even mice breed, were also found to have certain impacts on it (Seegers, 2002).
Our rL. reuteri successfully elicited both serum IgG and fecal IgA after the oral application of rL. reuteri 20016 or rL. reuteri 3‐3 to mice. Notably, a rapid and substantially higher antibody response was seen in the latter group. To provide a possible reason for this observation, we inspected all in vitro analyses and were able to find two obvious differences existing between rL. reuteri 20016 and rL. reuteri 3‐3: the plasmid stability of the vaccine vector pNIES‐GFP:STLTB in rL. reuteri and the persistent CFU number of the rL. reuteri in the gastrointestinal tract. Apparently, these two differences eventually would lead to a higher level of the GFP:STLTB antigen being presented in the gastrointestinal tract by the rL. reuteri 3‐3. This therefore confirmed the previous description that the level of the immune response is intimately associated with the level of the antigen expressed by an oral vaccine carrier (Seegers, 2002).
The suckling mouse model (SMM) had been well explored and found to be a simple, rapid, and reproducible assay for ST toxicity (Giannella, 1976). To judge the ST toxicity in an SMM, the positive threshold of G/C ratio was determined to be ≥0.083 (Giannella, 1976) and ≥0.09 (Cárdenas & Clements, 1993), in Swiss albino and CD‐1 suckling mice, respectively. The BALB/c mice (3 days old) used in our study, and for the first time in an SMM, was found to have a lower G/C ratio of 0.075 (Table 2) when 10 ng of ST (according to Sigma, supposed to produce a G/C ratio of ≥0.083 in Swiss albino mice) was orally inoculated, indicating that the degree of ST fluid response actually differs in different breeds of mice. On our visual and digital data, we therefore determined the positive threshold of G/C ratio in the BALB/c mice to be ≥0.09.
In the patent mouse gut (PMG) assay for the detection of enterotoxin neutralization, direct challenge with live ETEC instead of the purified LT was also tried for the first time in our study. A dose response between the G/C ratio and CFU of ETEC, ranging from 3 × 108 to 3 × 109, was observed. Notably, a significant fluid accumulation in bowel was visible in samples with a G/C ratio of ≥0.10, which therefore was determined to be a positive threshold for responding to an ETEC challenge in our study. This threshold was proved to be a fair criterion in our later enterotoxin neutralization study, in which all the rL. reuteri‐immunized mice with satisfactory protection from the ETEC challenge demonstrated a G/C ratio of <0.10.
In summary, in the present study a live oral ETEC vaccine, using L. reuteri as a delivery vehicle to present the GFP‐ST‐LTB fusion as an antigen, is described for the first time. Satisfactory serum IgG and IgA titers as well as full protection of the ETEC challenge were demonstrated in mice immunized with our rL. reuteri.
Supporting information
Fig. S1. Agarose gel electrophoresis loaded with the pNIES‐GFP:STLTB digested with SpeI/EcoRI (lane 1) and BamHI/EcoRI (lane 2), and the PCR product of its STLTB gene (lane 3). M1 and M2 indicate the linear form molecular weight marker of 1 kb and 100 bp, respectively. Fig. S2. Determination of appropriate concentration of live ETEC to induce fluid responses in patent mouse model. The dose responses of live ETEC strain ATCC 35401, ranging from 3 x 108 to3 x 109, to induce fluid influx in bowel were seen in our preliminary experiment. Values are the mean gut/carcass weight ratios ? S.E.M. for the groups at 3 hours after inoculation. The G/C ratios of ? 0.10 are considered positive in this study (dash line). * meant P < 0.05. ** meant P < 0.01. Fig. S3. Typical responses of experimental and control mice given cholera toxin (CT) or ETEC. The diagrams (A) and (C) are copied from the previous literature reported by Richardson et al. [24], in which the mice were orally challenged with 36 ?g of CT (A) or not (C) and examined after 6 hrs. In contrast, the mice in our study were orally administered with 3 ? 109 cfu of live ETEC (B) or buffer (D), and examine after a 3 hr of period. The gross lesions in our study were consistent with the description in Richardson et al [24].
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
This work was supported by Grants NSD91‐2313‐B‐005‐135 and NSC92‐2317‐B‐005‐025 from the National Science Council of the Republic of China.
Editor: Artur Ulmer
References
- Cárdenas L & Clements JD (1993) Development of mucosal protection against the heat‐stable enterotoxin (ST) of Escherichia coli by oral immunization with a genetic fusion delivered by a bacterial vector. Infect Immun 61: 4629–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy BM & Giuffrida A (1980) Method for the lysis of gram‐positive, asporogenous bacteria with lysozyme. Appl Environ Microbiol 39: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin SC, Abdullah N, Siang TW & Wan HY (2005) Plasmid profiling and curing of Lactobacillus strains isolated from the gastrointestinal tract of chicken. J Microbiol 43: 251–256. [PubMed] [Google Scholar]
- Chu H, Kang S, Ha S et al (2005) Lactobacillus acidophilus expressing recombinant K99 adhesive fimbriae has an inhibitory effect on adhesion of enterotoxigenic Escherichia coli . Microbiol Immunol 49: 941–948. [DOI] [PubMed] [Google Scholar]
- Chung TC, Axelsson L, Lindgren SE & Dobrogosz WJ (1989) In vitro studies on reuterin synthesis by Lactobacillus reuteri . Microbiol Ecol Health Dis 2: 137–144. [Google Scholar]
- Clements JD (1990) Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat‐labile and heat‐stable enterotoxins. Infect Immun 58: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy B, Boris S, Isler P, Grangette C & Mercenier A (2005) Oral immunization of mice with lactic acid bacteria producing Helicobacter pylori urease B subunit partially protects against challenge with Helicobacter felis . J Infect Dis 192: 1441–1449. [DOI] [PubMed] [Google Scholar]
- Cunliffe‐Beamer TL (1998) The Laboratory Mouse. CRC Press, Boca Raton, FL. [Google Scholar]
- Delgado S, Florez AB & Mayo B (2005) Antibiotic susceptibility of lactobacillus and bifidobacterium species from the human gastrointestinal tract. Curr Microbiol 50: 202–207. [DOI] [PubMed] [Google Scholar]
- Geoffroy MC, Guyard C, Quatannens B, Pavan S, Lange M & Mercenier A (2000) Use of green fluorescent protein to tag lactic acid bacterium strains under development as live vaccine vectors. Appl Environ Microbiol 66: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella RA (1976) Suckling mouse model for detection of heat‐stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun 14: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gory L, Montel MC & Zagorec M (2001) Use of green fluorescent protein to monitor Lactobacillus sakei in fermented meat products. FEMS Microbiol Lett 194: 127–133. [DOI] [PubMed] [Google Scholar]
- Grangette C, Muller‐Alouf H, Geoffroy M, Goudercourt D, Turneer M & Mercenier A (2002) Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: impact of strain viability and in vivo persistence. Vaccine 20: 3304–3309. [DOI] [PubMed] [Google Scholar]
- Guidry JJ, Cardenas L, Cheng E & Clements JD (1997) Role of receptor binding in toxicity, immunogenicity and adjuvanticity of Escherichia coli heat labile enterotoxin. Infect Immun 65: 4943–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman‐Verduzco LM & Kupersztoch YM (1987) Fusion of Escherichia coli heat‐stable enterotoxin and heat‐labile enterotoxin B subunit. J Bacteriol 169: 5201–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PS, Kwang J & Lee YK (2005) Intragastric administration of Lactobacillus casei expressing transmissible gastroenteritis coronavirus spike glycoprotein induced specific antibody production. Vaccine 23: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O, Stetter OK & Kohl R (1980) Lactobacillus reuteri so. Nov., a new species of heterofermentative lactobacilli. Zbl Bakt Hyg I Abt Orig C1: 264–269. [Google Scholar]
- Lin CF & Chung TC (1999) Cloning of erythromycin‐resistance determinants and replication origins from indigenous plasmids of Lactobacillus reuteri for potential use in construction of cloning vectors. Plasmids 42: 31–41. [DOI] [PubMed] [Google Scholar]
- Lionetti E, Miniello VL, Castellaneta SP, Magista AM, De Canio A, Maurogiovanni G, Ierardi E, Cavallo L & Francavilla R (2006) Lactobacillus reuteri therapy to reduce side‐effects during anti‐Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther 24: 1461–1468. [DOI] [PubMed] [Google Scholar]
- Martín MC, Fernández M, Martín‐Alonso JM, Parra F, Boga JA & Alvarez MA (2004) Nisin‐controlled expression of Norwalk virus VP60 protein in Lactobacillus casei . FEMS Microbiol Lett 237: 385–391. [DOI] [PubMed] [Google Scholar]
- Mathur S & Singh R (2005) Antibiotic resistance in food lactic acid bacteria – a review. Int J Food Microbiol 105: 281–295. [DOI] [PubMed] [Google Scholar]
- Oozeer R, Goupil‐Feuillerat N, Alpert CA, Van De Guchte M, Anba J, Mengaud J & Corthier G (2002) Lactobacillus casei is able to survive and initiate protein synthesis during its transit in the digestive tract of human. Appl Environ Microbiol 68: 3570–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveneau N, Geoffroy MC, Locht C, Chagnaud P & Mercenier A (2002) Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 20: 1769–1777. [DOI] [PubMed] [Google Scholar]
- Richardson SH, Giles JC & Kruger KS (1984) Sealed adult mice: a new model for enterotoxin evaluation. Infect Immun 43: 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Chamberlain LM, Schofield KM, Wells JM & Le Page RW (1997) Oral vaccination of mice against tetanus with recombinant Lactococcus lactis . Nat Biotechnol 15: 653–657. [DOI] [PubMed] [Google Scholar]
- Scheppler L, Vogel M, Zuercher AW, Zuercher M, Germond JE, Miescher SM & Stadler BM (2002) Recombinant Lactobacillus johnsonii as a mucosal vaccine delivery vehicle. Vaccine 20: 2913–2920. [DOI] [PubMed] [Google Scholar]
- Seegers JF (2002) Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol 20: 508–515. [DOI] [PubMed] [Google Scholar]
- Shaw DM, Gaerthe B, Leer RJ, Van Der Stap JG, Smittenaar C, Heijne Den Bak‐Glashouwer M, Thole JE, Tielen FJ, Pouwels PH & Havenith CE (2000) Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 100: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slos P, Dutot P, Reymund J, Kleinpeter P, Prozzi D, Kieny MP, Delcour J, Mercenier A & Hols P (1998) Production of cholera toxin B subunit in Lactobacillus . FEMS Microbiol Lett 169: 29–36. [DOI] [PubMed] [Google Scholar]
- Tubelius P, Stan V & Zachrisson A (2005) Increasing work‐place healthiness with the probiotic Lactobacillus reuteri : a randomized, double-blind placebo-controlled study. Environ Health 4: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CM & Chung TC (2006) Green fluorescent protein is a reliable reporter for screening signal peptides functional in Lactobacillus reuteri . J Microbiol Methods 67: 181–186. [DOI] [PubMed] [Google Scholar]
- Wu CM, Lin CF, Chung YC & Chung TC (2006) Construction and characterization of nisin‐controlled expression vectors for use in Lactobacillus reuteri . Biosci Biotech Biochem 70: 757–767. [DOI] [PubMed] [Google Scholar]
- Zegers ND, Kluter E, Van Der Stap H, Van Dura E, Van Dalen P, Shaw M & Baillie L (1999) Expression of the protective antigen of Bacillus anthracis by Lactobacillus casei : towards the development of an oral vaccine against anthrax. J Appl Microbiol 87: 309–314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Agarose gel electrophoresis loaded with the pNIES‐GFP:STLTB digested with SpeI/EcoRI (lane 1) and BamHI/EcoRI (lane 2), and the PCR product of its STLTB gene (lane 3). M1 and M2 indicate the linear form molecular weight marker of 1 kb and 100 bp, respectively. Fig. S2. Determination of appropriate concentration of live ETEC to induce fluid responses in patent mouse model. The dose responses of live ETEC strain ATCC 35401, ranging from 3 x 108 to3 x 109, to induce fluid influx in bowel were seen in our preliminary experiment. Values are the mean gut/carcass weight ratios ? S.E.M. for the groups at 3 hours after inoculation. The G/C ratios of ? 0.10 are considered positive in this study (dash line). * meant P < 0.05. ** meant P < 0.01. Fig. S3. Typical responses of experimental and control mice given cholera toxin (CT) or ETEC. The diagrams (A) and (C) are copied from the previous literature reported by Richardson et al. [24], in which the mice were orally challenged with 36 ?g of CT (A) or not (C) and examined after 6 hrs. In contrast, the mice in our study were orally administered with 3 ? 109 cfu of live ETEC (B) or buffer (D), and examine after a 3 hr of period. The gross lesions in our study were consistent with the description in Richardson et al [24].
Supporting info item
Supporting info item
Supporting info item


