Abstract
To identify clusters of canine parvoviral related disease occurring in Australia during 2010 and investigate the role of socio-economic factors contributing to these clusters, reported cases of canine parvovirus were extracted from an on-line disease surveillance system. Reported residential postcode was used to locate cases, and clusters were identified using a scan statistic. Cases included in clusters were compared to those not included in such clusters with respect to human socioeconomic factors (postcode area relative socioeconomic disadvantage, economic resources, education and occupation) and dog factors (neuter status, breed, age, gender, vaccination status).
During 2010, there were 1187 cases of canine parvovirus reported. Nineteen significant (P < 0.05) disease clusters were identified, most commonly located in New South Wales. Eleven (58%) clusters occurred between April and July, and the average cluster length was 5.7 days. All clusters occurred in postcodes with a significantly (P < 0.05) greater level of relative socioeconomic disadvantage and a lower rank in education and occupation, and it was noted that clustered cases were less likely to have been neutered (P = 0.004). No significant difference (P > 0.05) was found between cases reported from cluster postcodes and those not within clusters for dog age, gender, breed or vaccination status (although the latter needs to be interpreted with caution, since vaccination was absent in most of the cases). Further research is required to investigate the apparent association between indicators of poor socioeconomic status and clusters of reported canine parvovirus diseases; however these initial findings may be useful for developing geographically- and temporally-targeted prevention and disease control programs.
Keywords: Canine parvovirus, Spatio-temporal analysis, Socio-economic factors, Health disparities, Surveillance, Australia
Introduction
Canine parvovirus (CPV) is widely distributed in the global canine population and remains an important cause of morbidity and mortality despite extensive vaccination (Goddard and Leisewitz, 2010). The clinical presentation of the disease is most commonly acute enteritis, with severe leukopenia in young dogs up to 6 months of age; however in recent years a number of cases have been reported in older dogs (Goddard and Leisewitz, 2010, Decaro et al., 2008, Decaro et al., 2009, Lamm and Rezabek, 2008). Survival rates have been reported to be as high as 80–95% when cases are treated early and aggressively, but as low as 9.1% without treatment (Goddard and Leisewitz, 2010, Prittie, 2004).
The persistence CPV in dog populations is attributed to its environmental resilience, virulence in susceptible populations, and the ability to mutate and avoid recognition by the immune system even in vaccinated individuals (Pereira et al., 2007). There are currently three widely recognised strains of canine parvovirus, namely, CPV-2a, CPV-2b and the recently characterised CPV-2c, although other strains have also been documented. The most recent study of Australian strains suggests that CPV-2a remains the most prevalent strain; CPV-2b was found uncommonly, and there was no evidence of CPV-2c infection (Meers et al., 2007).
Predisposing factors associated with the development of clinical parvovirus disease include stressors (such as weaning, overcrowding and parasite load), insufficient passive or active immunity, geographical region and the presence of co-pathogens (including canine coronavirus and intestinal parasites) (Goddard and Leisewitz, 2010, Kalli et al., 2010). Some of these factors are thought to increase the likelihood of developing clinical canine parvoviral disease by increasing the mitotic activity of mucosal cells (Goddard and Leisewitz, 2010).
The role of season and breed in the development of CPV is debatable with discrepancies in findings between studies; however, it is possible that the importance of these factors may vary geographically due to local factors such as extremes in weather, environmental viral loads, population density, and breed popularity (Goddard and Leisewitz, 2010, Kalli et al., 2010, Roth and Spickler, 2010, Houston et al., 1996, Godsall et al., 2010). Warmer months have been associated with increased reporting of cases (Goddard and Leisewitz, 2010, Houston et al., 1996). The role of health disparities in infectious disease spread in public health has been recognised for many years, and is one consideration when planning disease control programs (Mbah and Gilligan, 2011). Similar studies in veterinary medicine are rare.
Studies in companion animal epidemiology have been limited by a lack of reliable and suitable data. Companion animal disease surveillance has mainly focused on zoonotic diseases such as rabies, for which case reporting is mandatory in most jurisdictions. Some specific research projects have been conducted, using data collected within the veterinary medical database (source data contributed by veterinary teaching hospitals across the United States) (Moore and Lund, 2009, Ward, 2002, Ward et al., 2002, Blanton et al., 2010). More recently a National Companion Animal Surveillance program for emerging and exotic diseases was established, with coverage of 2% of the entire dog and cat population of the US (Moore et al., 2005, Glickman et al., 2006), but was subsequently discontinued.
Until very recently, epidemiological data on diseases of companion animals in Australia could only be obtained by questionnaires and surveys used for a specific research objective (Sabine et al., 1982, Toribio et al., 2009). The introduction by Virbac Australia of a disease surveillance system, Disease WatchDog, 1 presents opportunities for the veterinary community to accumulate data (both temporally and spatially) on important diseases of dogs and cats relevant to their veterinary patients (Ward and Kelman, 2011). Evidence of spatio-temporal disease clustering indicates that some common factor(s) are contributing to disease propagation in specific areas and that targeted prevention programs will probably be effective in reducing disease occurrence (Ward and Carpenter, 2000). Analysis of data from the Disease WatchDog database may fill some of the current deficits in companion animal disease epidemiology (Ward and Kelman, 2011).
The objective of this study was to identify clusters of canine parvovirus-related disease that occurred in Australia during 2010 and to investigate potential factors contributing to these clusters. Specific aims were to analyse data from Disease WatchDog and describe the role that human socioeconomic indicators (relative socioeconomic disadvantage, access to economic resources, level of education, and occupation status) and dog factors (neuter status, breed, age, gender and vaccination status) might have played in the development of canine parvovirus-related disease clusters. We also wished to assess the role of geographical distribution of registered clinics in the formation of these clusters. The study endeavoured to further characterise canine parvoviral disease occurrence in Australia and provide insights into potential target areas for disease prevention.
Materials and methods
Data source
All case data for the study was acquired via the Disease WatchDog database, which was launched in January 2010 to log cases of diseases of dogs (including parvovirus) and cats occurring in Australia (Ward and Kelman, 2011). The database relies on veterinary practitioners and nurses entering case details; in exchange, practices gain access to real-time maps and data specific to their practice area. Such access to up-to-date epidemiological data enables practitioners to make more informed decisions regarding vaccination schedules and health prevention protocols relevant to their veterinary patients.
Records of all cases reported during 2010 were extracted. All cases of parvovirus-related disease reported were screened for duplicate entries to ensure that case reports were only included once in analyses. Each record entered was counted as one case report, even though there may have been more than one disease case when a litter was involved in the report. We assumed that a litter of puppies reported represented a single parvovirus infection event; because of the highly contagious nature of this disease, all puppies within a litter would presumably have been infected if the litter was infected and therefore represented one epidemiologic study unit. Each report (record) was allocated a case identification number and contained the following generic data fields: clinic name, veterinarian name, case occurrence date, animal name, suburb, postcode, state, species, breed, age (years, months, weeks), gender (male, female or unknown), neuter status (neutered, entire or unknown), disease (including canine parvovirus), case diagnosis (clinical presentation, ELISA snap test, PCR, immunofluorescence or other), case outcome (died, recovered, euthanased, tested positive but not clinically affected or treatment ongoing), vaccination status (vaccinated, unvaccinated or unknown), vaccine given and vaccine date. In addition, there was an optional field to record litters infected (number of animals in litter, number of animals in litter infected) although this additional data was not analysed in the current study.
Socio-economic data was sourced from the 2006 Australian census, made available by the Australian Bureau of Statistics. 2 Census data for each Australian postcode was obtained in summarised format from the Socio-economic Indexes for Areas (SEIFA) data cube. Indices recorded in the data set included education and occupation, economic resources, relative socio-economic disadvantage, and relative socio-economic advantage and disadvantage. The usual human population of each postal area code was also recorded. The index of socio-economic disadvantage is measured using financial and overall liveability factors, and can only be used as an indication of disadvantage (i.e. while a low score indicates greater relative disadvantage, a higher score does not necessarily indicate advantage; Pink, 2008).
The economic resources index is a ranking of postcodes based on indicators of high and low income and variables that correlate with high or low wealth, with higher scores indicating greater access to economic resources. Low education and occupation index scores represent postal areas with a high proportion of the population without tertiary qualifications, without jobs or with low skilled jobs; in contrast, a high score for this index suggests that a greater proportion of postcode residents are qualified and employed in skilled jobs (Pink, 2008). In addition, we used the relative socio-economic disadvantage index. A lower score for a postal area indicated greater relative disadvantage, with deciles also recorded for each postal area in relation to these scores (i.e. the lowest 10% of all postcode scores were allocated a decile of 1, while the highest 10% of all postcode scores were allocated a decile of 10; Pink, 2008).
Data management
Dog factors extracted from the recorded data in Disease WatchDog and analysed were neuter status, breed, age, gender and vaccination status. Neuter status was categorised as neutered or entire. Breeds were allocated to one of seven categories based on the Australian National Kennel Council breed standards. 3 Any cases recorded as crossbreeds or mixed breed were coded as mixed; the remainder of the dogs were classified by breed as toy, terrier, gundog, hound, working, utility or non-sporting. Three breeds reported in the extracted data are not recognised by the ANKC; these were subsequently classified as working (Bull Arab, Koolie) and non-sporting (Pit-bull).
Vaccination status was reported as vaccinated, unvaccinated or unknown. Vaccinated dogs were those that were recorded as having received at least one vaccination in their life. Based on reported information regarding date of vaccination, dogs classified as vaccinated were further categorised as vaccination incomplete (i.e. last recorded vaccination before 16 weeks of age), vaccinated within the previous 12 months, or non-recent vaccination (last recorded vaccination greater than 3 years prior to infection). Eleven cases classified initially as vaccinated were excluded from analysis of vaccination category due to errors (inconsistencies) in the reported dates of vaccine given.
The age of dogs was transformed from a years–months–weeks format to weeks only. For this transformation, it was assumed that 1 month consisted of 4 weeks and that 1 year consisted of 52 weeks. Gender was categorised as male, female or unknown. All clinic data was sorted according to postcode and month of registration in the database. Duplicate clinic entries (based on clinic name, postcode and state) were excluded, as were registrants identified as businesses other than Australian veterinary practices, and non-practising veterinarians.
Once disease clusters were identified at a postcode level, all recorded parvovirus cases were divided (based on postcode) into two data sets, namely, those cases within a cluster, and those not within a cluster. Although clusters were identified using a scanning window of 25% of the population and 2 week time period (see below), all cases recorded for these postcodes during the year 2010 were included in the ‘within cluster’ data set.
Data analysis
Maps displaying disease clusters, canine parvovirus case locations, and registered clinic locations were generated using ArcGIS v. 10 (ESRI). A retrospective space–time analysis scanning for clusters with high rates of disease was performed using the Space–Time Permutation model (SaTScan v9.1.1 Kulldorf M. and Information Management Services 2009). Space–time canine parvovirus case clusters were identified using a maximum spatial cluster size of 25% of the population at risk (i.e. 297 cases), and a maximum temporal cluster size of 4% of study period (1 January to 31 December 2010) i.e. 2 weeks. Clusters identified were postal code areas where a significantly (P < 0.05) greater number of cases were reported within the spatio-temporal window than would be expected based on the total number of canine parvovirus cases reported in Disease WatchDog during the study period (1 January to 31 December, 2010).
A Wilcoxon rank-sum test by cluster (outside vs. within cluster) was performed for age of reported cases, and also for both decile and score of postcode education and occupation, economic resources, and relative socio-economic disadvantage indices. Significance was reported using a two-tailed P-value for normal approximation. In addition, a χ 2-squared test for independence was performed by cluster (outside vs. within cluster) for reported dog neuter status, breed, gender and vaccination status. An overall χ 2-squared statistic and P-value were reported (Statistix v 8.0. Analytical Software).
Results
Extraction of reported cases of canine parvovirus in Disease WatchDog between 1 January and 31 December 2010 yielded 1187 cases (individual dogs and litters) from 169 clinics across Australia. Of these cases 916 (77%) were reported to be diagnosed by an ELISA antigen test, 233 (20%) by clinical presentation, and 38 (3%) by other methods (including PCR, immunofluorescence, and other unspecified CPV test). Overall, cases were reported from 357 (14.2%) Australian postal codes (Fig. 1 ).
Fig. 1.
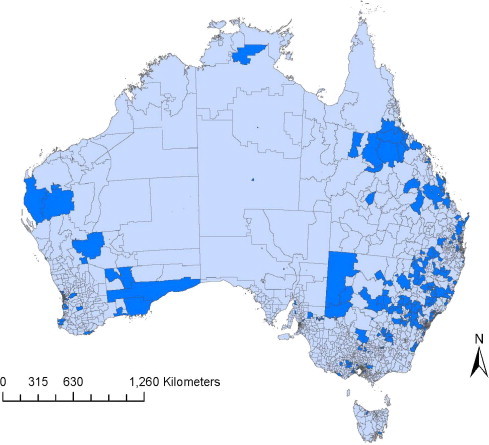
Distribution of Australian postcodes that reported one or more cases of canine parvovirus within the Disease WatchDog database in 2010.
Clinic results
During 2010, 622 Australian veterinary practices across all States and Territories became registered users of Disease WatchDog (approximately 30% of all registered practices in Australia). Of these clinics 27% (n = 168) of all registrations occurred during February (Fig. S1, Appendix A). Registrations during the remainder of 2010 ranged from 13 to 72 per month. In addition, 37.8% of all registered clinics were located in NSW, accounting for 37.2% of all registered veterinary practices in this state (n = 632). Overall, registered clinics were located in 481 Australian postal codes (Fig. S2, Appendix A) and distributed throughout most of the populated regions of Australia.
Cluster results
Nineteen significant (P < 0.05) space–time disease clusters were identified with a mean radius of 42.3 km (range 0–223 km). Clusters with a radius of 0 km represent clusters of disease reports within a single post code. The median number of cases within these clusters was 7 (range 4–22). Clusters were identified in both rural and urban areas of New South Wales (9), Victoria (3), Queensland (3), South Australia (1), and Western Australia (3; Fig. 2 ). Eleven (58%) clusters occurred between April and July (Fig. 3 ). The average cluster lasted for 5.7 days. A complete list of identified clusters is presented in Table 1 .
Fig. 2.
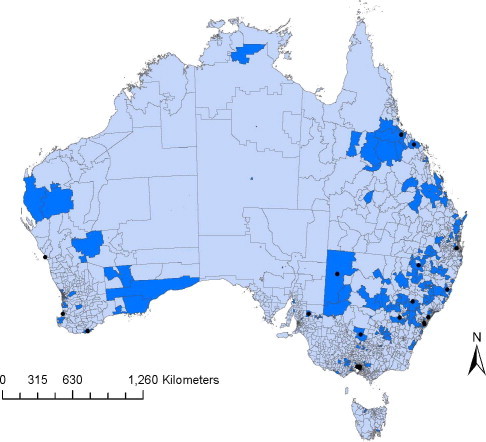
Postcodes from which canine parvovirus was reported within the Disease WatchDog database in 2010 (dark blue) and the centres of clusters (black dots) identified using a space–time permutation test.
Fig. 3.
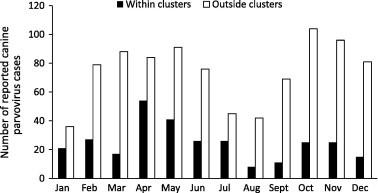
Distribution of new parvovirus cases reported in Australia within the Disease WatchDog database by month within clusters identified using a space–time permutation scan test and in areas outside identified clusters.
Table 1.
Clusters of parvovirus disease in Australia reported in Disease WatchDog (www.diseasewatchdog.org) during 2010 and identified using the space–time permutation model.
| Cluster | Start | End | Number of cases | Expected cases | Radius (km) | Location | Epicentre |
|---|---|---|---|---|---|---|---|
| 1 | 04/02/10 | 04/02/10 | 9 | 0.13 | 0 | Regional QLD (North-East) | Bowen |
| 2 | 21/04/10 | 30/04/10 | 22 | 2.61 | 52 | Regional NSW (Central) | Cowra |
| 3 | 13/11/10 | 13/11/10 | 7 | 0.08 | 0 | North-West Sydney | Blacktown |
| 4 | 02/12/10 | 03/12/10 | 6 | 0.05 | 0 | Regional SA (South-East) | Morgan |
| 5 | 03/01/10 | 14/01/10 | 12 | 0.92 | 18 | Regional WA (South-West) | Bunbury |
| 6 | 18/07/10 | 19/07/10 | 7 | 0.18 | 68 | Regional NSW (Central) | Mudgee |
| 7 | 01/05/10 | 11/05/10 | 15 | 1.79 | 173 | Regional NSW (North-East) | Taree, Tamworth, Coffs Harbour |
| 8 | 03/05/11 | 03/05/11 | 5 | 0.044 | 0 | South-West Melbourne | Altona |
| 9 | 20/05/10 | 21/05/10 | 5 | 0.04 | 223 | Regional WA (Southern coast) | Albany |
| 10 | 04/10/10 | 16/10/10 | 12 | 1.04 | 0 | Regional NSW (Central-South) | Deniliquin |
| 11 | 14/08/10 | 14/08/10 | 5 | 0.05 | 0 | Regional QLD (North-East) | Kelso |
| 12 | 02/04/10 | 02/04/10 | 5 | 0.05 | 3 | Western Sydney | Cabramatta |
| 13 | 06/04/10 | 18/04/10 | 15 | 1.96 | 21 | South-West Brisbane; Regional QLD (South-East) | Collingwood Park, Ipswich, Marsden |
| 14 | 01/02/10 | 08/02/10 | 8 | 0.42 | 63 | Regional NSW (Central-North) | Moree |
| 15 | 23/12/10 | 31/12/10 | 8 | 0.42 | 183 | Regional NSW (West) | Broken Hill |
| 16 | 26/06/10 | 09/07/10 | 8 | 0.45 | 0 | Regional WA (Central coast) | Geraldton |
| 17 | 10/05/10 | 10/05/10 | 4 | 0.04 | 0 | South-East Melbourne | Cheltenham |
| 18 | 28/07/10 | 29/07/10 | 4 | 0.04 | 0 | Regional NSW (Central coast) | Gosford |
| 19 | 06/07/10 | 10/07/10 | 4 | 0.04 | 0 | South-East Melbourne | Chadstone |
Dog factors
No significant difference (P = 0.5216) was observed between the age distributions of those cases reported from within clusters (median age 140 days) vs. those cases reported from areas not included within a cluster (median age 133 days). No significant difference (χ 2 = 13.55; P = 0.0599) was observed in the number of reported cases in dog breed categories between those CPV cases reported from within clusters vs. those cases reported from areas not included within a cluster (Fig. 4 ). The gender of cases reported from within clusters vs. those cases reported from areas not included within a cluster was not significantly different (χ 2 = 2.88; P = 0.2369) (Table 2 ). However, reported cases within clusters were less likely to be neutered than those cases reported outside identified clusters (2.36% vs. 6.85%; χ 2 = 8.26; P = 0.0040).
Fig. 4.
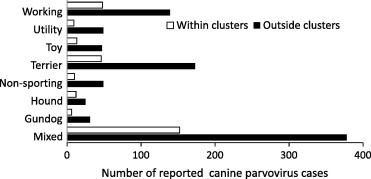
Canine parvovirus cases reported in the Disease WatchDog database by breed category within identified clusters compared to those reported from areas not within a cluster.
Table 2.
Gender and vaccine status of parvovirus cases reported within clusters vs. those reported outside clusters, Australia in Disease WatchDog. A status of ‘vaccinated’ indicates that the dog had received at least one vaccination.
| Variable | Outside clusters | Within clusters | Total |
|---|---|---|---|
| Gendera | |||
| Male | 486 | 161 | 654 |
| Female | 390 | 119 | 509 |
| Unknown | 15 | 9 | 24 |
| Vaccination statusb | |||
| Vaccinated | 210 | 64 | 274 |
| Unvaccinated | 460 | 169 | 629 |
| Unknown | 221 | 63 | 284 |
P = 0.2369.
P = 0.2505.
No significant difference (χ 2 = 2.77; P = 0.2505) was observed in the vaccination status (ever vaccinated vs. never vaccinated) reported from within clusters vs. those cases reported from areas not included within a cluster (Table 2). Comparison of vaccination category (unknown/never vaccinated, incomplete vaccination, vaccinated within previous 12 months) between the two groups was also non-significant (χ 2 = 1.11; P = 0.5745). Both groups reported 18% of dogs with an incomplete vaccination status, and 78% and 79% unknown/unvaccinated dogs outside and within clusters, respectively. Only 0.04% of cases reported outside clusters, and 0.02% within clusters were vaccinated within the 12 months prior to infection.
Socio-economic indices
All socioeconomic indices were significantly (P < 0.05) lower for postcodes included in identified parvovirus clusters compared to postcodes from which parvovirus was reported but which were not included in identified clusters (Table 3 ).
Table 3.
Median score and median decile ranking of socio-economic indices of postcodes included within identified clusters of parvovirus cases reported in Disease WatchDog in Australia during 2010 and postcodes from which reported cases were not within clusters.
| Socio-economic index | Reported cases within clusters | Reported cases outside clusters | P-value |
|---|---|---|---|
| Median score | |||
| Index of relative socioeconomic disadvantage | 955 (741–1055) | 987 (639–1132) | 0.0001 |
| Index of economic resources | 966 (831–1086) | 991 (659–1199) | 0.0004 |
| Index of education and occupation | 927 (774–1059) | 954 (772–1206) | 0.0002 |
| Median decile ranking | |||
| Index of relative socioeconomic disadvantage | 3 (1–9) | 5 (1–10) | 0.0001 |
| Index of economic resources | 3 (1–10) | 5 (1–10) | 0.0004 |
| Index of education and occupation | 3 (1–9) | 5 (1–10) | 0.0001 |
Population of postcodes
No significant difference (P > 0.05) was observed between the median human population size of postcodes within clusters (11,587) and postcodes outside clusters (14,199) of canine parvovirus reports.
Discussion
Contagious diseases such as those caused by canine parvovirus are often clustered in time or space, and the presence of clusters usually indicates the contribution of common factor(s) to disease occurrence in these canine patients (Ward and Carpenter, 2000). The recognition of such clusters allows targeted disease prevention and control practices to be implemented. In the current study, 19 significant space–time disease clusters were identified and occurred in both rural and urban communities and in five States of Australia.
Postal code areas that were included within these clusters on average had a significantly greater level of relative socio-economic disadvantage than postal areas that also reported cases of canine parvovirus in 2010 but which were not included in the identified clusters. In addition, postcodes within clusters on average had significantly lower economic resources scores and level of education and occupation. A lower level of ‘occupation’ refers to a greater proportion of unskilled workers and unemployed, vs. a greater proportion of qualified professionals within higher score areas.
The Australian Bureau of Statistics has summarised socioeconomic data and reported that areas of high or low disadvantage tend to be clustered, and that people in areas with a low economic resources score (i.e. limited access to economic resources) are more likely to report fair or poor health status than those living in the highest decile. In addition, those with lower education levels and those living in greater relative socio-economic disadvantage are more likely to be obese (Pink, 2008, Courcier et al., 2010). Studies reporting similar findings in companion animals are limited. However, previous research has indicated that owners with a higher education level (especially those with a professional qualification) generally provide a better standard of housing, feeding, and veterinary care for dogs than other owners and have greater knowledge of basic animal care based on survey responses from both pet owners and households that did not own a pet (Anene et al., 1996, Balassiano et al., 2009).
As the socio-economic status of an area increases, so too does the proportion of dogs that receive preventive veterinary care such as neutering, intestinal parasite prophylaxis and vaccination (Ramón et al., 2010, Rubel and Wisnivesky, 2005). Although Australian data are inadequate, it is feasible that attention to pet health follows a similar trend, as has been reported elsewhere. Supporting this idea is the finding that reported cases of parvovirus that were located within clusters were three times less likely (P = 0.0040) to be neutered than those cases outside clusters. There are probably many reasons for neutering pets, but socio-economic factors (reduced financial resources and lower education level) are likely involved and further suggest that socio-economic status plays a role in local parvovirus outbreaks.
Dog factors examined in this study included vaccination status, gender, age and breed. No significant difference was detected between cases within clusters and those outside clusters for any of these variables. Kalli et al. (2010) found that in some geographical areas certain breeds, especially purebreds, may be at increased risk of developing canine parvovirus. This finding may be biased by dog breed distribution and seasonal conditions, because it has also been argued that breed does not play a significant role in predisposition to canine parvovirus (Twark and Dodds, 2000). Houston et al. (1996) determined that male dogs older than 6 months of age were more likely to develop CPV related disease than females, and intact dogs were at a greater risk of disease than neutered dogs.
Based on infectious disease epidemiology principles, there are several possible explanations for the occurrence of the parvovirus disease outbreaks detected in this study, namely, (1) greater contact between dogs within affected areas; (2) inadequate herd immunity; and (3) reporting bias. Inadequate herd immunity seems a likely explanation and may arise because of a lack of regular virus transmission within the areas, or inadequate levels of vaccination. Several studies have found vaccination to be protective against development of the clinical disease but vaccination in these studies refers to compliance with a vaccination protocol capable of inducing protective antibody titres (Goddard and Leisewitz, 2010, Houston et al., 1996, Godsall et al., 2010, Twark and Dodds, 2000). Less than 1% of dogs in the current study were reported as having received adequate vaccination against canine parvovirus as determined by the WSAVA vaccination guidelines. 4
Although the findings in our study suggest that vaccination status (as well as gender, age and breed) did not play a significant role in the formation of disease clusters, information on vaccination status was limited (0.04% or less of all cases – whether within or outside clusters – had been vaccinated within 12 months of infection). The association between clusters and dog neuter status might represent the influence of socioeconomic factors on general veterinary cases and reduced financial resources and lower education level probably influenced an owner’s decision whether to neuter and/or to vaccinate their pet. Areas of lower socio-economic status should be targeted in programs to control canine parvovirus.
There were a number of limitations to this study, many of which are being overcome as Disease WatchDog evolves as a disease surveillance system. The main constraint to this study was that the baseline population demographics for dogs in Australia were largely unknown, and the small amount of data that is available is usually specific to a local area (Toribio et al., 2009). The absence of a control population makes it difficult to draw conclusions regarding analysis of diseased populations because there is no baseline data against which to make comparisons. Thus, all comparisons in this study were made between clinical cases within cluster areas and cases not within a cluster.
Clusters (cases reported to occur within 2 weeks within relatively small areas) were assumed to represent potential epidemics of disease transmission. Arising from this we expected that age, gender and breed could explain the formation of clusters (Goddard and Leisewitz, 2010, Kalli et al., 2010, Houston et al., 1996, Godsall et al., 2010). More specifically, any one of these factors might result in local parvovirus disease outbreaks – for example, an area (several postcodes) in which there are a greater proportion of young dogs could be more susceptible to an outbreak of parvovirus, irrespective of the various socioeconomic factors we assessed. However, there was no evidence of age, gender or breed explaining the clusters identified. Another major limitation of the study was the use of data collected within a passive surveillance system. Moore and Lund (2009) commented that passive surveillance systems have a number of drawbacks, including a high rate of underreporting, incomplete reporting, increased chance of reporting bias, and commonly the lack of a population at risk. Underreporting is a concern in any surveillance system; Godsall et al. (2010) observed that reporting compliance (5.9%) of staff was significantly lower than expected in a study in which data came from one group of hospitals under the same management in the UK. Reporting bias is difficult to assess because reporting of canine parvovirus is not mandatory and some clinics may be more diligent at reporting than others.
Conclusions
Canine parvovirus occurs in disease clusters where a greater than expected number of cases are reported. These clusters tend to occur in areas of greater relative socioeconomic disadvantage, lower levels of education and skilled occupations, and with reduced access to economic resources. Dog age, gender, breed and vaccination status do not appear to play a significant role in the formation of these clusters. Additional research is needed to further characterise these disease clusters and potential factors contributing to their formation.
Conflict of interest statement
Dr. Mark Kelman is an employee of Virbac Australia. None of the other authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgements
The many regular contributors of reports to the Disease WatchDog database (‘disease surveillance champions’) are gratefully acknowledged. Thank you to Simon Firestone for his time and assistance in the GIS aspects of the study. This study represents a BVSc. Honours dissertation submitted to the Faculty of Veterinary Science, The University of Sydney by S.B.
Footnotes
See: www.diseasewatchdog.org.
See: www.abs.gov.au.
Australian National Kennel Council Breeds; see: http://www.ankc.org.au/Breeds.aspx (accessed 26 January 2012).
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tvjl.2012.01.025.
Appendix A. Supplementary material
Fig. S1. Distribution of new veterinary clinic registration, by month, within the Disease WatchDog database (www.diseasewatchdog.org). Fig. S2. Distribution of all Australian veterinary clinics registered within the Disease WatchDog database (www.diseasewatchdog.org), 2010.
References
- Anene B.M., Nnaji T.O., Chime A.B. Intestinal parasitic infections of dogs in the Nsukka area of Enugu State, Nigeria. Preventative Veterinary Medicine. 1996;27:89–94. [Google Scholar]
- Balassiano B.C.C., Campos M.R., Alcantara de Menezes R., Pereira M.J.S. Factors associated with gastrointestinal parasite infection in dogs in Rio de Janeiro, Brazil. Preventative Veterinary Medicine. 2009;91:234–240. doi: 10.1016/j.prevetmed.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Blanton J.D., Palmer D., Rupprecht C.E. Rabies surveillance in the United States during 2009. Journal of the American Veterinary Medical Association. 2010;237:646–657. doi: 10.2460/javma.237.6.646. [DOI] [PubMed] [Google Scholar]
- Courcier E.A., Thomson R.M., Mellor D.J., Yam P.S. An epidemiological study of environmental factors associated with canine obesity. Journal of Small Animal Practice. 2010;51:362–367. doi: 10.1111/j.1748-5827.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Martella V., Mari V., Lavazza A., Nardi M., Buonavoglia C. Evidence for immunisation failure in vaccinated adult dogs infected with canine parvovirus type 2c. New Microbiologica. 2008;31:125–130. [PubMed] [Google Scholar]
- Decaro N., Cirone F., Desario C., Elia G., Lorusso E., Colaianni M.L., Martella V., Buonavoglia C. Severe parvovirus in a 12-year-old dog that had been repeatedly vaccinated. Veterinary Record. 2009;164:595. doi: 10.1136/vr.164.19.593. [DOI] [PubMed] [Google Scholar]
- Glickman L.T., Moore G.E., Glickman N.W., Caldanaro R.J., Aucoin D., Lewis H.B. Purdue University-Banfield national companion animal surveillance program for emerging and zoonotic diseases. Vector-Borne and Zoonotic Diseases. 2006;6:14–23. doi: 10.1089/vbz.2006.6.14. [DOI] [PubMed] [Google Scholar]
- Goddard A., Leisewitz A.L. Canine parvovirus. Veterinary Clinics Small Animal Practice. 2010;40:1041–1053. doi: 10.1016/j.cvsm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Godsall S.A., Clegg S.R., Stavisky J.H., Radford A.D., Pinchbeck G. Epidemiology of canine parvovirus and coronavirus in dogs presented with severe diarrhoea to PDSA PetAid hospitals. Veterinary Record. 2010;167:196–201. doi: 10.1136/vr.c3095. [DOI] [PubMed] [Google Scholar]
- Houston D.M., Ribble C.S., Head L.L. Risk factors associated with parvovirus enteritis in dogs: 283 cases (1982–1991) Journal of the American Veterinary Medical Association. 1996;208:542–546. [PubMed] [Google Scholar]
- Kalli I., Leontides L.S., Mylonakis M.E., Adamama-Moraitou K., Rallis T., Koutinas A.F. Factors affecting the occurrence, duration of hospitalization and final outcome in canine parvovirus infection. Research in Veterinary Science. 2010;89:174–178. doi: 10.1016/j.rvsc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Lamm C.G., Rezabek G.B. Parvovirus infection in domestic companion animals. Veterinary Clinics Small Animal Practice. 2008;38:837–850. doi: 10.1016/j.cvsm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Mbah M.L.N., Gilligan C.A. Resource allocation for epidemic control in metapopulations. PLoS ONE. 2011:e24577. doi: 10.1371/journal.pone.0024577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers J., Kyaw-Tanner M., Bensink Z., Zwijnenberg R. Genetic analysis of canine parvovirus from dogs in Australia. Australian Veterinary Journal. 2007;85:392–396. doi: 10.1111/j.1751-0813.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Moore G.E., Lund E. Disease reporting and surveillance: Where do companion animal diseases fit in? Veterinary Clinics Small Animal Practice. 2009;39:225–240. doi: 10.1016/j.cvsm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Moore G.E., Ward M.P., Kulldorff M., Caldanaro R.J., Guptill L.F., Lewis H.B., Glickman L.T. A space–time cluster of adverse events associated with canine rabies vaccine. Vaccine. 2005;23:5557–5562. doi: 10.1016/j.vaccine.2005.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C.A.D., Leal E.S., Durigon E.L. Selective regimen shift and demographic growth increase associated with the emergence of high-fitness variants of canine parvovirus. Infection, Genetics and Evolution. 2007;7:399–409. doi: 10.1016/j.meegid.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Pink, B., 2008. In: Statistics ABo (Ed.), Socio-Economic Indexes for Areas (SEIFA) – Technical Paper 2006. Australian Bureau of Statistics, Canberra.
- Prittie J. Canine parvoviral enteritis: A review of diagnosis, management, and prevention. Journal of Veterinary Emergency and Critical Care. 2004;13:167–176. [Google Scholar]
- Ramón M.E., Slater M.R., Ward M.P. Companion animal knowledge, attachment and pet cat care and their associations with household demographics for residents of a rural Texas town. Preventative Veterinary Medicine. 2010;94:251–263. doi: 10.1016/j.prevetmed.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Roth J.A., Spickler A.R. Duration of immunity induced by companion animal vaccines. Animal Health Research Reviews. 2010;11:165–190. doi: 10.1017/S1466252310000150. [DOI] [PubMed] [Google Scholar]
- Rubel D., Wisnivesky C. Magnitude and distribution of canine fecal contamination and helminth eggs in two areas of different urban structure, Greater Buenos Aires, Argentina. Veterinary Parasitology. 2005;133:339–347. doi: 10.1016/j.vetpar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Sabine M., Herbert L., Love D.N. Canine parvovirus infection in Australia during 1980. Veterinary Record. 1982;110:551–553. doi: 10.1136/vr.110.24.551. [DOI] [PubMed] [Google Scholar]
- Toribio J.L.M., Norris J.M., White J.D., Dhand N.K., Hamilton S.A., Malik R. Demographics and husbandry of pet cats living in Sydney, Australia: Results of cross-sectional survey of pet ownership. Journal of Feline Medicine and Surgery. 2009;11:449–461. doi: 10.1016/j.jfms.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twark L., Dodds W.J. Clinical use of serum parvovirus and distemper virus antibody titres for determining revaccination strategies in healthy dogs. Journal of the American Veterinary Medical Association. 2000;217:1021–1024. doi: 10.2460/javma.2000.217.1021. [DOI] [PubMed] [Google Scholar]
- Ward M.P. Clustering of reported cases of leptospirosis among dogs in the United States and Canada. Preventative Veterinary Medicine. 2002;56:215–226. doi: 10.1016/s0167-5877(02)00160-5. [DOI] [PubMed] [Google Scholar]
- Ward M.P., Carpenter T.E. Analysis of time-space clustering in veterinary epidemiology. Preventative Veterinary Medicine. 2000;43:225–237. doi: 10.1016/s0167-5877(99)00111-7. [DOI] [PubMed] [Google Scholar]
- Ward M.P., Glickman L.T., Guptill L.F. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998) Journal of the American Veterinary Medical Association. 2002;220:53–58. doi: 10.2460/javma.2002.220.53. [DOI] [PubMed] [Google Scholar]
- Ward M.P., Kelman M. Companion animal disease surveillance: A new solution to an old problem? Spatial and Spatio-temporal Epidemiology. 2011;2:147–158. doi: 10.1016/j.sste.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Distribution of new veterinary clinic registration, by month, within the Disease WatchDog database (www.diseasewatchdog.org). Fig. S2. Distribution of all Australian veterinary clinics registered within the Disease WatchDog database (www.diseasewatchdog.org), 2010.


