Abstract
Arterial stiffness, represented as carotid-femoral pulse wave velocity (cfPWV), predicts cardiovascular disease (CVD). In older populations, however, this association appears attenuated. Moreover, the prognostic values of PWV at different arterial segments and newer parameters like cardio-ankle vascular index (CAVI) remain unclear, especially in US older adults.
In 3034 ARIC participants (66–90 years) without CVD, we examined the associations of four PWV measures (cfPWV, heart-femoral [hfPWV], brachial-ankle, heart-ankle [haPWV]) and two new measures of arterial stiffness (CAVI and cardio-femoral vascular index [CFVI] derived from haPWV and hfPWV, respectively) with incident CVD (coronary disease, stroke, and heart failure) and all-cause mortality.
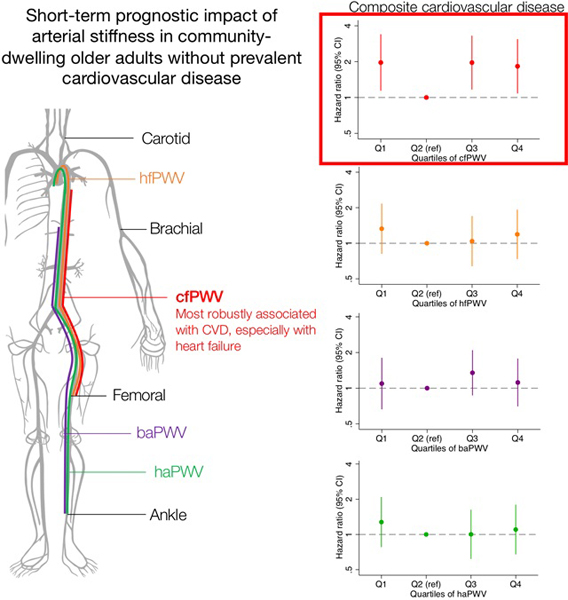
Over a median follow-up of 4.4 years, there were 168 incident CVD events and 244 deaths. Overall, stiffness measures did not show strong associations with CVD, except cfPWV, which demonstrated a J-shaped association even after adjusting for potential confounders (hazard ratio 1.83 [95% CI 1.08, 3.09] in top quartile and 1.97 [1.14, 3.39] in bottom quartile vs. second bottom quartile). When each CVD was examined separately, heart failure was most robustly associated with higher cfPWV, and stroke was strongly associated with lower cfPWV. There were no significant associations with all-cause mortality.
Among older adults without CVD, cfPWV showed the strongest associations with CVD, especially heart failure. Other PWV measures had no strong associations. Our findings further support cfPWV as the index measure of arterial stiffness and the link of arterial stiffness to heart failure development, but also suggest somewhat limited prognostic value of arterial stiffness in older adults overall.
Keywords: Arterial stiffness, pulse wave velocity, cardio-ankle vascular index, cardiovascular disease
Summary
Central arterial stiffness as measured by cfPWV may play an important role in incident cardiovascular disease, especially heart failure; however, overall, arterial stiffness may have limited prognostic value in older adults.
Graphical abstract
INTRODUCTION
Increased arterial stiffness or reduced compliance was observed to be an independent predictor of cardiovascular disease and mortality.1 While several parameters of arterial stiffness are available, carotid-femoral pulse wave velocity (cfPWV) is generally considered the standard measure of arterial stiffness, and has been associated with cardiovascular events and mortality in clinical and populations-based studies.1–3 In older populations, however, these associations appear to be attenuated.3 Also, the prognostic value of PWV measures at different arterial segments such as brachial-ankle, heart-ankle, and heart-femoral PWV remains unclear, as most studies involving these segments have included a small sample size and limited clinical populations.4
Furthermore, the American Heart Association recently introduced an additional parameter of arterial stiffness, the cardio-ankle vascular index (CAVI), which has some unique properties.4 First, CAVI is based on the arterial stiffness parameter β, therefore unlike pulse wave velocity (PWV), it is reportedly not much affected by changes in blood pressure during measurement.5 Second, CAVI reflects stiffness of various arteries including the aorta and femoral, popliteal, and tibial arteries, and has been demonstrated to be associated with cardiovascular risk factors and the presence of atherosclerosis.5,6 Third, CAVI does not require the use of probes on the carotid or femoral arteries and is therefore easier to measure compared to cfPWV which often requires exposure to the femoral area. However, unlike cfPWV,7 there have only been a handful of studies that assessed whether CAVI is predictive of future adverse health outcomes. Most of these studies were small, from Asia, and included high-risk groups such as individuals with kidney disease, diabetes, or hypertension, limiting its use in clinical practice and research especially in the US.8–11
We therefore assessed whether various segment-specific PWV and CAVI derived from PWV are associated with cardiovascular outcomes and total mortality in a large US community-based cohort of older adults. We also derived and examined a measure, cardio-femoral vascular index (CFVI), which is based on stiffness parameter β like CAVI but reflects only central arterial stiffness. We hypothesized that there would be positive associations between arterial stiffness and outcomes, and that central arterial stiffness would be more strongly associated with cardiovascular disease than peripheral arterial stiffness.
METHODS
Data availability and detailed policies for requesting ARIC data can be found at https://www2.cscc.unc.edu/aric/pubs-policies-and-forms-pg. ARIC data can be also obtained from the NHLBI BioLINCC repository (https://biolincc.nhlbi.nih.gov/home/).
Study design
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing cohort study that recruited participants from four US communities: Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD. Detailed study design and rationale have been described previously.12 Visit 1 was conducted during 1987–1989; visit 2 during 1990‐1992; visit 3 during 1993‐1995; visit 4 during 1996‐1998; and visit 5 during 2011‐2013. Participants were followed up with annual telephone interviews and hospitalization records to obtain updated medical history. For this analysis, the study baseline (i.e., time of origin) was set at visit 5 and participants were followed up until the endpoints of interest (i.e., cardiovascular event or mortality, as detailed below) or end of follow-up (December 31, 2016). Visit 5 study examination included blood and urine collection, assessment of cardiovascular risk factors and cardiovascular phenotypes, including PWV. The ARIC Study was approved by the institutional review board at each study center, and informed consent was obtained from participants at each study visit.
Study population
Eligible participants were 6,538 men and women who attended visit 5. Participants who were missing all measures of PWV, derived CAVI, and derived CFVI were excluded (n=857). Participants who had any clinical conditions influencing the reliability of PWV values (augmentation index >100, body mass index ≥40 kg/m2, severe arrhythmia, aortic or peripheral revascularization, aortic stenosis, or ejection fraction <30%) were further excluded (n=840). We also excluded 14 non-black and non-white participants, and participants missing the following covariates: education, body mass index, current smoking, systolic blood pressure, antihypertensive medication, diabetes, total cholesterol, history of coronary heart disease, heart failure and stroke, measures of kidney disease including serum creatinine, cystatin C, urine albumin-creatinine ratio, and ankle-brachial index (n=791). To examine incident outcomes, we also excluded those with prevalent coronary heart disease, heart failure, or stroke (n=1,002), which led to the final analytical sample of 3,034 participants (Figure S1).
Pulse wave velocity and derived CAVI
PWV was calculated by dividing the distance between two arterial recording sites by transit time and was assessed at four arterial segments: carotid-femoral (cfPWV), heart-femoral (hfPWV), heart-ankle (haPWV), and brachial-ankle (baPWV). For cfPWV, the distance from the carotid to the femoral artery was directly measured with a segmometer (Rosscraft, Surray, Canada) and calculated as the carotid to femoral distance minus the distance between the suprasternal notch to the carotid bulb.13 For haPWV and hfPWV, PWV from the heart was determined using heart sounds by phonocardiogram. cfPWV and hfPWV were considered measures of central arterial stiffness, and haPWV and baPWV were considered as a composite measure of both central and peripheral stiffness. Higher values of PWV indicated greater arterial stiffness. Pulse waves were measured with a vascular testing device (VP‐1000 plus, Omron Healthcare, Kyoto, Japan).14 Arterial pressure waveforms were recorded for 30 seconds by applanation tonometry sensors attached on the left common carotid and femoral artery. Bilateral brachial and post‐tibial arterial pressure waveforms were recorded for 10 seconds by extremities cuffs that were wrapped on both arms and ankles and connected to a plethysmographic sensor and an oscillometric pressure sensor. The validity and reliability of the automatic device for measuring PWV have been described previously.14,15
CAVI derived from PWV was considered another measure of composite arterial stiffness. The technical principles of CAVI measurement and calculation have been published previously.16 CAVI was calculated using haPWV and the following equation: CAVI = a[(2ρ/ΔP) x ln(Ps/Pd) x PWV2] + b, where Ps and Pd are systolic and diastolic blood pressures, respectively, ΔP is the difference of Ps and Pd, PWV is haPWV, ρ is blood density of 1.05 g/ml, and a and b are constants used to convert CAVI values to that from the Hasegawa method.16 Using the same equation, we also used hfPWV instead of haPWV to calculate CFVI that reflects central arterial stiffness from the aorta to the femoral artery. Higher values of derived CAVI and CFVI indicated greater arterial stiffness.
Cardiovascular events and all-cause mortality
The primary outcome of interest was a composite cardiovascular disease that comprised coronary heart disease, heart failure, and stroke. Secondary outcomes included coronary heart disease, heart failure, and stroke, all examined separately. We also investigated all-cause mortality since cardiovascular disease is a leading cause of death in the US. All cardiovascular outcomes were adjudicated by physician reviewers.12 Coronary heart disease was defined as definite or probable myocardial infarction, coronary heart disease death, or coronary revascularization procedure. Heart failure was adjudicated as definite or probable acute decompensated heart failure based on hospitalization record review.17 Stroke was defined as definite or probable cases of ischemic or hemorrhagic strokes based on stroke hospitalization and death records. All-cause mortality was defined as death due to any cause, and was ascertained through annual cohort follow-up, hospital surveillance, and linkage to the National Death Index.12 All outcomes were assessed through December 31, 2016.
Covariate measurements
All covariates were collected at visit 5 except for achieved education, which was collected at visit 1. Age was calculated from date of birth. Sex and race were self-reported. Education level was categorized as basic (either no education or incomplete high school education), intermediate (graduated high school or vocational school), or college or equivalent. Body mass index was calculated by dividing measured weight (kg) by height2 (m2). Systolic and diastolic blood pressure (SBP and DBP) was the average of the last two of the three readings that were recorded in a sitting position after five minutes of rest using a validated automatic sphygmomanometer (OMRON HEM-907 XL). Mean arterial pressure (MAP) was calculated using the following formula: MAP = (SBP + 2 * DBP)/3.18 Use of hypertension lowering medication in the past 4 weeks was recorded by self-report and confirmed by inspecting drug containers. History of diabetes was defined as fasting plasma glucose ≥126 mg/dL, fasting/non-fasting hemoglobin A1c ≥6.5%, using medication for diabetes, or self-report physician diagnosis of diabetes. History of smoking and alcohol consumption were self-reported and analyzed as dichotomous variables (current vs. non-current). Ankle-brachial index (ABI) was assessed alongside PWV using the same device. Total and high-density lipoprotein (HDL) cholesterol concentrations were determined using an enzymatic method and the Olympus HDL-Cholesterol test.19 Two measures of kidney disease included eGFR, an indicator of kidney function, and urine albumin-to-creatinine ratio (ACR), a marker of kidney damage. eGFR was calculated using the creatinine and cystatin C-based CKD-EPI equation.20
Statistical analysis
Baseline characteristics were summarized in the total analytical sample and by quartiles of segment-specific PWV measures, CAVI, and CFVI. Normally distributed continuous variables were described and compared across groups using means (standard deviation [SD]) and ANOVA; for variables with non-normal distributions, we used median (interquartile interval [IQI]) and Kruskal-Wallis test; and for categorical variables, we used frequency (percentage) and Pearson’s chi-squared test.
To allow for potential non-linear associations, PWV, derived CAVI, and derived CFVI measurements were categorized into quartiles (detailed cutoff values described in Results). Given a J-shaped association between several stiffness measures and outcomes and the lack of steep monotonic associations (as noted below), the second lowest quartile (Q2) served as the reference category in all analyses. PWV, CAVI, and CFVI values deviating 3 standard deviations from their respective mean were set to missing as outliers. For all analysis involving baPWV, haPWV, and derived CAVI, which incorporate a leg artery segment, we excluded participants with ABI ≤0.9 (n=119), as lower-limb stenosis or occlusion can interfere with measurements of PWV or CAVI in the lower extremities.21
The relationship between arterial stiffness measures and the cumulative incidence of the outcomes were visually assessed using Kaplan-Meier curves. Log-rank tests were used to compare the survival distributions. Cox proportional hazards models were used to estimate the associations of PWV, derived CAVI, and derived CFVI with cardiovascular events and all-cause mortality after adjusting for multiple potential confounders, which were selected based on their known associations with arterial stiffness, cardiovascular disease, and mortality. Model 1 was unadjusted, and Model 2 adjusted for demographic factors (i.e., age, sex, and race). Model 3 additionally adjusted for education, current smoking, MAP, antihypertensive medication, history of diabetes, and total and HDL cholesterol. Model 4 further accounted for ABI, eGFR, and ACR.
We conducted a few sensitivity analyses. For models examining cardiovascular events as the outcome, as a sensitivity analysis, we also conducted a competing risk analysis using subdistribution hazard models by Fine and Gray22 that considered death from non-cardiovascular causes as a competing event. The results were similar to those from the main models, and thus our data presentation is based on Cox models. We also performed a subgroup analysis to examine whether the associations of cfPWV with cardiovascular events were modified by sex or race. We selected cfPWV based on the consistent positive associations found with cardiovascular outcomes. We tested statistical significance for interactions using a likelihood ratio test. In addition, we examined the main associations after restricting our study population to those who had all six PWV measures (n=2,691).
We checked for the proportional hazards assumption using Schoenfeld residuals. There were no missing covariate values in the final analytical sample. A two-tailed p value of <0.05 was considered statistically significant. All analyses were performed using Stata/SE 14.2 (StataCorp, College Station, TX).
RESULTS
Baseline Characteristics
Overall, the mean age of the participants was 75 (SD 5) years, 39% were male, 79% were white, 32% had diabetes, and the majority were taking antihypertensive medication (Table 1). Participants with higher cfPWV values were more likely to be older, black, less educated, have higher SBP, DBP, MAP, diabetes, lower eGFR, and higher ACR. Comparisons across other measures of central arterial stiffness (hfPWV and CFVI) showed similar patterns (S1-S2). Measures of composite arterial stiffness (baPWV, haPWV, and derived CAVI) were associated with less evident difference in baseline characteristics but showed similar patterns overall: participants with higher values were more likely to be older and have lower body mass index, higher SBP and DBP, lower eGFR, and higher ACR (S3-S5).
Table 1:
Baseline characteristics by quartiles of carotid-femoral pulse wave velocity (cfPWV) in 2,755 participants
| Characteristics | cfPWV quartile 1 (n=689) | cfPWV quartile 2 (n=689) | cfPWV quartile 3 (n=691) | cfPWV quartile 4 (n=686) | Total with cfPWV (n=2755) | P |
|---|---|---|---|---|---|---|
| cfPWV range, cm/s | 325.0–949.0 | 949.5–1119.0 | 1120.0–1323.5 | 1324.0–2316.0 | 325.0–2316.0 | |
| Age, years | 74 (5) | 74 (5) | 75 (5) | 77 (5) | 75 (5) | <0.001 |
| Male sex | 34.7 | 38.8 | 39.1 | 40.4 | 39 | 0.15 |
| White race | 82.7 | 82.1 | 78.3 | 69.7 | 79 | <0.001 |
| Education | ||||||
| Basic | 8 | 9.4 | 9.7 | 16.5 | 10.7 | <0.001 |
| Intermediate | 37.9 | 42.2 | 43.7 | 42 | 41.2 | |
| College or equivalent | 54.1 | 48.3 | 46.6 | 41.5 | 48.1 | |
| Body mass index, kg/m2 | 27.7 (4.4) | 28.2 (4.6) | 27.9 (4.3) | 27.4 (4.6) | 27.9 (4.6) | 0.011 |
| Systolic blood pressure, mmHg | 123.0 (16.0) | 127.5 (14.9) | 132.9 (16.2) | 139.1 (17.8) | 130.7 (17.4) | <0.001 |
| Diastolic blood pressure, mmHg | 64.5 (9.8) | 66.8 (9.6) | 67.7 (10.2) | 68.4 (10.6) | 67.0 (10.3) | <0.001 |
| Mean arterial pressure, mmHg | 84.0 (10.5) | 87.0 (10.2) | 89.4 (10.8) | 92.0 (11.4) | 88.1 (11.1) | <0.001 |
| Antihypertensive drugs | 58.3 | 66.6 | 69.5 | 75.8 | 68.2 | <0.001 |
| Total cholesterol, mmol/L | 4.92 (1.03) | 4.87 (1.02) | 4.90 (1.07) | 4.81 (1.07) | 4.87 (1.05) | 0.26 |
| HDL cholesterol, mmol/L | 1.44 (0.37) | 1.38 (0.37) | 1.38 (0.35) | 1.37 (0.38) | 1.39 (0.37) | 0.002 |
| Diabetes | 21.9 | 28.6 | 32.6 | 40.1 | 31.6 | <0.001 |
| Current smoker | 6.4 | 5.7 | 5.9 | 4.8 | 5.8 | 0.64 |
| Current drinker | 57.8 | 57.5 | 53.3 | 43.8 | 53.5 | <0.001 |
| Ankle-brachial index | 1.17 (0.10) | 1.18 (0.11) | 1.17 (0.11) | 1.16 (0.12) | 1.17 (0.11) | 0.003 |
| eGFRcrcys, mL/min/1.73 m2 | 69.6 (58.8, 81.7) | 69.7 (58.0, 81.3) | 68.9 (57.1, 80.0) | 65.4 (53.1, 76.9) | 68.2 (56.4, 80.0) | <0.001 |
| Albumin-to-creatinine ratio, mg/g | 8.3 (5.6, 14.8) | 8.8 (5.8, 14.9) | 10.1 (6.3, 20.0) | 12.5 (7.1, 27.1) | 9.7 (6.1, 19.4) | <0.001 |
Values are % or mean (SD), or median (interquartile interval).
HDL=high density lipoprotein; eGFR=estimated glomerular filtration rate
When we compared the correlation between all measures of arterial stiffness, PWV measures of central arterial stiffness (cfPWV and hfPWV) were strongly correlated with each other and weakly correlated with derived CAVI, haPWV, and baPWV; however, derived CFVI was moderately correlated with CAVI and haPWV. As anticipated, derived CAVI was most strongly correlated with haPWV, followed by baPWV, and most weakly associated with cfPWV (S6).
Composite cardiovascular events
The median follow-up time was 4.4 years (IQI 4.2, 5.1 years, maximum 5.6 years). There were 168 incident cardiovascular events (47 coronary heart disease, 72 heart failure, 59 stroke events; incidence rate: 12.5 per 1,000 person-years). The cumulative incidence of cardiovascular disease significantly differed by quartiles of two central arterial stiffness measures, cfPWV and CFVI, but not hfPWV (Figures 1A, 1C, 1E). When we examined PWV measures reflecting composite arterial stiffness, there were no statistically significant differences by quartiles of baPWV (although borderline significant), haPWV, or derived CAVI (Figures 1B, 1D, 1F).
Figure 1: Cumulative probability of cardiovascular events by quartiles of PWV measures (A-D), derived CFVI (E), and derived CAVI (F).
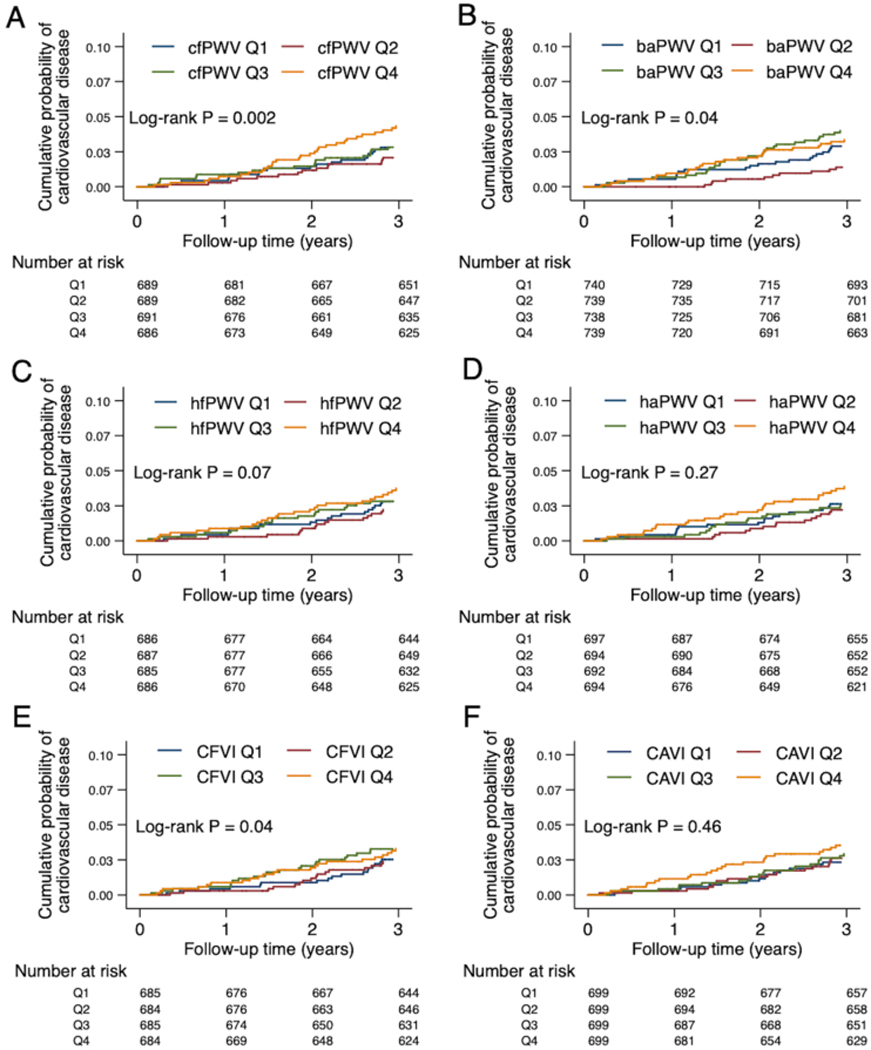
cfPWV, hfPWV, and CFVI indicate central arterial stiffness (A, C, E). baPWV, haPWV, and CAVI indicate composite arterial stiffness (B, D, F). Log-rank test was used to test for any difference across the quartiles.
The relationships of central arterial stiffness measures (cfPWV, hfPWV, and CFVI) with cardiovascular events were J-shaped, and of the three measures, cfPWV demonstrated the most evident results (Table 2). Higher and lower cfPWV showed consistent associations with a higher risk of cardiovascular disease even after adjusting for more potential confounders (Models 3–4 in Table 2). Specifically, compared to Q2, hazard ratio (HR) was 1.83 (95% CI 1.08, 3.09) in Q4 and 1.97 (1.14, 3.39) in Q1. The top quartile of hfPWV and CFVI was significantly associated with cardiovascular events only in unadjusted Model 1. None of baPWV, haPWV, and derived CAVI demonstrated significant associations even in Model 1 (Table 2).
Table 2:
Associations of PWV measures, derived CAVI, and derived CFVI with the risk of cardiovascular events
| Characteristics | Q1 | Q2 | Q3 | Q4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P* | HR (95% CI) | P* | HR (95% CI) | P* | |||
| Central arterial stiffness | ||||||||
| cfPWV | Model 1 | 1.66 (0.96, 2.84) | 0.07 | ref | 2.20 (1.31, 3.69) | 0.003 | 2.54 (1.53, 4.21) | <0.001 |
| Model 2 | 1.80 (1.05, 3.10) | 0.03 | ref | 2.01 (1.20, 3.39) | 0.01 | 2.05 (1.23, 3.43) | 0.01 | |
| Model 3 | 2.03 (1.17, 3.50) | 0.01 | ref | 2.02 (1.20, 3.41) | 0.01 | 1.91 (1.13, 3.23) | 0.02 | |
| Model 4 | 1.97 (1.14, 3.39) | 0.02 | ref | 1.96 (1.16, 3.31) | 0.01 | 1.83 (1.08, 3.09) | 0.02 | |
| hfPWV | Model 1 | 1.16 (0.71, 1.88) | 0.56 | ref | 1.21 (0.75, 1.97) | 0.44 | 1.74 (1.11, 2.73) | 0.02 |
| Model 2 | 1.28 (0.78, 2.08) | 0.33 | ref | 1.09 (0.67, 1.78) | 0.72 | 1.37 (0.86, 2.18) | 0.19 | |
| Model 3 | 1.33 (0.81, 2.17) | 0.26 | ref | 1.04 (0.63, 1.69) | 0.89 | 1.26 (0.78, 2.03) | 0.35 | |
| Model 4 | 1.33 (0.81, 2.17) | 0.26 | ref | 1.04 (0.64, 1.70) | 0.87 | 1.19 (0.73, 1.93) | 0.48 | |
| Derived CFVI | Model 1 | 1.38 (0.83, 2.28) | 0.21 | ref | 1.49 (0.91, 2.46) | 0.12 | 1.97 (1.23, 3.17) | 0.01 |
| Model 2 | 1.46 (0.88, 2.41) | 0.15 | ref | 1.29 (0.78, 2.13) | 0.32 | 1.47 (0.90, 2.40) | 0.13 | |
| Model 3 | 1.51 (0.91, 2.50) | 0.11 | ref | 1.25 (0.75, 2.07) | 0.39 | 1.36 (0.83, 2.24) | 0.22 | |
| Model 4 | 1.51 (0.91, 2.51) | 0.11 | ref | 1.24 (0.75, 2.06) | 0.39 | 1.30 (0.79, 2.14) | 0.30 | |
|
Composite arterial stiffness | ||||||||
| baPWV | Model 1 | 0.89 (0.54, 1.45) | 0.63 | ref | 1.51 (0.98, 2.34) | 0.06 | 1.45 (0.93, 2.25) | 0.10 |
| Model 2 | 1.02 (0.62, 1.67) | 0.94 | ref | 1.40 (0.90, 2.17) | 0.13 | 1.21 (0.77, 1.90) | 0.40 | |
| Model 3 | 1.10 (0.67, 1.82) | 0.70 | ref | 1.34 (0.86, 2.08) | 0.19 | 1.13 (0.71, 1.81) | 0.59 | |
| Model 4 | 1.09 (0.66, 1.80) | 0.73 | ref | 1.35 (0.87, 2.09) | 0.18 | 1.12 (0.70, 1.78) | 0.64 | |
| haPWV | Model 1 | 1.09 (0.67, 1.77) | 0.73 | ref | 1.16 (0.72, 1.87) | 0.55 | 1.52 (0.96, 2.40) | 0.07 |
| Model 2 | 1.21 (0.75, 1.98) | 0.44 | ref | 1.07 (0.66, 1.73) | 0.79 | 1.21 (0.76, 1.92) | 0.43 | |
| Model 3 | 1.28 (0.78, 2.09) | 0.33 | ref | 1.03 (0.63, 1.67) | 0.91 | 1.14 (0.70, 1.85) | 0.61 | |
| Model 4 | 1.28 (0.78, 2.09) | 0.33 | ref | 1.00 (0.62, 1.63) | 0.99 | 1.10 (0.67, 1.80) | 0.70 | |
| Derived CAVI | Model 1 | 1.07 (0.66, 1.74) | 0.77 | ref | 1.30 (0.82, 2.07) | 0.26 | 1.38 (0.87, 2.18) | 0.17 |
| Model 2 | 1.12 (0.69, 1.81) | 0.66 | ref | 1.10 (0.69, 1.75) | 0.69 | 0.97 (0.60, 1.56) | 0.89 | |
| Model 3 | 1.13 (0.69, 1.84) | 0.62 | ref | 1.08 (0.67, 1.72) | 0.76 | 0.95 (0.59, 1.54) | 0.84 | |
| Model 4 | 1.12 (0.68, 1.82) | 0.66 | ref | 1.07 (0.67, 1.72) | 0.76 | 0.94 (0.58, 1.52) | 0.80 | |
Model 1: Crude
Model 2: Age, sex, race
Model 3: Model 2 + education, current smoking, body mass index, history of diabetes, mean arterial pressure, antihypertensive medication, total cholesterol, high-density lipoprotein
Model 4: Model 3 + ankle brachial index, estimated glomerular filtration rate, albumin-creatinine ratio
HR=hazard ratio; Q1, Q2, Q3, Q4=quartile 1, quartile 2, quartile 3, quartile 4
P-values test quartile-specific estimates vs. the referent (quartile 2)
Individual cardiovascular outcomes
When each cardiovascular disease was examined separately, the highest quartiles of cfPWV and CFVI compared to the second lowest quartiles were associated with a higher risk of heart failure in the unadjusted model (S7). The two highest quartiles of cfPWV were also consistently associated with a higher risk of heart failure after adjusting for more potential confounders (hazard ratio [HR] of Q4 vs. Q2 2.72 [1.15, 6.45], HR of Q3 vs. Q2 2.44 [1.01, 5.87] in Model 4). The lowest and highest quartiles of cfPWV were also associated with a higher risk of stroke in the demographic-adjusted model (S8). The lowest quartile of cfPWV remained significantly associated with stroke even after further adjustment (HR of Q1 vs. Q2 3.06 [1.18, 7.95] in Model 4). No measure of central arterial stiffness was associated with the risk of coronary heart disease (S9).
Higher quartiles of composite arterial stiffness measures (baPWV, haPWV, and derived CAVI) were not associated with coronary heart disease, heart failure, or stroke (S7-S9). The higher quartiles of baPWV were associated with a higher risk of heart failure compared to the second lowest quartile, but this was not statistically significant after adjusting for cardiovascular disease risk factors (S7).
All-cause mortality
There were 244 deaths (incidence rate: 17.9 per 1,000 person-years) in our study. Unlike cardiovascular events, higher quartiles of all parameters of arterial stiffness were significantly associated with a higher cumulative incidence of all-cause mortality (Figure 2). However, once we accounted for demographic variables, the relationships of arterial stiffness measures with all-cause mortality were attenuated and no measures remained statistically significant (Table 3).
Figure 2: Cumulative probability of all-cause mortality by quartiles of PWV measures (A-D), derived CFVI (E), and derived CAVI (F).
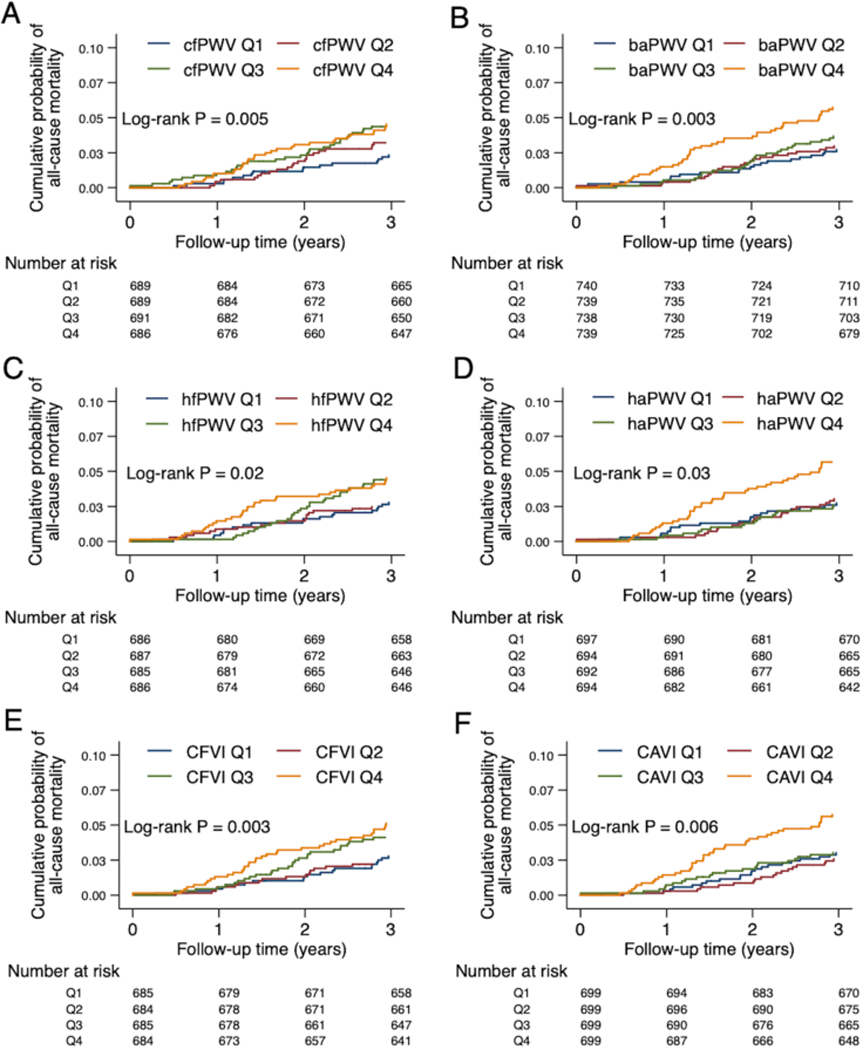
cfPWV, hfPWV, and CFVI indicate central arterial stiffness (A, C, E). baPWV, haPWV, and CAVI indicate composite arterial stiffness (B, D, F). Log-rank test was used to test for any difference across the quartiles.
Table 3:
Associations of PWV measures, derived CAVI, and derived CFVI with the risk of all-cause mortality
| Characteristics | Q1 | Q2 | Q3 | Q4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P* | HR (95% CI) | P* | HR (95% CI) | P* | |||
| Central arterial stiffness | ||||||||
| cfPWV | Model 1 | 1.01 (0.65, 1.57) | 0.96 | ref | 1.76 (1.19, 2.62) | 0.01 | 1.55 (1.04, 2.32) | 0.03 |
| Model 2 | 1.11 (0.71, 1.73) | 0.65 | ref | 1.60 (1.08, 2.39) | 0.02 | 1.24 (0.82, 1.87) | 0.30 | |
| Model 3 | 1.13 (0.72, 1.76) | 0.59 | ref | 1.55 (1.04, 2.32) | 0.03 | 1.13 (0.74, 1.72) | 0.57 | |
| Model 4 | 1.07 (0.69, 1.68) | 0.75 | ref | 1.44 (0.96, 2.15) | 0.07 | 0.99 (0.65, 1.51) | 0.96 | |
| hfPWV | Model 1 | 1.08 (0.71, 1.66) | 0.71 | ref | 1.39 (0.92, 2.09) | 0.11 | 1.71 (1.15, 2.53) | 0.01 |
| Model 2 | 1.21 (0.79, 1.86) | 0.39 | ref | 1.26 (0.84, 1.90) | 0.27 | 1.38 (0.92, 2.06) | 0.12 | |
| Model 3 | 1.28 (0.83, 1.96) | 0.27 | ref | 1.21 (0.80, 1.82) | 0.37 | 1.21 (0.80, 1.83) | 0.37 | |
| Model 4 | 1.25 (0.81, 1.93) | 0.31 | ref | 1.19 (0.79, 1.80) | 0.40 | 1.02 (0.67, 1.55) | 0.94 | |
| Derived CFVI | Model 1 | 1.24 (0.80, 1.93) | 0.33 | ref | 1.51 (0.99, 2.30) | 0.06 | 2.03 (1.36, 3.03) | 0.001 |
| Model 2 | 1.31 (0.85, 2.04) | 0.22 | ref | 1.30 (0.85, 2.00) | 0.22 | 1.56 (1.03, 2.36) | 0.04 | |
| Model 3 | 1.42 (0.92, 2.21) | 0.12 | ref | 1.26 (0.82, 1.93) | 0.29 | 1.40 (0.92, 2.14) | 0.11 | |
| Model 4 | 1.40 (0.90, 2.18) | 0.13 | ref | 1.24 (0.81, 1.90) | 0.32 | 1.23 (0.81, 1.89) | 0.33 | |
|
Composite arterial stiffness | ||||||||
| baPWV | Model 1 | 0.92 (0.62, 1.37) | 0.69 | ref | 1.08 (0.73, 1.59) | 0.69 | 1.65 (1.16, 2.35) | 0.01 |
| Model 2 | 1.06 (0.71, 1.59) | 0.76 | ref | 0.99 (0.67, 1.45) | 0.95 | 1.33 (0.93, 1.90) | 0.12 | |
| Model 3 | 1.06 (0.70, 1.59) | 0.78 | ref | 0.98 (0.66, 1.45) | 0.91 | 1.27 (0.87, 1.85) | 0.21 | |
| Model 4 | 1.02 (0.68, 1.54) | 0.92 | ref | 0.99 (0.67, 1.46) | 0.95 | 1.28 (0.88, 1.86) | 0.21 | |
| haPWV | Model 1 | 1.06 (0.70, 1.60) | 0.79 | ref | 1.02 (0.67, 1.55) | 0.93 | 1.59 (1.08, 2.34) | 0.02 |
| Model 2 | 1.18 (0.78, 1.79) | 0.44 | ref | 0.94 (0.61, 1.43) | 0.76 | 1.25 (0.85, 1.85) | 0.26 | |
| Model 3 | 1.26 (0.83, 1.92) | 0.29 | ref | 0.88 (0.58, 1.35) | 0.57 | 1.07 (0.71, 1.61) | 0.74 | |
| Model 4 | 1.22 (0.80, 1.86) | 0.36 | ref | 0.87 (0.57, 1.33) | 0.51 | 1.06 (0.70, 1.60) | 0.79 | |
| Derived CAVI | Model 1 | 1.30 (0.84, 2.03) | 0.24 | ref | 1.63 (1.07, 2.49) | 0.02 | 1.98 (1.32, 2.99) | 0.001 |
| Model 2 | 1.37 (0.88, 2.14) | 0.16 | ref | 1.39 (0.91, 2.13) | 0.13 | 1.42 (0.93, 2.16) | 0.11 | |
| Model 3 | 1.54 (0.99, 2.41) | 0.06 | ref | 1.37 (0.89, 2.10) | 0.15 | 1.24 (0.81, 1.91) | 0.32 | |
| Model 4 | 1.45 (0.93, 2.27) | 0.11 | ref | 1.34 (0.87, 2.06) | 0.18 | 1.25 (0.81, 1.92) | 0.32 | |
Model 1: Crude
Model 2: Age, sex, race
Model 3: Model 2 + education, current smoking, body mass index, history of diabetes, mean arterial pressure, antihypertensive medication, total cholesterol, high-density lipoprotein
Model 4: Model 3 + ankle brachial index, estimated glomerular filtration rate, albumin-creatinine ratio
HR=hazard ratio; Q1, Q2, Q3, Q4=quartile 1, quartile 2, quartile 3, quartile 4
P-values test quartile-specific estimates vs. the referent (quartile 2)
Sensitivity analysis
When the association between cfPWV and cardiovascular disease was examined by race, the J-shaped association was more prominent in whites than in blacks; however, there was no statistically significant interaction between cfPWV and race (S10). The association between cfPWV and composite cardiovascular disease was not significantly modified by sex. When we restricted our study population to only those who had all six PWV measures (n=2,691), the associations of PWV measures with the risk of cardiovascular disease remained similar (S11).
DISCUSSION
In a large community-based cohort of older adults, we found that cfPWV, a widely used reference measure of central arterial stiffness, was most consistently associated with a higher risk of cardiovascular disease, especially heart failure. Conversely, composite measures of arterial stiffness including derived CAVI, a relatively new measure of arterial stiffness, were not independently associated with cardiovascular disease. Arterial stiffness parameters were not evidently associated with all-cause mortality independently of confounders. These results were robust even after restricting our analytical sample to those who have all six PWV measurements.
Similar to previous studies reporting a relationship between higher central arterial stiffness and cardiovascular disease, we demonstrate that in older adults, PWV measures of central stiffness, cfPWV and hfPWV, as well as CFVI are more strongly associated with cardiovascular disease as compared to composite measures of composite arterial stiffness. Of all measures reflecting central arterial stiffness, cfPWV was the strongest predictor and most consistently associated with cardiovascular events, especially with heart failure. This suggests the pathophysiological importance of central stiffness over peripheral stiffness in the development of cardiovascular disease including heart failure. Indeed, central arteries are hemodynamically more relevant to the heart. For example, central arterial stiffening increases pulsatile shear stress and pressure of blood vessels, which elevates the ventricular afterload and induce cardiac remodeling and dysfunction (a concept recognized as ventricular-vascular coupling).4,23 cfPWV is therefore a plausibly strong predictor of cardiovascular outcomes. Our findings also further support the use of cfPWV as a reference standard measure of arterial stiffness in studies of cardiovascular disease even in older adults.
Interestingly, we observed a J-shaped association between cfPWV and composite cardiovascular disease, which has not been commonly reported in past studies. Even after adjustment for many potential confounders, we found that lower cfPWV is associated with an almost 2-fold increase in cardiovascular disease. It is uncertain whether this observation reflects pathophysiology, but the J-shaped relationship may be biologically plausible as cfPWV has been implicated in a similar J-shaped relationship with myocardial stress and heart rate.24
Of the different types of cardiovascular disease, the lowest quartile of cfPWV was most strongly associated with stroke. While the underlying mechanisms behind this observation are unknown, it is possible that stenoses that are present in the brachiocephalic artery or proximal carotid artery may interfere with the assessment of cfPWV and contribute to higher stroke risk. It is also possible that lower cfPWV reflects higher carotid artery stiffness since if the PWV reaches carotid artery faster cfPWV will be lower. Importantly, a past study reported that carotid stiffness is associated with an increased incidence of stroke, independent of aortic stiffness and other cardiovascular risk factors.25 Nonetheless, more studies are required to confirm our finding and if so, to investigate relevant mechanisms.
With the exception of the associations between cfPWV and some cardiovascular outcomes, overall, we observed weak or no associations between measures of arterial stiffness with cardiovascular outcomes, which suggests limited value in predicting cardiovascular endpoints from arterial stiffness in older adults. Indeed, a recently published individual participant meta-analysis demonstrated that the associations of aortic PWV with coronary heart disease, stroke, and the composite measure of the two events were significantly weaker in the older age group (>70 years).3 Considering that arterial stiffness is common in older adults (e.g., 71% of our participants exceeds a threshold of high cfPWV of 10 m/s4) the ability to discriminate cardiovascular disease events from it may be constrained. Our results do not rule out the possibility that arterial stiffness measures are associated with subclinical end-organ damage or are important predictors of health events in younger individuals.3
CAVI is a relatively new measure of arterial stiffness and only a few cohort studies have examined its prognostic value. Consequently, the results from past studies examining the association of CAVI with cardiovascular disease or total mortality were inconclusive.8,9,26 We found that in participants with ABI >0.9, derived CAVI was not a strong predictor of cardiovascular disease or all-cause mortality, which corroborates findings from recent studies that examined similar associations in smaller clinical populations in Japan, China, and Lithuania.8,9,26–29 Even when we used an alternative measure of CAVI referred to as CAVI0, which is derived from CAVI to correct for any residual blood pressure dependence,30 we found that the associations were similar (results not shown). However, it is possible that CAVI may be a stronger marker of cardiovascular disease in younger populations, as a few studies of CAVI that demonstrated significant associations with cardiovascular outcomes included relatively younger participants (mean age of 52–64 years).8,10,11
Some limitations of this study should be mentioned. Most of the participants are white, and the black participants were predominantly recruited from a single study site in Jackson, Mississippi; therefore, care should be taken to generalize to other racial groups or blacks in other regions. Moreover, our analytical sample included ARIC participants who could come to research clinics and may be healthier than average community-dwelling older adults. Our study sample is informative however, since we were interested in older adults without a history of cardiovascular disease. Our study median follow-up time of 4 years may also constrain our statistical power. Despite these limitations, this study is the largest community-based cohort study to evaluate newer and understudied parameters of arterial stiffness such as CAVI, CFVI, hfPWV, and haPWV and compare their prognostic values with more established measures of PWV (i.e., cfPWV and baPWV) that have previously been assessed with cardiovascular disease or mortality.
In conclusion, among older adults residing in the US, cfPWV, which reflects central arterial stiffness, is most strongly and consistently associated with cardiovascular events, especially heart failure. This observation suggests the importance of central arterial stiffness in the development of cardiovascular outcomes even among older adults, and confirms cfPWV as a representative measure of arterial stiffness. The association of cfPWV with cardiovascular events was modest and other parameters of arterial stiffness showed weak or no associations with cardiovascular disease, suggesting a somewhat limited prognostic value of arterial stiffness in older adults.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1). What is new?
Of PWV measures at different arterial segments and newer arterial stiffness parameters, cfPWV is the most strongly and consistently associated with incident cardiovascular disease in older adults.
cfPWV demonstrates the strongest relationship with heart failure out of all CVDs.
Other PWV measures and new parameters such as CAVI and CFVI are only weakly associated with outcomes.
2). What is relevant?
Findings further support cfPWV as the reference standard measure of arterial stiffness in studies of cardiovascular disease.
Given the weak relationships of other PWV measures and new parameters with outcomes, arterial stiffness may have limited prognostic value in older adults.
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
SOURCES OF FUNDING
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Pulse wave velocity data collection was supported by grant R01AG053938. This analytic study was supported by Fukuda Denshi.
Footnotes
PERSPECTIVES
The prognostic values of arterial stiffness at different arterial segments and newer parameters such as CAVI and CFVI remain unclear, especially in older adults residing in the US. Our study demonstrates that among older adults, cfPWV was the most consistently associated with incident CVD, especially heart failure, whereas other PWV measures and newer arterial stiffness parameters did not demonstrate strong relationships with CVD outcomes. Our findings further support cfPWV as the standard measure of arterial stiffness but also suggest somewhat limited prognostic value of arterial stiffness in older adults overall.
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Tillin T, Chambers J, Malik I, et al. Measurement of pulse wave velocity: site matters. J Hypertens. 2007;25(2):383–389. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–107. [DOI] [PubMed] [Google Scholar]
- 6.Hu H, Cui H, Han W, et al. A cutoff point for arterial stiffness using the cardio-ankle vascular index based on carotid arteriosclerosis. Hypertens Res. 2013;36(4):334–341. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20 Suppl 12:S45–50. [PubMed] [Google Scholar]
- 8.Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH. Coronary Artery Calcium Score Compared with Cardio-Ankle Vascular Index in the Prediction of Cardiovascular Events in Asymptomatic Patients with Type 2 Diabetes. J Atheroscler Thromb. 2015;22(12):1255–1265. [DOI] [PubMed] [Google Scholar]
- 9.Kato A, Takita T, Furuhashi M, Maruyama Y, Miyajima H, Kumagai H. Brachial-ankle pulse wave velocity and the cardio-ankle vascular index as a predictor of cardiovascular outcomes in patients on regular hemodialysis. Ther Apher Dial. 2012;16(3):232–241. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Nagayama D, Saiki A, et al. Cardio-Ankle Vascular Index is Independently Associated with Future Cardiovascular Events in Outpatients with Metabolic Disorders. J Atheroscler Thromb. 2016;23(5):596–605. [DOI] [PubMed] [Google Scholar]
- 11.Satoh-Asahara N, Kotani K, Yamakage H, et al. Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242(2):461–468. [DOI] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 13.Manual 2 Home and Field Center Procedures. ARIC Visit 5 and NCS Study Protocol. Version 1. 2013; https://www2.cscc.unc.edu/aric/cohort-manuals.
- 14.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol. 2003;91(12):1519–1522, A1519. [DOI] [PubMed] [Google Scholar]
- 15.Meyer ML, Tanaka H, Palta P, et al. Repeatability of Central and Peripheral Pulse Wave Velocity Measures: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2016;29(4):470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirai K, Hiruta N, Song M, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18(11):924–938. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cywinski J. The essentials in pressure monitoring. Martinus Nijhoff Publishers b.v; Boston; 1980. [Google Scholar]
- 19.ARIC Visit 5/NCS ANALYSIS MANUAL. In:2015: https://www2.cscc.unc.edu/aric/sites/default/files/public/listings/V5%20NCS%20Analysis%20Manual_150901%20v1.pdf.
- 20.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M, Ishii H, Aoyama T, et al. Ankle brachial pressure index but not brachial-ankle pulse wave velocity is a strong predictor of systemic atherosclerotic morbidity and mortality in patients on maintenance hemodialysis. Atherosclerosis. 2011;219(2):643–647. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943. [DOI] [PubMed] [Google Scholar]
- 24.Logan JG, Kim SS. Resting Heart Rate and Aortic Stiffness in Normotensive Adults. Korean Circ J. 2016;46(6):834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Sloten TT, Sedaghat S, Laurent S, et al. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. J Am Coll Cardiol. 2015;66(19):2116–2125. [DOI] [PubMed] [Google Scholar]
- 26.Kato A, Takita T, Furuhashi M, Kumagai H, Hishida A. A small reduction in the ankle-brachial index is associated with increased mortality in patients on chronic hemodialysis. Nephron Clin Pract. 2010;114(1):c29–37. [DOI] [PubMed] [Google Scholar]
- 27.Kusunose K, Sato M, Yamada H, et al. Prognostic Implications of Non-Invasive Vascular Function Tests in High-Risk Atherosclerosis Patients. Circ J. 2016;80(4):1034–1040. [DOI] [PubMed] [Google Scholar]
- 28.Laucevicius A, Ryliskyte L, Balsyte J, et al. Association of cardio-ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina (Kaunas). 2015;51(3):152–158. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita K, Ding N, Kim ED, et al. Cardio-ankle vascular index and cardiovascular disease: Systematic review and meta-analysis of prospective and cross-sectional studies. J Clin Hypertens (Greenwich). 2019;21(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spronck B, Mestanik M, Tonhajzerova I, et al. Direct means of obtaining CAVI0-a corrected cardio-ankle vascular stiffness index (CAVI)-from conventional CAVI measurements or their underlying variables. Physiol Meas. 2017;38(10):N128–N137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


