Abstract
No previous study has explored the structure of self-rated health (SRH), a measure holding strong predictive value for future health events, in the oldest old or in individuals with dementia. The aim was to construct a structural equation model of SRH for oldest old in general and for oldest old with dementia, and to explore direct and indirect associations between health-related factors and SRH. Cross-sectional data from the Vitality 90+, a population-based study in the city of Tampere, Finland, was used. Data were gathered by a mailed questionnaire in 2014. Altogether 1299 nonagenarians, of which 408 had self-reported dementia or cognitive decline, were included. Structural equation models were constructed for all participants and separately for participants with dementia. Diseases (heart disease, stroke, diabetes, arthritis, hip fracture, cancer and dementia for the model for all), dizziness, hearing, vision, mobility, activities of daily living, fatigue, depression and SRH were included in the models. Among all participants, fatigue, depression, problems in mobility, dizziness, deficits in vision and heart disease were directly associated with poor SRH. Among participants with dementia, only fatigue, dizziness and deficits in vision were directly associated with poor SRH. Among all participants, dementia and arthritis were indirectly associated with poor SRH through problems in mobility, depression and fatigue. Among the oldest old, the effects of diseases on SRH were mainly manifested through the consequences of diseases, namely fatigue, dizziness, deficits in vision and problems in mobility. Depression has an important direct and indirect role, and dementia and arthritis an important indirect role in the structure of SRH. Dementia weakens many of the direct and indirect associations for SRH.
Keywords: Finland, Self-assessed health, Perceived health, Structural equation modelling, Cognition, Nonagenarians
Highlights
-
•
First study to explore structure of self-rated health in oldest old and persons with dementia.
-
•
Fatigue, depression, mobility, dizziness, vision and heart disease directly affect health-rating in oldest old.
-
•
Dementia, depression and arthritis affect health rating indirectly through various routes in oldest old.
-
•
Dementia weakens many of the associations between objective indicators of health with self-rated health.
Introduction
Self-rated health (SRH) is an intriguing measure at the crossroads of culture and biology, reflecting the states of both human body and mind (Jylhä, 2009). With the simple question, “How is your health in general? Is it excellent, very good, good, fair, or poor?” (de Bruin, Picavet, & Nossikov, 1996, p. 161), or with a variant of this question, SRH has consistently shown its association with clinical diagnoses, physical functioning, well-being and mortality across variety of populations and ages (Bamia et al., 2017; Fayers & Sprangers, 2002; Idler & Benyamini, 1997; Jylhä, 2009; Nybo et al., 2003). Thus, the inclusion of SRH in numerous studies and questionnaires to indicate overall health status is certainly justified.
SRH allows us to capture elements that more guided questions are not able to (Jylhä, 2009) but our understanding on which health-related factors exactly direct a person to give a rating of poor or excellent health is limited. Which are the factors that health care professionals and policy makers should pay special attention to in which populations? One step forward in unwinding this issue has been the development of a conceptual model for SRH by Jylhä (2009). First, this model takes into account health-related factors, such as medical diagnoses, functional status and experienced bodily symptoms. Second, the model takes into account the contextual frameworks of evaluation, such as age, cultural conventions, reference group and disposition (Jylhä, 2009). Based on this model, Au and Johnston have highlighted vitality – feeling full of life and energetic – as an important component of SRH throughout all ages (Au & Johnston, 2014).
In order to get a comprehensive view on SRH, the structure of SRH needs to be explored thoroughly to unravel which factors are not only directly but also indirectly associated with it. For this, the approach of a structural equation model is appropriate but only a small fraction of the research on SRH has applied this model. Among previous studies, Stoller (Stoller, 1984), Jylhä and coworkers (Jylhä, Leskinen, Alanen, Leskinen, & Heikkinen, 1986), Liang and coworkers (Liang, Bennett, Whitelaw, & Maeda, 1991), Whitelaw and Liang (Whitelaw & Liang, 1991), Fylkesnes and Førde (Fylkesnes & Førde, 1992), Johnson and Wolinsky (Johnson & Wolinsky, 1993), Alonso and coworkers (Alonso et al., 2013), Hirve and coworkers (Siddhivinayak Hirve et al., 2014), and Golini and Egidi (Golini & Egidi, 2016) have investigated how the constructs of health-related factors and SRH interrelate. Findings among older adults have highlighted the importance of direct and indirect associations of chronic diseases in explaining SRH (Golini & Egidi, 2016; Johnson & Wolinsky, 1993; Jylhä et al., 1986; Whitelaw & Liang, 1991). In addition, the most recent findings focusing on poor SRH in older adults point out the importance of psychological and emotional health, such as depression and anxiety, on SRH (Golini & Egidi, 2016). However, all the previous models on SRH are focused on either young or younger old age groups. To the best of our knowledge, no previous studies have investigated SRH using structural equation modelling focusing on the oldest old, i.e., those aged 85 years and older.
As individuals get older, they lower their standards on what they consider as good health and adjust their health ratings accordingly (Johnson & Wolinsky, 1993; Jylhä, Guralnik, Balfour, & Fried, 2001; Leinonen, Heikkinen, & Jylhä, 1998). Thus, results from younger old people may not apply to the oldest old. Studies on both nonagenarians (Nybo et al., 2001) and centenarians (Araújo, Teixeira, Ribeiro, & Paúl, 2018) have shown a discrepancy between objective health and SRH. Though, the findings of Galenkamp and colleagues show that among nonagenarians SRH is still sensitive to changes in physical functioning and number of chronic conditions (Galenkamp et al., 2013). Furthermore, Enroth and colleagues have demonstrated that socioeconomic differences in SRH persist among nonagenarians (Enroth, Raitanen, Hervonen, & Jylhä, 2013). The oldest old are the fastest-growing segment of population in developed countries (Christensen, Doblhammer, Rau, & Vaupel, 2009) and a major population group using health care services (Forma et al., 2017). Therefore, there is a need to better understand what are the most meaningful health aspects among the oldest old and how SRH is structured among them.
While dealing with SRH among the oldest old, it is essential to acknowledge cognitive impairment and dementia. Studies show that approximately 25–30% of people in their early 90s, 50% of those in their late 90s, and 60% of those aged 100 years and over have some form of dementia (Yang, Slavin, & Sachdev, 2013). However, not many studies have investigated SRH in persons with cognitive impairment or dementia, and those that have, show conflicting results. Walker and coworkers showed that poor SRH still predicted mortality among older adults with mild or moderate cognitive impairment but not among those with severe cognitive impairment (Walker, Maxwell, Hogan, & Ebly, 2004). However, in the study by Nielsen and coworkers, poor SRH did not predict mortality in persons with mild Alzheimer's disease (Nielsen, Siersma, Waldemar, & Waldorff, 2016). Similarly, Damián and coworkers found no significant associations between number of chronic conditions or level of functional dependency with SRH among institutionalized older adults with cognitive impairment (Damián, Pastor-Barriuso, & Valderrama-Gama, 2008). Thus, there is a need to further explore the role of SRH in persons with cognitive impairment or dementia.
The aim of this study is to explore the path model of self-rated health among individuals aged 90+ years by looking at the whole population and those with dementia. Specifically, we explore the direct and indirect associations of physical functioning, morbidity, sensory functions and bodily symptoms with SRH by using structural equation modelling.
Materials and Methods
Design and sample
The study uses cross-sectional data from the Vitality 90+, a population-based study conducted in the area of Tampere, Finland (Jylhä, Enroth, & Luukkaala, 2013). The data for this study are derived from a mailed questionnaire that was sent in 2014 to all persons (n = 2157) aged 90 years and older, living in the area. Information on names, addresses and places of residence were derived from the Tampere City Population Register. Altogether 99 persons had died and two persons had moved away from Tampere by the time the questionnaire was sent. Another 419 persons did not answer to the questionnaire, leaving 1637 persons participating in the survey (response rate 80%). However, 296 persons were excluded from the analyses because of proxy answers – one cannot rate the self-rated health of another person (Knäuper & Turner, 2003). In addition, 42 persons were excluded because of missing data in the model variables. Thus, the final analytic sample in this study is 1299 persons, including both community-dwelling and institutionalized participants.
The study protocol was approved by the Ethics Committee of the Pirkanmaa Hospital District. All participants or their legal representatives (in case of proxy respondents, not included in this study) gave their written informed consent.
Self-rated health
The question for SRH was: “How is your health in general?” The response options were (i) very good, (ii) fairly good, (iii) average, (iv) fairly poor, and (v) poor.
Physical functioning
Mobility and activities of daily living (ADL) were used as measures for physical functioning. Mobility was based on questions (i) “Are you able to walk at least 400 m?” and (ii) “Are you able to climb stairs?”. ADL was based on questions (i) “Are you able to get in and out of bed?”, (ii) “Are you able to dress and undress yourself?”, and (iii) “Can you move inside?”. For both mobility and ADL disability the options were (i)“Yes, without difficulty” (ii) “Yes, but it's difficult”, (iii) “Only if somebody helps”, and (iv) “No”.
Diseases
The presence of diseases was assessed in the questionnaire by asking “Has a doctor ever told you that you have (a) heart disease, (b) cancer, (c) dementia or cognitive impairment, (d) stroke, (e) diabetes, (f) osteoarthritis, (g) hip fracture, (h) depression or depressiveness?”. The answer on dementia and cognitive decline was used in identifying the group for dementia.
Symptoms
Questions on fatigue and dizziness were used to describe bodily symptoms. The question for fatigue was “Do you feel yourself tired?” and for dizziness “Do you feel dizzy or your balance as weak?”. In both questions the options were (i) yes, often, (ii) yes, sometimes, (iii) never.
Sensory functions
Questions on vision and hearing were used to describe sensory functions. Vision was asked as “Is your vision good enough to read newspaper (with eyeglasses if you use them)?” The answers were (i) yes, (ii) partly (for example bigger headings) (iii) no. Hearing was asked as “Can you hear what another person is saying if you are alone with him/her (with a hearing device if you use it)?” The answers were (i) yes, (ii) partly (for example if the voice is raised), and (iii) no. Only 6 persons reported “no” to the question on hearing, and in the analyses the answers partly (ii) and no (iii) in hearing were combined.
Statistical analyses
Structural equation models using weighted least square mean and variance (WLSMV) adjusted estimator were constructed to assess the direct and indirect associations between physical functioning, morbidity, sensory functions, symptoms and SRH. Separate models were constructed for (i) all participants (Fig. 1) and for (ii) participants with dementia (Fig. 2). We did not use any weighting in the models.
Fig. 1.
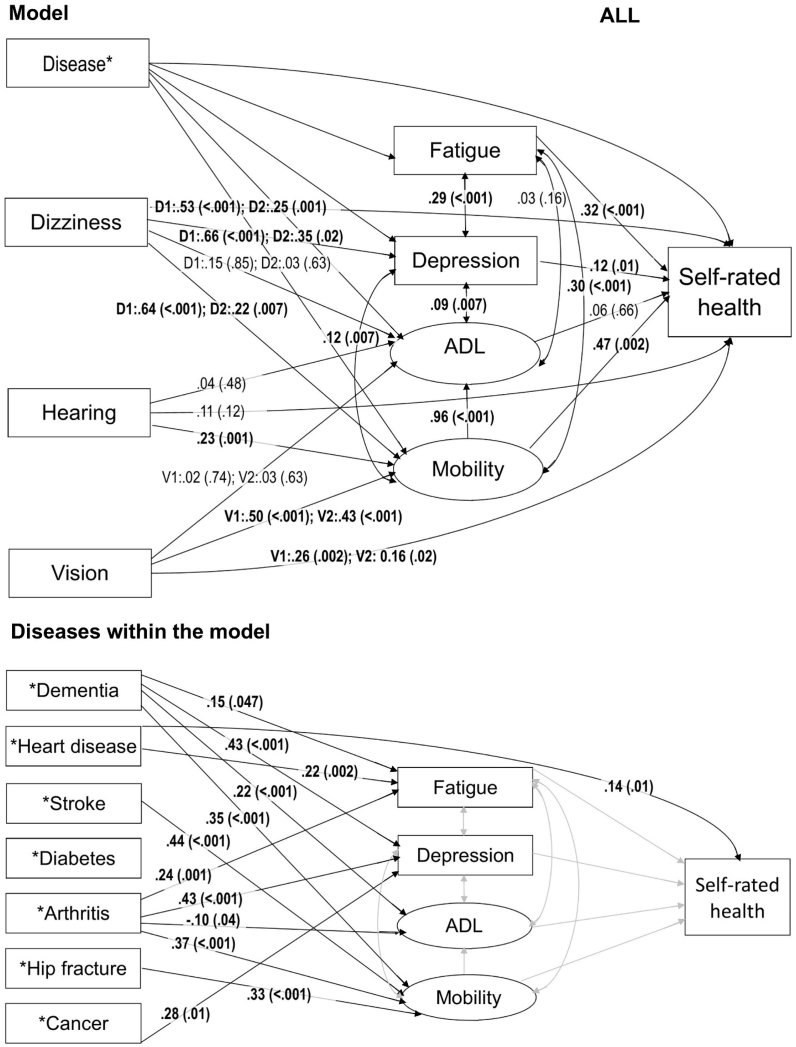
Path model for self-rated health among all participants in the Vitality 90+ Study. In the model the arrows represent all the associations included in the model. Path coefficient estimates with p values in parenthesis are presented on the related arrow. Statistically significant results are bolded in the model. Where diseases are presented separately within the model, associations from each disease to fatigue, depression, ADL, mobility and self-rated health are included in the model but only the associations that were statistically significant and the related arrows in black are presented. Regarding diseases, grey arrows represent association in the model for which the results are already shown. In all the variables, the reference group is the lowest value which represents the best option with no deficit or no disease. Notes: ADL = Activities of Daily Living; D1 = Dizziness, often; D2 = Dizziness, sometimes, V1 = Poor vision; V2 = Moderate vision.
Fig. 2.
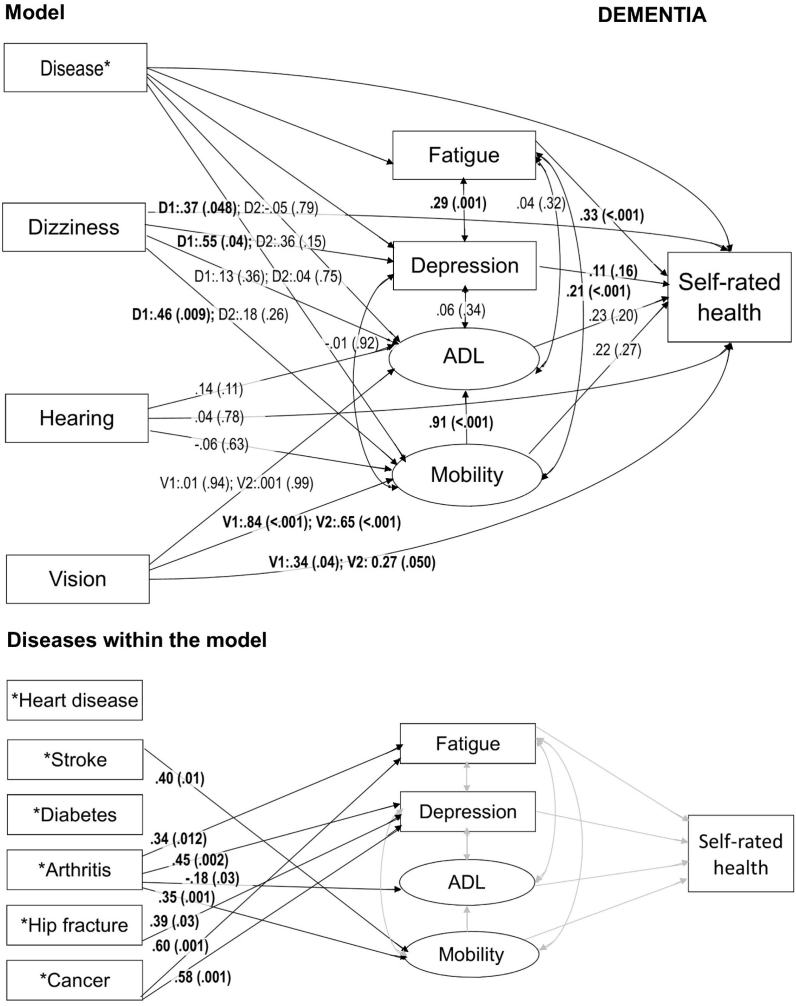
Path model for self-rated health among participants with dementia in the Vitality 90+ Study. In the model the arrows represent all the associations included in the model. STDYX standardized path coefficient estimates with p values in parenthesis are presented on the related arrow. Statistically significant results are bolded in the model. Where diseases are presented separately within the model, associations from each disease to fatigue, depression, ADL, mobility and self-rated health are included in the model but only the associations that were statistically significant and the related arrows in black are presented. Regarding diseases, grey arrows represent association in the model for which the results are already shown. In all the variables, the reference group is the lowest value which represents the best option with no deficit or no disease. Notes: ADL = Activities of Daily Living; D1 = Dizziness, often; D2 = Dizziness, sometimes, V1 = Poor vision; V2 = Moderate vision.
To a large extent, our analysis was informed by the conceptual model by Jylhä (2009) which suggests that when asked to evaluate their general health status, respondents will take into account any individual relevant information that they think describes their “health”. Empirical studies show that individuals will mainly take into account their medical diagnoses and physical functioning, but also experienced symptoms (Jylhä, 2009). This information is then considered in the context of the social and psychological situation.
According to this conceptual model, we constructed a structural model where we specified fatigue, depression, ADL and mobility as variables with direct effect on SRH. Diseases (heart disease, stroke, diabetes, arthritis, hip fracture, cancer and dementia; dementia was included only in the model for all), dizziness, hearing and vision were specified as variables with either direct and/or indirect effect on SRH. Fatigue, depression and ADL were specified as mutually correlated variables. Mobility was set to predict ADL and to correlate with fatigue and depression. Mobility and ADL were formed as latent variables. Depression, unlike other diseases, was specified in the model as a variable comparable to fatigue, mobility and ADL, because of its central role in self-ratings (Han & Jylhä, 2006; Schnittker, 2005). The model included only categorical variables, as described earlier in Materials and Methods section. In all the variables, the reference group was the lowest value which represents the best health option with no deficit or no disease. The path coefficient estimate indicates the amount of change (positive or negative) in the outcome value associated with moving from a healthy predictor category to a worse health category.
These theoretical associations were tested using likelihood ratio tests on empirical data to see which of them would survive to the final model. Furthermore, we explored which kind of associations, one-directional or bidirectional, fitted the model the best, although, for SRH we exploited only one-directional associations. However, if all mutual correlations between the variables were included, the models would become very complex. Therefore, we left associations between diseases, dizziness, hearing and vision out of the models. Age is known to be an important factor in self-rated health and most earlier studies have taken it into account. However, in our study all participants belonged to an exceptionally old group, being aged 90 years or older (age range 90–105 years; 88.8% aged 90–95 years), and for a majority of them, the age difference was max. 5 years. Therefore, we decided not to include age in our models. Our main analyses were conducted for both genders together, partly because of the rather small number of men particularly in the dementia group (n = 99). As supplementary analyses we did run additional models also for men and women separately, to have a rough understanding whether they fundamentally differ from each other. Due to low number of men, we had to combine two lowest categories in all mobility and ADL variables.
The analyses were performed using Mplus version 7 (Muthén & Muthén, 2012) and we report the STDYX standardized path coefficient estimates.
Results
Altogether 31% of the participants reported of having dementia (or cognitive impairment) (Table 1). The mean age of the participants was 92.5 years for all and 92.6 years for those with dementia. Both among all participants and among those with dementia, 76% were women. Among all participants and those with dementia, 3% and 1% rated their health very good, 26% and 18% fairly good, 46% and 44% average, 20% and 28% fairly poor and 6% and 9% poor, respectively. Heart disease and arthritis were the most frequently reported diseases. Among all participants, 54% reported heart disease and 46% arthritis. Among persons with dementia, 55% reported heart disease and 42% arthritis. Regarding mobility, climbing stairs was the most difficult task. Altogether, 28% of all participants and 19% of participants with dementia were able to climb stairs without difficulty, whereas 23% of all participants and 31% of participants with dementia were unable to climb stairs even with help. Regarding ADL, dressing and undressing was the most difficult task. Altogether 58% of all participants and 39% of those with dementia reported that they were able to dress and undress without difficulty, as for 5% of all participants and 12% of those with dementia reported that they were unable to dress and undress even with help (Table 1).
Table 1.
Characteristics of the participants.
| Variable | All |
Dementia |
|---|---|---|
| n = 1299 | n = 408 | |
| Age, year, mean (standard deviation) | 92.5 (2.4) | 92.6 (2.6) |
| Women, n (%) | 982 (75.6) | 309 (75.7) |
| Self-rated health (n = 1282), n (%) | ||
| Very good | 32 (2.5) | 5 (1.3) |
| Fairly good | 328 (25.6) | 72 (18.1) |
| Average | 591 (46.1) | 174 (43.8) |
| Fairly bad | 257 (20.0) | 109 (27.5) |
| Bad | 74 (5.8) | 37 (9.3) |
| Dementia, n (%) | 408 (31.4) | 408 (100.0) |
| Heart disease, n (%) | 701 (54.0) | 224 (54.9) |
| Stroke, n (%) | 109 (8.4) | 52 (12.7) |
| Diabetes, n (%) | 196 (15.1) | 67 (16.4) |
| Arthritis, n (%) | 591 (45.5) | 172 (42.2) |
| Hip fracture, n (%) | 214 (16.5) | 79 (19.4) |
| Cancer, n (%) | 217 (16.7) | 70 (17.2) |
| Depression (n = 1248), n (%) | 200 (15.4) | 97 (23.8) |
| Fatigue (n = 1279), n (%) | ||
| Never | 464 (36.8) | 175 (44.1) |
| Sometimes | 750 (58.6) | 204 (51.4) |
| Often | 65 (5.1) | 18 (4.5) |
| Dizziness, n (%) | ||
| Never | 211 (16.2) | 55 (13.5) |
| Sometimes | 721 (55.5) | 209 (51.2) |
| Often | 211 (28.3) | 144 (35.3) |
| Hearing, n (%) | ||
| Good | 1013 (78.0) | 283 (69.4) |
| Moderate or poor | 286 (22.1) | 125 (30.6) |
| Vision, n (%) | ||
| Good | 926 (71.3) | 261 (64.0) |
| Moderate | 218 (16.8) | 88 (21.6) |
| Poor | 155 (11.9) | 59 (14.5) |
| Mobility | ||
| Walking 400 m (n = 1288), n (%) | ||
| Yes, without difficulty | 489 (38.0) | 100 (24.7) |
| Yes, with difficulty | 322 (25.0) | 100 (24.7) |
| Only if someone helps | 168 (13.0) | 74 (18.3) |
| No | 309 (24.0) | 131 (32.3) |
| Climbing stairs (n = 1289), n (%) | ||
| Yes, without difficulty | 362 (28.1) | 75 (18.5) |
| Yes, with difficulty | 422 (32.7) | 120 (29.6) |
| Only if someone helps | 210 (16.3) | 86 (21.2) |
| No | 295 (22.9) | 125 (30.8) |
| Activities of Daily Living | ||
| Moving inside (n = 1291), n (%) | ||
| Yes, without difficulty | 774 (60.0) | 192 (47.3) |
| Yes, with difficulty | 422 (32.7) | 153 (37.7) |
| Only if someone helps | 57 (4.4) | 37 (9.1) |
| No | 38 (2.9) | 24 (5.9) |
| Dressing and undressing (n = 1293), n (%) | ||
| Yes, without difficulty | 747 (57.8) | 160 (39.4) |
| Yes, with difficulty | 375 (29.0) | 128 (31.5) |
| Only if someone helps | 107 (8.3) | 71 (17.5) |
| No | 64 (4.9) | 47 (11.6) |
| Getting in and out of bed (n = 1295), n (%) | ||
| Yes, without difficulty | 939 (72.5) | 237 (58.4) |
| Yes, with difficulty | 258 (19.9) | 99 (24.4) |
| Only if someone helps | 56 (4.3) | 40 (9.9) |
| No | 42 (3.2) | 30 (7.4) |
The model for all participants explained 59% and the model for participants with dementia 54% of the variability in SRH (Table 2). Among all participants, fatigue (p < 0.001), depression (p = 0.01), problems in mobility (p = 0.002), dizziness (p < 0.001 for often; p = 0.001 for sometimes), and deficits in vision (p = 0.002 for poor; p = 0.02 for moderate) were statistically significantly associated with poorer SRH (Fig. 1). Dementia was not directly associated with SRH but was associated with fatigue (p = 0.047), depression (p < 0.001), problems in ADL (p < 0.001) and problems in mobility (p < 0.001). Of the specific indirect effects between dementia and SRH, the results for depression (p = 0.027) and problems in mobility (p = 0.007) were statistically significant and for fatigue nearly significant (p = 0.052) (Table 3).
Table 2.
Information on the path models for self-rated health: Weighted least square mean and variance (WLSMV) adjusted estimator.
| Variable | Model for | Model for |
|---|---|---|
| All | Dementia | |
| Chi2 Test of Model Fit | 445 | 337 |
| Chi2 Test of Model Fit for the Baseline Model | 16 091 | 17 782 |
| Root Mean Square Error of Approximation | 0.072 | 0.043 |
| Weighted Root Mean Square Residual | 1.275 | 0.773 |
| Comparative Fit Index | 0.976 | 0.989 |
| Pseudo-R2 for self-rated health | 0.594 | 0.536 |
Table 3.
Path model for self-rated health (SRH): Path coefficient estimates for specific indirect effects with a p value < 0.10
| Associationa | 95% Confidence Interval |
|||
|---|---|---|---|---|
| Estimate | Lower | Upper | p Value | |
| All Participants | ||||
| Dementia → Fatigue → SRH | 0.047 | 0.024 | 1.944 | 0.052 |
| Dementia → Depression → SRH | 0.052 | 0.024 | 2.215 | 0.027 |
| Dementia → Mobility → SRH | 0.164 | 0.061 | 2.706 | 0.007 |
| Dizziness, sometimes → Mobility → SRH | 0.101 | 0.050 | 2.042 | 0.041 |
| Dizziness, often → Depression → SRH | 0.080 | 0.038 | 2.124 | 0.034 |
| Dizziness, often → Mobility → SRH | 0.298 | 0.105 | 2.828 | 0.005 |
| Hearing → Mobility → SRH | 0.107 | 0.047 | 2.272 | 0.023 |
| Vision, moderate → Mobility → SRH | 0.201 | 0.075 | 2.689 | 0.007 |
| Vision, poor → Mobility → SRH | 0.235 | 0.085 | 2.774 | 0.006 |
| Heart disease → Fatigue → SRH | 0.070 | 0.024 | 2.957 | 0.003 |
| Stroke → Mobility → SRH | 0.206 | 0.083 | 2.492 | 0.013 |
| Arthritis → Fatigue → SRH | 0.076 | 0.024 | 3.138 | 0.001 |
| Arthritis → Depression → SRH | 0.053 | 0.024 | 2.212 | 0.027 |
| Arthritis → Mobility → SRH | 0.175 | 0.062 | 2.832 | 0.005 |
| Hip fracture → Mobility → SRH | 0.154 | 0.061 | 2.503 | 0.012 |
| Participants with Dementia | ||||
| Cancer → Fatigue → SRH | 0.197 | 0.067 | 2.922 | 0.003 |
| Arthritis → Fatigue → SRH | 0.113 | 0.049 | 2.324 | 0.020 |
In all the variables the reference group is the lowest value which represents the best option with no deficit or no disease.
Among all participants, heart disease was the only disease in addition to depression that had a statistically significant direct association with poor SRH (p = 0.01) (Fig. 1). Especially arthritis, in addition to dementia, had statistically significant indirect associations with SRH (Table 3). Among all participants, arthritis was associated with fatigue (p = 0.001), depression (p < 0.001) and problems mobility (p < 0.001), and with better ADL (p = 0.04) (Fig. 1). Of the specific indirect effects between arthritis and SRH, the results for fatigue (p = 0.001), depression (p = 0.027) and problems in mobility (p = 0.005) were statistically significant (Table 3).
Among participants with dementia, only fatigue (p < 0.001), dizziness (p < 0.048 for often) and deficits in vision (p = 0.04 for poor; p = 0.050 for moderate) were directly associated with poor SRH (Fig. 2). Unlike among all participants, mobility and depression were not directly associated with SRH among participants with dementia. None of the diseases had a statistically significant direct association with SRH among those with dementia. However, similarly to all participants, arthritis was associated with fatigue (p = 0.01), depression (p = 0.002) and problems in mobility (p = 0.001), and with better ADL (p = 0.03) (Fig. 2). However, of the specific indirect effects between arthritis and SRH, only the result for fatigue (p = 0.020) was statistically significant (Table 3).
Supplement 1 presents specific model results for all the studied direct associations among all participants and among participants with dementia.
Our main analyses were conducted in the combined group of men and women, also because the male group was rather small (among all, n = 317; among individuals with dementia, n = 99). As supplementary analyses we also constructed the models separately for men and women (Supplement 2 and Supplement 3). Mainly, the models were similar to both genders. In women, both among all participants and among those with dementia, fatigue was fairly strongly and significantly (path coefficient estimate for all 0.320 and for individuals with dementia 0.374, respectively; p < 0.001 in both groups) associated with SRH while in men, the associations were not significant (path coefficient estimate for all 0.125, p = 0.272, and for individuals with dementia 0.105, p = 0.530, respectively).
Discussion
This study used cross-sectional design to explore the direct and indirect associations between health-related measures and SRH among the oldest old and separately among the subgroup with dementia through structural equation modelling. The findings showed that among all participants, fatigue, dizziness, depression, problems in mobility, deficits in vision and heart disease were directly associated with poor SRH. Dementia and arthritis were indirectly associated with poor SRH. Moreover, the results illustrated that the effects of diseases on SRH were manifested mainly through the consequences of diseases, not directly. Dementia appeared to weaken many of the direct and indirect associations found for SRH. Among individuals with dementia, only fatigue, dizziness and deficits in vision were directly associated with poor SRH. In both groups, arthritis was statistically significantly associated with fatigue, depression and problems in mobility. Separate analyses for men and women implied largely similar structures with the exception of the importance of fatigue in women. Yet, due to the small number of men, comparisons between these groups are problematic.
Different from the findings among older adults in general (Golini & Egidi, 2016; Johnson & Wolinsky, 1993; Jylhä et al., 1986), among the oldest old in general and in the subgroup living with dementia, the direct role of diseases on SRH appears to be small. Basically the oldest old seem to take into account similar factors as younger older adults while rating their health. However, it appears that among the oldest old, the effects of diseases on SRH is not emerging from the awareness of the disease per se but rather through the consequences of the disease, namely fatigue, dizziness, deficits in vision and problems in mobility. There are not many earlier studies that have investigated the effects of bodily symptoms on SRH. However, there are findings showing that fatigue and dizziness (de Moraes, Soares, Ferriolli, & Perracini, 2013; Engberg, Segerstedt, Waller, Wennberg, & Eliasson, 2017; Gassmann & Rupprecht, 2009; Taloyan, Leineweber, Hyde, & Westerlund, 2015), as well as deficits vision (Yiengprugsawan, Seubsman, & Sleigh, 2015), are associated with poor SRH.
Depression had an important direct and indirect role in the model for SRH among all participants. The result is in line with earlier findings (Han & Jylhä, 2006; Schnittker, 2005). Earlier studies also support the significance of osteoarthritis on SRH (Ogunbode, Adebusoye, Olowookere, & Alonge, 2014; Perruccio, Power, & Badley, 2005; Riddle & Dumenci, 2013). The results of this study suggest that the association is not direct but indirect. Our results extend the earlier findings on SRH and arthritis to apply also the oldest old and persons with dementia. In all, we demonstrated that the indirect association between arthritis and SRH is conveyed through various routes, which stresses the importance of arthritis as a factor behind deteriorating health.
Our results indicate that among the oldest old, dementia weakens many of the direct and indirect associations found for SRH. The traditional and strong determinants of SRH, diseases and physical functioning (Arnadottir, Gunnarsdottir, Stenlund, & Lundin-Olsson, 2011; Singh-Manoux et al., 2006), were not directly associated with SRH among oldest old with dementia. These results are similar with the ones of Damián and coworkers, who found no statistically significant determinants for SRH among institutionalized persons with cognitive impairment (Damián et al., 2008). Though, we cannot exclude the possibility that, in our study, some of the differences between total group and those with dementia that were based on the p value could be due to lower power in the analyses in the dementia group.
As cognitive impairment is included in the definition of dementia in this study, persons with dementia represent most likely mild and moderate cognitive impairment here. With severe cognitive impairment, the answers must have been given primarily by a proxy. Regarding all the individuals with dementia who participated in the survey, 38% of the answers were given by proxy. These individuals are not included in the results of this study and the results for dementia apply only for those who are able to answer themselves. Because of dementia, and because the existence of dementia was self-reported, one may question the reliability of the data. However, earlier studies (Goebeler, Jylhä, & Hervonen, 2007; Jylhä et al., 2013; Walker et al., 2004) support the view that persons with some degree of cognitive impairment or mild dementia are able to provide sufficiently reliable information on their health status, including the existence of dementia. As the data were collected by mailed survey, we cannot confirm whether the information given in the questionnaire on the respondent (the participant or a proxy) is correct, but our long-term experiences in the project implies that it is highly reliable. All the other potential reliability problems in survey studies also apply for this study particularly because of the high age of the participants. Yet cumulating evidence (Jylhä et al., 2013; Kelfve et al., 2013; Tiainen et al., 2013; Vuorisalmi, Sarkeala, Hervonen, & Jylhä, 2012) suggests that survey data on health and functioning among the oldest old has acceptable validity and reliability. However, even if the response rate in our study was high, it is likely that the findings underestimate the prevalence of health problems (Jylhä, 2020), yet we do not expect that the associations between the health variables would be distorted. It is important to recognize that our study did not have information on disease history or severity of diseases. For example, cancer was not directly associated with self-rated health but if current cancer cases were examined, the results may well have been different.
The results showed that dementia had no direct association with SRH. However, dementia had indirect effects on SRH and individuals with dementia experienced poorer SRH through depression, fatigue and problems in mobility. Oddly, in both models, persons with problems in ADL were less likely to report arthritis. This might be explained by the fact that if a person has severe problems in ADL, it is possible that arthritis is no longer regarded as a relevant disease for the person or relevant disease to report about.
An interesting feature in the results of this study was that among all participants, mobility was important in terms of SRH but ADL was not. ADL had no direct association with SRH. However, ADL was highly correlated with mobility (r = 0.93) and in our model the effect of mobility had an overlapping component with ADL. Thus, although we observed no direct effect of ADL, the overlapping share of variance was associated with SRH through mobility. On the other hand, among persons with dementia, not even mobility was statistically significantly associated with SRH. Yet, when we explored the models by using only one latent variable for physical functioning that combined mobility and ADL (data not shown), physical functioning was significantly associated with SRH among both individuals with dementia and among all individuals (for both p < 0.001). Overall the findings on the relation of mobility and ADL on SRH probably reflect the fact that with aging, individuals lower the threshold for what is considered as good health or physical functioning (Jylhä, 2009). A person may have severe restrictions in physical functioning but still give a high health rating. The results demonstrate the discrepancy between objective health indicators and SRH that has been found also in other studies among the oldest old (Araújo et al., 2018; Nybo et al., 2001). Altogether, our results support earlier research in that SRH cannot be considered as a valid measure for specific diagnosed diseases or physical performance, it may be a valid and important summary indicator in describing health - also in a very advanced age.
The models used in the analyses explained only 59% of the variability in SRH among all and 54% among persons with dementia. The meaning of psychosocial factors in the structure of SRH among older adults has shown to be important (Golini & Egidi, 2016). Furthermore, positive attitudes and emotions along with SRH have been emphasized in mental health outcomes of the oldest old (Kato, Zweig, Schechter, Barzilai, & Atzmon, 2016). Unfortunately, besides depression, we did not have other data for psychosocial measures to be added in our model. However, psychosocial and emotional health are closely reflected by diseases and functional health (Golini & Egidi, 2016), which in turn we were able to model well. Pain is also a measure that would have most likely improved our model (Mäntyselkä et al., 2003). However, in a study by Perruccio and colleagues, worsening pain fully explained the effect of arthritis onset on worsening SRH (Perruccio et al., 2005). This indicates that, at least to some extent, arthritis served as a proxy for pain in our study.
In conclusion, the results of this study broaden the understanding of SRH by introducing its structure among the oldest old population in general and among oldest old with dementia. The study uses data from the Vitality 90+ Study, which is unique with the high age of the participants and exceptionally high representativeness of the population. Even though the data are not optimal for unravelling all the components of the structure of SRH, we were able to demonstrate various novel aspects in the factors either directly or indirectly associated with SRH. With multiple studied associations, the study provides grounds for diverse future studies. The results of this study suggest that for the oldest old individuals themselves, instead of diseases as such, the most important factors are the consequences of diseases, such as dizziness, fatigue and problems in physical functioning. In future, additional measures should be introduced to improve the model for SRH among the oldest old.
Ethics approval
The study protocol was approved by the Ethics Committee of the Pirkanmaa Hospital District. All participants or their legal representatives gave their written informed consent.
CRediT authorship contribution statement
Inna Lisko: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Timo Törmäkangas: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Writing - review & editing. Marja Jylhä: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - review & editing.
Declarations of cometing interest
None.
Acknowledgements
This study was financially supported by the Academy of Finland (the Centre of Excellence in Research of Ageing and Care and the project number 287372 to MJ; grant number 286536 to TT) and by the Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital to MJ.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2020.100567.
Contributor Information
Inna Lisko, Email: inna.lisko@ki.se.
Timo Törmäkangas, Email: timo.tormakangas@jyu.fi.
Marja Jylhä, Email: marja.jylha@tuni.fi.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alonso J., Vilagut G., Adroher N.D., Chatterji S., He Y., Andrade L.H. Disability mediates the impact of common conditions on perceived health. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0065858. [Electronic version] e65858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo L., Teixeira L., Ribeiro O., Paúl C. Objective vs. subjective health in very advanced ages: Looking for discordance in centenarians. Frontiers of Medicine. 2018;5:189. doi: 10.3389/fmed.2018.00189. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnadottir S.A., Gunnarsdottir E.D., Stenlund H., Lundin-Olsson L. Determinants of self-rated health in old age: A population-based, cross-sectional study using the international classification of functioning. BMC Public Health. 2011;11:670. doi: 10.1186/1471-2458-11-670. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au N., Johnston D.W. Self-assessed health: What does it mean and what does it hide? Social Science & Medicine. 2014;121(1982):21–28. doi: 10.1016/j.socscimed.2014.10.007. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Bamia C., Orfanos P., Juerges H., Schöttker B., Brenner H., Lorbeer R. Self-rated health and all-cause and cause-specific mortality of older adults: Individual data meta-analysis of prospective cohort studies in the CHANCES consortium. Maturitas. 2017;103:37–44. doi: 10.1016/j.maturitas.2017.06.023. [Electronic version] [DOI] [PubMed] [Google Scholar]
- de Bruin A., Picavet H.S., Nossikov A. Vol. 58. WHO Regional Publications; 1996. (Health interview surveys. towards international harmonization of methods and instruments). [Electronic version] Euro. Ser. [PubMed] [Google Scholar]
- Christensen K., Doblhammer G., Rau R., Vaupel J.W. Ageing populations: The challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damián J., Pastor-Barriuso R., Valderrama-Gama E. Factors associated with self-rated health in older people living in institutions. BMC Geriatrics. 2008;8:5. doi: 10.1186/1471-2318-8-5. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Segerstedt J., Waller G., Wennberg P., Eliasson M. Fatigue in the general population- associations to age, sex, socioeconomic status, physical activity, sitting time and self-rated health: The northern Sweden MONICA study 2014. BMC Public Health. 2017;17(1):654–659. doi: 10.1186/s12889-017-4623-y. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth L., Raitanen J., Hervonen A., Jylhä M. Do socioeconomic health differences persist in nonagenarians? Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;68(5):837–847. doi: 10.1093/geronb/gbt067. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Fayers P.M., Sprangers M.A.G. Understanding self-rated health. Lancet. 2002;359(9302):187–188. doi: 10.1016/S0140-6736(02)07466-4. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Forma L., Aaltonen M., Pulkki J., Raitanen J., Rissanen P., Jylhä M. Long-term care is increasingly concentrated in the last years of life: A change from 2000 to 2011. The European Journal of Public Health. 2017;27(4):665–669. doi: 10.1093/eurpub/ckw260. [DOI] [PubMed] [Google Scholar]
- Fylkesnes K., Førde O.H. Determinants and dimensions involved in self-evaluation of health. Social Science & Medicine. 1992;35(3):271–279. doi: 10.1016/0277-9536(92)90023-j. [Electronic version] 1982. [DOI] [PubMed] [Google Scholar]
- Galenkamp H., Deeg D.J.H., Huisman M., Hervonen A., Braam A.W., Jylhä M. Is self-rated health still sensitive for changes in disease and functioning among nonagenarians? Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;68(5):848–858. doi: 10.1093/geronb/gbt066. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Gassmann K.G., Rupprecht R. Dizziness in an older community dwelling population: A multifactorial syndrome. The Journal of Nutrition, Health & Aging. 2009;13(3):278–282. doi: 10.1007/s12603-009-0073-2. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Goebeler S., Jylhä M., Hervonen A. Self-reported medical history and self-rated health at age 90. agreement with medical records. Aging Clinical and Experimental Research. 2007;19(3):213–219. doi: 10.1007/BF03324692. [DOI] [PubMed] [Google Scholar]
- Golini N., Egidi V. The latent dimensions of poor self-rated health: How chronic diseases, functional and emotional dimensions interact influencing self-rated health in Italian elderly. Social Indicators Research. 2016;128(1):321–339. [Electronic version] [Google Scholar]
- Han B., Jylhä M. Improvement in depressive symptoms and changes in self-rated health among community-dwelling disabled older adults. Aging & Mental Health. 2006;10(6):599–605. doi: 10.1080/13607860600641077. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Hirve S., Johan H.L.O., Sambhudas S., Juvekar S., Blomstedt Y., Stephen T. Unpacking self-rated health and quality of life in older adults and elderly in India: A structural equation modelling approach. Social Indicators Research. 2014;117(1):105–119. [Electronic version] from the EconLit database. [Google Scholar]
- Idler E.L., Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38(1):21–37. [Electronic version] [PubMed] [Google Scholar]
- Johnson R.J., Wolinsky F.D. The structure of health status among older adults: Disease, disability, functional limitation, and perceived health. Journal of Health and Social Behavior. 1993;34(2):105–121. [Electronic version] [PubMed] [Google Scholar]
- Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science & Medicine. 2009;69(3):307–316. doi: 10.1016/j.socscimed.2009.05.013. [Electronic version] 1982. [DOI] [PubMed] [Google Scholar]
- Jylhä M. New ages of life – emergence of the oldest-old. In: Rattan S., editor. Encyclopedia of Biomedical Gerontology. Elsevier; 2020. pp. 479–488. [Google Scholar]
- Jylhä M., Enroth L., Luukkaala T. Trends of functioning and health in nonagenarians- the vitality 90+ study. In: Robine J.M., Jagger C., editors. Annual review of gerontology and geriatrics, “healthy longevity”. Springer Publishing Company; 2013. pp. 313–332. [Google Scholar]
- Jylhä M., Guralnik J.M., Balfour J., Fried L.P. Walking difficulty, walking speed, and age as predictors of self-rated health: The women's health and aging study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2001;56(10):609. doi: 10.1093/gerona/56.10.m609. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Jylhä M., Leskinen E., Alanen E., Leskinen A.L., Heikkinen E. Self-rated health and associated factors among men of different ages. Journal of Gerontology. 1986;41(6):710–717. doi: 10.1093/geronj/41.6.710. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Kato K., Zweig R., Schechter C.B., Barzilai N., Atzmon G. Positive attitude toward life, emotional expression, self-rated health, and depressive symptoms among centenarians and near-centenarians. Aging & Mental Health. 2016;20(9):930–939. doi: 10.1080/13607863.2015.1056770. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelfve S., Thorslund M., Lennartson C. Sampling and non-response bias on health-outcomes in surveys of the oldest old. European Journal of Ageing. 2013;10:237–245. doi: 10.1007/s10433-013-0275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knäuper B., Turner P.A. Measuring health: Improving the validity of health assessments. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2003;12(Suppl 1):81–89. doi: 10.1023/a:1023589907955. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Leinonen R., Heikkinen E., Jylhä M. Self-rated health and self-assessed change in health in elderly men and women--a five-year longitudinal study. Social Science & Medicine. 1998;46(4–5):591–597. doi: 10.1016/s0277-9536(97)00205-0. [Electronic version] 1982. [DOI] [PubMed] [Google Scholar]
- Liang J., Bennett J., Whitelaw N., Maeda D. The structure of self-reported physical health among the aged in the United States and Japan. Medical Care. 1991;29(12):1161–1180. doi: 10.1097/00005650-199112000-00001. [Electronic version] [DOI] [PubMed] [Google Scholar]
- de Moraes S.A., Soares W.J.de S., Ferriolli E., Perracini M.R. Prevalence and correlates of dizziness in community-dwelling older people: A cross sectional population based study. BMC Geriatrics. 2013;13:4. doi: 10.1186/1471-2318-13-4. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntyselkä P.T., Turunen J.H.O., Ahonen R.S., Kumpusalo E.A. Chronic pain and poor self-rated health. Jama. 2003;290(18):2435–2442. doi: 10.1001/jama.290.18.2435. [DOI] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. 7th ed. Muthén & Muthén; Los Angeles, CA: 2012. Mplus user's guide: Statistical analysis with latent variables. [Google Scholar]
- Nielsen A.B.S., Siersma V., Waldemar G., Waldorff F.B. Poor self-rated health did not increase risk of permanent nursing placement or mortality in people with mild alzheimer's disease. BMC Geriatrics. 2016;16:87. doi: 10.1186/s12877-016-0262-x. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo H., Gaist D., Jeune B., McGue M., Vaupel J.W., Christensen K. Functional status and self-rated health in 2,262 nonagenarians: The Danish 1905 cohort survey. Journal of the American Geriatrics Society. 2001;49(5):601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- Nybo H., Petersen H.C., Gaist D., Jeune B., Andersen K., McGue M. Predictors of mortality in 2,249 nonagenarians--the Danish 1905-cohort survey. Journal of the American Geriatrics Society. 2003;51(10):1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- Ogunbode A.M., Adebusoye L.A., Olowookere O.O., Alonge T.O. Physical functionality and self-rated health status of adult patients with knee osteoarthritis presenting in a primary care clinic. Ethiopian Journal of Health Sciences. 2014;24(4):319–328. doi: 10.4314/ejhs.v24i4.7. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruccio A.V., Power J.D., Badley E.M. Arthritis onset and worsening self-rated health: A longitudinal evaluation of the role of pain and activity limitations. Arthritis & Rheumatism. 2005;53(4):571–577. doi: 10.1002/art.21317. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Riddle D.L., Dumenci L. Self-rated health and symptomatic knee osteoarthritis over three years: Data from a multicenter observational cohort study. Arthritis Care & Research. 2013;65(2):169–176. doi: 10.1002/acr.21661. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittker J. When mental health becomes health: Age and the shifting meaning of self-evaluations of general health. The Milbank Quarterly. 2005;83(3):397–423. doi: 10.1111/j.1468-0009.2005.00407.x. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A., Martikainen P., Ferrie J., Zins M., Marmot M., Goldberg M. What does self rated health measure? Results from the british whitehall II and French gazel cohort studies. Journal of Epidemiology & Community Health. 2006;60(4):364–372. doi: 10.1136/jech.2005.039883. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller E.P. Self-assessments of health by the elderly: The impact of informal assistance. Journal of Health and Social Behavior. 1984;25(3):260–270. [Electronic version] [PubMed] [Google Scholar]
- Taloyan M., Leineweber C., Hyde M., Westerlund H. Self-rated health amongst male and female employees in Sweden: A nationally representative study. International Archives of Occupational and Environmental Health. 2015;88(7):849–859. doi: 10.1007/s00420-014-1014-x. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Tiainen K., Luukkaala T., Hervonen A., Jylhä M. Predictors of mortality in men and women aged 90 years and older: nine-year follow-up. Age & Ageing. 2013;42:468–475. doi: 10.1093/ageing/aft030. [DOI] [PubMed] [Google Scholar]
- Vuorisalmi M., Sarkeala T., Hervonen A., Jylhä M. Among nonagenarians, congruence between self-rated and proxy-rated health was low but both predicted mortality. Journal of Clinical Epidemiology. 2012;65(5):553–559. doi: 10.1016/j.jclinepi.2011.11.001. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Walker J.D., Maxwell C.J., Hogan D.B., Ebly E.M. Does self-rated health predict survival in older persons with cognitive impairment? Journal of the American Geriatrics Society. 2004;52(11):1895–1900. doi: 10.1111/j.1532-5415.2004.52515.x. [Electronic version] [DOI] [PubMed] [Google Scholar]
- Whitelaw N.A., Liang J. The structure of the OARS physical health measures. Medical Care. 1991;29(4):332–347. doi: 10.1097/00005650-199104000-00003. [Electronic version] Retrieved Oct 11, 2018. [DOI] [PubMed] [Google Scholar]
- Yang Z., Slavin M.J., Sachdev P.S. Dementia in the oldest old. Nature Reviews Neurology. 2013;9(7):382–393. doi: 10.1038/nrneurol.2013.105. [DOI] [PubMed] [Google Scholar]
- Yiengprugsawan V., Seubsman S., Sleigh A.C. Association between vision impairment and health among a national cohort of 87,134 Thai adults. Asia-Pacific Journal of Public Health. 2015;27(2):202. doi: 10.1177/1010539511433049. [Electronic version] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.