Abstract
The main source of Leishmania infantum infection in humans is a naturally infected dog. This study reports on the infectivity to phlebotomine sandflies (Lutzomyia longipalpis) of serologically positive mongrel dogs that differed in clinical status, haematology and humoral responses to immunoglobulin (Ig) GT (total anti-Leishmania IgG), IgG1 and IgG2 subclasses of antibody to crude antigen of L. infantum. Forty-five female L. longipalpis were allowed to feed directly on the ears of dogs classified as asymptomatic, oligosymptomatic or symptomatic before being dissected five days later. Promastigotes were detected in 88% of the dissected sandflies. The highest rate of infectivity to sandflies was found in symptomatic dogs, followed by oligosymptomatic and asymptomatic animals. The results suggest that dogs naturally infected with L. infantum with higher total IgG and IgG2 concentrations and lower haematocrit levels were able to infect the highest proportion of L. longipalpis. No correlation was observed between anaemia and the intensity of clinical signs. Symptomatic dogs presented the highest infection rate and intensity of infection.
Keywords: Canine visceral leishmaniasis, Xenodiagnosis, IgG, IgG1, IgG2, Clinical status, Haematology
1. Introduction
Visceral leishmaniasis (VL) is a zoonosis caused by Leishmania (Leishmania) infantum that is found around the Mediterranean area as well throughout the neotropics. The human disease in these regions is associated with the presence of domestic dogs and the parasite is transmitted by phlebotomine sandflies (Diptera: Psychodidae), the main neotropical vector being Lutzomyia longipalpis (Lutz and Neiva, 1912).
VL has an important role in public health in Brazil due to its high incidence, wide geographical distribution, marked increase in transmission associated with urbanisation, and following the emergence of the disease as an opportunistic infection in human immunodeficiency virus (HIV)-infected individuals. Control measures are currently based on the treatment of human cases, spraying of houses and animal shelters with residual insecticides, and the culling of dogs identified as seropositive by the indirect immunofluorescent antibody test (IFAT) or enzyme-linked immunosorbent assays (ELISAs). These measures do not however represent a permanent solution and new VL foci continue to appear throughout the country.
In urban areas, the domestic dog is the main reservoir of L. infantum and canine VL is associated with human cases of disease, acquired when sandflies infected by feeding on infected animals take a subsequent blood meal from humans (Moreno and Alvar, 2002). Infected dogs may present with a wide range of clinical profiles, from apparently healthy to critically diseased (Ciaramella et al., 1997), depending on the balance between cellular and humoral responses (Pinelli et al., 1994, Ferrer, 1999, Pinnelli et al., 1999). Mancianti et al. (1998) suggested that these animals could be classified as: asymptomatic, with no clinical signs of Leishmania infection; oligosymptomatic, presenting lymphadenopathy, slight weight loss and/or alopecia; or symptomatic, showing all or some of the severe signs of the disease, including cutaneous lesions, onychogryphosis, keratoconjunctivitis and rigidity of the hind limbs.
Immunoglobulin (Ig)G production by infected dogs may involve IgG1, IgG2, IgG3 and IgG4 subclasses of immunoglobulins (Deplazes et al., 1995, Quinnell et al., 2003). Bourdoiseau et al. (1997a) detected IgG1 and IgG2 subclasses in serum samples from symptomatic and asymptomatic dogs. The IgG2 levels were always predominant but no differences were observed that could be correlated with categories of clinical signs. Vercammen et al. (2002) found that dogs naturally infected with L. infantum presented great variations in IgG1 and IgG2 levels with IgG2 always present at high levels but IgG1 detected in only 6/11 animals examined. In a study of Brazilian dogs naturally infected with L. infantum, Quinnell et al. (2003) obtained very different results from those of the previous study with animals in which the parasite could be detected showing higher levels of IgG1 than IgG2.
Haematological and serum biochemical measurements in L. infantum-infected dogs have limited applications for disease diagnosis but can be very important in evaluating the clinical status of the animal, as well as in the understanding of VL pathogenesis (Ikeda et al., 2002, Reis et al., 2006a). Although not universally accepted (Moreno et al., 1998, Amusategui et al., 2003), anaemia is a frequent clinical sign in canine VL, occurring in 50–70% of patients as normocytic, normochromic and non-regenerative (Abranches et al., 1991, Ciaramella et al., 1997, Koutinas et al., 1999, Ikeda et al., 2002, Reis et al., 2006a). The possible causes of the anaemia are blood loss due to epistaxis and skin ulcerations, haemolysis, generalised inflammation, renal insufficiency and bone marrow hypoplasia or aplasia (Anosa and Idowu, 1983, Slappendel and Greene, 1990, Koutinas et al., 1999).
Decreased lipid fluidity of erythrocytes was found in 17 dogs with anaemia caused by L. infantum infection by De Luna et al. (2000). Sequestration of erythrocytes in the spleen due to cell rigidity, alterations in erythrocyte receptor ligands or both may result from decreased membrane fluidity and contribute to anaemia in these dogs. According to some authors (Amusategui et al., 2003, Reis et al., 2006a) anaemia in dogs with VL is related to the severity of clinical signs, with symptomatic dogs presenting with lower erythrocyte counts, haematocrit levels and haemoglobin concentrations.
Xenodiagnosis cannot be recommended as a routine diagnostic technique, since it requires a ready supply of laboratory-colonised sandflies. However, it can be used to answer certain epidemiological questions, especially those related to the clinical status of the animal and its infectivity after treatment (Alvar et al., 1994, Gradoni, 2002). Using serial xenodiagnosis to assess the infectivity of dogs naturally infected with L. infantum, Travi et al. (2001) showed that asymptomatic individuals were unable to infect L. longipalpis females, while oligosymptomatic animals were infective at very low rates. On the other hand, symptomatic animals were able to infect large numbers of females at a very high intensity. These authors also showed the skin of the ear to be more heavily parasitised than that of the abdomen. Courtenay et al. (2001) demonstrated that dogs became infective to L. longipalpis only after serum antibodies to the parasite could be detected and suggested that antibody titre could be used as a predictive factor.
In the present study, xenodiagnosis was used as a tool to assess the reservoir competence of dogs with distinct clinical presentations and based on their serum levels of total IgG, IgG1 and IgG2. Since dogs play a central role in the maintenance of VL foci, basic knowledge of infectivity to sandflies of dogs with different clinical presentations is important in order to generate epidemiological data from areas to which the disease has spread and where the prevalence of canine and human VL is increasing.
2. Materials and methods
2.1. Animals
The University Ethical Committee on Animal Experimentation sanctioned all experimental procedures.
Forty-two male mongrel dogs obtained from the Belo Horizonte Municipal Zoonotic Diseases Control Department and weighing 5–35 kg were used in the experiments. The animals had been diagnosed as Leishmania-seropositive and were destined for compulsory euthanasia, as required by Brazilian health regulations. The dogs received anti-parasitic medication during a 40 day settling-in period and were vaccinated against rabies, distemper, parvovirus, coronavirus, parainfluenza and two leptospirosis strains. During this period, as well as for the duration of the experiments, the animals were kept in communal kennels and received water and commercial dog food ad libitum. They were then given a complete clinical examination and classified as asymptomatic, oligosymptomatic or symptomatic (Mancianti et al., 1998).
2.2. Collection of blood and serum samples
Blood for haematological analysis and serum for ELISA tests were obtained by jugular venepuncture from each dog, with 3 mL blood being collected into a vial containing ethylenediamine tetraacetic acid (EDTA). A further 7 mL aliquot was collected into tubes without anticoagulant (Sarstedt), centrifuged at 200 g and the serum divided into three 2 mL samples, which were stored at −20 °C until analysed.
2.3. Haematological analysis
The blood samples collected into EDTA were submitted to automatic analysis (ABCVet, ABX) to obtain complete blood cell (CBC) and platelet counts. Morphological characteristics of the blood cells and differential leucocyte counts were obtained by blood smear analysis after prior staining by routine methods. Reference values from the Clinical Pathology Laboratory of the Veterinary School were used to analyse the CBC results.
2.4. Antibody ELISA
Total IgGT, IgG1 and IgG2 serum concentrations were detected using a technique modified from that of Voller et al. (1979). The antigen was produced from cultured promastigotes of L. infantum strain MHOM/BR/1967/BH46, previously ruptured by ultrasound (40 ω) and centrifuged at 150 g for 10 min. Individual wells of a 96-well microplate were coated with soluble antigen at a final concentration of 2 μg/mL in 0.05 M carbonate buffer (pH 9.6). A volume of 100 μL per well was left overnight at 4 °C and then washed five times in phosphate buffered saline (PBS) containing 0.2% Tween-20. Antigenic sites were saturated for 30 min at 37 °C with 150 μL PBS containing 0.2% Tween-20 and 2% casein (Sigma; C0376).
The wells were washed again three times with PBS containing 0.2% Tween-20 and 100 μL per well of dog sera diluted 1:400 for detection of IgGT and IgG subclasses. After incubation for 45 min at 37 °C, the plates were washed five times, followed by the addition of 100 μL per well of rabbit anti-dog IgG labelled with peroxidase (Sigma; P6782) diluted 1:2000. Peroxidase-labelled goat anti dog-specific chain immunoglobulins (Bethyl Laboratories; A120p, A121p) were used for IgG1 and IgG2 fraction detection.
Enzyme-labelled antibody was diluted to 1:2000 and 1:1200 for IgG1 and IgG2 assays, respectively. These conjugates were incubated for 45 min at 37 °C and the plates washed five times before 100 μL of a mixture of a 40% solution (w/v) of ortho-phenylenediamine (OPD) in phosphate/citrate buffer (pH 5) and 30 volumes of H2O2 was added to each well. After incubation for 10 min in darkness, the reaction was stopped by the addition of 25 μL of 4N H2SO4 to each well. Absorbance values were read at 492 nm in an automatic ELISA reader (BioRad Model 550). The cut-off point for each plate was determined as being the average absorbance reading plus twice the standard deviation of values for Brazilian dog samples obtained from areas not endemic for L. infantum.
2.5. Sandflies
2.5.1. Source of sandflies
Five-day-old male and female L. longipalpis from a closed laboratory colony were used in the experiments. This colony was initiated from sandflies collected in Teresina, in the North-Eastern Brazilian State of Piauí. The insects used in this study belonged to generations 46-51. The colony was maintained according to the protocol of Modi and Tesh (1983).
2.5.2. Exposure of sandflies to L. infantum infection
Sandflies were fed on dogs by putting the insects in a specially designed receptacle (FleboContainer), consisting of a semi-transparent PVC container 10 cm high and with a diameter of 8.7 cm. The screw lid had a 6 cm diameter nylon mesh window (80 apertures) cut in the centre, secured with silicon cement (Fig. 1 ). A 15 mm hole was made in the wall of the container about 10 cm from the bottom and sealed with a cork. Between 40–45 females and about the same number of males were used in each experimental replicate.
Fig. 1.
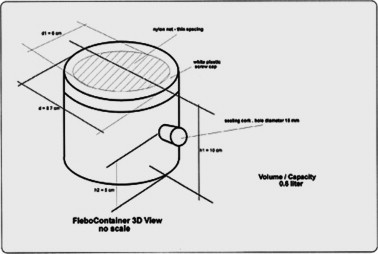
FleboContainer.
The FleboContainer netting was placed in direct contact with the medial skin of the dog’s right ear (Travi et al., 2001). In order to prevent excessive movement, dogs were previously sedated with 0.8 or 1.2 mL/kg of acepromazine (Univet). Sandflies were allowed to feed directly on the ears of infected dogs for 40 min in a darkened room. After feeding, the insects were returned to the insectary for five days. Twenty-four hours after the meal, fed flies were provided with a solution of fructose in distilled water (50:50 v/v) and kept at 28 °C. Survival of the flies was monitored at least twice daily until they could be dissected and examined.
2.5.3. Sandfly dissection
Five days after feeding, live male and female sandflies were counted and the engorged females dissected in a drop of PBS. The head of each engorged female fly was severed with a mounted needle and the gut removed. The guts were examined under interference microscopy (Johnson et al., 1963) and the proportions of infected females and appearance of flagellates within the digestive tract of each sandfly recorded. The approximate numbers of promastigotes and their distribution in different parts of the gut were estimated based on observations using the 40× objective of a microscope.
The intensity of the sandfly infections showed great variability with regard to motility and localisation of promastigotes in the gut. Intensity of infections was classified as “low” when few promastigotes were seen in the sandfly midgut with little or no motility. Intensity was classified as “medium” when many motile promastigotes where seen in the fore- and midgut. In “high” intensity infections, large numbers of promastigotes were seen throughout the gut and rosettes of parasites were visible.
The unruptured guts of sandflies containing promastigotes were transferred to a drop of inactivated bovine fetal serum and dissected to liberate parasites, which were photographed immediately or after staining with Giemsa.
2.6. Statistical analysis
The data were submitted to statistical analysis using the Instat program (GraphPad). Statistical methods were chosen according to the data features. A χ 2 test was performed when data were parametric and a contingency table could be prepared, as in the following comparisons: anaemia vs. category of clinical signs; anaemia vs. sandfly infection rate; IgGT, IgG1 and IgG2 concentrations vs. category of clinical signs; sandfly infection rate vs. category of clinical signs; and IgGT, IgG1 and IgG2 levels vs. sandfly infection rate. Parametric data on neutrophils or lymphocyte numbers vs. category of clinical signs were analysed by ANOVA When data were non-parametric or did not show normality after transformation, the Kruskal–Wallis test was used, as in comparisons between eosinophil and monocyte numbers and category of clinical signs. Correlation between IgG1 and IgG2 levels was analysed by paired-sample t test.
3. Results
3.1. Correlation of clinical categories with haematology results
When the 42 dogs were classified according to their clinical signs, as described by Mancianti et al. (1998), 13 (31%) were symptomatic, 22 (52%) oligosymptomatic and 7 (17%) asymptomatic. The most common clinical sign presented by the dogs was lymphadenopathy, mainly of the superficial cervical lymph nodes. Red blood cell counts revealed a high frequency of anaemia: 20/42 dogs (47%) presented with erythrocyte counts (mean value 5.09 × 106/μL; standard error 1.09 × 106/μL; reference range 5.5–8.5 × 106/μL), haemoglobin concentrations (mean value 11.07 g/dL; standard error 2.59 g/dL; reference range 12–18 g/dL) and haematocrit (mean value 34–43%; standard error: 6.21%; reference range 37–55%) all below reference values.
When these parameters were analysed on the basis of reticulocyte counts, the anaemia was shown to be normocytic, normochromic and non-regenerative. As it was considered to be a more reliable red blood cell index than total erythrocyte count or haemoglobin concentration (Willard et al., 1994), only the haematocrit value was used to obtain correlations between anaemia and the clinical signs of each dog, as well as between anaemia and sandfly infection rates. Dogs with low haematocrit values infected more female sandflies than those presenting with normal values. On the other hand, no correlation was observed between haematocrit values and clinical signs presented by dogs.
Twenty-nine dogs presented with normal leucocyte counts, while nine had leucocytosis (always with degenerative left shift) and four had leucopenia. No correlation was found between leucocyte count and clinical signs in these animals. This observation was also true for each leucocyte category, except for lymphocytes; asymptomatic dogs presented with the highest lymphocyte counts, followed by oligosymptomatic and symptomatic animals (P < 0.05).
Significant correlations were found when the categories were compared (P < 0.05). Although no positive correlation could be elicited between clinical category and eosinophil counts, 5/9 dogs with exfoliative dermatitis had eosinophilia. Only 7/42 dogs had thrombocytopenia, but none of these presented with haemorrhage. One dog had epistaxis but a normal platelet count. No correlation was found between clinical category and platelet counts.
3.2. Correlation of clinical categories with serology results
The cut off point for total IgG ELISA was 0.05 and the absorbance values ranged from 0.069 to 0.559. To permit statistical analysis, absorbance values were approximately divided into three parts of the range values and then categorised as “low” (0.096–0.190), “medium” (0.191–0.380) and “high” (0.381–0.559). When the clinical category was compared with total IgG ELISA absorbance, no significant difference was found between absorbance values for symptomatic and oligosymptomatic dogs (P > 0.05), although asymptomatic dogs showed significantly lower absorbance values than the other two clinical categories (P < 0.05), as can be seen in Fig. 2 . All dogs presented positive results to IgG1 and IgG2 ELISA tests. The values for the second immunoglobulin subclass were significantly higher than those of the first (P < 0.0001).
Fig. 2.
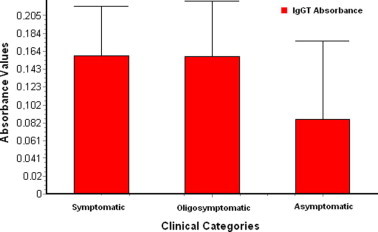
Values of ELISA absorbance for total IgG (IGgT) in each clinical category.
Absorbance values of the IgG subclasses were categorised as IgGT to permit further statistical analysis. Thus for IgG1, in which the cut-off point was 0.05, the optical density (OD) values were categorised as “low” (0.055–0.3), “medium” (0.301–0.6) or “high” (0.601–0.96) based on antibody levels. The cut-off point for IgG2 was 0.084 and values for low, medium and high antibody levels were set at 0.092–0.320, 0.321–0.640 and 0.641–0.961, respectively.
No statistically significant differences were observed when the clinical categories were compared with IgG1 absorbance values. On the other hand, when this comparison was made for OD values of IgG2, significant differences were seen between dogs of different categories. The difference was more pronounced when asymptomatic and oligosymptomatic animals (P < 0.001) were compared than when the former were compared with symptomatic ones (P < 0.05). The variations for each category are shown in Fig. 3 .
Fig. 3.
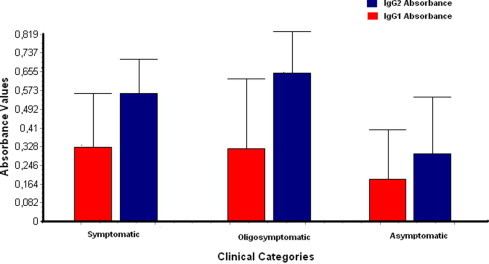
Values of ELISA absorbance for IgG1 and IgG2 for each clinical category.
3.3. Correlation of clinical categories with infectivity for sandflies
Xenodiagnosis was positive in 37/42 (88%) dogs. Interestingly, no female sandflies could be infected from some dogs, while other dogs infected all insects that bit them. When the number of infected sandflies was correlated with the intensity of infection for each dog, a statistically significant association (P < 0.001) was detected; dogs that infected a large number of sandflies did so with high intensity. Low intensity infections were observed in a few dogs.
A positive correlation between the number of positive sandflies and clinical category showed an interesting progression; symptomatic dogs showed higher infection rates (51.9%) than oligosymptomatic (41.9%) and asymptomatic (18.3%) animals. Data were however qualitative and insufficient to permit statistical correlations between the intensity of infection and clinical signs. Only one symptomatic dog infected no sandflies at all; six oligosymptomatic and five asymptomatic dogs infected no sandflies or did so at very low intensities of infection.
Promastigotes in the sandfly gut can be observed in Fig. 4 . A significant positive association was observed between sandfly infection rates and IgGT ELISA absorbance values; the dogs whose sera showed highest absorbance values were those that infected more L. longipalpis females. Once again, data insufficiency after categorization, as well as its qualitative nature, meant that no statistical correlations could be established between the intensity of infection and the IgGT ELISA.
Fig. 4.
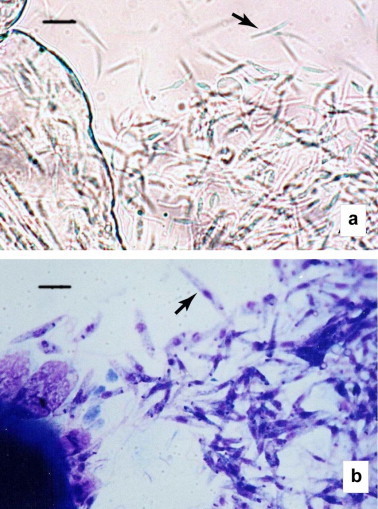
Promastigotes of L. infantum (arrows) from the intestine of L. longipalpis fed on a symptomatic dog. (a) Unstained. (b) Giemsa-stained. Original magnifications 400× (a) and 1000× (b). Bar = 10 μm (a) and 6 μm (b).
When the absorbance values obtained from the ELISA IgG1 and IgG2 subclasses were compared to the sandfly infection rates, it was found that dogs with lower IgG1 values infected more L. longipalpis females while those with higher IgG2 values infected greater numbers of sandflies (P < 0.0001).
4. Discussion
The group of dogs studied was not representative of the general canine VL population in Belo Horizonte. The most common clinical sign in the infected dogs studied was lymphadenopathy. Dogs already known to be seropositive were chosen based on their gross clinical findings and those with severe dermatological signs were rejected. These data thus differ from earlier reports in the literature, where skin lesions were reported to be the most common clinical sign in canine VL (Ciaramella et al., 1997, Ferrer, 2002, Strauss-Ayali and Baneth, 2001, Amusategui et al., 2003, Solano-Gallego et al., 2004).
The results obtained when categories of clinical signs were compared with ELISA absorbance values agree with other work (Cabral et al., 1992, Pinelli et al., 1994, Quinnell et al., 2001, Solano-Gallego et al., 2001, Fernendez-Perez et al., 2003, Mendes et al., 2003, Reis et al., 2006b) and corroborate the view that hypergammaglobulinaemia due to excessive antibody production in canine VL may be responsible for some of the clinical signs presented by dogs through complement activation and immune complex formation. A common feature reported in various studies (Gradoni et al., 1987, Alvar et al., 1994, Molina et al., 1994) is a strong positive correlation between infectivity to sandflies and serological response, although none of these authors used ELISA to determine this correlation.
The finding of IgG subclasses 1 and 2 in dogs with VL is consistent with other reports (Deplazes et al., 1995, Bourdoiseau et al., 1997a, Vercammen et al., 2002, Quinnell et al., 2003). In fact, given that polyclonal B lymphocyte activation occurs in L. infantum infection, the presence of both immunoglobulin subclasses is to be expected. Other authors have also described IgG2 predominance (Deplazes et al., 1995, Vercammen et al., 2002). The lack of association between clinical signs in affected dogs and IgG1 levels has been observed by others, as has a positive correlation with IgG2 levels (Vercammen et al., 2002). When considered together with the observation that parasites could be demonstrated in all dogs (data not shown), it appears that these IgG subclasses may not play a role in the development of clinical signs or control of the disease as in the murine model.
The results obtained from xenodiagnosis disagree with the findings of previous workers (Molina et al., 1994, Guarga et al., 2000) who could not demonstrate significant associations between clinical signs in dogs and sandfly infection rates. These differences may be attributed to the fact that these authors used Phlebotomus perniciosus females in their studies. On the other hand, differences in infection rates when sandflies were fed on oligosymptomatic and symptomatic dogs have been found by others (Travi et al., 2001, Travi et al., 2002), although asymptomatic dogs from these experiments were not able to infect the insects. Although Travi et al., 2001, Travi et al., 2002 employed L. longipalpis in their experiments, the absence of infection in this clinical category can be explained by the fact that a different feeding method was used with sandflies being fed directly on the blood of asymptomatic dogs. This method proved to be less efficient, since the same authors obtained positive results by xenodiagnosis in 2/7 oligosymptomatic and 4/8 symptomatic dogs. The small number of dogs used in those experiments, compared to the study reported here, may also have contributed to the discrepancy between the two sets of results.
If one considers that dogs showing more clinical signs are less able to control the disease so that there is greater parasite dissemination in the skin, the number of amastigotes in the skin could be equivalent to the quantity of promastigotes within the insect gut, although in the gut promastigotes are able to divide. A significant positive correlation between the numbers of infected L. longipalpis females and the intensity of infection has been observed previously (Travi et al., 2001), although the criteria chosen by these authors differed from those we used in the present study.
No published studies linking total IgG antibody levels and sandfly infection rates have been found. It has been demonstrated here that dogs with more clinical signs presented higher levels of total IgG and IgG2 and were able to infect large numbers of female sandflies. From these observations it can be surmised that dogs with higher antibody levels are able to infect more sandflies. This conclusion addresses a crucial epidemiological question, linking these two parameters and supporting the findings of Courtenay et al. (2001) who observed that female sandflies can be infected by dogs only after they show antibodies in their sera; they considered antibody titres to be the best predictor of infectivity.
Forty-five percent of the dogs we studied presented normocytic and normochromic anaemia, as has been observed by other authors (Abranches et al., 1991, Ciaramella et al., 1997, Koutinas et al., 1999, Ikeda et al., 2002, Reis et al., 2006a). The non-regenerative feature of the anaemia can be attributed to infection of the bone marrow by L. infantum, inducing infiltration by lymphocytes, plasma cells and macrophages that could contribute to a decrease in erythrocyte production. The elevated blood urea nitrogen (BUN) levels encountered in most of the animals (data not shown) could also contribute to the anaemia, as this would alter erythropoietin function and reduce erythrocyte life span by its toxic action. More studies are needed to examine further the pathology of anaemia in canine VL.
Other workers have observed that symptomatic dogs showed more severe anaemia (Amusategui et al., 2003, Reis et al., 2006a), although the results of the present study did not confirm these findings. Anaemia would be responsible for some of the classic clinical signs of canine VL, such as apathy, weakness and emaciation, although the clinical presentation is also the sum of disturbances caused by presence of the parasite and inflammatory reactions produced by the immune response. The differences observed can be attributed to the larger number of dogs used in our study.
No published work has been found that examines the relationship between the haematological status of dogs with VL and sandfly infection rates. In order to complete its blood meal on an anaemic animal, a sandfly might have to feed for a longer period or ingest more blood, both being factors that could contribute to higher infection rates.
The great variability in the leucocyte count of dogs with VL was confirmed in this study (Anosa and Idowu, 1983, Moreno et al., 1998, Koutinas et al., 1999, Jüttner et al., 2001, Ikeda et al., 2002, Amusategui et al., 2003). The high number of dogs presenting with normal leucocyte counts, as well as the absence of relationships between clinical category and the counts of total leucocytes (or subcategories of leucocytes) showed that the disease has little influence on this parameter. On the other hand, lymphocyte counts do appear to be influenced by the disease, as lymphocytopenia increased in severity with manifestation of clinical disease. This may be attributed to the immunosuppressive nature of VL (Bourdoiseau et al., 1997b, Reis et al., 2006b).
Even though the number of animals presenting with eosinophilia and dermatological signs was low and statistical analyses could not be performed, a trend towards an association between these two factors was observed, as has been noted previously by others (Amusategui et al., 2003). Moreover, thrombocytopenia was rarely observed among animals used in the present study, as others have also found (Slappendel and Greene, 1990, Alvar et al., 1994, Ciaramella et al., 1997).
5. Conclusion
From the data presented here, it can be concluded that dogs naturally infected with L. infantum may present with a wide range of clinical signs, the severity of which is related to high levels of total IgG and IgG2 antibodies. It was also found that anaemic dogs have a higher infectivity for L. longipalpis sandflies, although anaemia was not related to the severity of clinical signs. Lymphocytes seemed to be the only leucocyte category influenced by the disease.
Acknowledgements
The authors acknowledge Mrs Elza Soares, Mrs Soraia de Oliveira Silva and Mrs Rosângela Fátima Gomes for technical support and Arina Lopes Lima, Andréa Barbosa and Sandra Marinho for helping to care for the dogs. Financial support was provided from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) Brazil – Grant CBB# 39401.
References
- Abranches P., Santos-Gomes G., Rachamim N., Campino L., Schnur L.F., Jaffe C.L. An experimental model for canine visceral leishmaniasis. Parasite Immunology. 1991;13:537–550. doi: 10.1111/j.1365-3024.1991.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Alvar J., Molina R., San-Andrés M., Tesouro M., Nieto J., Vitutia M., Gonzalez F., San-Andres M.D., Boggio J., Rodriguez F., Sainz A., Escacena C. Canine leishmaniasis: clinical and entomological follow-up after chemotherapy. Annals of Tropical Medicine and Parasitology. 1994;88:371–378. doi: 10.1080/00034983.1994.11812879. [DOI] [PubMed] [Google Scholar]
- Amusategui I., Sainz A., Rodriguez F., Tesouro M.A. Distribution and relationships between clinical and biopathological parameters in canine leishmaniasis. European Journal of Epidemiology. 2003;18:147–156. doi: 10.1023/a:1023090929302. [DOI] [PubMed] [Google Scholar]
- Anosa V.O., Idowu A. The clinical–haematological features and pathology of leishmaniasis in a dog in Nigeria. Zentralblatt für Veterinärmedizin Reihe B. 1983;30:600–608. doi: 10.1111/j.1439-0450.1983.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Bourdoiseau G., Bonnefont C., Hoareau E., Boehringer C., Stolle T., Chabanne L. Specific IgG1 and IgG2 antibody and lymphocyte subset in naturally Leishmania infantum infected treated and untreated dogs. Veterinary Immunology and Immunopathology. 1997;59:21–30. doi: 10.1016/s0165-2427(97)00072-x. [DOI] [PubMed] [Google Scholar]
- Bourdoiseau G., Bonnefont C., Magnol J.P., Saint-Andre I., Chabanne L. Lymphocyte subset abnormalities in canine leishmaniasis. Veterinary Immunology and Immunopathology. 1997;56:345–351. doi: 10.1016/s0165-2427(96)05768-6. [DOI] [PubMed] [Google Scholar]
- Cabral M., Grady J.O., Alexander J. Demonstration of Leishmania specific cell mediated and humoral immunity in asymptomatic dogs. Parasite Immunology. 1992;14:531–539. doi: 10.1111/j.1365-3024.1992.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Ciaramella P., Oliva G., De Luna L., Gradoni R., Ambrosio L., Cortese A., Scalone A., Persechino A. A retrospective clinical study of canine leishmaniasis in 150 naturally infected by Leishmania infantum. Veterinary Record. 1997;141:539–543. doi: 10.1136/vr.141.21.539. [DOI] [PubMed] [Google Scholar]
- Courtenay, O, Quinnell, R.J., Dye, C., 2001. Infection and infectiousness in a cohort of sentinel dogs naturally exposed to Leishmania infantum: implications for control. In: Proceedings of the Second World Congress on Leishmaniasis, Hersonissos, Crete, p. 39.
- De Luna R., Ferrante M., Severino L., Ambrosio R., Piantedosi D., Gradoni L., Lucisano A., Persechino A. Decreased lipid fluidity of the erythrocyte membrane in dogs with leishmaniasis associated anaemia. Journal of Comparative Pathology. 2000;122:213–216. doi: 10.1053/jcpa.1999.0357. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Smith N.C., Arnold P., Lutz H., Eckert J. Specific IgG1 and IgG2 antibody responses of dogs to Leishmania infantum and other parasites. Parasite Immunology. 1995;17:451–458. doi: 10.1111/j.1365-3024.1995.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Fernendez-Perez F.J., Gómez-Munoz M.T., Mendez S., Alunda J.M. Leishmania-specific lymphoproliferative responses and IgG1/IgG2 immunodetection patterns by Western blot in asymptomatic, symptomatic and treated dogs. Acta Tropica. 2003;86:83–91. doi: 10.1016/s0001-706x(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Ferrer, L., 1999. Clinical aspects of canine leishmaniasis. In: Canine Leishmaniasis: An Update. Proceedings of the International Canine Leishmaniasis Forum, Barcelona, Spain, pp. 6–10.
- Ferrer, L., 2002. The pathology of canine leishmaniasis. In: Canine Leishmaniasis: Moving Towards a Solution. Proceedings of the Second International Canine Leishmaniasis Forum, Sevilla, Spain, pp. 21–24.
- Gradoni, L., 2002. The diagnosis of canine leishmaniasis. In: Canine Leishmaniasis: Moving Towards a Solution. Proceedings of the Second International Canine Leishmaniasis Forum, Sevilla, Spain, pp. 7–14.
- Gradoni L., Maroli M., Gramiccia M., Mancianti F. Leishmania infantum rates in Phlebotomus perniciosus fed on naturally infected dogs under antimonial treatment. Medical and Veterinary Entomology. 1987;1:339–342. doi: 10.1111/j.1365-2915.1987.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Guarga J.L., Lucientes J., Peribanez M.A., Molina R., Gracia M.J., Castillo A. Experimental infection of Phlebotomus perniciosus and determination of the natural infection rates of Leishmania infantum in dogs. Acta Tropica. 2000;77:203–207. doi: 10.1016/s0001-706x(00)00141-8. [DOI] [PubMed] [Google Scholar]
- Ikeda F.A., Ciarlini P.C., Feitosa M.M., Gonçalves M.E., Luvizotto M.C.R., Lima V.M.F. Perfil hematolólogico de cães infectados por Leishmania chagasi no município de Araçatuba-SP: estudo restrospectivo de 191 casos. Clïnica Veterinária. 2002;47:42–48. [Google Scholar]
- Johnson P.T., McConnell E., Hertig M. Natural infection of leptomonad flagellates in Panamanian Phlebotomus sandflies. Experimental Parasitology. 1963;14:107–122. doi: 10.1016/0014-4894(63)90015-8. [DOI] [PubMed] [Google Scholar]
- Jüttner C., Rodriguez Sanchez M., Rollan Landeras E., Slappendel R.J., Arnold F.C. Evaluation of the potential causes of the epistaxis in dogs with natural visceral leishmaniasis. Veterinary Record. 2001;149:176–179. doi: 10.1136/vr.149.6.176. [DOI] [PubMed] [Google Scholar]
- Koutinas A.F., Polizopoulou Z.S., Saridomichelakis M.N., Argyriadis D., Fytianou A., Plevraki K. Journal of the American Animal Hospital Association. 1999;35:376–383. doi: 10.5326/15473317-35-5-376. [DOI] [PubMed] [Google Scholar]
- Lutz A., Neiva A. Contribuição para o conhecimento das espécies do gênero Phlebotomus existentes no Brasil. Memórias do Instituto Oswaldo Cruz Mem Inst Oswaldo Cruz. 1912;4:84–95. [Google Scholar]
- Mancianti F., Gramiccia M., Gradoni L., Pieri S. Studies on canine leishmaniasis control: 1. Evolution of different clinical forms of canine leishmaniasis following antimonial treatment. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;82:566–567. doi: 10.1016/0035-9203(88)90510-x. [DOI] [PubMed] [Google Scholar]
- Mendes C.O., Souza E.P., Borja-Cabrera G.P., Batista L.M.M., Santos M.A., Parra L.E., Menz I., de Souza C.B.P. IgG1/IgG2 dichotomy in sera of vaccinated or naturally infected dogs with visceral leishmaniasis. Vaccine. 2003;21:2589–2597. doi: 10.1016/s0264-410x(03)00046-x. [DOI] [PubMed] [Google Scholar]
- Modi G.B., Tesh R.B. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. Journal of Medical Entomology. 1983;5:568–569. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- Molina R., Amela C., Nieto J., San-Andres M., Gonzalez F., Castillo J.A., Lucientes J., Alvar J. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88:491–493. doi: 10.1016/0035-9203(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Moreno J., Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends in Parasitology. 2002;18:399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- Moreno P., Lucena R., Ginel P.J. Evaluation of primary haemostasis in canine leishmaniasis. Veterinary Record. 1998;142:81–83. doi: 10.1136/vr.142.4.81. [DOI] [PubMed] [Google Scholar]
- Pinelli E., Killick-Kendrick R., Wagenaar J., Bernardina W., del Ral G., Ruitenberg J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infection and Immunity. 1994;62:229–235. doi: 10.1128/iai.62.1.229-235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnelli, E., Rutten, V.P.M.G, Ruitenberg, E.J., 1999. Cellular immune responses in canine leishmaniasis. In: Canine Leishmaniasis: An Update. Proceedings of the International Canine Leishmaniasis Forum, Barcelona, Spain, pp. 60–64.
- Quinnell R.J., Couternay O., Shaw M.A., Day M., Garcez L.M., Dye C., Kaye P.M. Tissue cytokine responses in canine visceral leishmaniasis. Journal of Infectious Diseases. 2001;183:1421–1424. doi: 10.1086/319869. [DOI] [PubMed] [Google Scholar]
- Quinnell R.J., Courtenay O., Garcez L.M., Kaye P.M., Shaw M.A., Dye C., Day M.J. IgG subclass responses in a longitudinal study of canine visceral leishmaniasis. Veterinary Immunology and Immunopathology. 2003;91:161–168. doi: 10.1016/s0165-2427(02)00311-2. [DOI] [PubMed] [Google Scholar]
- Reis A.B., Martins-Filho O.A., Teixeira-Carvalho A., Carvalho M.G., Mayrink W., França-Silva J.C., Giunchetti R.C., Genaro O., Corrêa-Oliveira R. Parasite density and impaired biochemical/hematological status are associated with severe clinical aspects of canine visceral leishmaniasis. Research in Veterinary Science. 2006;81:68–75. doi: 10.1016/j.rvsc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Reis A.B., Teixeira-Carvalho A., Vale A.M., Marques M.J., Giunchetti R.C., Mayrink W., Guerra L.L., Andrade A.R., Corrêa-Oliveira R., Martins-Filho O.A. Isotype patterns of immunoglobulins: hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Veterinary Immunology and Immunopathology. 2006;112:102–116. doi: 10.1016/j.vetimm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Slappendel R.J., Greene C.E. Leishmaniasis. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. Second ed. W.B. Saunders; St Louis: 1990. pp. 769–777. [Google Scholar]
- Solano-Gallego L., Riera C., Roura X., Iniesta L., Gallego M., Valladares J.E., Fisa R., Castillejo S., Alberola J., Ferrer L., Arboix M., Portus M. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Veterinary Parasitology. 2001;96:265–276. doi: 10.1016/s0304-4017(00)00446-5. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L., Fernandez-Bellon H., Morell P., Fondevila D., Alberola J., Ramis A., Ferrer L. Histological and immunohistochemical study of clinically normal skin of Leishmania infantum-infected dogs. Journal of Comparative Pathology. 2004;130:7–12. doi: 10.1016/s0021-9975(03)00063-x. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali, D., Baneth, G., 2001. Canine visceral leishmaniasis. In: Carmichael, L. (Ed.), Recent Advances in Canine Infectious Diseases. International Veterinary Information Service, Ithaca. http://www.ivis.org.
- Travi B.L., Tabares C.J., Cadena H., Ferro C., Osorio Y. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitological status and infectivity for sandflies. American Journal of Tropical Medicine and Hygiene. 2001;64:119–124. doi: 10.4269/ajtmh.2001.64.119. [DOI] [PubMed] [Google Scholar]
- Travi B.L., Ferro C., Cadena H., Montoya-Lerma J., Adler G.H. Canine visceral leishmaniasis: dog infectivity to sandflies from non-endemic areas. Research in Veterinary Science. 2002;72:83–86. doi: 10.1053/rvsc.2001.0527. [DOI] [PubMed] [Google Scholar]
- Vercammen F., Fernandez-Perez F.J., del Amo C., Alunda J.M. Follow up of Leishmania infantum naturally infected dogs treated with allopurinol: immunofluorescence antibody test, ELISA and Western blot. Acta Tropica. 2002;84:175–181. doi: 10.1016/s0001-706x(02)00178-x. [DOI] [PubMed] [Google Scholar]
- Voller, A., Bidwell, D. Bartlett, A., 1979. The enzyme linked immunosorbent assay (ELISA): A guide with abstracts of microplate applications, Dynatech Europe, p. 124.
- Willard M.D., Tvedten H., Turnwald G.H. Second ed. W.B. Saunders; St Louis: 1994. Small Animal Diagnosis by Laboratory Methods. p. 377. [Google Scholar]


