Abstract
Patients with acute upper respiratory tract infection (URTI) have been shown to be hyperreactive to inhaled tussigens such as citric acid and capsaicin, and the authors propose that this may be due to an increased sensitivity of airway receptors that mediate cough. In recent studies we have demonstrated that cough may be induced by vibration of the airway at the level of the throat or chest in patients with URTI but that the same stimuli induce little or no cough in healthy subjects. The difference between the patients with URTI and healthy subjects in their response to airway vibration may be explained on the basis of hyperreactivity of airway sensory receptors. We propose that the model of cough induced by airway vibration may be useful for studies on the pathophysiology and pharmacology of airway hyperreactivity in acute cough. The airway vibration model of cough may have some advantages over inhaled tussigens as the stimulus is easily controlled and the method is safe for use in children.
Keywords: Hyperreactivity, Common cold, Airway vibration, Percussor
1. Introduction
Cough associated with acute upper respiratory tract infection (URTI) is a common and troublesome problem and it is surprising that very little is known about the aetiology of this type of cough. It is assumed that the normal protective reflex of cough is exaggerated in some way during URTI so that cough occurs spontaneously instead of in response to food or fluid coming in contact with the airway epithelium. It has been proposed that during URTI there is an increased sensitivity of the sensory nerves in the airway epithelium that mediate cough due to the presence of mediators of inflammation [1], [2]. In a prospective study on healthy volunteers it was shown that cough sensitivity to inhaled capsaicin was increased during periods of URTI [2]. A similar increase in cough sensitivity associated with URTI has been reported when cough was induced with an aerosol of inhaled citric acid [1] or nebulized distilled water [3]. Empey et al. [1] have proposed that the increase in cough sensitivity during URTI may be due to increased sensitivity of the rapidly adapting airway receptors (RARs). The RARs are particularly sensitive to mechanical stimulation [4], [5] and therefore it should be possible to induce cough with an adequate mechanical stimulus to the airway. Mechanical induction of cough has been achieved in anaesthetized animals by intra-tracheal stimulation of the upper airway with bristles or iron slugs [6], [7] and airway vibration has been shown to induce cough in humans [8], [9].
Studies on the induction of cough in humans by vibration of the throat or chest have reported that cough can be induced in patients with URTI but that little or no cough occurs in healthy volunteers [8], [9]. The difference in cough sensitivity between patients with URTI and healthy volunteers as demonstrated by airway vibration supports the hypothesis that cough associated with URTI is caused by hyperreactivity of the cough reflex, perhaps related to an increased sensitivity of airway sensory receptors such as RARs. The cough model associated with airway vibration may allow measurement of the degree of airway hyperreactivity associated with URTI since cough is not readily induced in healthy volunteers by airway vibration. The present paper will discuss the mechanism of airway hyperreactivity associated with URTI and the usefulness of the cough model of airway vibration in clinical trials of new treatments for cough associated with URTI.
2. Cough associated with URTI and airway hyperreactivity
URTI is associated with viral infection of the upper airways. The immune response to infection causes the generation of a complex mixture of mediators such as bradykinin, prostaglandins, neuropeptides and cytokines that trigger the process of inflammation [10]. The symptoms of URTI such as runny nose, nasal congestion and sore throat are nearly always present but cough is a less common symptom and many cases of URTI do not develop cough to a degree that causes discomfort.
2.1. Cough and viruses
In an epidemiological study of common cold symptoms in 131 persons over a 1 year period it was found that the group suffered an average of 2.2 colds, with runny nose being the most common symptom (87%) and cough occurring in only 44% of subjects [11]. In general, patterns of symptom development are not substantially different with different viruses [12] but there is evidence that some viruses are more likely to cause the symptom of cough. Comparison of the percentages of subjects that experienced various symptoms after viral challenge showed that the incidence of cough varies from 9 to 64% depending on the virus used for challenge [12]. This finding indicates that some viruses have the capacity to induce cough whereas others do not. A simple explanation for this difference may be differences in the novelty of the viruses and previous immune history of the patient. Viruses that are commonly circulating in the community tend to cause mild symptoms and sub-clinical infections because of ‘herd’ immunity to the virus whereas the introduction of a new virus to a previously unexposed community can cause severe illness. Epidemics and pandemics of influenza result because of the novelty of the virus and absence of herd immunity, and severe illness with cough and fever as major symptoms occurs in the population. In the recent cases of severe acute respiratory syndrome (SARS) the major self-reported symptoms were fever (99%) and non-productive cough (69%) [13] and the best predictors for the diagnosis of influenza in the community are cough and fever with a positive predictive value of 79% [14].
2.2. Trigeminal and vagus nerves
Mild URTI may not be associated with cough because the infection is restricted to the nose and generates rhinitis with symptoms of runny nose, sneezing and nasal congestion. The sensory nerve supply to the nose is from the branches of the trigeminal nerve and stimulation of this pathway results in reflex sneezing and secretion but not cough [15]. Cough is said to be mediated exclusively by the vagus nerves [16] and therefore it is not surprising that a mild common cold is not associated with cough as the infection is restricted to the part of the airway supplied by the trigeminal nerves. With more severe URTI the infection and inflammation may spread down the airway to the larynx, trachea and bronchi, and thus involve parts of the airway supplied by the vagus nerves and therefore trigger cough. The restriction of the vagus nerve to the parts of the airway from the larynx downwards may explain why cough is a dominant symptom in severe respiratory infections such as influenza and SARS but not a major symptom with common cold. In the course of URTI cough develops as a late symptom [12] and this may be because the infection starts at the level of the nose and gradually spreads down the airway to the larynx. Only when the inflammation involves the structures of the larynx and below does cough develop as a symptom.
2.3. Cough threshold
Cough is a threshold phenomenon and the generation of cough depends on the intensity of the stimulus. Studies on cough with nebulized chemical irritants such as citric acid and capsaicin clearly demonstrate the threshold nature of cough. Low concentrations of citric acid or capsaicin may not induce cough but as the concentration of the irritant is increased so a cough threshold is reached. The concentration of citric acid or capsaicin needed to induce cough is lower when subjects are suffering from URTI compared with when they are healthy [1], [2] and this demonstrates the threshold nature of cough.
Patients suffering from URTI may not have cough as a symptom but may have a lowered threshold to stimuli that induce cough. Cold air is one of the most common cough-inducing irritants and many persons suffering from cough complain that cough is induced on contact with cold air [17]. In a population of patients suffering from URTI it is possible to induce cough by a stimulus such as airway vibration even though this group of patients do not have cough as a symptom [8], [9]. In this case one can speculate that the patients with URTI have a lowered threshold to cough and the stimulus of airway vibration is sufficient to induce cough when suffering from URTI but not in the absence of URTI.
3. Cough induced by airway vibration
Mechanical stimulation of the lower airway is a potent stimulus for the induction of cough in man as demonstrated by the inhalation of any foreign body into the lower airway. One would therefore expect that vibration of the airway would induce cough, as vibration will cause physical deformation of airway sensory receptors such as RARs that mediate cough. Mechanical stimulation of the airway experimentally induces cough in anaesthetized animals [6], [7] but there are no reports in the literature to support the idea that airway vibration will induce cough in man apart from recent publications from this Centre [8], [9].
3.1. Vibration of the throat
Vibration of the throat at the level of the jugular notch with a modified battery-operated shaver induces cough in patients with URTI but little or no cough in healthy subjects. A detailed report of these results has been previously published [9]. The modified battery shaver is illustrated in Fig. 1 , and its application to the throat in Fig. 2 . Mean cough counts for six consecutive episodes of vibration, in healthy subjects and patients with URTI, are illustrated in Fig. 3 . The figure shows that there was a progressive increase in the number of coughs over the first three periods of vibration in the patients with URTI. In total, 7/15 healthy subjects coughed at least once during the periods of airway vibration whereas 28/30 of the patients with URTI coughed. There was a significant difference in the mean total cough counts for the six episodes of cough between the healthy subjects (0.73) and the patients with URTI (5.63) (P=0.001) [9]. These results demonstrate that vibration of the upper airway causes cough in patients with URTI but little cough in healthy subjects.
Fig. 1.

Modified battery-operated shaver used to vibrate the throat.
Fig. 2.
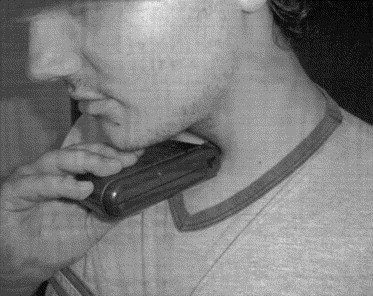
Illustration of how the vibrator was applied to the throat by the subject.
Fig. 3.
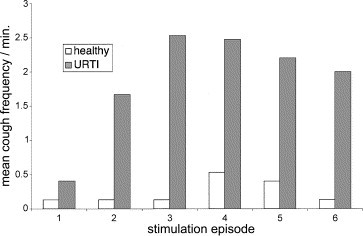
Mean cough frequency in 15 healthy subjects (empty columns) and 30 patients with URTI (filled columns) induced by vibration of the throat at the level of the jugular notch with a modified battery-operated shaver. The stimulus was applied for around 2 s, with six stimulation episodes separated by a rest of 2 min [9]. Coughs were counted in the 30 s period immediately after the stimulus.
3.2. Vibration of the chest wall
Chest percussion is often used to facilitate drainage of respiratory secretions in patients with chronic obstructive pulmonary disease or cystic fibrosis but there are no reports that vibration of the chest wall induces cough. Chest wall vibration in healthy volunteers and patients with asthma has been reported to create a sensation of breathlessness but these studies have not shown any cough [18], [19]. Similarly, studies on chest wall vibration in anaesthetized dogs showed an increased clearance of tracheal mucus but no cough [20], [21].
Vibration of the chest over the manubrium sternum with a chest percussor (model G5 Variko percussor, Physiotherapie Generale, France S.A.) induces cough in patients with URTI but little or no cough in healthy subjects. A detailed report of these results has been previously published [8]. An illustration of the application of the chest percussor is shown in Fig. 4 . The percussor was applied to the chest and the vibration applied for a maximum of 1 min. The vibration was switched off when the subject first coughed, and the number of coughs in the 2 min period from the start of the vibration was counted. Mean cough counts for three consecutive episodes of vibration are shown in Fig. 5 . In total, 2/15 healthy subjects coughed at least once during the periods of airway vibration whereas 25/29 of the patients with URTI coughed. There was a significant difference in the mean total cough counts for the three episodes of cough between the healthy subjects (0.47) and the patients with URTI (7.48) (P=0.01) [8]. These results demonstrate that vibration of the chest with a percussor causes cough in patients with URTI but little cough in healthy subjects.
Fig. 4.
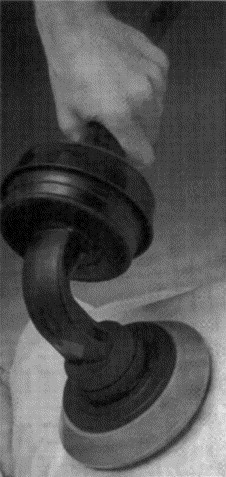
Illustration of how the G5 Variko percussor was applied to the chest by the investigator. The percussor was used with a polyurethane sponge applicator (number 212, 100 mm diameter and 30 mm thickness).
Fig. 5.
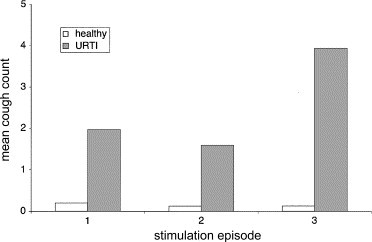
Mean number of coughs in 15 healthy subjects (empty columns) and 29 patients with URTI (filled columns), induced by vibration of the chest with a percussor. The stimulus was applied to the chest for up to a maximum of 1 min or until the subject coughed. Coughs were counted over a 2 min period from the start of the stimulus.
3.3. Cough recruitment
The studies on cough induction by airway vibration described above demonstrated that there was a progressive increase in the number of coughs with each period of airway vibration. This recruitment of cough is clear in Fig. 3. where the number of coughs reached a maximum after three periods of throat vibration, and is also apparent in Fig. 5 where the number of coughs is greatest after three periods of chest percussion. These results indicate that there is some recruitment or facilitation of cough with repeated airway vibration. The cough recruitment could be due to cough itself traumatizing the airway and causing the release of inflammatory mediators, or it could be due to a central rather than a peripheral mechanism. Brainstem mechanisms such as cough exhibit plasticity and short- and long-term memories that affect the response [22] and it is possible that this type of memory would lead to an enhancement of the cough reflex.
4. Airway vibration as a cough model
In a recent review on the development of new cough medicines O'Connell [23] stated that:
Preliminary studies in healthy human volunteers may continue to be useful but ultimately these studies should also be carried out in patients with cough who show enhanced cough sensitivity to inhaled tussigens. Based on current knowledge, the challenge is to find an antitussive agent which can return the abnormal sensitivity of the cough reflex to normal…
A case can be made that airway vibration as described above may prove to be a useful model for future clinical trials on cough medicines that can return the sensitivity of the cough reflex towards normal, and that airway vibration may have some advantages for inducing cough when compared with methods using inhaled tussigens.
4.1. Ease of use of airway vibration
Compared with methods using inhaled tussigens airway vibration offers a much easier means of inducing cough. The Variko G5 percussor is a standard clinical instrument that is marketed world wide for the mobilisation of secretions in patients with chronic respiratory disease. In comparison with methods using inhaled tussigens the use of the percussor offers a much simpler method of inducing cough as minimal cooperation is required from the patient, there is no need to make up and store tussigenic solutions, and the duration and intensity of the stimulus is easily controlled.
4.2. Safety
Airway vibration is a non-invasive and safe method of inducing cough, and chest percussion is widely used in children as a means of mobilizing airway secretions in conditions such as cystic fibrosis [24]. Although there are no reported safety issues with inhaled tussigens such as citric acid and capsaicin the fact that they are administered directly into the airway and that they may induce bronchoconstriction means that they need to be used with care in patients with respiratory disease.
4.3. Standardisation
Inhaled tussigens have been used for decades and there is a large literature on this method of inducing cough. However, there is no standardization of methods for induction of cough with inhaled tussigens and Morice et al. [25] state that results from different centres are difficult to compare or reproduce as a wide range of different methods are used, and absence of standardization is a major problem. Airway vibration is a new method of inducing cough but it has the potential for easy standardization as standard medical equipment such as the Variko G5 percussor is available for use.
4.4. Discrimination between healthy and hyperreactive subjects
It is important that any method of inducing cough should be able to discriminate between healthy and hyperreactive subjects as future research on cough medicines will be directed towards returning the sensitivity of the cough reflex back towards normal as described above [23].
It is possible to demonstrate airway hyperreactivity with inhaled tussigens but airway vibration may be a more sensitive method. In a study on citric acid-induced cough the threshold concentration that caused cough in patients with URTI was 2% whereas the threshold concentration in healthy subjects was 3.75% [1]. In a study on capsaicin-induced cough the log concentration of capsaicin (micromoles) needed to induce two coughs (C2) in patients with URTI was 0.27 whereas in healthy subjects the C2 was 0.89 [2]. These studies on inhaled tussigens indicate that patients with URTI need only 30–50% of the concentration of tussigen in order to induce cough compared with healthy subjects. The studies on airway vibration described above, indicate that this method may be better able to discriminate between healthy and hyperreactive patients than methods using inhaled tussigens. A great advantage is that airway vibration induces very little cough in healthy subjects and therefore there is a great difference in the total number of coughs induced in healthy and URTI patients as described above. The large difference in cough sensitivity between healthy and URTI patients demonstrated with airway vibration means that this method could be useful in studying cough medicines that may return the sensitivity of the cough reflex back towards normal, as normal in this case would be the absence of cough or a very long latency to cough.
5. Conclusions
Airway vibration is a safe, non-invasive method of inducing cough in patients with URTI. The relatively low level of cough induced by airway vibration in healthy subjects indicates that this method may be useful for studies on airway hyperreactivity as the method readily discriminates between health and disease.
References
- 1.Empey D.W., Laitinen L.A., Jacobs L., Gold W.M., Nadel J.A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976;113:131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- 2.O'Connell F., Thomas V.E., Studham J.M., Pride N.B., Fuller R.W. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med. 1996;90:279–286. doi: 10.1016/s0954-6111(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 3.Lowry R., Wood A., Higenbottam T. The effect of anticholinergic bronchodilator therapy on cough during upper respiratory tract infections. Br J Clin Pharmacol. 1994;37:187–191. doi: 10.1111/j.1365-2125.1994.tb04259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widdicombe J.G. Afferent receptors in the airways and cough. Respir Physiol. 1998;114:5–15. doi: 10.1016/s0034-5687(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 5.Sant'Ambrogio G., Widdicombe J. Reflexes from airway rapidly adapting receptors. Respir Physiol. 2001;125:33–45. doi: 10.1016/s0034-5687(00)00203-6. [DOI] [PubMed] [Google Scholar]
- 6.Tedeschi R., Tedeschi D., Hitchens J., Cook L., Mattis P., Fellows E. A new antitussive method involving mechanical simulation in unanaesthetised dogs. J Pharmacol Exp Ther. 1959;126:338–344. [PubMed] [Google Scholar]
- 7.Widdicombe J. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol. 1954;123:55–70. doi: 10.1113/jphysiol.1954.sp005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee P.C., Eccles R. Cough induction by high-frequency chest percussion in healthy volunteers and patients with common cold. Respir Med. 2004;98(8):771–776. doi: 10.1016/j.rmed.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Lee P., Eccles R. Cough induced by mechanical stimulation of the upper airway in man. Acta Otolaryngol (Stockholm) 2004;124(6):720–725. doi: 10.1080/00016480410017251. [DOI] [PubMed] [Google Scholar]
- 10.Eccles R. Pathophysiology of nasal symptoms. Am J Rhinol. 2000;14:335–338. doi: 10.2500/105065800781329528. [DOI] [PubMed] [Google Scholar]
- 11.Reid D., Williams R., Hirch A. Colds among office workers. An epidemiological study. Lancet. 1953:1303–1306. doi: 10.1016/s0140-6736(53)91373-7. [DOI] [PubMed] [Google Scholar]
- 12.Tyrrell D.A., Cohen S., Schlarb J.E. Signs and symptoms in common colds. Epidemiol Infect. 1993;111:143–156. doi: 10.1017/s0950268800056764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth C.M., Matukas L.M., Tomlinson G.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 14.Monto A.S., Gravenstein S., Elliott M., Colopy M., Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 15.Eccles R. Neurological and pharmacological considerations. In: Proctor D.F., Andersen I., editors. The nose, upper airways physiology and the atmospheric environment. Elsevier; Amsterdam: 1982. pp. 191–214. [Google Scholar]
- 16.Widdicombe J.G. A brief overview of the mechanisms of cough. In: Chung K.F., Widdicombe J.G., Boushey H.A., editors. Cough, causes, mechanisms and therapy. Blackwell; Massachusetts: 2003. pp. 17–23. [Google Scholar]
- 17.Cho Y.S., Park S.Y., Lee C.K. Enhanced cough response to hyperpnea with cold air challenge in chronic cough patients showing increased cough sensitivity to inhaled capsaicin. Allergy. 2003;58:486–491. doi: 10.1034/j.1398-9995.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 18.Manning H.L., Basner R., Ringler J. Effect of chest wall vibration on breathlessness in normal subjects. J Appl Physiol. 1991;71:175–181. doi: 10.1152/jappl.1991.71.1.175. [DOI] [PubMed] [Google Scholar]
- 19.Homma I., Obata T., Sibuya M., Uchida M. Gate mechanism in breathlessness caused by chest wall vibration in humans. J Appl Physiol. 1984;56:8–11. doi: 10.1152/jappl.1984.56.1.8. [DOI] [PubMed] [Google Scholar]
- 20.King M., Phillips D.M., Gross D., Vartian V., Chang H.K., Zidulka A. Enhanced tracheal mucus clearance with high frequency chest wall compression. Am Rev Respir Dis. 1983;128:511–515. doi: 10.1164/arrd.1983.128.3.511. [DOI] [PubMed] [Google Scholar]
- 21.King M., Phillips D.M., Zidulka A., Chang H.K. Tracheal mucus clearance in high-frequency oscillation. II: chest wall versus mouth oscillation. Am Rev Respir Dis. 1984;130:703–706. doi: 10.1164/arrd.1984.130.5.703. [DOI] [PubMed] [Google Scholar]
- 22.Morris K.F., Baekey D.M., Nuding S.C., Dick T.E., Shannon R., Lindsey B.G. Plasticity in respiratory motor control: invited review: neural network plasticity in respiratory control. J Appl Physiol. 2003;94:1242–1252. doi: 10.1152/japplphysiol.00715.2002. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell F. Central pathways for cough in man—unanswered questions. Pulm Pharmacol Ther. 2002;15:295–301. doi: 10.1006/pupt.2002.0344. [DOI] [PubMed] [Google Scholar]
- 24.Varekojis S.M., Douce F.H., Flucke R.L. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respir Care. 2003;48:24–28. [PubMed] [Google Scholar]
- 25.Morice A.H., Kastelik J.A., Thompson R. Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol. 2001;52:365–375. doi: 10.1046/j.0306-5251.2001.01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


