Abstract
Viral diseases of rabbits have been used historically to study oncogenesis (e.g. rabbit fibroma virus, cottontail rabbit papillomavirus) and biologically to control feral rabbit populations (e.g. myxoma virus). However, clinicians seeing pet rabbits in North America infrequently encounter viral diseases although myxomatosis may be seen occasionally. The situation is different in Europe and Australia, where myxomatosis and rabbit hemorrhagic disease are endemic. Advances in epidemiology and virology have led to detection of other lapine viruses that are now recognized as agents of emerging infectious diseases. Rabbit caliciviruses, related to rabbit hemorrhagic disease, are generally avirulent, but lethal variants are being identified in Europe and North America. Enteric viruses including lapine rotavirus, rabbit enteric coronavirus and rabbit astrovirus are being acknowledged as contributors to the multifactorial enteritis complex of juvenile rabbits. Three avirulent leporid herpesviruses are found in domestic rabbits. A fourth highly pathogenic virus designated leporid herpesvirus 4 has been described in Canada and Alaska. This review considers viruses affecting rabbits by their clinical significance. Viruses of major and minor clinical significance are described, and viruses of laboratory significance are mentioned.
Keywords: Borna disease virus, Caliciviridae infections, Coronavirus infections, Herpesviridae infections, Papillomavirus infections, Poxviridae infections, Rabbits, Rabies virus, Rotavirus infections
Key points
-
•
Rabbit hemorrhagic disease is caused by a calicivirus and is characterized by fulminant hepatitis with a fatality rate of more than 90% in adult rabbits. It is not present in the United States. It is spread by direct contact between rabbits, by flies feeding on carcasses, and experimentally by fleas and mosquitoes. A single-dose vaccination of inactivated virus at 8 to 12 weeks followed by an annual booster is generally protective.
-
•
Myxomatosis is a lethal, generalized viral disease of rabbits. It is endemic in wild rabbit populations and in Sylvilagus spp. It commonly occurs in the Pacific states of the United States in warmer months. The virus is spread passively on the mouthparts of mosquitoes and fleas. Outbreaks in farmed, laboratory, and pet rabbits result from spillover in wild rabbit populations. A single-dose vaccination of inactivated virus at 8 to 12 weeks followed by an annual booster is generally protective.
-
•
Rabbit fibroma virus is endemic in Sylvilagus spp in North America. It is spread by mosquitoes and causes a cutaneous fibroma at the inoculation site with no systemic signs of disease. The fibroma generally regresses within 3 to 4 weeks of infection. It is one of the most common causes of cutaneous neoplasms in pet rabbits.
-
•
Cottontail rabbit papillomavirus, also known as Shope papillomavirus, is a cause of papillomas (warts) on nonhaired or thinly haired skin of rabbits. In 66% to 80% of infected rabbits, papillomas develop into carcinomas 8 to 14 months later. The virus is spread from Sylvilagus spp to rabbits by mosquitoes. Infection is uncommon.
-
•
Rabbit oral papillomavirus occurs naturally in rabbits and is widespread among domestic rabbits in Europe and the Americas, particularly young animals. The papillomas are localized mostly on the underside of the tongue and usually regress spontaneously within a few weeks to a few months.
-
•
The multifactorial enteritis complex of juvenile rabbits can be caused by bacteria, viruses, and parasites. Several different viruses have been isolated from rabbits with diarrhea, such as rotavirus, coronavirus, and astrovirus. Whether natural outbreaks of enteritis can be caused by these viral agents alone or in conjunction with other pathogens (eg, Clostridia spp, Escherichia coli, and coccidia) is not clear.
Introduction
Viral diseases in rabbits are infrequently encountered by clinicians seeing pet rabbits in North America. Occasionally myxomatosis may be seen. The situation is different in Europe and Australia where myxomatosis and rabbit hemorrhagic disease, the major viral diseases affecting European rabbit (Oryctolagus cuniculus) populations are endemic. This review considers viruses affecting rabbits by their clinical significance. Viruses of major and minor clinical significance are described, and viruses of laboratory significance are mentioned.
Viral infections of major clinical significance
Rabbit Hemorrhagic Disease Virus: Rabbit Hemorrhagic Disease
Introduction
Rabbit hemorrhagic disease (RHD) was first described in China in 1984 in a shipment of Angora rabbits (Oryctolagus cuniculus) from East Germany. The disease is characterized by fulminant hepatitis with a case fatality rate of more than 90% in adult rabbits. RHD is caused by a calicivirus termed Rabbit Hemorrhagic Disease Virus (RHDV) (Family: Caliciviridae; Genus: lagovirus), which since 1985 has spread to or emerged in Europe in wild and domestic rabbits, and in domestic rabbits in Asia, the Middle East, North Africa, and the Americas. RHDV was deliberately released in Australia and New Zealand as a biologic control for European rabbits and is now established in the wild in these countries (Table 1 ).1
Table 1.
Viral infections of rabbits
| Disease Caused | |
|---|---|
| • Viruses of Major Clinical Significance | |
| Rabbit hemorrhagic disease virus | Rabbit hemorrhagic disease |
| Myxoma virus | Myxomatosis |
| • Viruses of Minor Clinical Significance | |
| Rabbit fibroma virus (Shope fibroma virus) | Rabbit fibromatosis |
| Lapine rotavirus | Rotaviral infection (Rotaviral diarrhea) |
| Cottontail rabbit papillomavirus (Shope papillomavirus) | Rabbit papillomatosis |
| Rabbit oral papillomavirus | Oral papillomatosis |
| Herpesvirus: Herpes simplex | Herpes encephalitis |
| Herpesvirus: Leporid-4 | Systemic herpesvirus infection |
| • Viruses of Laboratory Significance | |
| Astrovirus | Enteric disease (?) |
| Bornavirus | Borna disease |
| Rabies virus | Rabies |
| Vaccinia virus | Rabbitpox (Rabbit plague) |
| Pleural effusion disease virus (infectious cardiomyopathy virus) | Pleural effusion disease and cardiomyopathy |
| Rabbit enteric coronavirus | Coronaviral enteritis |
The United States has experienced 4 sporadic incursions of RHDV, the first of which occurred in Iowa in 2000.2 In 2001, an outbreak of RHD was reported in Utah and was traced to a shipment and subsequent outbreak in Illinois.3 A second isolated outbreak occurred in 2001 in New York and is suspected to have resulted from the importation of rabbit meat from China.4 The most recent outbreak of RHD was in 2005 and occurred in Indiana.5 Each outbreak was contained, and was the result of a separate but indeterminable introduction of RHDV rather than from a single virus lineage.4
Caliciviruses closely related to RHDV, but generally avirulent or of low virulence, have been identified in domestic and wild rabbits in Europe6, 7 and Australia8, 9, 10 and domestic rabbits in the United States (Michigan rabbit calicivirus)11 and their presence inferred by serology in rabbit populations on various islands12, 13 and New Zealand.14 These viruses have been termed rabbit caliciviruses (RCVs) to distinguish them from the lethal RHDV. Viral monitoring indicates that more virulent variants of RCVs, which are genetically distinct from RHDV, are emerging in Europe.15, 16 A related lagovirus, European Brown Hare Syndrome virus (EBHSV), emerged in Europe around the same time as RHDV but does not cause disease in European rabbits. EBHSV may not be as species-specific as RHDV.1
Epidemiology
RHDV is specific for European rabbits (Oryctolagus cuniculus). Probably all ages are susceptible to infection but young rabbits less than 4 weeks of age rarely develop lethal disease. This age-based protection is lost between 4 and 12 weeks of age.17 There is no gender predisposition. Young rabbits may be more susceptible to an emergent virulent variant of RCV.15
RHDV is infectious by virtually all routes of administration but oronasal infection is probably the most common natural route of infection; conjunctival inoculation by flies may also be important.
Infected rabbits shed RHDV in all secretions. The virus is robust and persists for prolonged periods particularly in rabbit carcasses or on fomites.18, 19 RHDV is spread by direct contact between rabbits or indirectly between susceptible rabbits and carcasses or contaminated environments. It is spread by flies feeding on carcasses, which contain very high concentrations of virus particularly in the liver, and experimentally can be spread mechanically by fleas and mosquitoes.20, 21 Because of the extreme concentrations of virus in carcasses, predator or scavenger species of birds and mammals may also aid spread in the wild, as virus can pass both unchanged and infectious through the gut, and be shed in the feces.
Strong seasonality may be present in wild populations, where most adults have survived infection and are subsequently immune. During the breeding season, as young rabbits gradually lose protection provided by maternal antibody, they provide a susceptible population for RHDV to spread. Epidemics in wild rabbits provide the potential for virus to spread into farmed or domestic rabbits via insects, fomites, or direct contact.
Epidemiology of RHDV, both in wild and farmed rabbits, may be strongly influenced by the circulation of RCV strains, which in some circumstances can provide cross-protection to RHDV.6, 8, 22, 23, 24, 25, 26
Clinical presentation
Peracute disease
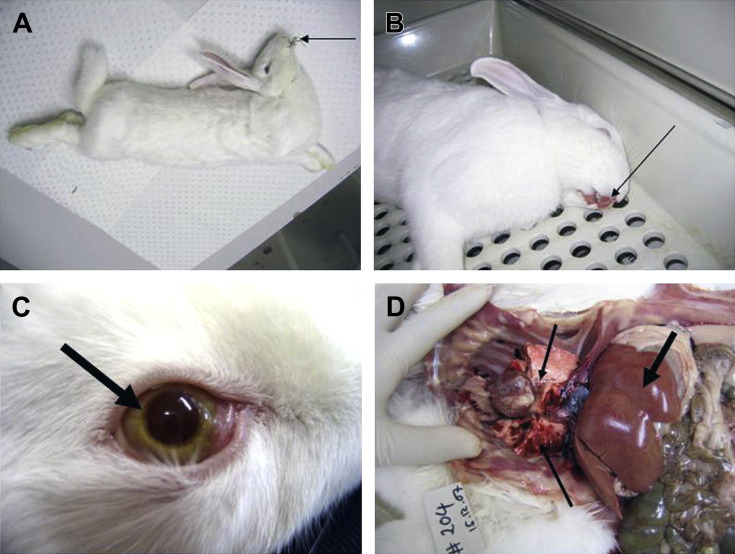
There is sudden death with no premonitory signs; rabbits may be observed grazing normally immediately before death (Fig. 1 A). Death can occur within 30 to 36 hours of oral infection and as early as 24 hours after injection of virus.
Fig. 1.
Rabbits with rabbit hemorrhagic disease. (A) Rabbit that died of RHD during a convulsion. Notice how food is still in the mouth (arrow). (B) Blood from nose and mouth (arrow) of a rabbit that died of acute RHD. (C) Icteric iris (arrow) in albino rabbit with chronic RHD. (D) Necropsy of a rabbit that died of RHD. Notice the pale liver (thick arrow) and hemorrhages in the lungs (thin arrows).
(Courtesy of Tanja Strive, PhD, CSIRO Entomology, Black Mountain, Australia.)
Acute disease
Rabbits appear quiet and reluctant to move; they may have an elevated temperature and raised heart and respiratory rates for 24 hours before death. Death usually occurs within 48 to 72 hours after infection.
Subacute disease
In subacute disease, there are signs of liver failure, including icterus (see Fig. 1C); death occurs over days to several weeks.
Subclinical disease
A small proportion of infected adult rabbits clears the virus and seroconverts with few or no clinical signs of disease. Young rabbits less than 4 to 5 weeks of age rarely develop disease following infection, although they shed virus, may have a raised temperature, and deaths do occur.
History
There is sudden death in adult or subadult rabbits, possibly preceded by 24 hours of quietness/depression with elevated respiratory rate and temperature. Lateral recumbency, coma, and convulsions may be observed before death. Blood from the nose or hematuria may be present (see Fig. 1B). In rabbitries, an epidemic with extreme mortality rates in adult and subadult rabbits but not in very young rabbits is typical.
Clinical examination
Depression, elevated temperature (up to 42°C), raised respiratory rate, convulsions, ataxia, posterior paresis, and central nervous system depression may occur. Hematuria, bloody diarrhea, and blood from the nares or mouth may be present (see Fig. 1B). Pronounced jaundice may be seen in rabbits that have survived more than a few days after infection.
Pathophysiology
RHDV replicates to high titers in the liver, inducing acute hepatic necrosis and fulminant liver failure (see Fig. 1D); disseminated intravascular coagulation, hepatic encephalopathy and nephrosis may occur because of the acute hepatic necrosis.27, 28
Diagnosis
Initial database
Leukopenia (lymphopenia and neutropenia) with moderate reduction in thrombocytes but normal erythrocyte numbers29; elevated liver enzymes: serum alanine amino transferase (ALT), aspartate amino transferase (AST), lactate dehydrogenase (LDH); alkaline phosphatase (AP) and γ-glutamyltransferase (GGT) are dramatically elevated by 36 to 48 hours after infection; increased serum bilirubin; hypoglycemia; hyperlipidemia; significantly elevated blood urea nitrogen (BUN) and creatinine; increased prothrombin time and decreased factor V and factor VII are all present by 36 to 48 hours after infection.28, 29, 30, 31, 32
Advanced/confirmatory testing
Necropsy
Pale, swollen liver with pronounced lobular pattern and possible focal hemorrhages may be the only major necropsy finding (see Fig. 1D). Additional findings include very enlarged, dark spleen; dark-colored kidneys; congested or hemorrhagic lungs with fluid/froth in the trachea (see Fig. 1D); hyperemic tracheal mucosa; and ecchymotic and petechial hemorrhages in intestinal and bladder walls and in subcutaneous tissues.
Histopathology
Histopathology includes coagulative necrosis of hepatocytes at the periphery of the lobule; thrombi in renal and pulmonary blood vessels (disseminated intravascular coagulation); nephrosis; and lymphocyte depletion from spleen. Subacute cases may show signs of liver regeneration with connective tissue and bile duct proliferation and large, pale-staining binucleate hepatocytes.27, 33
Virology
RHDV cannot be grown in tissue culture. Reverse transcriptase polymerase chain reaction (RT-PCR) is the most common diagnostic tool. Viral RNA can be detected in most tissues, including blood, but the highest concentration is in the liver. Low levels of RHDV RNA can persist for prolonged periods in rabbits that survive infection34, 35, 36 and, depending on the specificity of the assay, nonpathogenic RCV strains may cross-react, so care is needed in interpretation of RT-PCR results in the absence of liver pathology. Hemagglutination of human red blood cells is used as a diagnostic test for RHDV, but not all strains hemagglutinate, and the assay is much less sensitive than RT-PCR; capture enzyme-linked immunosorbent assay (ELISA) using clarified liver homogenates can also be used to demonstrate virus but again is much less sensitive than RT-PCR. Immunostaining of liver sections or impression smears using specific monoclonal antibodies can be used to detect RHDV antigen.37 Electron microscopy on liver homogenates can demonstrate calicivirus particles in negative stained preparations.37
Serology
Infected juvenile rabbits or surviving adults develop very high titers of antibody to RHDV capsid protein. Indirect and competition ELISAs are used to demonstrate specific serum antibody to RHDV and isotype ELISAs can be used to demonstrate IgG, IgM and IgA.37 Hemagglutination inhibition tests can also be used but have largely been replaced by ELISA because of the increased sensitivity and convenience. Serologic cross-reaction with avirulent RCVs occurs, so care is needed when interpreting serologic results.38 Competition ELISA is more specific than indirect or isotype ELISAs.
Treatment
There is no specific treatment; supportive care only. Experimentally, treatment with melatonin (starting at the time of infection) or cardiotrophin (starting at 12 hours post infection) reduced liver damage, and cardiotrophin also increased survival rates.39, 40, 41, 42 Vaccination in the face of an outbreak is used in rabbitries to bring the disease under control, as vaccinated animals quickly develop protective immunity. Infected rabbits should be isolated to prevent transmission.
Prognosis and outcome
Prognosis is extremely guarded. Most clinically affected rabbits will die; subacutely infected animals may survive depending on the degree of liver damage and damage to other tissues; chronic liver disease including cirrhosis may result.
Prevention
Vaccination using an adjuvanted, inactivated whole virus vaccine is generally protective. A single dose at 8 to 12 weeks is followed by an annual booster. The vaccine manufacturer’s recommendations should be followed. It is not clear if maternal antibody interferes with earlier vaccination but if this is necessary, it may be advisable to give a booster at 10 to 12 weeks of age. Antigenic variants of RHDV/RCV that overcome vaccination have been reported.15, 16, 43
A combined inactivated RHDV vaccine plus a live myxoma virus vaccine in a single-dose formulation is available (Dercunimix, Merial, Lyons, France). A recombinant Myxoma virus vaccine expressing the RHDV capsid protein has recently been released (Nobivac Myxo-RHD, MSD-Animal Health, Hoddesdon, Hertfordshire, UK) as a combined live vaccine to provide protection against myxomatosis and RHDV.44 A single dose is recommended in rabbits older than 5 weeks of age.
Incoming rabbits should always be quarantined to prevent introduction of disease.
Myxoma Virus: Myxomatosis
Introduction
Myxoma virus (MYXV) (Subfamily: Chordopoxvirinae; Genus: leporipoxvirus) causes the lethal, generalized disease myxomatosis in domestic and wild European rabbits (Oryctolagus cuniculus). The virus naturally circulated in tapeti (Sylvilagus brasiliensis) in South America; however, following deliberate introductions into Australia and Europe as a biologic control for wild European rabbits, MYXV now is endemic in wild European rabbit populations and can spill over into farmed, laboratory, and pet rabbits. MYXV has also been successfully introduced into Chile and Argentina to control feral European rabbits. A closely related virus, often called Californian myxoma virus, is found in Sylvilagus bachmani (brush rabbit) in the Pacific states of the United States and the Baja peninsula of Mexico (Table 2 ). This virus is also highly lethal in European rabbits.45
Table 2.
Lagomorph viruses: transmission between Oryctolagus and Sylvilagus and severity of induced disease
| Virus |
Oryctolagus European Rabbit |
Sylvilagus American Cottontail |
|
|---|---|---|---|
| • Poxviridae | |||
| Myxoma virus | Myxoma | Fibromaa | |
| Rabbit (Shope) fibroma virus | Fibroma, mild | Fibroma, severea | |
| • Papillomaviridae | |||
| Cottontail rabbit (Shope) papilloma virus | Papilloma, SQC 75% | Papilloma, SQC 25%a | |
| Oral papilloma virus | Oral papillomaa | Oral papilloma | |
Abbreviation: SQC, squamous cell carcinoma.
Natural host.
Epidemiology
Myxomatosis is essentially a disease of European rabbits, although European hares may rarely develop generalized disease.46 There is no age or sex predilection but very young rabbits may die without obvious myxomatosis. Passively transmitted maternal antibody may modify the disease outcome in young rabbits born to immune does.47
The virus is spread passively on the mouthparts of biting arthropods, predominantly mosquitoes and fleas (but also Culicoides midges and lice), that probe through the virus-rich cutaneous lesions when seeking a blood meal. The virus does not replicate in the vector.48
Contact spread from rabbit to rabbit may also occur from virus shed in ocular and nasal secretions or from the surface of eroded skin lesions46; virus is also potentially present in semen and genital secretions. Oral infection is very inefficient so conjunctival or nasal inoculation is probably necessary for transmission. Aerosol transmission is inefficient but virus is readily transmitted on fomites, such as water bottles or feeders, or by handlers.
In Europe and Australia, wild European rabbits act as reservoirs for MYXV and mosquitoes can transmit the virus to domestic rabbits. Where there is close proximity between wild and domestic rabbits, fleas and direct contact may also transmit virus.
In South America, vector transmission from S brasiliensis to farmed, laboratory, or pet rabbits occurs.46 Similar spillover occurs from S bachmani in the Pacific states of the United States and Mexico.49, 50, 51, 52, 53
Seasonality is driven by the availability of vectors and the epidemiology of the disease in wild rabbits. In rabbitries, introduction of rabbits carrying the disease (including vaccinated rabbits) has led to epidemics.54 Introduced semen may also pose a risk.55
Clinical presentation
Disease forms
Peracute myxomatosis
Peracute myxomatosis may present as sudden deaths with only mild or no clinical signs of myxomatosis, particularly with Californian MYXV infections. Neurologic signs, such as convulsions, have been described.46 In Australia, some field strains cause acute pulmonary edema in domestic rabbits. Death typically occurs 7 to 15 days after infection.
Acute myxomatosis
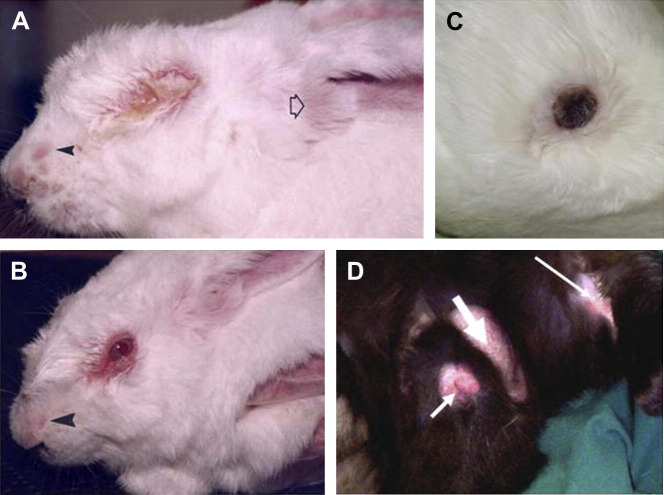
Acute myxomatosis is the classic mucocutaneous form of myxomatosis with multiple skin lesions (Fig. 2 A); a second amyxomatous form with fewer skin lesions has been described in Europe.55, 56, 57, 58 Australian field isolates may also present as essentially amyxomatous in domestic rabbits. Case fatality rates are nearly 100%, but in some individuals, the disease course may be prolonged. Death typically occurs 10 to 15 days after infection.
Fig. 2.
Clinical appearance of rabbits with myxomatosis. (A) Acute myxomatosis caused by virulent myxoma virus. Skin lesions are present on the nose (solid arrowhead). The base of the ear is swollen (open arrowhead) and the eyes are closed with mucopurulent conjunctivitis. (B) Rabbit infected with attenuated strain of myxoma virus. The eyelid margins are red and swollen and serous discharge is present. Swelling is noted around the face and nose (arrowhead). (C) Scabbed skin lesion (approximately 2.5-cm diameter) at primary infection site. (D) Swollen testis (broad arrow); swollen genital opening (short arrow); secondary skin lesions (narrow arrow).
([A, B] From Best SM, Kerr PJ. Coevolution of host and virus: the pathogenesis of virulent and attenuated strains of myxoma virus in resistant and susceptible European rabbits. Virology 2000;267(1):36–48; with permission.)
Subacute myxomatosis
Natural selection in European rabbits in Australia and Europe has seen the emergence of attenuated field strains of MYXV.59, 60 These viruses cause typical myxomatosis but with a protracted course (see Fig. 2B); case fatality rates may be less than 50% to more than 95%, with deaths occurring from 10 to 30 plus days after infection. Survivors may be left with chronic respiratory disease.
History and physical examination
Very early cases present with slight eyelid swelling, conjunctivitis, and thickened ears (see Fig. 2A); temperatures may be elevated above 40°C but this is variable. Depending on the virulence of the virus, this may be 5 to 10 days after infection. Careful examination may reveal a 1-cm to 2-cm diameter, raised, reddened primary cutaneous lesion, but the size and thickness of this varies enormously with the strain of virus (see Fig. 2C).
Later in the disease course, rabbits present with swollen eyelids and mucopurulent blepharoconjunctivitis in which the eyes may be partially or completely closed by the eyelid swelling (there is no involvement of the eye itself). The face is swollen and the ears swollen and drooping or just thickened, particularly around the base (see Fig. 2A).
Mucopurulent nasal discharge is common and the nasal passages may be occluded causing a gasping, stertorous respiration with extension of the head and neck.
Severe swelling of the anogenital region is typical and in males, orchitis and epididymitis together with gross swelling of the scrotum occur (see Fig. 2D).61
In the classic mucocutaneous form of myxomatosis, cutaneous lesions ranging from a few millimeters to 5 to 6 cm in diameter and from 1 to 20 mm high occur over the eyelids, face, ears, and scrotum and late in the disease can be palpated over the body, legs, and feet. In late infections, or in recovering rabbits, the surface of the cutaneous lesions may be hemorrhagic, black, or scabbing (see Fig. 2C).
Popliteal and prescapular lymph nodes are grossly enlarged and readily palpable.
Temperatures may be as high as 41°C but often drop below normal before death.
Rabbits may continue to eat and drink until quite late in the disease course but severe weight loss is common and dehydration may occur.
Pathophysiology
Virus inoculated into the dermis/epidermis by biting arthropods replicates locally in macrophages/dendritic cells and then in epidermal cells.62 Proliferation of the epidermal cells and mucoid swelling of the underlying dermis forms the raised primary lesion that is sometimes referred to as a myxoma or tumor. From the primary site, MYXV is transported to the draining lymph node where it replicates in T cells, macrophages, and other cells, causing almost complete loss of T cells. From the draining lymph node, it disseminates in lymphocytes and possibly macrophage/monocytes to distal tissues, such as lungs, spleen, testes, skin, and mucocutaneous sites, such as eyelids. Virus replication at distal skin sites causes the secondary cutaneous lesions found over the body.62, 63, 64
The cause of death in acute classic myxomatosis is obscure; major organs are typically not severely damaged.65 MYXV profoundly suppresses innate and adaptive immune responses, although low titers of IgM and IgG and even neutralizing antibody can be detected before death.45, 64, 66, 67, 68, 69, 70 Secondary bacterial infections of the conjunctivae, upper respiratory tract, and lungs are typical in rabbits that survive longer than 10 to 14 days after infection and may be the major cause of death in rabbits infected with subacute strains of MYXV. Rabbits free of Pasteurella multocida and Bordetella bronchiseptica appear to have fewer complications before death, but even these may have lobar pneumonia at necropsy.
Diagnosis
Clinical signs of classic myxomatosis are fairly clear-cut, although bacterial upper respiratory tract infections can cause confusion and misdiagnosis.55 Hematology is generally unremarkable: neutrophilia, lymphopenia, and lymphocytosis can all be seen at different stages depending on the virulence of the virus.
Necropsy findings are largely limited to the external lesions and features already described, and depend on the time of death and the underlying virulence of the virus. The cutaneous lesions are clearly separated from the underlying tissue and have a glistening, mucoid appearance on the cut surface. Lymph nodes are grossly enlarged, sometimes hemorrhagic, and may have a watery consistency on section. The spleen is generally 2 to 3 times normal size and often dark-colored. In males, scrotal edema of 0.5 to 1.0 cm may be obvious on incising the skin. In peracute cases, pulmonary edema may be the cause of death with the trachea and bronchi full of froth and fluid, and the lungs dripping fluid. Hemorrhages on serosal membranes and intestinal and stomach walls may be present in some cases.
Confirmatory testing
Histopathology of cutaneous lesions is characterized by cellular proliferation and cell death.71 In the epidermis, the epidermal cells proliferate, enlarge, and undergo ballooning degeneration; the underlying dermis is edematous with complete disruption of the connective tissue architecture; the endothelium of the small blood vessels is disrupted by large stellate or polygonal “myxoma cells.” These cells appear to migrate from the blood vessels into the dermis where they are often surrounded by polymorphs. In infections with virulent virus, there may be an influx of polymorphs into the dermis, particularly at the base but lymphocytes are rarely present.62 In lymphoid tissues, complete loss of lymphocytes from both B-cell and T-cell zones can occur together with proliferation of reticular cells that obliterate the sinuses; disruption of the small blood vessels by myxoma cells is similar to that in the epidermis and polymorphs may be prominent. The presence of virus can be confirmed by immunostaining of epidermal sections from the nodular lesions or eyelids. Negative-stained electron microscopy can be used to identify virus eluted from skin sections.72
PCR, using specific oligonucleotide primers for MYXV, can be done on DNA extracted from biopsies, conjunctival swabs, or tissues collected at necropsy. Virus titers in blood are generally low but the white cell fraction could be used for PCR in early/acute cases. A 1-mm dermal punch biopsy from a lesion yields sufficient DNA for diagnostic PCR.
MYXV can be readily cultured from cutaneous lesions or eyelids (biopsied or collected at necropsy) or from conjunctival swabs. The virus grows in various rabbit cell lines, including RK-13 and SIRC, and other mammalian cell lines, such as Vero and BGMK, and on the chorioallantoic membrane of embryonated chicken eggs. Tissues and swabs in 1:1 glycerol/saline or glycerol/phosphate-buffered saline can be stored at –20°C for some weeks before processing but for prolonged storage should be kept at –80°C.
Specific antibody can be detected as early as 6 to 10 days after infection using ELISA and persists for at least 12 months. Other serologic assays include complement fixation, virus neutralization, and gel immunodiffusion; these are less sensitive than ELISA.72 Gel immunodiffusion can also be used to detect viral antigen.
Differential diagnosis
-
•
Bacterial respiratory tract infections (eg, pasteurellosis)
-
•
Bacterial conjunctivitis/keratoconjunctivitis
-
•
Rabbit systemic herpesvirus infection, a recently described virus (leporid 4 herpesvirus) in North America causing swollen head, mucopurulent conjunctivitis, nodular hemorrhagic skin lesions, and respiratory distress.73
Treatment
At present, no specific treatment exists for myxomatosis. If the decision is made to attempt treatment, careful monitoring is necessary to avoid prolonging suffering. The aims of treatment should be to provide nursing support, control secondary infections, and minimize distress. Cessation of food and water intake, ongoing severe weight loss, or rectal temperature below 38°C is grounds for euthanasia.
Infected rabbits should be kept warm. There is evidence that high temperatures ameliorate the effects of attenuated viruses74; food intake and hydration should be carefully monitored. Broad-spectrum antibiotics, such as fluoroquinolones, potentiated sulfonamides, or tetracyclines, could be used but there is no clear evidence supporting their use and antibiotic treatment had no impact on the disease caused by virulent virus.75 For analgesia, buprenorphine 0.03 mg/kg twice daily has been used in acute myxomatosis but appeared to have little impact on rabbit behavior compared with untreated rabbits.76
An orally active derivative of cidofovir, CMX001, has activity against MYXV in vitro (G. McFadden, personal communication, 2009) and against the orthopoxvirus rabbitpox in rabbits in vivo but is available only experimentally.45, 77, 78
Prognosis and outcome
Highly virulent strains of MYXV have essentially 100% case fatality rates in domestic rabbits. Attenuated strains of MYXV may have case fatality rates of less than 50% to more than 95%, but the clinical course of the disease is prolonged. Rabbits infected with attenuated strains may recover over 2 to 3 weeks; in these rabbits, the cutaneous lesions become circumscribed and clearly demarcated from the surrounding skin, scab, and dry out, often leaving a scarred “moth-eaten” appearance of the face and ears. Chronic respiratory disease, such as snuffles, is common in surviving rabbits; some surviving rabbits do not gain weight and may have more serious underlying secondary infections, such as bacterial pneumonia. Even in apparently recovered rabbits, it is not unusual to find complete consolidation of cranial lung lobes at necropsy.
Prevention
Vaccination
Immunization with live attenuated strains of MYXV (e.g. Dervaximyo SG33, Merial, Lyons, France) or the heterologous rabbit fibroma virus (RFV) (Nobivac Myxo, MSD-Animal Health, Hoddesdon, Hertfordshire, UK) is used to protect rabbits against myxomatosis in Europe.79 The homologous live vaccines appear to provide longer lasting protection than vaccination with RFV but some have been associated with immunosuppression in young rabbits. This has led to recommendations to vaccinate initially with RFV followed by a boost with attenuated MYXV.80, 81 The Australian federal government does not permit commercial use of myxomatosis vaccines in domestic rabbits in Australia.
Neither type of vaccine provides 100% protection against high-dose challenge and protection can be quite short-lived (3–12 months). Vaccinated rabbits can become infected on challenge and shed virus.
Other preventive measures
-
•
Prevention of contact with wild rabbits and screening of cages/buildings to prevent mosquito transmission. Elimination of other vectors, such as rabbit fleas, lice, and mites.
-
•
Quarantine of incoming rabbits to prevent introduction of rabbits incubating the disease. Even vaccinated rabbits should be quarantined.
-
•
Isolation of suspected clinical cases and in-contact rabbits until diagnosis is confirmed.
Viral infections of minor clinical significance
Rabbit Fibroma Virus (Shope Fibroma Virus): Rabbit Fibromatosis
Introduction
Rabbit fibroma virus (RFV) (Subfamily: Chordopoxvirinae; Genus: leporipoxvirus) circulates in Eastern cottontail rabbits (Sylvilagus floridanus) in North America (see Table 2). It is genetically and antigenically closely related to myxoma virus, but in European rabbits (Oryctolagus cuniculus) RFV normally causes only a cutaneous fibroma at the inoculation site with no systemic signs of disease. In suckling rabbits, more generalized disease and death usually occur.82, 83 RFV is used as a live virus heterologous vaccine against myxomatosis.79, 84
Epidemiology
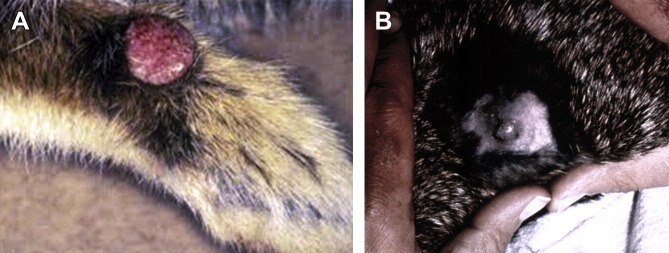
RFV causes cutaneous fibromas in S floridanus usually on the feet (Fig. 3 A), legs, or muzzle85; virus is spread passively on the mouthparts of biting arthropods (predominantly mosquitoes) that probe through the fibroma seeking a blood meal; fleas can transmit the virus but experimentally seemed relatively inefficient.86, 87 European rabbits may be infected by mosquitoes (or possibly by fleas if in close contact with Eastern cottontail rabbits) from this natural reservoir of the virus. The highest risk is during the autumn when large populations of infected cottontail rabbits and mosquitoes may coexist. The virus is reported in the Eastern and Midwest states of the United States, including Texas, and in Ontario, Canada.46, 88 Experimentally, mosquito transmission from immunocompetent European rabbits is inefficient, despite high titers of virus in the fibroma, so the European rabbit is essentially a dead-end host and virus spread from either naturally infected or vaccinated rabbits would be unlikely.48, 89, 90 However, suckling rabbits or immunosuppressed adult rabbits in which the immune response fails to clear the fibroma can act as sources for mosquito transmission.90, 91 Direct rabbit-to-rabbit spread does not occur,82 but mechanical spread between rabbits is theoretically possible. Virus dropped into the conjunctivae or nose will infect rabbits. Natural outbreaks in commercial rabbitries have been reported in the United States.88, 92 In a 16-year retrospective study of cutaneous neoplasms in pet rabbits from the University of Pennsylvania veterinary school, 10.5% of skin tumors were RFV-induced fibromas.93
Fig. 3.
(A) Nodular fibromatous growth on forepaw of Eastern cottontail rabbit naturally infected with rabbit (Shope) fibroma virus. (B) Experimentally induced Shope fibroma on the dorsum of a pigmented rabbit 21 days post inoculation. The fibroma is freely movable in the subcutaneous tissue.
([A] Image from Department of Veterinary Pathology, Armed Forces Institute of Pathology, Washington, DC.)
Clinical presentation
Skin nodules 0.5 to 6.0 cm in diameter are typically found on the forepaws, ears, and head. There may be multiple discrete nodules, freely moveable with no underlying tissue connections (see Fig. 3B). Usually no systemic signs of illness occur except in suckling rabbits where more generalized disease and death may ensue.82, 83, 88 The fibromas generally resolve within 3 to 4 weeks of infection.84
Pathophysiology
There is a solid tumorlike nodule, glistening and mucoid on cut surface; it may be necrotic in the center with scabbing of overlying epidermis. Histologically, it is described as a fibroxanthosarcomalike tumor.94, 95
Diagnosis
Diagnosis is made from clinical appearance and history; histopathology; virus isolation (RFV is readily cultured in rabbit cell lines, such as RK-13 or SIRC, and on the chorioallantoic membrane of embryonated chicken eggs); demonstration of poxvirus particles using thin-section electron microscopy on fibroma sections; or amplification of RFV DNA using PCR on DNA extracted from fibroma tissue collected at necropsy or biopsy.
Treatment
No treatment is normally necessary.
Prognosis and outcome
In all but suckling rabbits or experimentally immunosuppressed rabbits, the disease is not normally significant.
Prevention
Shield lactating does and their litters from mosquitoes.
Lapine Rotavirus: Rotaviral Enteritis
Introduction
Rotaviruses (Family: Reoviridae) are a major cause of diarrhea in intensively reared animals throughout the world. Essentially, every species of domestic animal and bird harbors at least one indigenous rotavirus that typically is responsible for causing diarrhea in newborn animals. The clinical signs, diagnosis, and epidemiology of disease are similar in all species; the severity of disease ranges from subclinical, through enteritis of varying severity, to death.
The classification of rotaviruses is based on genotypic and serologic analyses. Variation in the group-specific capsid antigen on VP6 defines the 6 major groups A to F. Rabbit rotaviruses are typically group A rotaviruses.96, 97, 98 Differentiation into serotypes is based on neutralization tests. Because both outer capsid proteins (VP4 and VP7) carry type-specific epitopes recognized by neutralizing antibodies, a binary system of classification of serotypes has developed, similar to that used for influenza viruses. For example, in group A rotaviruses, 14 G serotypes have been defined on the basis of differences in VP7, and 14 P serotypes based on differences in VP4.99
Epidemiology
Rotavirus is an important and common disease agent in commercial rabbitries. It is rarely diagnosed in pet rabbits. Serologic surveys of commercial rabbitries in Europe100, 101, 102, 103, 104 and North America,105, 106 laboratory research rabbits,107 Eastern cottontail rabbits (S floridanus),108 and hares (Lepus spp)108, 109 indicate lapine rotaviruses are widespread. In many commercial rabbitries, rotavirus infection is likely enzootic, as rotaviruses can survive in feces for several months.
Rotaviruses are excreted in the feces of infected animals in high titer (up to 1011 viral particles per gram); shedding of virus can occur for 6 to 8 days from the second to fifth day postinfection.110 Some rotaviruses are highly resistant to chlorination, and can survive for long periods in water, so that water-borne transmission is also a risk.111
Most viruses with multisegmented double-stranded RNA genomes, such as rotavirus, are included in the family Reoviridae. Because of their segmented RNA genomes, reassortment of genome segments among different strains of rotavirus is common, as is a high rate of RNA mutations in individual genes.112 The resulting genetic shift and drift leads to a remarkable diversity of rotaviruses, reflected by the numerous serotypes and strains of virus. Consequently, infection with new strains of rotavirus is common in breeding facilities.
Clinical presentation
Rotavirus infection in rabbits younger than 2 weeks old is characterized by voluminous, soft to liquid feces.113 Some affected animals are only moderately depressed, and often continue to suckle or drink milk.106, 110 Rabbits older than 2 weeks of age typically do not show diarrhea because of the fluid absorptive capability of the cecum.113 Other factors, particularly reduced colostrum intake, but also infections with other enteric pathogens, such as Escherichia coli, poor hygiene, chilling, and overcrowding, contribute to the severity of disease.104, 105 Young animals may die because of dehydration or secondary bacterial infection.
During epizootic infections, suckling rabbits may experience high mortality. During enzootic infections, suckling rabbits receive maternal antibody transplacentally, and experience low mortality but high morbidity.114
Pathophysiology
Rotavirus infections cause intestinal malabsorption and maldigestion by destruction of the terminally differentiated enterocytes lining the tips of the intestinal villi. Rotaviruses replicate in mature enterocytes lining intestinal villi, especially in the jejunum and ileum. Damaged villi become shortened and covered with undifferentiated epithelial cells that migrate from the crypts. These cells secrete reduced levels of disaccharidases (eg, lactase); lactose and other disaccharides accumulate in the lumen causing an osmotic drain and attracting fluid into the lumen.113, 115, 116 The neonatal bowel is especially susceptible to infection, because of the slow epithelial turnover rate and the high proportion of terminally differentiated epithelium, and is less able to carry out glucose-coupled sodium transport116 and chloride secretion.115
Undigested lactose in the milk promotes bacterial growth and exerts a further osmotic effect; both mechanisms contribute to the diarrhea. In young rabbits, bacterial superinfection with E coli and other bacteria (eg, Clostridia spp) and coccidia have an additive effect and contribute to the multifactorial enteritis complex of juvenile rabbits.103, 105, 117 Mortality can often be high in commercial rabbitries.
Diagnosis
Diagnosis is based on history, age, and characteristic gross and microscopic features. Electron microscopy still allows rapid diagnosis, as virus particles are plentiful in the feces of affected animals, and have a highly distinctive wheel-like appearance (from the Latin rota = wheel). ELISA is a more practicable and more sensitive method for detection of rotaviruses in feces in most laboratories. A commercial human rotavirus antigen detection kit has been shown to be effective in laboratory rabbits because of its ability to detect rotavirus antigen in feces.118 The specificity of enzyme immunoassays can be manipulated by selecting either group-specific or serotype-specific or broadly cross-reactive antibodies as capture and/or indicator antibodies in an antigen-capture assay. Rotaviruses are difficult to isolate in cell culture.119
Diagnostic tests that identify the viral genome in RNA extracted directly from feces include polyacrylamide gel electrophoresis, which can distinguish rotavirus groups A, B, and C by RNA electropherotype pattern alone.112 RT-PCR assay allows the use of primer pairs appropriate for the degree of specificity desired (rotavirus groups based on VP6, or G and P genotypes based on VP7 and VP4).112 These tests are generally used to make necessary distinctions in molecular epidemiologic studies to identify reassortant viruses and potential interspecies transmission. The rate of success of any diagnostic test for rotavirus is significantly affected by the time of sample collection; samples collected beyond 48 hours after onset of diarrhea are of limited value.101
Treatment
Supportive care, including warmth, is critical. Recovery in severely dehydrated rabbits can be aided by administering oral electrolyte solutions containing glucose shortly after the onset of diarrhea.104 In severe cases that are likely to be “enteritis complex,” the value of antibiotics is questionable.
Prognosis and outcome
Prognosis is affected by superinfection with other intestinal pathogens.103, 113, 117 Rotavirus antigen has been detected from healthy rabbits (specimens taken one day after weaning and 1 week later) in about 15% of commercial Italian rabbitries.103
Prevention
Although the management of intensive rearing units for farmed rabbits can be improved to reduce the incidence of disease, there is little likelihood that improved hygiene alone can completely control rotavirus infections. Local immunity in the small intestine is more important than systemic immunity in providing resistance to infection; rotavirus antibodies present in immune colostrum and milk are critical in protecting neonates.114, 120 Although much of the colostral antibody enters the circulation, serum antibody levels are not as critical for protection. Inoculation of the dam with inactivated or attenuated rotavirus vaccines promotes higher levels of antibody in the colostrum and milk, and a longer period of antibody secretion in milk, with a corresponding decrease in the incidence of disease in neonates.121
Cottontail Rabbit Papillomavirus (Shope Papillomavirus): Rabbit Papillomatosis
Introduction
Cottontail rabbit papillomavirus (CRPV), also commonly known as Shope papillomavirus (Family: Papillomaviridae) is a cause of papillomas (warts) in rabbits. Classic studies on viral oncogenesis were performed in the 1930s122, 123, 124 with CRPV.
Epidemiology
The natural host is the cottontail rabbit (Sylvilagus spp) (see Table 2).125 CRPV causes natural disease uncommonly in domestic rabbits.126, 127 Insect vectors (eg, mosquitoes) are probably the cause of mechanical spread of virus from cottontail rabbits to domestic rabbits.
Clinical presentation
CRPV papillomas occur most frequently on nonhaired or thinly haired skin, such as eyelids and ears. In CRPV papillomas, the normal process of keratinization is altered, as evidenced by the hyperkeratosis, parakeratosis, and fragmentation of the horny layer. Consequently, lesions range from a pedunculated, cornified surface overlying a fleshy central area (early papilloma) to multiple conical/cylindrical hornlike masses of firm keratin 3 to 5 mm in diameter and 0.5 to 2.0 cm in length (cutaneous horns) (Fig. 4 ).
Fig. 4.
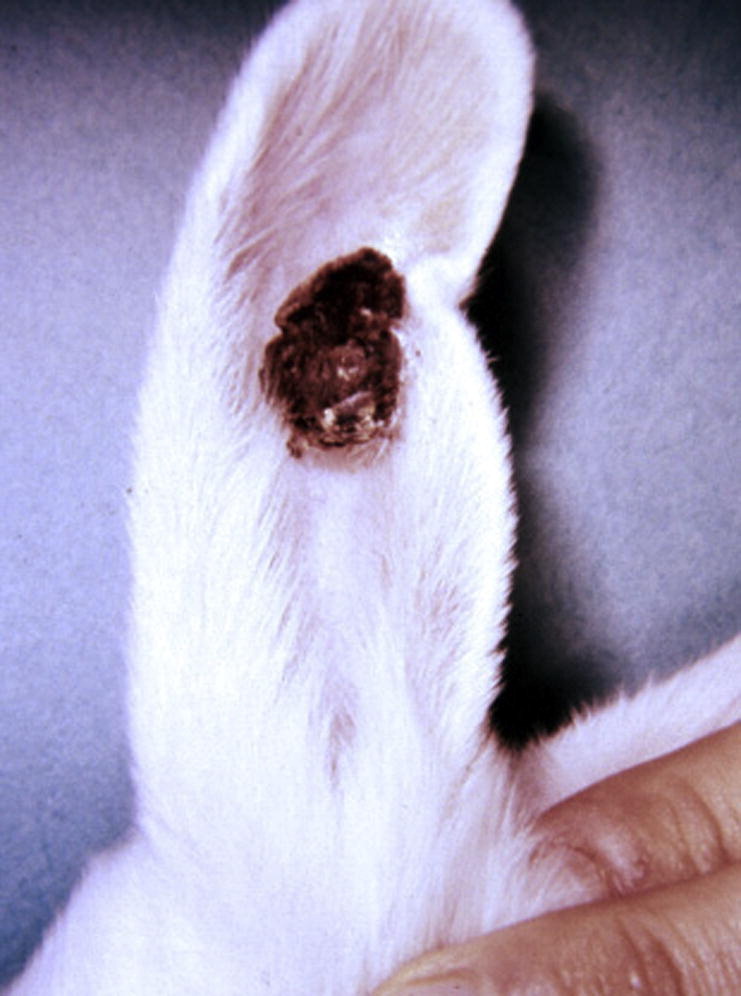
CRPV-induced papilloma on the ear of a New Zealand white rabbit. Notice how the papilloma shows multiple hornlike masses of firm, protruding keratin.
Pathophysiology
Warts induced by CRPV in both cottontail rabbits125 and European rabbits often progress to carcinomas. Virus replication occurs only in the cottontail rabbit,128 and not the European rabbit, although one strain of CRPV has been shown to replicate at low levels in European rabbits.129 In domestic rabbits, the tissue surrounding an inoculation site (either experimentally or naturally by mosquitoes) is clinically and histologically normal, but contains viral DNA at low levels detectable by PCR. The latent virus is able to form warts from mild skin irritation.130 Ultraviolet irradiation will also induce warts, which is why the lesions are typically found on the ears, lips and eyelids: areas without much fur that are exposed to sunlight.131 Different mRNA transcripts are present (E1 protein, E2 protein, E6 protein, E7 protein, L1 protein, and L2 protein transcripts) in latent viruses. Only E6 and E7 oncoprotein transcripts can be induced to form papillomas.132 The papillomas develop into carcinomas 8 to 14 months later in 25% of cottontail rabbits and in 66% to 80% of European rabbits.
Diagnosis
The histologic diagnosis is consistent with a squamous papilloma. In contrast to papillomas induced by CRPV, spontaneous nonviral squamous papillomas develop in haired skin. Other causes of skin lesions in rabbits that should be considered in the differential diagnosis include rabbit oral papillomavirus induced conjunctival papilloma and rabbit (Shope) fibroma virus infection. Shope fibromas are typically flattened to nodular tumors 0.5 to 6.0 cm in diameter that tend to occur on the forepaws, ears, and head.
Treatment
Intralesional 1% (wt/vol) (0.036 M) cidofovir treatment of rabbit papillomas led to elimination, or “cure” of large papillomas over a 6-week to 8-week treatment period.133 However, recurrences at periods from 1 to 8 weeks after treatment cessation were observed in approximately 50% of cured sites. Tumor reappearance occurred because of latent virus around the inoculation sites.
Immune stimulation with unmethylated dinucleotides of cytosine and guanine (CpG) have shown efficacy against papillomas as monotherapy, as vaccine adjuvants, and in combination with chemotherapies.134 Despite the potency of CpG in triggering host immunity, CpG oligodeoxynucleotide has experimentally not shown a therapeutic effect against experimentally induced CRPV papilloma in rabbits.
Surgical excision of papillomas can be attempted, but a wide margin is required. PCR of the edges of excised skin can be used to detect CRPV DNA and determine if all latent infected cells were removed. However, even if one lesion is completely removed, there are likely to be other latent infected inoculation sites in surrounding skin that have the potential to develop into papillomas and eventually carcinomas if E6 and/or E7 oncoproteins are present. If a papilloma occurs on the ear, amputation of the ear is a potential but drastic treatment.
Prognosis and outcome
CRPV papillomas develop into carcinomas 8 to 14 months later in 66% to 80% of infected rabbits. Papillomas undergo immune-mediated regression if they do not progress to carcinomas. Infection is uncommon. Only 2 CRPV papillomas (1.1%) were identified in a 16-year retrospective study of cutaneous neoplasms in pet rabbits from the University of Pennsylvania veterinary school.93
Prevention
Keep rabbits indoors or in insect-protected enclosures to avoid transmission of virus from cottontail rabbits. This is an issue only in North America where cottontail rabbits occur naturally.
If a papilloma is identified, and PCR identifies CRPV, advise owners to keep their rabbit out of direct sunlight and not to irritate (eg, removing incrustations) the papilloma or surrounding skin, as it will induce the occurrence of new papillomas.
Rabbit Oral Papillomavirus: Oral Papillomatosis
Introduction
Rabbit oral papillomavirus (Family: Papillomaviridae) (ROPV) occurs naturally in domestic rabbits (see Table 2). The causative papillomavirus is distinct from the cottontail rabbit papillomavirus (Shope papillomavirus).
Epidemiology
ROPV is widespread among domestic rabbits in Europe and the Americas, particularly young animals.135, 136, 137, 138 Lesions typically occur in rabbits between 2 and 18 months of age. The oral papillomas are not highly contagious. Lesions are found more frequently in young rabbits whose mothers have papillomas; transmission from the mother to offspring during suckling is common.139 ROPV can be recovered from the mouth washings of rabbits having no oral papillomas, as it is latent in the mouth, and does not proliferate unless the mucous membrane is injured.
Clinical presentation
Tumors are small, gray-white, filiform or pedunculated nodules (5 mm in diameter and 4 mm in height) and are localized mostly on the underside of the tongue (Fig. 5 )140; however, one report describes a conjunctival papilloma.141 The papillomas usually regress spontaneously within a few weeks to a few months. Most pet rabbit owners do not notice the lesions.
Fig. 5.

Rabbit oral papillomavirus tumor presenting as a white pedunculated nodule on the underside of a New Zealand white rabbit tongue (arrow).
(Image from Department of Veterinary Pathology, Armed Forces Institute of Pathology, Washington, DC.)
Pathophysiology
ROPV causes papillomas in the oral mucosa of several species of rabbits and hares but fails to cause lesions when inoculated into other rabbit tissues and into the oral mucosa of other species. ROPV differs from the cottontail rabbit papilloma virus (Shope papillomavirus) and homologies between the open reading frames of the 2 viruses vary between 23% and 68%.142 Rabbits immune to the oral papillomavirus are fully susceptible to cottontail rabbit papilloma virus and vice versa.142 There is little sequence homology between the genomes of papillomaviruses from different species.143
Diagnosis
Lesions are typical squamous papillomas on fibrovascular stalks. Basophilic intranuclear inclusions may be present in the stratum spinosum.136 Virus particles from lesions may be seen under electron microscopy. Differential diagnosis of oral lesions includes sialoceles.144
Treatment and prognosis
Oral papillomas are typically not treated, as they regress spontaneously and show no tendency to malignancy.
Herpes Simplex Virus: Herpes Encephalitis
Introduction
Although rabbits have served as an animal model for herpesvirus encephalitis and keratitis following experimental inoculation with human herpes simplex virus (HSV) (Subfamily: Alphaherpesvirinae; Genus: simplexvirus) 4 reports exist of naturally occurring fatal encephalitis in rabbits due to HSV infection.145, 146, 147, 148
Epidemiology
Owners of rabbits with clinical facial herpesvirus infection are suspected to be the origin of infection. A similar situation has been reported in 2 pet chinchillas (Chinchilla lanigera) diagnosed with fatal HSV encephalitis.149, 150
Viral isolates derived from 2 marmosets and 1 domestic rabbit that died from HSV encephalitis revealed different genotypes, suggesting that certain HSV genotypes with a higher potential of being transmitted to animals do not exist.147 The infrequency of natural cross-infection between humans and other species has led some researchers to postulate a nonimmunologic protective mechanism against HSV infection in animals.151
Clinical presentation
Affected rabbits present with severe signs of central nervous system dysfunction, such as incoordination, intermittent myoclonic seizures, and opisthotonus. In one case, results of hematologic and serum biochemical analyses revealed only lymphopenia, a relative monocytosis, and an increase in serum activity of creatine phosphokinase and serum concentration of total protein.146
Diagnosis
All cases described have been diagnosed at necropsy. Histologic evaluation of brain tissue reveals lesions characteristic of severe, diffuse, nonsuppurative meningoencephalitis and a few large, eosinophilic, intranuclear inclusion bodies in neurons and glial cells. In situ hybridization and/or PCR detect HSV DNA in the nuclei of glial cells, lymphocytes, and neurons.152
Treatment
In one case, despite intravenous administration of crystalloid fluids and treatment with antimicrobials, vitamin B complex, nutritional support, and prednisolone, the condition of the rabbit deteriorated and it was euthanized 7 days after admission.146
Prognosis and outcome
All cases in rabbits and chinchillas have resulted in death.
Prevention
Owners with active HSV facial lesions should avoid close contact, such as kissing their pet rabbits.
Leporid-4 Herpes Virus: Systemic Herpes Virus Infection
Introduction
A herpes virus designated as leporid herpesvirus 4 (LHV-4) (Subfamily: Alphaherpesvirinae; Genus: simplexvirus) that is highly pathogenic for domestic rabbits has been recently described in rabbits in Alaska and Canada.73, 153, 154, 155 Analysis of virus samples indicates that the virus is most closely related to bovine herpesvirus-2. The next most closely related viruses are human HSV 1 and 2, and a number of cercopithecine herpesviruses.156
Three naturally occurring herpesviruses of rabbits and hares, called leporid herpesviruses 1, 2, and 3 (LHV-1, LHV-2, and LHV-3), have been identified.156 They have been classified tentatively as belonging to the subfamily Gammaherpesvirus.156 The best characterized is LHV-3 (Herpesvirus sylvilagus), which is endemic in Eastern cottontail rabbits (S floridanus) and causes tumorlike lesions in lymph nodes, kidney, spleen, and liver157; however, it does not cause disease in domestic rabbits.156 LHV-2 (Herpesvirus cuniculi) causes asymptomatic infections of domestic rabbits.156 LHV-1 is found in cottontail rabbits158, 159 and no disease has been reported to be associated with it in domestic rabbits.156 The novel herpes virus identified in Alaska and Canada is referred to as LHV-4.153
Epidemiology
Reports to date of LHV-4 infection are limited to commercial rabbitries. Experimental exposure of domestic rabbits to virus isolates results in severe clinical disease and necrosis in the spleen and lymph nodes.156 Viral DNA has been identified in a variety of tissues by PCR, consistent with a systemic infection.
It is possible that LHV-4 is present in an animal reservoir found in northwestern North America, in which it causes asymptomatic infections and infrequently comes in contact with domestic rabbits. It is also possible that LHV-4 is present in wild rabbit populations but is not often transmitted to domestic rabbits.158
Clinical presentation
The primary lesions are conjunctivitis and periocular swelling, multifocal hemorrhagic/ulcerative dermatitis on the face and dorsum.154 Clinical signs include progressive weakness, anorexia, respiratory distress, and abortion.73 Frequently animals are found dead with no previous evidence of disease.
Pathophysiology
Death is due to cardiovascular and respiratory failure.155
Diagnosis
At necropsy there is massive necrosis and fibrin deposition within red pulp of the spleen.73 Large eosinophilic, intranuclear inclusion bodies are observed microscopically in tissue sections of skin, spleen, and lung.154 Hemorrhagic dermatitis and panniculitis are associated with epidermal microvesicular degeneration, dermal and subcutaneous vascular necrosis, and thrombosis. Other findings include hemorrhagic necrosis of the myocardium with rare intranuclear inclusions within stromal cells, multifocal pulmonary hemorrhage, and hemorrhage with sinus erythrophagocytosis in lymph nodes.73
Viral infections of laboratory significance
Astrovirus: Probable Factor in Enteritis Complex
A novel astrovirus (Family: Astroviridae; Genus: mamastrovirus) was recently identified by screening rabbits with enteritis complex and healthy rabbits.160 Rabbit astrovirus was found in 10 (43%) of 23 samples from rabbits with enteric disease and in 25 (18%) of 139 samples from healthy rabbits in Italy during 2005 to 2008. The median titers of virus in the rabbits with enteric disease were 103 greater than in the healthy rabbits.
Astroviruses are a family of RNA viruses with 2 genera: mamastrovirus and avastrovirus. The genus mamastrovirus is associated with gastroenteritis in most animal species and humans.161 Astrovirus infections are regarded as the second most common cause of viral diarrhea in children after rotavirus infection, but in animals, their association with enteric diseases is not well documented, with the exception of turkey and mink astrovirus infection.161
The multifactorial enteritis complex of juvenile rabbits103, 105, 117 can be caused by bacteria, viruses, and parasites. Several different viruses have been isolated from rabbits with diarrhea, such as rotavirus, coronavirus, and now astrovirus. Whether natural outbreaks of enteritis can be caused by these viral agents alone or in conjunction with other pathogens is not clear. Rabbit astroviruses should be included in the diagnostic algorithm of rabbit enteritis complex. Further experiments to increase information about their epidemiology and potential pathogenic role are required.
Bornavirus: Borna Disease
Borna disease virus (Family: Bornaviridae; Genus: bornavirus) (BDV) is the cause of a fatal neurologic disease primarily of horses and sheep that occurs sporadically in central Europe. Incidence is highest during spring and summer. Arthropods have been discussed as a potential vector, but BDV has never been isolated from insects in Europe.162 A definite virus reservoir for BDV has not been found; various rodents most likely represent such a reservoir.162 Natural infections in other Equidae, ruminants, rabbits, and cats have also been described in Europe, North Africa, and the Middle East162, 163; however, the virus exists worldwide. The infection can be fatal, but most carriers are persistently infected without showing clinical signs. Recently, avian bornavirus was identified as the cause of proventricular dilatation disease in parrots, and, like Borna disease virus, has been detected worldwide.164
BDV is assumed to be transmitted through salivary, nasal, or conjunctival secretions. Animals become infected by direct contact with these secretions or by exposure to contaminated food or water. A minimum incubation period of 4 weeks is estimated for horses and sheep. In rabbits, natural infections have been reported only in Germany.163 Clinical signs in naturally and experimentally infected rabbits are neurologic. Although Borna disease is rare in rabbits, the worldwide existence of the virus means the infection must be differentiated from rabies virus infection and Encephalitozoon cuniculi infection.165 Histopathologically, Borna disease is characterized by a nonpurulent inflammation of the brain and the spinal cord.166 The detection of BDV in diseased animals, mainly sheep and horses, is achieved by histologic, immunohistochemical, and serologic approaches and/or PCR-based technologies.167
Rabies Virus: Rabies
Rabies virus (Family: Rhabdoviridae; Genus: lyssavirus) infection is relatively rare in both pet and feral rabbits. Of the 87,700 cases of animal rabies reported in the United States from 1992 to 2002, only 621 occurred in rodents or lagomorphs.168, 169 The majority (559 cases) occurred in groundhogs (Marmota monax) and were most likely because of den contact from infected skunks or raccoons.169 Despite its rarity, rabies has been reported in pet rabbits in Western Europe and North America.170, 171, 172, 173, 174 All cases have been in outdoor-housed pet rabbits. Most cases have come in contact with wildlife carrying rabies (eg, raccoon in North America, fox in Europe) but the source of infection in some rabbits remains unknown.
Both furious and dumb forms of rabies have been reported in naturally infected rabbits; however, the dumb form seems more common. Unilateral pelvic limb paresis or paralysis has been reported as an early clinical sign in domestic rabbits.171 The one case of furious rabies described the rabbit biting at inanimate objects.172
Currently, there is not a rabies vaccine approved for use in rabbits. The only way to prevent rabies infection in rabbits is to prevent exposure. Veterinarians in rabies-enzootic areas should be familiar with the clinical signs of rabies in rabbits and should caution rabbit owners about the need to protect their pets from contact with wildlife.
Vaccinia Virus: Rabbitpox (Rabbit Plague)
Because of its widespread use as a smallpox vaccination in humans, and its wide host range, vaccinia virus (Subfamily: Chordopoxvirinae; Genus: orthopoxvirus) sometimes has caused naturally spreading diseases in domestic animals (eg, teat infections of cattle) and also in laboratory rabbits (rabbitpox). Epidemics of rabbitpox occurring in isolated animal rooms were reported in the United States and Netherlands.175, 176
The disease is acute and rapidly fatal. Confluent papules on the skin, sometimes accompanied by necrosis and hemorrhage, characterize rabbitpox.177, 178 Papular lesions may occur in the oropharynx, respiratory tract, spleen, and liver.179 In the so-called “pockless” form, a few pocks were present in the oral cavity, and focal hepatic necrosis, pleuritis, and splenomegaly were observed.180 The primary site for replication in the naturally occurring disease is the respiratory tract.181 There is a subsequent viremia with replication in lymphoid tissues and skin.181
Now that smallpox vaccination has been discontinued for the civilian populations of all countries, rabbitpox is unlikely to be seen. However, the use of aerosolized rabbitpox infection as an animal model for evaluation of antivirals under development for the therapeutic treatment of human smallpox still continues. Consequently, rabbitpox may be seen in laboratory rabbits if the experimental virus is not confined to the biocontainment research area.182
Pleural Effusion Disease Virus (Infectious Cardiomyopathy Virus): Pleural Effusion Disease and Cardiomyopathy
Pleural effusion, right-sided heart enlargement, mesenteric lymphadenopathy, and multifocal necrosis of multiple organs have been associated with infection by a coronavirus referred to as pleural effusion disease virus (PEDV) (Family: Coronaviridae; Genus: coronavirus) in laboratory rabbits in North America and Europe.183 PEDV was discovered as a contaminant of Treponema pallidum (the causative agent of syphilis), which is maintained by intratesticular inoculation of laboratory rabbits.184 PEDV infection is not seen in pet rabbits. Different PEDV isolates vary in pathogenicity and range from subclinical infection to infection causing more than 50% mortality.185 Lymphoid depletion of splenic follicles, focal degenerative changes in lymph nodes and thymus, proliferative changes in glomerular tufts, and uveitis characterized fatal infections.183, 186 In one infection, multifocal myocardial degeneration and necrosis were seen.187 Antibodies to 2 human strains of coronavirus have been demonstrated in convalescent rabbit sera and antigen to human 229E coronavirus (one of the 4 human coronaviruses circulating worldwide and a proven common cold virus in healthy adults) was detected in myocardial lesions.188, 189 The evidence suggests that the causative agent is not a natural pathogen of rabbits.
Rabbit Enteric Coronavirus: Coronaviral Enteritis
Rabbit enteric coronavirus (RECV) (Family: Coronaviridae; Genus: coronavirus) induces disease in juvenile rabbits that is characterized by intestinal villus attenuation, malabsorption, and diarrhea. Infection may predispose rabbits to, or be obscured by, the enteritis complex. Although RECV has been isolated, it has not been characterized.190
Coronavirus-associated enteritis has been reported in commercial rabbitries but generally with low mortality.191 In one report, an outbreak of fatal enteritis in 3 to 8-week old rabbits occurred in a barrier-maintained breeding colony in Germany.192 The prevalence of detectable antibody in a serologic survey of North American commercial rabbitries ranged from 3% to 40%.193 The presence of coronaviral particles in feces of young diarrheic rabbits indicates the virus may play a role in enteritis complex. However, coronaviral particles have also been observed in gastrointestinal contents of healthy rabbits. Differential diagnoses include E coli infections, coccidiosis, rotavirus infection, and clostridial enteropathies.
Experimentally and naturally infected young rabbits may be emaciated and dehydrated and show perineal fecal staining.192, 194 The cecum may be distended, with a milky to tan liquid.192, 194 Microscopic changes are confined to the small and large intestines.194 Distinctive findings include villous blunting, vacuolation and necrosis of enterocytes, mucosal edema, and polymorphonuclear and mononuclear cell infiltration.
Confirmation of diagnosis requires demonstration of coronaviral particles from gastrointestinal contents by electron microscopy.190 The virus has not been grown in cell culture.
References
- 1.Cooke B.D., Fenner F. Rabbit haemorrhagic disease and the biological control of wild rabbits, Oryctolagus cuniculus, in Australia and New Zealand. Wildl Res. 2002;29(6):689–706. [Google Scholar]
- 2.Anonymous, AVMA News Rabbit calicivirus infection confirmed in Iowa rabbitry. J Am Vet Med Assoc. 2000;216(10):1537. [PubMed] [Google Scholar]
- 3.Campagnolo E.R., Ernst M.J., Berninger M.L. Outbreak of rabbit hemorrhagic disease in domestic lagomorphs. J Am Vet Med Assoc. 2003;223(8):1151–1155. doi: 10.2460/javma.2003.223.1151. 1128. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh M.T., Behan S.C., Mohamed F.M. A pandemic strain of calicivirus threatens rabbit industries in the Americas. Virol J. 2007;4:96. doi: 10.1186/1743-422X-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Emerging Issues, USDA. Rabbit hemorrhagic disease, Indiana, June 15, 2005. Impact Worksheet. Available at: http://www.aphis.usda.gov/animal_health/emergingissues/impactworksheets/iw_2005_files/domestic/rhdindiana061505.htm. Accessed December 3, 2012.
- 6.Capucci L., Fusi P., Lavazza A. Detection and preliminary characterization of a new rabbit calicivirus related to rabbit hemorrhagic disease virus but nonpathogenic. J Virol. 1996;70(12):8614–8623. doi: 10.1128/jvi.70.12.8614-8623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester N.L., Trout R.C., Gould E.A. Benign circulation of rabbit haemorrhagic disease virus on Lambay Island, Eire. Virology. 2007;358(1):18–22. doi: 10.1016/j.virol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Strive T., Wright J., Kovaliski J. The non-pathogenic Australian lagovirus RCV-A1 causes a prolonged infection and elicits partial cross-protection to rabbit haemorrhagic disease virus. Virology. 2010;398(1):125–134. doi: 10.1016/j.virol.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Strive T., Wright J.D., Robinson A.J. Identification and partial characterisation of a new Lagovirus in Australian wild rabbits. Virology. 2009;384(1):97–105. doi: 10.1016/j.virol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Jahnke M., Holmes E.C., Kerr P.J. Evolution and phylogeograph of the non-pathogenic calicivirus RCV-A1 in wild rabbits in Australia. J Virol. 2010;84(23):12397–12404. doi: 10.1128/JVI.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergin I.L., Wise A.G., Bolin S.R. Novel calicivirus identified in rabbits, Michigan, USA. Emerg Infect Dis. 2009;15(12):1955–1962. doi: 10.3201/eid1512.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke B.D., Chapuis J.L., Magnet V. Potential use of myxoma virus and rabbit haemorrhagic disease virus to control feral rabbits in the Kerguelen Archipelago. Wildl Res. 2004;31(4):415–420. [Google Scholar]
- 13.Marchandeau S., Bertagnoli S., Leonard Y. Serological evidence for the presence of non-pathogenic rabbit haemorrhagic disease virus-like strains in rabbits (Oryctolagus cuniculus) of the Kerguelen archipelago. Polar Biol. 2010;33(7):985–989. [Google Scholar]
- 14.O'Keefe J.S., Tempero J.E., Motha M.X. Serology of rabbit haemorrhagic disease virus in wild rabbits before and after release of the virus in New Zealand. Vet Microbiol. 1999;66(1):29–40. doi: 10.1016/s0378-1135(98)00307-1. [DOI] [PubMed] [Google Scholar]
- 15.Dalton K.P., Nicieza I., Balseiro A. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg Infect Dis. 2012;18(2):2009–2012. doi: 10.3201/eid1812.120341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Gall-Recule G., Zwingelstein F., Boucher S. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet Rec. 2011;168(5):137–138. doi: 10.1136/vr.d697. [DOI] [PubMed] [Google Scholar]
- 17.Robinson A.J., So P.T., Muller W.J. Statistical models for the effect of age and maternal antibodies on the development of rabbit haemorrhagic disease in Australian wild rabbits. Wildl Res. 2002;29(6):663–671. [Google Scholar]
- 18.McColl K.A., Morrissy C.J., Collins B.J. Persistence of rabbit haemorrhagic disease virus in decomposing rabbit carcasses. Aust Vet J. 2002;80(5):298–299. doi: 10.1111/j.1751-0813.2002.tb10848.x. [DOI] [PubMed] [Google Scholar]
- 19.Henning J., Meers J., Davies P.R. Survival of rabbit haemorrhagic disease virus (RHDV) in the environment. Epidemiol Infect. 2005;133(4):719–730. doi: 10.1017/s0950268805003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McColl K.A., Merchant J.C., Hardy J. Evidence for insect transmission of rabbit haemorrhagic disease virus. Epidemiol Infect. 2002;129(3):655–663. doi: 10.1017/s0950268802007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenghaus C., Westbury H., Collins B. Overview of the RHD project in the Australian Animal Health Laboratory. In: Munro R.K., Williams R.T., editors. Rabbit haemorrhagic disease: issues in assessment for biological control. Bureau of Resource Sciences; Canberra (Australia): 1994. pp. 104–129. [Google Scholar]
- 22.Capucci L., Nardin A., Lavazza A. Seroconversion in an industrial unit of rabbits infected with a non-pathogenic rabbit haemorrhagic disease-like virus. Vet Rec. 1997;140(25):647–650. doi: 10.1136/vr.140.25.647. [DOI] [PubMed] [Google Scholar]
- 23.Mutze G., Sinclair R., Peacock D. Does a benign calicivirus reduce the effectiveness of rabbit haemorrhagic disease virus (RHDV) in Australia? Experimental evidence from field releases of RHDV on bait. Wildl Res. 2010;37(4):311–319. [Google Scholar]
- 24.Parkes J.P., Norbury G.L., Heyward R.P. Epidemiology of rabbit haemorrhagic disease (RHD) in the South Island, New Zealand, 1997-2001. Wildl Res. 2002;29(6):543–555. [Google Scholar]
- 25.McPhee S.R., Butler K.L., Kovaliski J. Antibody status and survival of Australian wild rabbits challenged with rabbit haemorrhagic disease virus. Wildl Res. 2009;36(5):447–456. [Google Scholar]
- 26.Marchandeau S., Le Gall-Recule G., Bertagnoli S. Serological evidence for a non-protective RHDV-like virus. Vet Res. 2005;36(1):53–62. doi: 10.1051/vetres:2004049. [DOI] [PubMed] [Google Scholar]
- 27.Teifke J.P., Reimann I., Schirrmeier H. Subacute liver necrosis after experimental infection with rabbit haemorrhagic disease virus (RHDV) J Comp Pathol. 2002;126(2–3):231–234. doi: 10.1053/jcpa.2001.0534. [DOI] [PubMed] [Google Scholar]
- 28.Tunon M.J., Sanchez-Campos S., Garcia-Ferreras J. Rabbit hemorrhagic viral disease: characterization of a new animal model of fulminant liver failure. J Lab Clin Med. 2003;141(4):272–278. doi: 10.1067/mlc.2003.30. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira P.G., Costa-e-Silva A., Oliveira M.J. Severe leukopenia and liver biochemistry changes in adult rabbits after calicivirus infection. Res Vet Sci. 2006;80(2):218–225. doi: 10.1016/j.rvsc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Campos S., Alvarez M., Culebras J.M. Pathogenic molecular mechanisms in an animal model of fulminant hepatic failure: rabbit hemorrhagic virus disease. J Lab Clin Med. 2004;144(4):215–222. doi: 10.1016/j.lab.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Chen S.Y., Chou C.C., Liu C.I. Impairment of renal function and electrolyte balance in rabbit hemorrhagic disease. J Vet Med Sci. 2008;70(9):951–958. doi: 10.1292/jvms.70.951. [DOI] [PubMed] [Google Scholar]
- 32.Chen S.Y., Shien J.H., Ooi H.K. Hyperlipidemia in rabbit hemorrhagic disease. Exp Anim. 2008;57(5):479–483. doi: 10.1538/expanim.57.479. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs A., Weissenbock H. Comparative histopathological study of rabbit haemorrhagic disease (RHD) and European brown hare syndrome (EBHS) J Comp Pathol. 1992;107(1):103–113. doi: 10.1016/0021-9975(92)90100-9. [DOI] [PubMed] [Google Scholar]
- 34.Shien J.H., Shieh H.K., Lee L.H. Experimental infections of rabbits with rabbit haemorrhagic disease virus monitored by polymerase chain reaction. Res Vet Sci. 2000;68(3):255–259. doi: 10.1053/rvsc.1999.0372. [DOI] [PubMed] [Google Scholar]
- 35.Forrester N.L., Boag B., Moss S.R. Long-term survival of New Zealand rabbit haemorrhagic disease virus RNA in wild rabbits, revealed by RT-PCR and phylogenetic analysis. J Gen Virol. 2003;84(Pt 11):3079–3086. doi: 10.1099/vir.0.19213-0. [DOI] [PubMed] [Google Scholar]
- 36.Gall A., Hoffmann B., Teifke J.P. Persistence of viral RNA in rabbits which overcome an experimental RHDV infection detected by a highly sensitive multiplex real-time RT-PCR. Vet Microbiol. 2007;120(1–2):17–32. doi: 10.1016/j.vetmic.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Lavazza A., Capucci L. Rabbit haemorrhagic disease. In: OIE Biological Standards Commission, editor. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 7th edition. Office International des Épizooties; Paris: 2012. Section 2:6:2. [Google Scholar]
- 38.Liu J., Kerr P.J., Wright J.D. Serological assays to discriminate rabbit haemorrhagic disease virus from Australian non-pathogenic rabbit calicivirus. Vet Microbiol. 2012;157(3–4):345–354. doi: 10.1016/j.vetmic.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Crespo I., Miguel B.S., Laliena A. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J Pineal Res. 2010;49(2):193–200. doi: 10.1111/j.1600-079X.2010.00787.x. [DOI] [PubMed] [Google Scholar]
- 40.Tuñón M.J., San Miguel B., Crespo I. Melatonin attenuates apoptotic liver damage in fulminant hepatic failure induced by the rabbit haemorrhagic disease virus. J Pineal Res. 2011;50(1):38–45. doi: 10.1111/j.1600-079X.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- 41.Tunon M.J., San Miguel B., Crespo I. Cardiotrophin-1 promotes a high survival rate in rabbits with lethal fulminant hepatitis of viral origin. J Virol. 2011;85(24):13124–13132. doi: 10.1128/JVI.05725-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laliena A., San Miguel B., Crespo I. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012;53(3):270–278. doi: 10.1111/j.1600-079X.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang X.L., Hao H.F., Qiu L. Phylogenetic analysis of rabbit hemorrhagic disease virus in China and the antigenic variation of new strains. Arch Virol. 2012;157(8):1523–1530. doi: 10.1007/s00705-012-1340-9. [DOI] [PubMed] [Google Scholar]
- 44.Spibey N., McCabe V.J., Greenwood N.M. Novel bivalent vectored vaccine for control of myxomatosis and rabbit haemorrhagic disease. Vet Rec. 2012;170(12):309. doi: 10.1136/vr.100366. [DOI] [PubMed] [Google Scholar]
- 45.Kerr P.J. Myxomatosis in Australia and Europe: a model for emerging infectious diseases. Antiviral Res. 2012;93(3):387–415. doi: 10.1016/j.antiviral.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Fenner F., Ratcliffe F.N. Cambridge University Press; Cambridge (England): 1965. Myxomatosis. [Google Scholar]
- 47.Fenner F., Marshall I.D. Passive immunity in myxomatosis of the European rabbit (Oryctolagus cuniculus): the protection conferred on kittens born by immune does. J Hyg (Lond) 1954;52(3):321–336. doi: 10.1017/s0022172400027534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Day M.F., Fenner F., Woodroofe G.M. Further studies on the mechanism of mosquito transmission of myxomatosis in the European rabbit. J Hyg (Lond) 1956;54(2):258–283. doi: 10.1017/s002217240004451x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall I.D., Regnery D.C. Myxomatosis in a California brush rabbit (Sylvilagus bachmani) Nature. 1960;188:73–74. doi: 10.1038/188073b0. [DOI] [PubMed] [Google Scholar]
- 50.Marshall I.D., Regnery D.C., Grodhaus G. Studies in the epidemiology of myxomatosis in California. I. Observations on two outbreaks of myxomatosis in coastal California and the recovery of myxoma virus from a brush rabbit (Sylvilagus bachmani) Am J Hyg. 1963;77(2):195–204. [PubMed] [Google Scholar]
- 51.Kessel J.F., Fisk R.T., Prouty C.C. Studies with the Californian strain of the virus of infectious myxomatosis. In: Cook S.J., editor. Proceedings of the 5th Pacific Science Congress, Canada, 1933. University of Toronto Press; 1934. pp. 2927–2939. [Google Scholar]
- 52.Patton N.M., Holmes H.T. Myxomatosis in domestic rabbits in Oregon. J Am Vet Med Assoc. 1977;171(6):560–562. [PubMed] [Google Scholar]
- 53.Licon Luna R.M. First report of myxomatosis in Mexico. J Wildl Dis. 2000;36(3):580–583. doi: 10.7589/0090-3558-36.3.580. [DOI] [PubMed] [Google Scholar]
- 54.Kritas S.K., Dovas C., Fortomaris P. A pathogenic myxoma virus in vaccinated and non-vaccinated commercial rabbits. Res Vet Sci. 2008;85(3):622–624. doi: 10.1016/j.rvsc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Marlier D., Mainil J., Sulon J. Study of the virulence of five strains of amyxomatous myxoma virus in crossbred New Zealand White/Californian conventional rabbits, with evidence of long-term testicular infection in recovered animals. J Comp Pathol. 2000;122(2–3):101–113. doi: 10.1053/jcpa.1999.0345. [DOI] [PubMed] [Google Scholar]
- 56.Arthur C.P., Louzis C. La myxomatose du lapin en France: une revue. Rev Sci Tech. 1988;7(4):937–957. doi: 10.20506/rst.7.4.385. [DOI] [PubMed] [Google Scholar]
- 57.Marlier D., Cassart D., Boucraut-Baralon C. Experimental infection of specific pathogen-free New Zealand White rabbits with five strains of amyxomatous myxoma virus. J Comp Pathol. 1999;121(4):369–384. doi: 10.1053/jcpa.1999.0335. [DOI] [PubMed] [Google Scholar]
- 58.Marlier D., Herbots J., Detilleux J. Cross-sectional study of the association between pathological conditions and myxoma virus seroprevalence in intensive rabbit farms in Europe. Prev Vet Med. 2001;48(1):55–64. doi: 10.1016/s0167-5877(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 59.Fenner F., Marshall I.D. A comparison of the virulence for European rabbits (Oryctolagus cuniculus) of strains of myxoma virus recovered in the field in Australia, Europe and America. J Hyg (Lond) 1957;55(2):149–191. doi: 10.1017/s0022172400037098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fenner F. The Florey lecture, 1983. Biological control, as exemplified by smallpox eradication and myxomatosis. Proc R Soc Lond B Biol Sci. 1983;218(1212):259–285. doi: 10.1098/rspb.1983.0039. [DOI] [PubMed] [Google Scholar]
- 61.Fountain S., Holland M.K., Hinds L.A. Interstitial orchitis with impaired steroidogenesis and spermatogenesis in the testes of rabbits infected with an attenuated strain of myxoma virus. J Reprod Fertil. 1997;110(1):161–169. doi: 10.1530/jrf.0.1100161. [DOI] [PubMed] [Google Scholar]
- 62.Best S.M., Collins S.V., Kerr P.J. Coevolution of host and virus: cellular localization of virus in myxoma virus infection of resistant and susceptible European rabbits. Virology. 2000;277(1):76–91. doi: 10.1006/viro.2000.0505. [DOI] [PubMed] [Google Scholar]
- 63.Fenner F., Woodroofe G.M. The pathogenesis of infectious myxomatosis; the mechanism of infection and the immunological response in the European rabbit (Oryctolagus cuniculus) Br J Exp Pathol. 1953;34(4):400–411. [PMC free article] [PubMed] [Google Scholar]
- 64.Best S.M., Kerr P.J. Coevolution of host and virus: the pathogenesis of virulent and attenuated strains of myxoma virus in resistant and susceptible European rabbits. Virology. 2000;267(1):36–48. doi: 10.1006/viro.1999.0104. [DOI] [PubMed] [Google Scholar]
- 65.Mims C. Aspects of the pathogenesis of viral diseases. Bacteriol Rev. 1964;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strayer D.S. Determinants of virus-related suppression of immune responses as observed during infection with an oncogenic poxvirus. In: Melnick J.L., editor. Progress in medical virology. Karger; Basel (Switzerland): 1992. pp. 228–255. [PubMed] [Google Scholar]
- 67.Cameron C.M., Barrett J.W., Liu L. Myxoma virus M141R expresses a viral CD200 (vOX-2) that is responsible for down-regulation of macrophage and T-cell activation in vivo. J Virol. 2005;79(10):6052–6067. doi: 10.1128/JVI.79.10.6052-6067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnston J.B., Barrett J.W., Nazarian S.H. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23(6):587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Jeklova E., Leva L., Matiasovic J. Characterisation of immunosuppression in rabbits after infection with myxoma virus. Vet Microbiol. 2008;129(1–2):117–130. doi: 10.1016/j.vetmic.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 70.Cameron C.M., Barrett J.W., Mann M. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology. 2005;337(1):55–67. doi: 10.1016/j.virol.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 71.Hurst E.W. Myxoma and the Shope fibroma. I. The histology of myxoma. Br J Exp Pathol. 1937;18(1):1–15. [Google Scholar]
- 72.Bertagnoli S. Myxomatosis. In: OIE Biological Standards Commission, editor. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 7th edition. Office International des Épizooties; Paris: 2012. Section 2:6.1. [Google Scholar]
- 73.Jin L., Valentine B.A., Baker R.J. An outbreak of fatal herpesvirus infection in domestic rabbits in Alaska. Vet Pathol. 2008;45(3):369–374. doi: 10.1354/vp.45-3-369. [DOI] [PubMed] [Google Scholar]
- 74.Marshall I.D. The influence of ambient temperature on the course of myxomatosis in rabbits. J Hyg (Lond) 1959;57:484–497. doi: 10.1017/s0022172400020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKercher D.G. Infectious myxomatosis. I. Vaccination. II. Antibiotic therapy. Am J Vet Res. 1952;13(48):425–429. [PubMed] [Google Scholar]
- 76.Robinson A.J., Muller W.J., Braid A.L. The effect of buprenorphine on the course of disease in laboratory rabbits infected with myxoma virus. Lab Anim. 1999;33(3):252–257. doi: 10.1258/002367799780578129. [DOI] [PubMed] [Google Scholar]
- 77.Adams M.M., Rice A.D., Moyer R.W. Rabbitpox virus and vaccinia virus infection of rabbits as a model for human smallpox. J Virol. 2007;81(20):11084–11095. doi: 10.1128/JVI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rice A.D., Adams M.M., Wallace G. Efficacy of CMX001 as a post exposure antiviral in New Zealand white rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses. 2011;3(1):47–62. doi: 10.3390/v3010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marlier D. Vaccination strategies against myxomavirus infections: are we really doing the best? Tijdschr Diergeneeskd. 2010;135(5):194–198. [PubMed] [Google Scholar]
- 80.Brun A., Godard A., Moreau Y. La vaccination contre la myxomatose. Vaccins heterologues et homologues. Bulletin de la Société des sciences vétérinaires de (Lyon) 1981;83(5):251–254. [Google Scholar]
- 81.Vautherot J.F., Milon A., Petit F. Vaccines for lagomorphs. In: Pastoret P.P., Blancou J., Vannier P., editors. Veterinary vaccinology. Elsevier; Amsterdam (Netherlands): 1997. pp. 406–410. [Google Scholar]
- 82.Hyde R.R., Gardner R.E. Transmission experiments with the fibroma (Shope) and myxoma (Sanarelli) viruses. Am J Hyg. 1939;30(1/3):57–63. [Google Scholar]
- 83.Smith J.W., Tevethia S.S., Levy B.M. Comparative studies on host responses to Shope fibroma virus in adult and newborn rabbits. J Natl Cancer Inst. 1973;50(6):1529–1539. doi: 10.1093/jnci/50.6.1529. [DOI] [PubMed] [Google Scholar]
- 84.Fenner F., Woodroofe G.M. Protection of laboratory rabbits against myxomatosis by vaccination with fibroma virus. Aust J Exp Biol Med Sci. 1954;32(5):653–668. doi: 10.1038/icb.1954.68. [DOI] [PubMed] [Google Scholar]
- 85.Shope R.E. A transmissible tumor-like condition in rabbits. J Exp Med. 1932;56(6):793–802. doi: 10.1084/jem.56.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kilham L., Dalmat H.T. Host-virus-mosquito relations of Shope fibromas in cottontail rabbits. Am J Hyg. 1955;61(1):45–54. doi: 10.1093/oxfordjournals.aje.a119737. [DOI] [PubMed] [Google Scholar]
- 87.Kilham L., Woke P.A. Laboratory transmission of fibromas (Shope) in cottontail rabbits by means of fleas and mosquitoes. Proc Soc Exp Biol Med. 1953;83(2):296–301. doi: 10.3181/00379727-83-20339. [DOI] [PubMed] [Google Scholar]
- 88.Joiner G.N., Jardine J.H., Gleiser C.A. An epizootic of shope fibromatosis in a commercial rabbitry. J Am Vet Med Assoc. 1971;159(11):1583–1587. [PubMed] [Google Scholar]
- 89.Dalmat H.T. Arthropod transmission of rabbit fibromatosis (Shope) J Hyg (Lond) 1959;57(1):1–30. doi: 10.1017/s0022172400019860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dalmat H.T., Stanton M.F. A comparative study of the Shope fibroma in rabbits in relation to transmissibility by mosquitoes. J Natl Cancer Inst. 1959;22(3):593–615. [PubMed] [Google Scholar]
- 91.Dalmat H.T. Effects of x-rays and chemical carcinogens on infectivity of domestic rabbit fibromas for arthropods. J Infect Dis. 1958;102(2):153–157. doi: 10.1093/infdis/102.2.153. [DOI] [PubMed] [Google Scholar]
- 92.Raflo C.P., Olsen R.G., Pakes S.P. Characterization of a fibroma virus isolated from naturally-occurring skin tumors in domestic rabbits. Lab Anim Sci. 1973;23(4):525–532. [PubMed] [Google Scholar]
- 93.von Bomhard W., Goldschmidt M.H., Shofer F.S. Cutaneous neoplasms in pet rabbits: a retrospective study. Vet Pathol. 2007;44(5):579–588. doi: 10.1354/vp.44-5-579. [DOI] [PubMed] [Google Scholar]
- 94.Hurst E.W. Myxoma and the Shope fibroma. IV. The histology of Shope fibroma. Aust J Exp Biol Med Sci. 1938;16(1):53–64. [Google Scholar]
- 95.Sell S., Scott C.B. An immunohistologic study of Shope fibroma virus in rabbits: tumor rejection by cellular reaction in adults and progressive systemic reticuloendothelial infection in neonates. J Natl Cancer Inst. 1981;66(2):363–373. [PubMed] [Google Scholar]
- 96.Rizzi V., Legrottaglie R., Cini A. Electrophoretic typing of some strains of enteric viruses isolated in rabbits suffering from diarrhoea. New Microbiol. 1995;18(1):77–81. [PubMed] [Google Scholar]
- 97.Tanaka T.N., Conner M.E., Graham D.Y. Molecular characterization of three rabbit rotavirus strains. Arch Virol. 1988;98(3):253–265. doi: 10.1007/BF01322173. [DOI] [PubMed] [Google Scholar]
- 98.Thouless M.E., DiGiacomo R.F., Neuman D.S. Isolation of two lapine rotaviruses: characterization of their subgroup, serotype and RNA electropherotypes. Arch Virol. 1986;89(1):161–170. doi: 10.1007/BF01309886. [DOI] [PubMed] [Google Scholar]
- 99.Martella V., Ciarlet M., Camarda A. Molecular characterization of the VP4, VP6, VP7, and NSP4 genes of lapine rotaviruses identified in Italy: emergence of a novel VP4 genotype. Virology. 2003;314(1):358–370. doi: 10.1016/s0042-6822(03)00418-5. [DOI] [PubMed] [Google Scholar]
- 100.Endre K., Iren H., Arpad A. Occurrence of rotavirus infection of rabbits in Hungary. Magy Allatorvosok Lapja. 1982;37(4):248–254. [Google Scholar]
- 101.Bányai K., Forgách P., Erdélyi K. Identification of the novel lapine rotavirus genotype P[22] from an outbreak of enteritis in a Hungarian rabbitry. Virus Res. 2005;113(2):73–80. doi: 10.1016/j.virusres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 102.Martella V., Ciarlet M., Lavazza A. Lapine rotaviruses of the genotype P[22] are widespread in Italian rabbitries. Vet Microbiol. 2005;111(1–2):117–124. doi: 10.1016/j.vetmic.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Badagliacca P., Letizia A., Candeloro L. Clinical, pathological and microbiological profiles of spontaneous enteropathies in growing rabbits. World Rabbit Sci. 2010;18(4):187–198. [Google Scholar]
- 104.Marlier D., Vindevogel H. Les maladies virales chez le lapin Européen (Oryctolagus cuniculus) Ann Med Vet. 1996;140(6):393–403. [Google Scholar]
- 105.Percy D.H., Muckle C.A., Hampson R.J. The enteritis complex in domestic rabbits: a field study. Can Vet J. 1993;34(2):95–102. [PMC free article] [PubMed] [Google Scholar]
- 106.Schoeb T.R., Casebolt D.B., Walker V.E. Rotavirus-associated diarrhea in a commercial rabbitry. Lab Anim Sci. 1986;36(2):149–152. [PubMed] [Google Scholar]
- 107.Iwai H., Machii K., Ohtsuka Y. Prevalence of antibodies to Sendai virus and rotavirus in laboratory rabbits. Jikken Dobutsu. 1986;35(4):491–494. doi: 10.1538/expanim1978.35.4_491. [DOI] [PubMed] [Google Scholar]
- 108.Petric M., Middleton P.J., Grant C. Lapine rotavirus: preliminary studies on epizoology and transmission. Can J Comp Med. 1978;42(1):143–147. [PMC free article] [PubMed] [Google Scholar]
- 109.Legrottaglie R., Mannelli A., Rizzi V. Isolation and characterization of cytopathic strains of rotavirus from hares (Lepus europaeus) New Microbiol. 1997;20(2):135–140. [PubMed] [Google Scholar]
- 110.Thouless M.E., DiGiacomo R.F., Deeb B.J. Pathogenicity of rotavirus in rabbits. J Clin Microbiol. 1988;26(5):943–947. doi: 10.1128/jcm.26.5.943-947.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li D., Gu A.Z., Zeng S. Evaluation of the infectivity, gene and antigenicity persistence of rotaviruses by free chlorine disinfection. J Environ Sci (China) 2011;23(10):1691–1698. doi: 10.1016/s1001-0742(10)60623-7. [DOI] [PubMed] [Google Scholar]
- 112.Saif L.J. Reoviridae. In: Maclachlan N.J., Dubovi E.J., editors. Fenner's veterinary virology. 4th edition. Academic Press; San Diego (CA): 2011. pp. 275–291. [Google Scholar]
- 113.Ciarlet M., Gilger M.A., Barone C. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology. 1998;251(2):343–360. doi: 10.1006/viro.1998.9406. [DOI] [PubMed] [Google Scholar]
- 114.DiGiacomo R.F., Thouless M.E. Epidemiology of naturally occurring rotavirus infection in rabbits. Lab Anim Sci. 1986;36(2):153–156. [PubMed] [Google Scholar]
- 115.Lorrot M., Martin S., Vasseur M. Rotavirus infection stimulates the Cl- reabsorption process across the intestinal brush-border membrane of young rabbits. J Virol. 2003;77(17):9305–9311. doi: 10.1128/JVI.77.17.9305-9311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Halaihel N., Lievin V., Alvarado F. Rotavirus infection impairs intestinal brush-border membrane Na(+)-solute cotransport activities in young rabbits. Am J Physiol Gastrointest Liver Physiol. 2000;279(3):G587–G596. doi: 10.1152/ajpgi.2000.279.3.G587. [DOI] [PubMed] [Google Scholar]
- 117.Thouless M.E., DiGiacomo R.F., Deeb B.J. The effect of combined rotavirus and Escherichia coli infections in rabbits. Lab Anim Sci. 1996;46(4):381–385. [PubMed] [Google Scholar]
- 118.Fushuku S., Fukuda K. Examination of the applicability of a commercial human rotavirus antigen detection kit for use in laboratory rabbits. Exp Anim. 2006;55(1):71–74. doi: 10.1538/expanim.55.71. [DOI] [PubMed] [Google Scholar]
- 119.Sato K., Inaba Y., Miura Y. Isolation of lapine rotavirus in cell cultures. Arch Virol. 1982;71(3):267–271. doi: 10.1007/BF01314878. [DOI] [PubMed] [Google Scholar]
- 120.Conner M.E., Estes M.K., Graham D.Y. Rabbit model of rotavirus infection. J Virol. 1988;62(5):1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ciarlet M., Crawford S.E., Barone C. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J Virol. 1998;72(11):9233–9246. doi: 10.1128/jvi.72.11.9233-9246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rous P., Kidd J.G. The carcinogenic effect of a papilloma virus on the tarred skin of rabbits: I. Description of the phenomenon. J Exp Med. 1938;67(3):399–428. doi: 10.1084/jem.67.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shope R.E. Immunization of rabbits to infectious papillomatosis. J Exp Med. 1937;65(2):219–231. doi: 10.1084/jem.65.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shope R.E., Hurst E.W. Infectious papillomatosis of rabbits: with a note on the histopathology. J Exp Med. 1933;58(5):607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weiner C.M., Rosenbaum M.D., Fox K. Cottontail rabbit papillomavirus in Langerhans cells in Sylvilagus spp. J Vet Diagn Invest. 2010;22(3):451–454. doi: 10.1177/104063871002200321. [DOI] [PubMed] [Google Scholar]
- 126.Griem W. Kaninchen Papillomatose: einen Bericht über drei spontane Fälle unter den Versuchstieren. Z Versuchstierkd. 1982;24(1/2):36. [Google Scholar]
- 127.Hagen K.W. Spontaneous papillomatosis in domestic rabbits. Bull Wildl Dis Ass. 1966;2(4):108–110. [Google Scholar]
- 128.Watts S.L., Ostrow R.S., Phelps W.C. Free cottontail rabbit papillomavirus DNA persists in warts and carcinomas of infected rabbits and in cells in culture transformed with virus or viral DNA. Virology. 1983;125(1):127–138. doi: 10.1016/0042-6822(83)90069-7. [DOI] [PubMed] [Google Scholar]
- 129.Hu J., Budgeon L.R., Cladel N.M. Detection of L1, infectious virions and anti-L1 antibody in domestic rabbits infected with cottontail rabbit papillomavirus. J Gen Virol. 2007;88(Pt 12):3286–3293. doi: 10.1099/vir.0.82879-0. [DOI] [PubMed] [Google Scholar]
- 130.Amella C.A., Lofgren L.A., Ronn A.M. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency. Am J Pathol. 1994;144(6):1167–1171. [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang P., Nouri M., Brandsma J.L. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation. Virology. 1999;263(2):388–394. doi: 10.1006/viro.1999.9950. [DOI] [PubMed] [Google Scholar]
- 132.Zeltner R., Borenstein L.A., Wettstein F.O. Changes in RNA expression pattern during the malignant progression of cottontail rabbit papillomavirus-induced tumors in rabbits. J Virol. 1994;68(6):3620–3630. doi: 10.1128/jvi.68.6.3620-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Christensen N.D., Han R., Cladel N.M. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob Agents Chemother. 2001;45(4):1201–1209. doi: 10.1128/AAC.45.4.1201-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Poetker D.M., Kerschner J.E., Patel N.J. Immune stimulation for the treatment of papilloma. Ann Otol Rhinol Laryngol. 2005;114(9):657–661. doi: 10.1177/000348940511400901. [DOI] [PubMed] [Google Scholar]
- 135.Sundberg J.P., Junge R.E., el Shazly M.O. Oral papillomatosis in New Zealand white rabbits. Am J Vet Res. 1985;46(3):664–668. [PubMed] [Google Scholar]
- 136.Mews A.R., Ritchie J.S., Romero-Mercado C.H. Detection of oral papillomatosis in a British rabbit colony. Lab Anim. 1972;6(2):141–145. doi: 10.1258/002367772781006310. [DOI] [PubMed] [Google Scholar]
- 137.Weisbroth S.H., Scher S. Spontaneous oral papillomatosis in rabbits. J Am Vet Med Assoc. 1970;157(11):1940–1944. [PubMed] [Google Scholar]
- 138.Dominguez J.A., Corella E.L., Auro A. Oral papillomatosis in two laboratory rabbits in Mexico. Lab Anim Sci. 1981;31(1):71–73. [PubMed] [Google Scholar]
- 139.Parsons R.J., Kidd J.G. Oral papillomatosis of rabbits: a virus disease. J Exp Med. 1943;77(3):233–250. doi: 10.1084/jem.77.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rdzok E.J., Shipkowitz N.L., Richter W.R. Rabbit oral papillomatosis: ultrastructure of experimental infection. Cancer Res. 1966;26(1):160–165. [PubMed] [Google Scholar]
- 141.Munday J.S., Aberdein D., Squires R.A. Persistent conjunctival papilloma due to oral papillomavirus infection in a rabbit in New Zealand. J Am Assoc Lab Anim Sci. 2007;46(5):69–71. [PubMed] [Google Scholar]
- 142.Christensen N.D., Cladel N.M., Reed C.A. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection. Virology. 2000;269(2):451–461. doi: 10.1006/viro.2000.0237. [DOI] [PubMed] [Google Scholar]
- 143.Parrish C.R. Papillomaviridae and Polyomaviridae. In: Maclachlan N.J., Dubovi E.J., editors. Fenner's veterinary virology. 4th edition. Academic Press; San Diego (CA): 2011. pp. 213–223. [Google Scholar]
- 144.Weisbroth S.H. Sialocele (ranula) simulating oral papillomatosis in a domestic (Oryctolagus) rabbit. Lab Anim Sci. 1975;25(3):321–322. [PubMed] [Google Scholar]
- 145.Grest P., Albicker P., Hoelzle L. Herpes simplex encephalitis in a domestic rabbit (Oryctolagus cuniculus) J Comp Pathol. 2002;126(4):308–311. doi: 10.1053/jcpa.2002.0548. [DOI] [PubMed] [Google Scholar]
- 146.Muller K., Fuchs W., Heblinski N. Encephalitis in a rabbit caused by human herpesvirus-1. J Am Vet Med Assoc. 2009;235(1):66–69. doi: 10.2460/javma.235.1.66. [DOI] [PubMed] [Google Scholar]
- 147.Sekulin K., Jankova J., Kolodziejek J. Natural zoonotic infections of two marmosets and one domestic rabbit with herpes simplex virus type 1 did not reveal a correlation with a certain gG-, gI- or gE genotype. Clin Microbiol Infect. 2010;16(11):1669–1672. doi: 10.1111/j.1469-0691.2010.03163.x. [DOI] [PubMed] [Google Scholar]
- 148.Weissenböck H., Hainfellner J.A., Berger J. Naturally occurring herpes simplex encephalitis in a domestic rabbit (Oryctolagus cuniculus) Vet Pathol. 1997;34(1):44–47. doi: 10.1177/030098589703400107. [DOI] [PubMed] [Google Scholar]
- 149.Wohlsein P., Thiele A., Fehr M. Spontaneous human herpes virus type 1 infection in a chinchilla (Chinchilla lanigera f. dom.) Acta Neuropathol. 2002;104(6):674–678. doi: 10.1007/s00401-002-0597-6. [DOI] [PubMed] [Google Scholar]
- 150.Goudas P., Giltoy J.S. Spontaneous herpes-like viral infection in a chinchilla (Chinchilla laniger) J Wildl Dis. 1970;6(3):175–179. doi: 10.7589/0090-3558-6.3.175. [DOI] [PubMed] [Google Scholar]
- 151.Skinner G.R., Ahmad A., Davies J.A. The infrequency of transmission of herpesviruses between humans and animals; postulation of an unrecognised protective host mechanism. Comp Immunol Microbiol Infect Dis. 2001;24(4):255–269. doi: 10.1016/s0147-9571(01)00014-5. [DOI] [PubMed] [Google Scholar]
- 152.Gruber A., Pakozdy A., Weissenbock H. A retrospective study of neurological disease in 118 rabbits. J Comp Pathol. 2009;140(1):31–37. doi: 10.1016/j.jcpa.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 153.Brash M.L., Nagy E., Pei Y. Acute hemorrhagic and necrotizing pneumonia, splenitis, and dermatitis in a pet rabbit caused by a novel herpesvirus (leporid herpesvirus-4) Can Vet J. 2010;51(12):1383–1386. [PMC free article] [PubMed] [Google Scholar]
- 154.Onderka D.K., Papp-Vid G., Perry A.W. Fatal herpesvirus infection in commercial rabbits. Can Vet J. 1992;33(8):539–543. [PMC free article] [PubMed] [Google Scholar]
- 155.Swan C., Perry A., Papp-Vid G. Alberta. Herpesvirus-like viral infection in a rabbit. Can Vet J. 1991;32(10):627–628. [PMC free article] [PubMed] [Google Scholar]
- 156.Jin L., Lohr C.V., Vanarsdall A.L. Characterization of a novel alphaherpesvirus associated with fatal infections of domestic rabbits. Virology. 2008;378(1):13–20. doi: 10.1016/j.virol.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 157.Medveczky P.G. Herpesvirus sylvilagus (Herpesviridae) In: Allan G., Robert G.W., editors. Encyclopedia of virology. Elsevier; Oxford (United Kingdom): 1999. pp. 703–706. [Google Scholar]
- 158.Schmidt S.P., Bates G.N., Lewandoski P.J. Probable herpesvirus-infection in an eastern cottontail (Sylvilagus floridanus) J Wildl Dis. 1992;28(4):618–622. doi: 10.7589/0090-3558-28.4.618. [DOI] [PubMed] [Google Scholar]
- 159.Hesselton R.M., Yang W.C., Medveczky P. Pathogenesis of herpesvirus sylvilagus infection in cottontail rabbits. Am J Pathol. 1988;133(3):639–647. [PMC free article] [PubMed] [Google Scholar]
- 160.Martella V., Moschidou P., Pinto P. Astroviruses in rabbits. Emerg Infect Dis. 2011;17(12):2287–2293. doi: 10.3201/eid1712.110967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.De Benedictis P., Schultz-Cherry S., Burnham A. Astrovirus infections in humans and animals—molecular biology, genetic diversity, and interspecies transmissions. Infect Genet Evol. 2011;11(7):1529–1544. doi: 10.1016/j.meegid.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Richt J.A., Pfeuffer I., Christ M. Borna disease virus infection in animals and humans. Emerg Infect Dis. 1997;3(3):343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Metzler A., Ehrensperger F., Wyler R. Naturliche Bornavirus-Infektion bei Kaninchen. Zentralbl Veterinarmed B. 1978;25(2):161–164. [PubMed] [Google Scholar]
- 164.Staeheli P., Rinder M., Kaspers B. Avian bornavirus associated with fatal disease in psittacine birds. J Virol. 2010;84(13):6269–6275. doi: 10.1128/JVI.02567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gosztonyi G., Dietzschold B., Kao M. Rabies and borna disease. A comparative pathogenetic study of two neurovirulent agents. Lab Invest. 1993;68(3):285–295. [PubMed] [Google Scholar]
- 166.Ludwig H., Kraft W., Kao M. Borna-Virus-Infektion (Borna-Krankheit) bei naturlich und experimentell infizierten Tieren: ihre Bedeutung fur Forschung und Praxis. Tierarztl Prax. 1985;13(4):421–453. [PubMed] [Google Scholar]
- 167.Schindler A.R., Vogtlin A., Hilbe M. Reverse transcription real-time PCR assays for detection and quantification of Borna disease virus in diseased hosts. Mol Cell Probes. 2007;21(1):47–55. doi: 10.1016/j.mcp.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Krebs J.W., Wheeling J.T., Childs J.E. Rabies surveillance in the United States during 2002. J Am Vet Med Assoc. 2003;223(12):1736–1748. doi: 10.2460/javma.2003.223.1736. [DOI] [PubMed] [Google Scholar]
- 169.Childs J.E., Colby L., Krebs J.W. Surveillance and spatiotemporal associations of rabies in rodents and lagomorphs in the United States, 1985–1994. J Wildl Dis. 1997;33(1):20–27. doi: 10.7589/0090-3558-33.1.20. [DOI] [PubMed] [Google Scholar]
- 170.Eidson M., Matthews S.D., Willsey A.L. Rabies virus infection in a pet guinea pig and seven pet rabbits. J Am Vet Med Assoc. 2005;227(6):932–935. doi: 10.2460/javma.2005.227.932. [DOI] [PubMed] [Google Scholar]
- 171.Karp B.E., Ball N.E., Scott C.R. Rabies in two privately owned domestic rabbits. J Am Vet Med Assoc. 1999;215(12):1824–1827. [PubMed] [Google Scholar]
- 172.Forest E. Un cas de rage chez un lapin a Montreal. Med Vet Quebec. 1990;20(1):33. [Google Scholar]
- 173.Weyhe D. Tollwut beim Hauskaninchen. Monatsh Veterinarmed. 1979;34(9):336–337. [Google Scholar]
- 174.Hohner L., Klotzer H.H., Heucke H. Zwei Falle von Lyssa beim Hauskaninchen. Monatsh Veterinarmed. 1978;33(14):550–551. [Google Scholar]
- 175.Rosahn P.D., Hu C.K. Rabbit pox: report of an epidemic. J Exp Med. 1935;62(3):331–347. doi: 10.1084/jem.62.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Verlinde J.D., Wensinck F. Manifestation of a laboratory epizootic of rabbit pox by non-specific stimuli. Antonie Van Leeuwenhoek. 1951;17(4):232–236. doi: 10.1007/BF02062268. [DOI] [PubMed] [Google Scholar]
- 177.Greene H.S. Rabbit pox: I. Clinical manifestations and course of disease. J Exp Med. 1934;60(4):427–440. doi: 10.1084/jem.60.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rosahn P.D., Hu C.K., Pearce L. Studies on the etiology of rabbit pox: II. Clinical characteristics of the experimentally induced disease. J Exp Med. 1936;63(2):259–276. doi: 10.1084/jem.63.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Greene H.S. Rabbit pox: II. Pathology of the epidemic disease. J Exp Med. 1934;60(4):441–455. doi: 10.1084/jem.60.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Christensen L.R., Bond E., Matanic B. “Pock-less” rabbit pox. Lab Anim Care. 1967;17(3):281–296. [PubMed] [Google Scholar]
- 181.Bedson H.S., Duckworth M.J. Rabbit pox: an experimental study of the pathways of infection in rabbits. J Pathol Bacteriol. 1963;85:1–20. [PubMed] [Google Scholar]
- 182.Roy C.J., Voss T.G. Use of the aerosol rabbitpox virus model for evaluation of anti-poxvirus agents. Viruses. 2010;2(9):2096–2107. doi: 10.3390/v2092096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Christensen N., Fennestad K.L., Bruun L. Pleural effusion disease in rabbits. Histopathological observations. Acta Pathol Microbiol Scand A. 1978;86(3):251–256. doi: 10.1111/j.1699-0463.1978.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 184.Fennestad K.L., Bruun L., Wedo E. Pleural effusion disease agent as passenger of Treponema pallidum suspensions from rabbits. Survey of laboratories. Br J Vener Dis. 1980;56(4):198–203. doi: 10.1136/sti.56.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fennestad K.L., Mansa B., Christensen N. Pathogenicity and persistence of pleural effusion disease virus isolates in rabbits. J Gen Virol. 1986;67(Pt 6):993–1000. doi: 10.1099/0022-1317-67-6-993. [DOI] [PubMed] [Google Scholar]
- 186.Fledelius H., Bruun L., Fennestad K.L. Uveitis in rabbits with pleural effusion disease. Clinical and histopathological observations. Acta Ophthalmol (Copenh) 1978;56(4):599–606. doi: 10.1111/j.1755-3768.1978.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 187.Osterhaus A.D., Teppema J.S., van Steenis G. Coronavirus-like particles in laboratory rabbits with different syndromes in the Netherlands. Lab Anim Sci. 1982;32(6):663–665. [PubMed] [Google Scholar]
- 188.Small J.D., Aurelian L., Squire R.A. Rabbit cardiomyopathy associated with a virus antigenically related to human coronavirus strain 229E. Am J Pathol. 1979;95(3):709–729. [PMC free article] [PubMed] [Google Scholar]
- 189.Small J.D., Woods R.D. Relatedness of rabbit coronavirus to other coronaviruses. Adv Exp Med Biol. 1987;218:521–527. doi: 10.1007/978-1-4684-1280-2_68. [DOI] [PubMed] [Google Scholar]
- 190.Descoteaux J.P., Lussier G., Berthiaume L. An enteric coronavirus of the rabbit: detection by immunoelectron microscopy and identification of structural polypeptides. Arch Virol. 1985;84(3–4):241–250. doi: 10.1007/BF01378976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Peeters J.E., Pohl P., Charlier G. Infectious agents associated with diarrhoea in commercial rabbits: a field study. Ann Rech Vet. 1984;15(3):335–340. [PubMed] [Google Scholar]
- 192.Eaton P. Preliminary observations on enteritis associated with a coronavirus-like agent in rabbits. Lab Anim. 1984;18(1):71–74. doi: 10.1258/002367784780864938. [DOI] [PubMed] [Google Scholar]
- 193.Deeb B.J., DiGiacomo R.F., Evermann J.F. Prevalence of coronavirus antibodies in rabbits. Lab Anim Sci. 1993;43(5):431–433. [PubMed] [Google Scholar]
- 194.Descoteaux J.P., Lussier G. Experimental infection of young rabbits with a rabbit enteric coronavirus. Can J Vet Res. 1990;54(4):473–476. [PMC free article] [PubMed] [Google Scholar]