Abstract
The epidemiology and clonality of methicillin-resistant Staphylococcus aureus (MRSA) has not been investigated, as not much research or surveillance has been undertaken to identify and characterize the circulating MRSA strains in Barbados. Prevalence rates, molecular characteristics and antimicrobial susceptibility pattern of MRSA infections in hospitalized and nonhospitalized patients were investigated. A total of 293 isolates were included in the study, with 100 from the hospital and 193 from the public health laboratory. Isolates were collected over a period of 1 (2015–2016) and 3 years (2013–2016) respectively. MRSA was identified using standard microbiologic techniques and was further analysed by multiplex PCR for the presence of the spa, mec gene complex typing and PVL genes (lukS-PV and lukF-PV). A prevalence rate of 19.7% was calculated for those hospitalized. All hospital isolates were sensitive to vancomycin, rifampin, linezolid and cotrimoxazole (trimethoprim/sulfamethoxazole), whilst 82% were sensitive to clindamycin. The PVL gene was detected in 76% of hospital isolates. In the community isolates, resistance was observed in erythromycin (100%), ciprofloxacin (97.4%), clindamycin (13%) and cotrimoxazole (5.7%). There was no resistance to vancomycin. The PVL gene was detected in 97.9% of the isolates, the mecA gene in only 2.1% and the mecC gene in 0%. Most MRSA isolates were community acquired in both settings, and the antimicrobial susceptibility profile was similar, suggesting transmission of community-associated MRSA into the hospital environment. Further harmonization of antimicrobial policy for the treatment of MRSA (and by extension other pathogens) should be implemented to quell ongoing transmission. We found that 93.4% of MRSA in Barbados treated in the primary healthcare system were sensitive to cotrimoxazole. By typing MRSA isolates and drawing interferences on transmission on the basis of genetic relatedness, transmission pathways may be tracked. Further studies must be performed for this high level of comprehensiveness so that with the surveillance of MRSA, effective strategies may be developed to prevent or limit transmission.
Keywords: Antimicrobial resistance, antimicrobial susceptibility, CA-MRSA, HA-MRSA, methicillin-resistant Staphylococcus aureus, molecular characteristics, MRSA
Introduction
Methicillin, a semisynthetic penicillin that is poorly hydrolyzed by penicillinase, was first used clinically in 1960. Only 1 year later, Staphylococcus aureus strains that showed resistance to methicillin were reported [1]. Resistance to methicillin confers resistance to all penicillinase-resistant penicillins and cephalosporins. Since then, methicillin-resistant Staphylococcus aureus (MRSA) strains have appeared in countries worldwide, and these strains continue to be one of the most common hospital pathogens [2]. It has been shown that methicillin-susceptible S. aureus (MSSA) strains become MRSA strains through the acquisition of a staphylococcal cassette chromosome mec (SCCmec) element carrying a mecA gene, which is responsible for methicillin resistance [3,4].
Barbados, a populous island country of 287 212 persons in the Eastern Caribbean, is 34 km (21 miles) long and 23 km (14 miles) wide, covering an area of 432 km2 (167 square miles) [5]. Historically, the economy of Barbados has been dependent on sugarcane cultivation and related activities but has diversified into the manufacturing and tourism since the late 1970s and early 1980s. The tourism industry generates more than 50% of the country's foreign exchange, where approximately 14 000 people are directly employed, and contributes 12% of GDP [6]. With globalization and the increasing rates of travel, dissemination of infectious agents is an important determinant in the healthcare—and the health of the public. In many parts of the world, epidemiologically and genotypically defined community-associated (CA) MRSA strains have emerged to become frequent causes of hospital infection. Until the emergence of CA-MRSA in the late 1990s [7,8], infection was predominantly due to healthcare-associated (HA) strains associated with advanced age, comorbidities, surgical procedures or indwelling medical devices [[9], [10], [11]]. CA-MRSA later emerged as a cause of infection in the community in previously healthy individuals of all ages, with no history of hospital contact and none of the risk profiles that are typical of healthcare exposure [12,13]. Recently, however, CA-MRSA strains have emerged as a cause of HA infection in some parts of the world [13], challenging definitions of CA-MRSA based on clinical epidemiology and where disease manifests [[14], [15], [16]] in favour of genotype-based definitions [[17], [18], [19]]. Nonetheless, CA-MRSA strains retain a number of important characteristics, notably the association with infection in previously healthy individuals in the community [7,8,12,16,20]. Outbreaks in hospitals and nursing homes are today caused by both HA-MRSA and CA-MRSA clones [[21], [22], [23]], and it has been suggested that it is no longer useful to regard HA-MRSA and CA-MRSA as separate entities [24].
Healthcare settings are regarded as the epicentre for MRSA transmission in many countries worldwide. Hospital admission of unknown MRSA carriers, lack of MRSA admittance screening and spread of MRSA among nursing home residents could all be part of the explanation of this mélange of HA-MRSA and CA-MRSA in hospitals [25]. This would increase our knowledge of the prevalence and management of such infections in light of increasing antibiotic resistance and the overuse of more potent antibiotics in the community clinics that could compromise future treatment regimens by creating antibiotic resistance isolates.
The aims of this study were to determine the antimicrobial susceptibility patterns of MRSA in the in Barbados healthcare system (polyclinics, hospital and satellite clinics); and to characterize by molecular techniques the strains of MRSA in hospitalized and nonhospitalized patients.
Patients and methods
Hospitalized patients
Isolates of MRSA were collected at the Department of Microbiology, The Queen Elizabeth Hospital Barbados, a 600-bed facility, from December 2014 to December 2015. One hundred isolates were collected from the following specimens: blood, bone, ear, fluids, surgical drains, tissue, urine and wounds. Samples were submitted to the microbiology department from wards in the hospital, including the intensive care units. They were categorized as HA or CA. HA was classified as a patient whose MRSA isolate was cultured more than 48 hours after admission, while CA was classified as a patient whose MRSA isolate was cultured within 48 hours after admission. This information was provided by the infection control department.
Community-based patients (nonhospitalized)
Samples were collected as convenience samples from routine testing that were suspected to be Staphylococcus aureus species from the period July 2013 to January 2016. These samples were received at the laboratory from the healthcare institutions on the island. These include the eight polyclinics, their satellite clinics and three other healthcare institutions (termed ‘other’ in this study).
Processing
Samples were received in the microbiology department and cultured according to standard operating procedures for each sample type. Cultures were incubated at 35°C for 18 to 24 hours. Culture plates were examined the next day, and isolates of Staphylococcus species were identified and subcultured on 5% sheep's blood agar and MacConkey agar, and incubated as described above. Gram staining as well as catalase and coagulase tests were performed for confirmation of the organism.
All hospital isolates were confirmed to be Staphylococcus aureus. These were speciated using the MicroScan Dried Gram Positive MIC/Combo panel with Cefoxitin Screen well (CfxS) using the MicroScan WalkAway system, which is a fully automated identification and susceptibility system. Amoxicillin/clavulanate acid, ampicillin, ceftriaxone, ciprofloxacin, clindamycin, erythromycin, gentamicin, levofloxacin, linezolid, moxifloxacin, nitrofurantoin, oxacillin, penicillin, rifampin, tetracycline, cotrimoxazole (trimethoprim/sulfamethoxazole) and vancomycin are included in the panel.
Antimicrobial susceptibilities of community isolates were determined by the Kirby-Bauer disc diffusion method on Müller-Hinton agar. The following antibiotic discs were applied to the plate: erythromycin (E), penicillin (P), oxacillin (OX), cephalothin (CF), cefuroxime (CXM), ceftazidime (CAZ), ciprofloxacin (CIP), cotrimoxazole (SXT), clindamycin (CC), cefoxitin (FOX), amoxicillin/clavulanic acid (AMC) and vancomycin (VAN). Susceptibilities determined by Clinical and Laboratory Standards Institute (CLSI) guidelines. Data were analysed by WHONET 5.6 (http://www.whonet.org/software.html).
All MRSA isolates were stored in brain–heart infusion broth with 20% glycerol at −20°C before extraction of DNA.
DNA extraction
Cultures that were stored in brain–heart infusion broth were inoculated on 5% sheep's blood agar and incubated overnight. Two to three colonies were picked and suspended in 100 μL of lysis buffer (Instagene Matrix; Bio-Rad, Hercules, CA, USA) in a 1.5 mL Eppendorf tube. The suspension was vortexed and incubated at 56°C for 1 hour. The suspension was further incubated at 95°C for 1 hour. After the second incubation, the suspension was vortexed and centrifuged at 13 200 rpm for 5 minutes. DNA extractions were stored at −20°C for PCR [26].
Polymerase chain reaction
A multiplex PCR method developed by the European Union Reference Laboratory–Antimicrobial Resistance was used for confirmation of methicillin resistance by amplification of both mecA and mecC, identification of S. aureus by amplification of the spa gene (also used for typing) and detection of the Panton-Valentine leukocidin (PVL or LukF-PV)-encoding gene [26]. A PCR run consisted of 15 patient samples and three controls; Staphylococcus aureus subsp. aureus Rosenbach ATCC 25923D-5TM, Staphylococcus aureus subsp. aureus Rosenbach ATCC 6538D-5TM and ATCC 1556D-5. A negative control was also included in each run, which consisted of the master mix and deionized water. After the preparation of the master mix, 2 μL of sample DNA was added to 23 μL of the master mix to give a PCR volume of 25 μL; this was amplified according to the above-mentioned protocol. Briefly, each PCR contained 0.4 μM of the primers as follows: mecA primers (mecA P4, 5′-TCCAGATTACAACTTCACCAGG-3′; mecA P7, 5′-CCACTTCATATCTTGTAACG-3′), spa primers (spa-1113F, 5′-TAAAGACGATCCTTCGGTGAGC-3′; spa-1514R, 5′-CAGCAGTAGTGCCGTTTGCTT-3′), 1 μM PVL primers (PVL-F, 5′-GCTGGACAAAACTTCTTGGAATAT-3′; PVL-R, 5′-GATAGGACACCAATAAATTCTGGATTG-3′) and mecALGA251 primers (mecALGA251 Multi FP, 5′-GAAAAAAAGGCTTAGAACGCCTC-3′; mecALGA251 Multi RP, 5′-GAAGATCTTTTCCGTTTTCAGC-3′) [26,27]. Amplification was performed in a PX 0.2 thermal cycler manufactured by Thermo Electric (West Chester, PA, USA) with 5 minutes at 94°C hot start, followed by 30 cycles of 30 seconds at 94°C, 1 minute at 59°C and 1 minute at 72°C, with a final 10 minutes at 72°C. Amplicons were electrophoresed in a 2% agarose gel and visualized under ultraviolet light [26,27].
This study was approved by the institutional review board of the University of the West Indies (Cave Hill) and by the Ministry of Health and the ethics committee of Queen Elizabeth Hospital. No patient information was disclosed.
Results
HA isolates
Over the 1-year period from December 2014 to December 2015, a total of 4696 hospital isolates were identified, of which 688 (19.7%) were MRSA positive, occurring in 44% in male and 56% female subjects ranging in age from 1 to 87 years. Patients were admitted from the accident and emergency department to the following units: surgical 35%, paediatric 15%, internal medicine 10%, orthopaedics 10% and urology 8%. Other patients were admitted to other wards on the basis of the availability of space. When analysed by site of infection, 25% were isolated from blood, 2% urine, 4% surgical drains, 30% tissue, 24% wounds, 9% bone, 4% fluid and 2% ear.
One hundred isolates of MRSA were identified over a period of 1 year. Ninety of these isolates were CA-MRSA, and the other ten were HA-MRSA. The 90 CA-MRSA isolates were made up of tissue (n = 30), wound swabs (n = 24) and blood (n = 17). Of the ten HA-MRSA, eight were blood and two surgical drains (Table 1).
Table 1.
HA-MRSA and CA-MRSA of hospital isolates
| Site of specimen | Total no. of isolates | CA-MRSA isolates, n (%) | HA-MRSA isolates, n (%) |
|---|---|---|---|
| Blood | 25 | 17 (18.9) | 8 (80) |
| Bone | 9 | 9 (10) | 0 |
| Ear | 2 | 2 (2.2) | 0 |
| Fluid | 4 | 4 (4.4) | 0 |
| Surgical drain | 4 | 2 (2.2) | 2 (20) |
| Tissue | 30 | 30 (33.3) | 0 |
| Urine | 2 | 2 (2.2) | 0 |
| Wound swab | 24 | 24 (26.7) | 0 |
| Total | 100 | 90 | 10 |
CA, community acquired; HA, hospital acquired; MRSA, methicillin-resistant Staphylococcus aureus.
Overall, 100% of isolates were resistant to oxacillin (MIC >2 μg/mL), with a positive cefoxitin screen well of MIC >4 μg/mL. Antimicrobial susceptibility testing revealed that all isolates were resistant to ceftriaxone and ciprofloxacin, and 90% of isolates were resistant to erythromycin. All isolates were sensitive to vancomycin, rifampin, gentamicin, linezolid and cotrimoxazole; 82% were sensitive to clindamycin, with 2% inducible clindamycin resistance.
Multiplex PCR analysis confirmed the presence of the spa, mecA gene in 77 of 100 MRSA isolates. The PVL gene was negative in one of 77 isolates. A further 15 isolates showed only spa and PVL genes, with the remaining eight isolates showing no spa, mecA/C and PVL gene but that were identified as MRSA by the MIC values (Table 2).
Table 2.
Demographic data and presence of PVL gene of hospital-acquired MRSA
| No. | PVL gene present | Ward, sex | Site | Age (years) | Sex |
|---|---|---|---|---|---|
| 1 | No | Medical, M | Surgical drain | 67 | M |
| 2 | Yes | Medical, M | Blood | 57 | M |
| 3 | Yes | Medical, F | Surgical drain | 29 | F |
| 4 | Yes | Medical, M | Blood | 58 | M |
| 5 | Yes | Medical, F | Blood | 60 | F |
| 6 | Yes | Surgical, mixed | Blood | 59 | F |
| 7 | Yes | Medical, M | Blood | 57 | M |
| 8 | Yes | Medical, M | Blood | 71 | M |
| 9 | Yes | Surgical, mixed | Blood | 60 | F |
| 10 | Yes | Surgical, mixed | Blood | 69 | F |
CA isolates
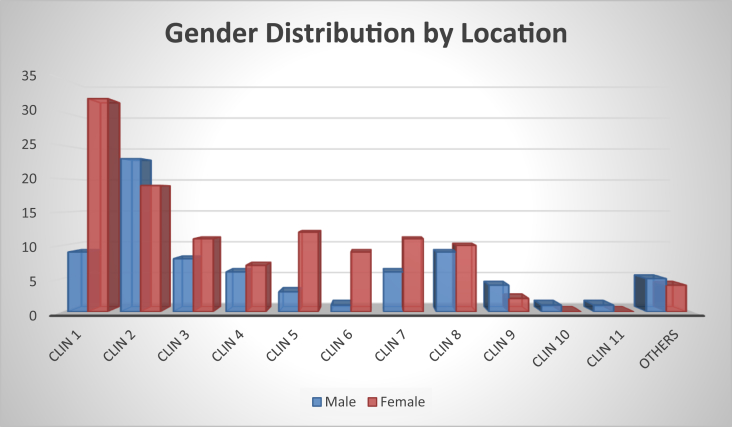
A total of 193 samples were collected from 12 clinical sites over the 3-year period 2013 to 2016 (Fig. 1). There were 117 female (60.6%) and 76 male (39.4%) subjects included in the study. Most of the isolates were from wound swabs (71%), followed by nasal swabs (8.8%), with penile (0.5%) and vaginal (0.5%) swabs being the least common specimen types submitted for testing (Table 3).
Fig. 1.
Sex distribution of nonhospitalized patients.
Table 3.
Specimen types collected in community
| Site of specimen | Swabs, n (%) | Cotrimoxazole | Vancomycin | Clindamycin | Ciprofloxacin |
|---|---|---|---|---|---|
| Abscess | 16 (8.3) | 16 | 16 | 15 | 0 |
| Aspirate | 1 (0.5) | 1 | 1 | 0 | 0 |
| Axilla | 4 (2.1) | 4 | 4 | 4 | 0 |
| Groin | 2 (1.0) | 2 | 2 | 2 | 0 |
| Eye | 2 (1.0) | 2 | 2 | 0 | 0 |
| Ear | 6 (3.1) | 6 | 6 | 6 | 0 |
| Nasal | 16 (8.3) | 12 | 13 | 16 | 0 |
| Penis | 1 (0.5) | 1 | 1 | 1 | 0 |
| Vaginal | 1 (0.5) | 1 | 1 | 1 | 0 |
| Pus | 5 (2.5) | 5 | 5 | 2 | 3 |
| Ulcer | 6 (3.1) | 5 | 6 | 4 | 0 |
| Wound | 132 (68.4) | 127 | 130 | 114 | 3 |
| Skin | 1 (0.5) | 0 | 1 | 0 | 0 |
| Total | 193 | 182 | 188 | 169 | 6 |
Of the 193 samples tested, nine, or 4.7%, gave D-zones for clindamycin induction. A total of 94.3% susceptibility was recorded to cotrimoxazole, in 92.3% female and 96.1% male subjects. A susceptibility to vancomycin of 97.4% was observed; clindamycin susceptibility averaged 89.6%. All isolates were resistant to the β-lactam antibiotics and macrolides. When analysed by age, of those aged ≤18 years (16.1%), 94.5% of the isolates were susceptible to cotrimoxazole, vancomycin (100%), clindamycin (93.5%) and ciprofloxacin (0). Of the study population aged 19 to 54 years (35.2%), 97.1% of the isolates were susceptible to cotrimoxazole, 100% to vancomycin, 86.8% to clindamycin and 1.5% to ciprofloxacin. Of the study population aged ≥55 years (39.3%), 92.1% of isolates were susceptible to cotrimoxazole, 100% to vancomycin, 80.3% to clindamycin and 1.3% to ciprofloxacin (Table 4).
Table 4.
Age distribution and antibiotic susceptibility in community isolates
| Age (years) (%) | Percentage susceptible to: |
|||
|---|---|---|---|---|
| Cotrimoxazole | Vancomycin | Clindamycin | Ciprofloxacin | |
| ≤18 (16.1%) | 94.5 | 100 | 93.5 | 0 |
| 19–54 (35.2%) | 97.1 | 99 | 86.8 | 1.5 |
| ≥55 (39.3%) | 92.1 | 97 | 80.3 | 1.3 |
| Average | 94.6 | 98.7 | 86.9 | 0.9 |
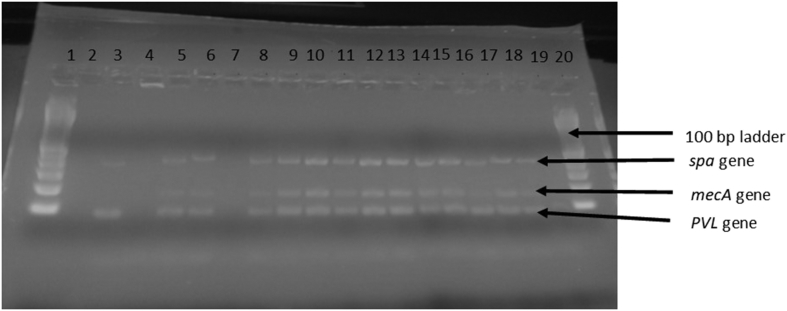
The PVL gene was amplified in 185 (95.9%) of the samples tested, along with the spa and mecA genes. The other eight isolates had amplification of the spa and mecA genes only and were obtained from clinic 1 (n = 1), clinic 2 (n = 1), clinic 3 (n = 2), clinic 4 (n = 2) and clinic 5 (n = 2) (Fig. 2).
Fig. 2.
Agarose gel electrophoresis of PCR-amplified products showing portions of spa (variable), mecA (162 bp) and PVL gene (83 bp). Lanes 1 and 20, 100 bp ladder; lanes 2–4, controls; lanes 5–19, isolates.
For the eight (4.2%) of 193 isolates where only the spa and mecA genes were amplified, four of these isolates were resistant to vancomycin, whilst one isolate was resistant to cotrimoxazole, ciprofloxacin and vancomycin. Six isolates were resistant to clindamycin. There was a missing age demographic for the single patient with an isolate with multiple resistances (Table 5).
Table 5.
Patient demographics, susceptibility and molecular characteristics of nonhospitalized patients with variant genotypic characteristics
| Patient No. | Clinic | Specimen | Age (years) | Sex | D test results | Antibiotic |
Amplified gene |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | SXT | CIP | VAN | spa | mecA | mecC | PVL | ||||||
| 9 | 5 | Ear | 40 | F | — | S | S | R | S | + | + | — | — |
| 13 | 2 | Wound | — | M | — | S | R | R | S | + | + | — | — |
| 14 | 5 | Wound | 58 | F | — | R | S | R | S | + | + | — | — |
| 16 | 1 | Wound | 38 | F | — | R | S | R | S | + | + | — | — |
| 47 | 3 | Nasal | 80 | F | — | R | S | R | S | + | + | — | — |
| 48 | 3 | Nasal | 67 | F | — | R | S | R | S | + | + | — | — |
| 49 | 4 | Wound | 41 | M | — | R | S | R | S | + | + | — | — |
| 70 | 4 | Wound | 48 | F | — | R | S | R | S | + | + | — | — |
CC, clindamycin; CIP, ciprofloxacin; R, resistant; S, susceptible; SXT, cotrimoxazole (trimethoprim/sulfamethoxazole); VAN, vancomycin.
Discussion
To our knowledge, our study is the first in Barbados to determine the prevalence and molecular characteristics of MRSA isolates from both hospitalized and nonhospitalized patients. To generate definitive ideas regarding the types and presence of MRSA circulating within the country, consecutive isolates submitted to the both the public health laboratory from 2012 to 2016 and the hospital laboratory from 2015 to 2016 (prospective) were analysed, with a total of 293 isolates analysed.
There is limited information available on the antimicrobial susceptibility and prevalence of MRSA in the Caribbean region, particularly their genotypic characterization. Several studies in the Caribbean showed that MRSA isolates were predominantly SCCmec types IV and V, but no antimicrobial resistance patterns were documented [28]. Another study demonstrated the presence of the circulating sequence type (ST) 239 MRSA III clone in Trinidadian patients from 294 clinical isolates by microarray hybridization. The mecA gene was found in 15.3% of isolates compared to 285 (97.3%) of 293 of MRSA isolates from our study. The PVL gene was detected in 261 (89%) of 293 of clinical isolates, comprising of 76% from hospitalized patients and nonhospitalized patients (261/293, 63.1%). This gene was not analysed in that Trinidadian study, so no comparisons can be made [29]. A high proportion of patients in St Kitts and Nevis were found to have MRSA, with a prevalence of 45% compared to 17.9% of Barbadian patients, which seems to be similar to other Caribbean islands [[29], [30], [31], [32], [33]]. No mecC genes were detected, indicating that none of the MRSA isolates from Barbados were associated livestock-associated MRSA lineages. Of the hospital isolates, no spa, mecA/mecC or PVL genes were detected in eight patients, and in 15, mecA was undetected; however, all isolates were cefoxitin-resistant strains (MIC >4 μg/mL). The latter phenomenon could be associated with improper characterization of the strain or challenges in the basic microbiologic investigation. However, spa-deficient S. aureus has been isolated from Danish patients, which was either truly missing the spa gene or was associated with deletions in the immunoglobulin G–binding domain C, where the upstream primer used for spa typing is located [34]. Hence, nontypeable spa genes might not have been detectable with the set of primers used.
A predominant feature of CA-MRSA is the presence of the PVL genes that encode a S. aureus exotoxin that induces lysis of monocytes and neutrophil granulocytes and harbours SCCmec types IV and V [[35], [36], [37]]; a relationship between CA-MRSA, SCCmec type IV and V, and PVL has been confirmed in some studies [[38], [39], [40]]. The resistance of S. aureus to methicillin is caused by the mecA gene, located on a mobile genetic element, the staphylococcal cassette chromosome mec (SCCmec). This lack of a mecA gene and β-lactam resistance has been attributed to mutations in various genes, including those encoding penicillin-binding proteins [39,41]. Eight nonhospitalized patients had a similar pattern; these patients had isolates that were susceptible to vancomycin, analogous to the hospitalized patients. In addition, SCCmec type IV and V are known to be small and highly mobile elements. Their dissemination in a community population is mostly by transfer of strains from carriers to other individuals; from MRSA strains to MSSA strains; or even from coagulase-negative staphylococci strain to an MSSA strain [42]. This was detected in the nares of two such patients, indicating the possibility of easy transfer within the community. These strains need further investigation to confirm the mechanisms of resistance due to the plausibility of detecting vancomycin-resistant Staphylococcus aureus, as seen with the strains exhibiting resistance.
In the present study, resistance to antibiotics both the community and hospital settings was high, particularly to fluoroquinolones (290/293, 98.9%), macrolides (community, 100%; hospitalized, 284/293, 97%) and β-lactams (100%) inclusive of methicillin. These findings are important because of the increasing antimicrobial resistance within the community and hospital settings. All hospital isolates were sensitive to vancomycin, linezolid, gentamicin and cotrimoxazole. Of the isolates from nonhospitalized subjects, 24 were resistant to clindamycin compared to 18 in hospitalized subjects, with positive inducible clindamycin results in 24 (8.2%) and two (0.7%) isolates respectively, indicating that clindamycin cannot be used in 4.5% of the population. Most isolates were susceptible to cotrimoxazole (186/193, 96.4%, of nonhospitalized and 100% of hospitalized patients). Some similarities in antibacterial susceptibility patterns to vancomycin, clindamycin, ciprofloxacin, erythromycin and cotrimoxazole have been observed in Trinidad, Jamaica, Antigua and the French territories [31,[43], [44], [45]].
Incongruous antibiotic usage, hygienic practices, ineffectual infection control and extensively used antimicrobials in agricultural practice has led to an increase in antimicrobial resistance in the community setting, which can be transferred to the hospital setting. Our findings show that the molecular characteristics and antimicrobial susceptibilities of isolates in hospitalized and nonhospitalized patients are similar. One isolate from a hospitalized patient was negative for the PVL gene and was designated a true HA-MRSA. This patient was first hospitalized in 2013 for diabetic complications, then returned in January 2015, where MRSA screening of nasal, axilla and groin was negative. Subsequently MRSA was isolated from surgical drains in February 2015, followed by positive blood culture, which remained positive until March 2015, upon this patient's death. The patient was administered vancomycin; however, because of an allergy, he was instead administered linezolid, but he never recovered. His isolates were sensitive to vancomycin, cotrimoxazole, gentamicin and linezolid and resistant to ceftriaxone, ciprofloxacin, clindamycin and erythromycin. This isolate could be classified as a true HA-MRSA due to the absence of the PVL gene. However, the remaining HA-MRSA isolates showed antimicrobial profiles similar to those characterized as CA-MRSA, so it can be surmised that the isolate of MRSA circulating is primarily from the community and that the incidence of true HA-MRSA is low and can be attributed to the stringent infection control measures set up by the Department of Infection Control. Proper surveillance, antibiogram development and molecular characterization should be implemented in the community and hospital environment to monitor and guide treatment of these infections. In addition, all types of MRSA were found to be cotrimoxazole sensitive, suggesting this drug's likely use within both populations.
Conflict of interest
None declared.
Acknowledgements
The research was conducted principally by D. Alleyne and E. Chase in fulfilment of their Caribbean Association of Medical Laboratory Technologists (CASMET) fellowship requirements. Funded by Staff Awards, University of the West Indies (Cave Hill), Barbados. The research was conducted at Leptospira Laboratory, Ministry of Health.
References
- 1.Jevons M.P. ‘Celbenin’-resistant staphylococci. Br Med J. 1961;1(5219):124–125. [Google Scholar]
- 2.Ayliffe G.A. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1997;24(Suppl. 1):S74–S79. doi: 10.1093/clinids/24.supplement_1.s74. [DOI] [PubMed] [Google Scholar]
- 3.Katayama Y., Ito T., Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito T., Katayama Y., Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre–methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999;43:1449–1458. doi: 10.1128/aac.43.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World population review. Barbados population; 2019. http://worldpopulationreview.com/countries/barbados-population/ Available at: [Google Scholar]
- 6.World Travel and Tourism Council Economic impact, 2018, Barbados. https://www.wttc.org/-/media/files/reports/economic-impact-research/archived/countries-2018/barbados2018.pdf Available at:
- 7.Otter J.A., French G.L. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect Dis. 2010;10:227–239. doi: 10.1016/S1473-3099(10)70053-0. [DOI] [PubMed] [Google Scholar]
- 8.Herold B.C., Immergluck L.C., Maranan M.C., Lauderdale D.S., Gaskin R.E., Boyle-Vavra S. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 9.Lowy F.D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 10.Coia J.E., Duckworth G.J., Edwards D.I., Farrington M., Fry C., Humphreys H. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect. 2006;63(Suppl. 1):S1–S44. doi: 10.1016/j.jhin.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Harbarth S., Sax H., Fankhauser-Rodriguez C., Schrenzel J., Agostinho A., Pittet D. Evaluating the probability of previously unknown carriage of MRSA at hospital admission. Am J Med. 2006;119:275. doi: 10.1016/j.amjmed.2005.04.042. e15–23. [DOI] [PubMed] [Google Scholar]
- 12.Naimi T.S., LeDell K.H., Como-Sabetti K., Borchardt S.M., Boxrud D.J., Etienne J. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 13.Egea A.L., Gagetti P., Lamberghini R., Faccone D., Lucero C., Vindel A. New patterns of methicillin-resistant Staphylococcus aureus (MRSA) clones, community-associated MRSA genotypes behave like healthcare-associated MRSA genotypes within hospitals, Argentina. Int J Med Microbiol. 2014;304:1086–1099. doi: 10.1016/j.ijmm.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Popovich K.J., Weinstein R.A. Commentary: the graying of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2009;30:9–12. doi: 10.1086/592709. [DOI] [PubMed] [Google Scholar]
- 15.Popovich K.J., Weinstein R.A., Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008;46:787–794. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 16.Otter J.A., French G.L. Community-associated meticillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated infection. J Hosp Infect. 2011;79:189–193. doi: 10.1016/j.jhin.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Chua K., Laurent F., Coombs G., Grayson M.L., Howden B.P. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis. 2011;52:99–114. doi: 10.1093/cid/ciq067. [DOI] [PubMed] [Google Scholar]
- 18.Millar B.C., Loughrey A., Elborn J.S., Moore J.E. Proposed definitions of community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA) J Hosp Infect. 2007;67:109–113. doi: 10.1016/j.jhin.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Otter J.A., French G.L. Community-associated meticillin-resistant Staphylococcus aureus: the case for a genotypic definition. J Hosp Infect. 2012;81:143–148. doi: 10.1016/j.jhin.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Zetola N., Francis J.S., Nuermberger E.L., Bishai W.R. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 21.DeLeo F.R., Otto M., Kreiswirth B.N., Chambers H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375(9725):1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Ruscio F., Bjørnholt J.V., Leegaard T.M., Moen A.E.F., de Blasio B.F. MRSA infections in Norway: a study of the temporal evolution, 2006–2015. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson A., Nimmo G.R. Control of healthcare- and community-associated MRSA: recent progress and persisting challenges. Br Med Bull. 2018;125:25–41. doi: 10.1093/bmb/ldx046. [DOI] [PubMed] [Google Scholar]
- 24.Bal A.M., Coombs G.W., Holden M.T.G., Lindsay J.A., Nimmo G.R., Tattevin P. Genomic insights into the emergence and spread of international clones of healthcare-, community- and livestock-associated meticillin-resistant Staphylococcus aureus: blurring of the traditional definitions. J Glob Antimicrob Resist. 2016;6:95–101. doi: 10.1016/j.jgar.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez B.E., Rueda A.M., Shelburne S.A., 3rd, Musher D.M., Hamill R.J., Hulten K.G. Community-associated strains of methicillin-resistant Staphylococcus aureus as the cause of healthcare-associated infection. Infect Control Hosp Epidemiol. 2006;27:1051–1056. doi: 10.1086/507923. [DOI] [PubMed] [Google Scholar]
- 26.National Food Institute . 2012. Protocol for PCR Amplification af mecA, mecC (mecALGA251), spa and PVL.https://www.eurl-ar.eu/CustomerData/Files/Folders/21-protocols/279_pcr-spa-pvl-meca-mecc-sept12.pdf 2nd version. Available at: [Google Scholar]
- 27.Stegger M., Andersen P.S., Kearns A., Pichon B., Holmes M.A., Edwards G. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA(LGA251) Clin Microbiol Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 28.Vire F.P., Akpaka P.E., Unakal C. Molecular characterization of methicillin-resistant Staphylococcus aureus isolates from rural community settings in Trinidad and Tobago. Niger J Clin Pract. 2018;21:1596–1601. doi: 10.4103/njcp.njcp_269_18. [DOI] [PubMed] [Google Scholar]
- 29.Monecke S., Stieber B., Roberts R., Akpaka P.E., Slickers P., Ehricht R. Population structure of Staphylococcus aureus from Trinidad and Tobago. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akpaka P.E., Kissoon S., Swanston W.H., Monteil M. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolates from Trinidad and Tobago. Ann Clin Microbiol Antimicrob. 2006;5:16. doi: 10.1186/1476-0711-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orrett F.A., Land M. Methicillin-resistant Staphylococcus aureus prevalence: current susceptibility patterns in Trinidad. BMC Infect Dis. 2006;6:83. doi: 10.1186/1471-2334-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlemann A.C., Dumortier C., Hafer C., Taylor B.S., Sanchez J., Rodriguez-Taveras C. Molecular characterization of Staphylococcus aureus from outpatients in the Caribbean reveals the presence of pandemic clones. Eur J Clin Microbiol Infect Dis. 2012;31:505–511. doi: 10.1007/s10096-011-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guardabassi L., Moodley A., Williams A., Stegger M., Damborg P., Halliday-Simmonds I. High prevalence of USA300 among clinical isolates of methicillin-resistant Staphylococcus aureus on St Kitts and Nevis, West Indies. Front Microbiol. 2019;10:1123. doi: 10.3389/fmicb.2019.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T., Okuma K., Ma X.X., Yuzawa H., Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist Updat. 2003;6:41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 35.Ito T., Ma X.X., Takeuchi F., Okuma K., Yuzawa H., Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–2651. doi: 10.1128/AAC.48.7.2637-2651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baum C., Haslinger-Loffler B., Westh H., Boye K., Peters G., Neumann C. Non-spa-typeable clinical Staphylococcus aureus strains are naturally occurring protein A mutants. J Clin Microbiol. 2009;47:3624–3629. doi: 10.1128/JCM.00941-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenesch F., Naimi T., Enright M.C., Lina G., Nimmo G.R., Heffernan H. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla S.K., Stemper M.E., Ramaswamy S.V., Conradt J.M., Reich R., Graviss E.A. Molecular characteristics of nosocomial and Native American community–associated methicillin-resistant Staphylococcus aureus clones from rural Wisconsin. J Clin Microbiol. 2004;42:3752–3757. doi: 10.1128/JCM.42.8.3752-3757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ba X., Harrison E.M., Edwards G.F., Holden M.T., Larsen A.R., Petersen A. Novel mutations in penicillin-binding protein genes in clinical Staphylococcus aureus isolates that are methicillin resistant on susceptibility testing, but lack the mec gene. J Antimicrob Chemother. 2014;69:594–597. doi: 10.1093/jac/dkt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee R., Gretes M., Harlem C., Basuino L., Chambers H.F. A mecA-negative strain of methicillin-resistant Staphylococcus aureus with high-level beta-lactam resistance contains mutations in three genes. Antimicrob Agents Chemother. 2010;54:4900–4902. doi: 10.1128/AAC.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miragaia M. Factors Contributing to the Evolution of mecA-mediated beta-lactam resistance in staphylococci: update and new insights from whole genome sequencing (WGS) Front Microbiol. 2018;9:2723. doi: 10.3389/fmicb.2018.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallin M., Denis O., Deplano A., De Mendonca R., De Ryck R., Rottiers S. Genetic relatedness between methicillin-susceptible and methicillin-resistant Staphylococcus aureus: results of a national survey. J Antimicrob Chemother. 2007;59:465–472. doi: 10.1093/jac/dkl535. [DOI] [PubMed] [Google Scholar]
- 43.Brown P.D., Ngeno C. Antimicrobial resistance in clinical isolates of Staphylococcus aureus from hospital and community sources in southern Jamaica. Int J Infect Dis. 2007;11:220–225. doi: 10.1016/j.ijid.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Martin T.C., Stranahan P., Rivero J.C. A fatal case of community acquired methicillin resistant Staphylococcus aureus brain abscess in a previously healthy adolescent. West Indian Med J. 2006;55:200–204. doi: 10.1590/s0043-31442006000300014. [DOI] [PubMed] [Google Scholar]
- 45.Chroboczek T., Boisset S., Rasigade J.P., Meugnier H., Akpaka P.E., Nicholson A. Major West Indies MRSA clones in human beings: do they travel with their hosts? J Travel Med. 2013;20:283–288. doi: 10.1111/jtm.12047. [DOI] [PubMed] [Google Scholar]