Abstract
Hantaviruses are the causative agents of HFRS and HCPS (hemorrhagic fever with renal syndrome and hantavirus cardiopulmonary syndrome), two severe, and often fatal human diseases. Mortality from HFRS varies between hantaviruses; Hantaan and Dobrava show the highest, Seoul intermediate, and Puumala low mortality. Saaremaa, genetically closely related to Dobrava, is also known to induce HFRS, with low or no mortality. In this study, mice were inoculated with Dobrava and Saaremaa viruses to test for infectibility, lethality, viremia, nitric oxide production and antibody responses. Out of suckling mice intracerebrally inoculated with 50, 500 and 5000 focus-forming units of Dobrava virus, respectively, 1/8, 2/8 and 7/8 died within 18–26 days. In all but one of the lethally infected mice high levels of replicating virus were detected, and most were positive for neutralizing antibodies and showed elevated levels of nitric oxide production. All suckling mice intracerebrally inoculated with 50, 500, or 5000 focus-forming units of Saaremaa virus survived and all seroconverted. Clearly lower viral titers were observed for the Saaremaa virus-inoculated mice, also when sacrificed at day 18 after infection, compared to those in mice that died following Dobrava virus infection. Dobrava, Saaremaa, Puumala and Hantaan virus infections of adult mice were asymptomatic, and the anti-nucleocapsid protein IgG2a/IgG1-titer ratio was higher in mice inoculated with Dobrava virus than in those inoculated with Saaremaa virus. Elevated nitric oxide production was not detected in asymptomatically infected mice, and iNOS−/− mice, like normal mice, cleared viremia. In conclusion, we show that Dobrava virus and Saaremaa virus induce distinct differences in terms of survival, viremia, nitric oxide production and antibody responses in mice.
Keywords: Hantavirus, Mice, Nitric oxide
Abbreviations: HFRS, hemorrhagic fever with renal syndrome; HCPS, hantavirus cardiopulmonary syndrome; HTNV, Hantaan virus; DOBV, Dobrava virus; SEOV, Seoul virus; PUUV, Puumala virus; SNV, Sin Nombre virus; ANDV, Andes virus; TULV, Tula virus; TOPV, Topografov virus; SAAV, Saaremaa virus; NO, nitric oxide; iNOS, inducible nitric oxide synthase; N, nucleocapsid protein; FRNT, focus reduction neutralizing test; OD405, optical density at 405 nm; FFU, focus-forming units; RT-PCR, reverse transcriptase PCR
1. Introduction
Hantaviruses cause two severe forms of human disease that are often lethal: hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS). The more severe forms of HFRS are caused by Hantaan virus (HTNV) in Asia and Dobrava virus (DOBV) in Europe, with reported mortalities of 5–12%, while Seoul virus (SEOV) is reported to cause an intermediate form, predominantly occurring in Asia and with a mortality of around 1% [1]. Puumala virus (PUUV) causes a milder form of HFRS in Europe, with less than 0.2% mortality [2]. Sin Nombre virus (SNV), Andes virus (ANDV), and related viruses cause HCPS in the Americas; these infections are associated with a mortality rate of around 40% [2], [3]. Other hantaviruses, like Tula virus (TULV), circulating in large areas of Europe, and Topografov virus (TOPV), have never been clearly associated with human disease.
Saaremaa virus (SAAV) is genetically very closely related to DOBV [4], and both exist in Europe, where DOBV is carried by Apodemus flavicollis [5], and SAAV by Apodemus podemus agrarius [6]. Interestingly, there seem to be clear differences in terms of pathogenicity for humans after DOBV and SAAV infections. The most severe HFRS cases, with high fatality rates, have been reported from the Balkans, where DOBV is dominant [7], [8], [9]. In contrast, in parts of Europe where SAAV dominates, no fatalities associated with DOBV-like viruses have been registered [2], [10], [11], [12], [13]. One prominent example is the large DOBV-like associated outbreak in central Russia in 1991–1992, when 130 HFRS patients were hospitalised, but no fatal cases occurred [10]. Taken together, those reports suggest that SAAV induces a disease more similar to PUUV than to DOBV infection [2].
Today, little is known regarding the mechanisms behind hantavirus pathogenesis, but immune mechanisms have been suggested to be involved in HFRS and HCPS pathogenesis [14]. Hantavirus infection per se does not directly damage endothelial cells in vitro [15], and specific immune responses are present at the onset of the symptomatic phase of the disease. In SNV-infected patients there is a correlation between a high frequency of SNV-specific cytotoxic T lymphocytes and severe disease, and these cytotoxic T lymphocytes are suggested to contribute to the disease [16]. Furthermore, histological studies of postmortem tissues show blood mononuclear cell infiltration that might play a role in functional organ failure [17], [18], [19]. Elevated levels of cytokines like IL-6, IL-10 and TNF-α have been reported, and elevated production of the free radical nitric oxide (NO) has been detected in hantavirus infected patients [20], [21], [22], as well as in monkeys infected with PUUV [23], indicating that it may contribute to hantavirus pathogenesis [22]. SNV infection of deer mice, the natural host, does not induce elevated NO production [22]. NO is known to contribute to the pathogenesis of certain virus infections in mice, like influenza virus [24] and neurotropic viruses [25], but NO is also known to have antiviral effects against some viruses, including ectromelia virus (mousepox), coxsackievirus, herpesviruses and SARS coronavirus [26], [27].
Recently, we showed that adult laboratory mice could be infected with SAAV and DOBV, however, without causing lethality or any apparent symptoms within 21 days of infection [28], [29]. Feral mice are not a natural reservoir for any known hantavirus, but infection of laboratory mice has been described in several studies. HTNV and SEOV infections of suckling mice have been reported to be lethal, although without reflecting the symptoms seen in humans [30], [31], while infections of adult mice with HTNV, ANDV and SNV are transient and asymptomatic [32], [33], [34], although a recent report showed that HTNV given via the intraperitoneal route can be lethal also for adult mice [35].
In this study we compared the infectibility, lethality and antibody responses of DOBV and SAAV and other hantaviruses in suckling and adult mice, and in adult inducible NO synthase (iNOS) knock-out mice. We also measured the level of replicating viruses, and the levels of the stable end products of NO, nitrite and nitrate, during infection.
2. Materials and methods
2.1. Mice
One- to five-day-old suckling NMRI mice, and 6- to 8-week-old adult NMRI, BALB/c, C57/BL6 and C57/BL6 iNOS−/− mice, generated by homologous recombination in embryonal stem cells and then backcrossed to C57BL/6 mice [36], were purchased from BHK, Stockholm, Sweden and/or MTC, Breeding unit, Karolinska Institutet, Stockholm, Sweden. Suckling mice were inoculated intracerebrally with 20 μl, and adult mice subcutaneously, intravenously and/or intraperitoneally with 100 μl/route diluted or undiluted virus as indicated in the text. Infected and non-infected control mice were kept in biological safety isolators.
Serum was stored at −20 °C until further use. Lungs, hearts and brains were removed aseptically; lungs and brains were minced in PBS, and stored at −70 °C until further use. Hearts were stored at −70 °C with PBS for 24 h, after thawing the supernatant was transferred to a new tube and used for detection of hantavirus specific antibodies. The care of all animals used in the present study was in compliance with the relevant guidelines and requirements of the Swedish Institute for Infectious Disease Control, Stockholm, Sweden.
2.2. Viruses
The hantaviruses used in this study were Vero E6 cell line adapted HTNV, strain 76–118 [37], DOBV, strain Slovenia [5], SAAV [6], TULV, strain Moravia/Ma5302V/94 [38], TOPV, strain TOP/Ls136V5/94 [39] and various PUUV strains as follows: PUUV Kazan-E6 [40], passage 6 (p6), PUUV Sot, strain Sotkamo [41], PUUV esc-5A2 (PUUV; strain Sotkamo, monoclonal antibody 5A2 escape mutant [42]) and PUUV Kazan-wt, strain Kazan wild-type, never passaged in cell culture [40]. Propagation and titration of the cell line adapted virus stocks were performed on Vero E6 cells (VERO C1008; American Type Culture Collection), as described earlier [43]. PUUV Kazan-wt was propagated in bank voles as described earlier [40].
2.3. Antibody assays
ELISAs for the determination of DOBV and PUUV nucleocapsid protein (N)-specific IgG were performed essentially as described earlier [29]. ELISA for the detection of DOBV or SAAV specific antibodies in suckling mice was performed on supernatants from hearts mixed with PBS. N-specific IgG subclass ELISAs were performed on pooled sera from groups of infected animals. Briefly, 1 μg/ml of recombinant DOBV or PUUV N [44] was coated on 96 well plates. After washing and blocking, sera as well as negative and positive controls, were three-fold diluted starting from 1:10 (for total IgG in suckling mice), four-fold diluted starting from 1:200 (for total IgG in adult mice), or two- or three-fold diluted starting from 1:100 (for IgG subclasses in adult mice), and added to the wells in duplicate. After washing, alkaline phosphatase (ALP)-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch) or goat anti-mouse IgG1, IgG2a, IgG2b or IgG3 (Sigma) followed by ALP-conjugated rabbit anti-goat IgG (Sigma) was added. P-nitrophenyl phosphate substrate (Sigma) was added after washing, and the optical densities at 405 nm (OD405) were determined. Focus reduction neutralization test (FRNT) was performed as described previously [40].
2.4. Detection and titration of hantavirus from brains
Minced brain in PBS (total volume 1 ml) was diluted ten-fold in HBSS supplemented with penicillin, streptomycin, 2% Hepes pH 7.0 and 2% FCS and added to confluent Vero E6 cells in 24 well plates. After 1 h of incubation, cells were overlaid with 0.5% agar-medium [45] and incubated for 10 days. Foci of infected cells were visualized and counted using polyclonal rabbit anti-DOBV, or anti-SAAV, sera followed by horseradish peroxidase-conjugated goat anti-rabbit IgG and 3,3′,5,5′-tetramethylbenzidin.
2.5. Viral RNA assay
Reverse transcription polymerase chain reaction (RT-PCR) and nested PCR of nt 389–741 of DOBV/SAAV S segment were performed essentially as described earlier [46] on experimentally infected animals. RNA was extracted using TriPure as described by the manufacturer (Roche Diagnostics). RT-PCR of the DOBV/SAAV S segment was performed with external primers: 5′ GGGAAAAACATGGGCAAGAAG 3′ (DOBS 256F) and 5′ AGGTAGGAGGRCAYCTATCAGG 3′ (DOBS 997R), followed by a nested PCR with primers 5′ CTGCAGACTGGCTGGCTAAGCATTGTGA 3′ (DOBS 389F) and 5′ CAAGCCATTCTTCAACCCTTCTG 3′ (DOBS 741L).
2.6. Nitric oxide assay
The production of NO in the brains of suckling mice, and in sera from adult mice, was assessed as the accumulation of nitrate and nitrite, stable end products of NO. Aliquots of brain/PBS suspension were centrifuged and the supernatants were boiled for 15 min to inactivate virus and denature protein. After centrifugation the amount of nitrite was measured for each sample after reduction of nitrate to nitrite using the total nitric oxide colorimetric assay (R&D systems), according to the manufacturer's instructions.
3. Results
3.1. Intracerebral inoculation of suckling mice with DOBV, but not SAAV, is lethal
To investigate if DOBV and/or SAAV were lethal for suckling mice, we inoculated groups of seven to eight suckling NMRI mice with 50, 500 or 5000 focus-forming units (FFU) of each virus. Of the mice inoculated with DOBV, one out of eight, two out of eight, and seven out of eight receiving 50, 500 or 5000 FFU, respectively, died. Time to death after DOBV inoculation correlated with the amount of virus given: of the mice that died, all mice in the group receiving 5000 FFU died within 18–20 days after infection, while those receiving 500 FFU died 20 days after infection and the one receiving 50 FFU died 26 days after infection ( Table 1). The mice that died showed ruffled fur, paralysis of the limbs, and progressively diminishing mobility, as described earlier for HTNV-infected suckling mice [47].
Table 1.
DOBV in suckling mice
| ID | Inoculated with | Outcome | Detection of virus/RNA in braina | Antibody titer |
|
|---|---|---|---|---|---|
| IgGb | Neut.c | ||||
| 1:1 | Inactivatedd | S | − | − | − |
| 1:2 | Inactivated | S | − | − | − |
| 1:3 | Inactivated | S | − | − | − |
| 1:4 | Inactivated | S | − | − | − |
| 1:5 | Inactivated | S | − | − | − |
| 1:6 | Inactivated | S | − | − | − |
| 1:7 | Inactivated | S | − | − | − |
| 1:8 | Inactivated | S | − | − | − |
| 3:1 | 5000 FFU | † (d. 18)e | + (1 × 106) | − | 80 |
| 3:2 | 5000 FFU | † (d. 18) | + (3 × 106) | − | 20 |
| 3:3 | 5000 FFU | † (d. 18) | + (3 × 106) | − | 20 |
| 3:4 | 5000 FFU | † (d. 18) | + (1.5 × 106) | − | − |
| 3:5 | 5000 FFU | S | − (+ in PCR) | 810 | 80 |
| 3:6 | 5000 FFU | † (d. 18) | + (5 × 106) | − | 40 |
| 3:7 | 5000 FFU | † (d. 18) | + (2 × 106) | − | − |
| 3:8 | 5000 FFU | † (d. 20) | + (1 × 106) | − | 20 |
| 4:1 | 500 FFU | S | − | − | − |
| 4:2 | 500 FFU | S | − | − | − |
| 4:3 | 500 FFU | S | − (+ in PCR) | 270 | 40 |
| 4:4 | 500 FFU | S | − | − | − |
| 4:5 | 500 FFU | S | − (+ in PCR) | 810 | 80 |
| 4:6 | 500 FFU | S | − | − | − |
| 4:7 | 500 FFU | † (d. 20) | + (2.5 × 106) | − | 40 |
| 4:8 | 500 FFU | † (d. 20) | + (3 × 106) | − | 40 |
| 5:1 | 50 FFU | S | − | − | − |
| 5:2 | 50 FFU | S | − | − | − |
| 5:3 | 50 FFU | S | − (+ in PCR) | 810 | 80 |
| 5:4 | 50 FFU | S | − | − | − |
| 5:5 | 50 FFU | S | − (+ in PCR) | 810 | 40 |
| 5:6 | 50 FFU | S | − | 270 | − |
| 5:7 | 50 FFU | S | − (+ in PCR) | 810 | 160 |
| 5:8 | 50 FFU | † (d. 26) | − (+ in PCR) | 90 | 40 |
S, survived.
Virus from brains was titrated on Vero E6 cells, FFU/brain in brackets, + in PCR, negative for replicating virus, but positive for RT-PCR on DOBV/SAAV S, −, negative for replicating virus and RT-PCR.
Reciprocal anti-DOBV N protein-IgG titer. −, Negative at 10× dilution of sample.
Neutralizing antibody titer. −, Negative at 20× dilution of sample.
Heat-inactivated DOBV, corresponding to 5000 FFU, was administered.
† d. 18, 20 or 26, died at day 18, 20 or 26 after infection.
All SAAV inoculated, as well as all mice receiving heat-inactivated DOBV or SAAV (corresponding to 5000 FFU), survived until the end of the experiment at day 34 after infection. None of the surviving mice, including those that survived DOBV infection, showed any symptoms during the experiment.
3.2. Seroconversion in DOBV and SAAV-infected suckling mice
We then tested for the presence of anti-N IgG and neutralizing antibodies in all inoculated mice. All mice that died following DOBV inoculation, except the one that died 26 days after infection, were negative for N-specific IgG, as were half of the mice that survived DOBV inoculation and all mice receiving heat-inactivated DOBV (Table 1). Half of the mice that survived DOBV inoculation were found to be IgG-positive (Table 1). As most mice that died following DOBV inoculation were negative for DOBV specific anti-N IgG, we then tested if the DOBV-inoculated mice had seroconverted at all by FRNT, since this method can detect neutralizing antibodies of all antibody classes. Eight out of the total 10 mice that died after DOBV inoculation and five out of six of the DOBV-inoculated mice that were found to be IgG-positive, but none that were IgG-negative and survived, developed neutralizing antibodies (Table 1). Thus, most of the mice that died following DOBV inoculation had developed specific antibody responses against the envelope proteins of DOBV, but not detectable levels of IgG against the N protein.
All mice inoculated with 50, 500 or 5000 FFU of SAAV, but none of the mice inoculated with heat-inactivated SAAV, developed anti-N IgG antibodies within 34 days after infection ( Table 2). FRNT showed that most of the SAAV inoculated mice were also positive for neutralizing antibodies (Table 2).
Table 2.
SAAV in suckling mice
| ID | Inoculated with | Outcome | Isolation of replicating virusa | Antibody titer |
|
|---|---|---|---|---|---|
| IgGb | Neut.c | ||||
| 2:1 | Inactivatedd | S | ND | − | − |
| 2:2 | Inactivated | S | ND | − | − |
| 2:3 | Inactivated | S | ND | − | − |
| 2:4 | Inactivated | S | ND | − | − |
| 2:5 | Inactivated | S | ND | − | − |
| 2:6 | Inactivated | S | ND | − | − |
| 2:7 | Inactivated | S | ND | − | − |
| 2:8 | Inactivated | S | ND | − | − |
| 6:1 | 5000 FFU | S | + (1 × 103) | 2430 | 80 |
| 6:2 | 5000 FFU | S | + (1 × 103) | 270 | 40 |
| 6:3 | 5000 FFU | S | − | 810 | − |
| 6:4 | 5000 FFU | S | − | 810 | 20 |
| 6:5 | 5000 FFU | S | − (+ in PCR) | 270 | 40 |
| 6:6 | 5000 FFU | S | − (+ in PCR) | 90 | 40 |
| 6:7 | 5000 FFU | S | + (0.5 × 103) | 90 | 40 |
| 6:8 | 5000 FFU | S | − (+ in PCR) | 270 | − |
| 7:1 | 500 FFU | S | − (+ in PCR) | 810 | 40 |
| 7:2 | 500 FFU | S | − (+ in PCR) | 810 | 40 |
| 7:3 | 500 FFU | S | − (+ in PCR) | 810 | 40 |
| 7:4 | 500 FFU | S | − (+ in PCR) | 270 | − |
| 7:5 | 500 FFU | S | − (+ in PCR) | 810 | 80 |
| 7:6 | 500 FFU | S | + (5 × 103) | 810 | 40 |
| 7:7 | 500 FFU | S | + (0.5 × 103) | 270 | 20 |
| 7:8 | 500 FFU | S | − | 810 | 20 |
| 8:1 | 50 FFU | S | + (1 × 103) | 810 | 160 |
| 8:2 | 50 FFU | S | − | 270 | 20 |
| 8:3 | 50 FFU | S | − (+ in PCR) | 810 | − |
| 8:4 | 50 FFU | S | − | 270 | 40 |
| 8:5 | 50 FFU | S | − | 270 | 80 |
| 8:6 | 50 FFU | S | − | 90 | − |
| 8:7 | 50 FFU | S | − | 270 | − |
S, survived, ND, not determined.
Virus from brains was titrated on Vero E6 cells, FFU/brain in brackets, + in PCR, negative for replicating virus, but positive for RT-PCR on DOBV/SAAV S, −, negative for replicating virus and RT-PCR.
Reciprocal anti-DOBV N protein-IgG titer. −, Negative at 10× dilution of sample.
Neutralizing antibody titer. −, Negative at 20× dilution of sample.
Heat-inactivated SAAV, corresponding to 5000 FFU, was administered.
3.3. High levels of replicating virus in brains of lethally infected suckling mice
Next, we tested for replicating virus in the brains of the inoculated mice. High titers of replicating virus (1 × 106–5 × 106 FFU/brain) were detected in all but one of the mice that died following DOBV inoculation, but in none of the DOBV-inoculated mice that were sacrificed at day 34 after inoculation (Table 1). Replicating SAAV, titers ranging from 0.5 × 103 to 5 × 103 FFU/brain (Table 2), could be detected in one out of seven, two out of eight and three out of eight mice given 50, 500, and 5000 FFU of SAAV, respectively, in mice sacrificed at day 34 after inoculation.
To test if higher titers of SAAV would be detected at an earlier time point after infection than 34 days, one group of suckling mice was inoculated with 5000 FFU of SAAV and sacrificed at day 18 after inoculation; the time point of sacrifice was chosen because this was the day when the highest titers were observed in the DOBV lethally infected mice. No symptoms were observed, and all mice were positive for replicating viruses, showing that they were successfully infected, with titers ranging from 1.5 × 103 to 5 × 105 FFU/brain ( Table 3). The results thus showed that the viral titers were at least 50% lower at day 18 after SAAV infection as compared to the titers in the DOBV-infected mice that died between days 18 and 20 after infection.
Table 3.
Virus titers in suckling mice infected with 5000 FFU SAAV and sacrificed at day 18 after infection
| ID | Isolation of replicating virusa |
|---|---|
| 9:1 | + (5 × 104) |
| 9:2 | + (5 × 105) |
| 9:3 | + (2.5 × 104) |
| 9:4 | + (1.5 × 105) |
| 9:5 | + (1.5 × 103) |
| 9:6 | + (1.5 × 105) |
| 9:7 | + (2.5 × 104) |
| 9:8 | + (5 × 104) |
Virus from brains was titrated on Vero E6 cells, FFU/brain in brackets.
To test if viral RNA could be detected in the mice that were negative for replicating virus we performed RT-PCR on the brains. All, except one, DOBV-infected mice that seroconverted, but were negative for replicating DOBV, were found to be positive in RT-PCR for DOBV S (Table 1). In the SAAV-infected mice half that were negative for replicating virus were found to be positive for viral RNA in PCR (Table 2).
3.4. Detection of elevated levels of NO in lethally infected suckling mice
The differences in the levels of replicating virus early after infection might explain, at least partly, why DOBV, but not SAAV, is lethal to suckling mice. Another possible mediator of the pathogenesis could be NO, earlier shown to be involved in the pathogenesis of neurotropic viruses in mice [25]. We indirectly measured the levels of NO in the brains of the suckling mice by measuring the level of nitrite, after conversion of nitrate to nitrite. In this experiment, we also included a group of suckling mice inoculated with inactivated DOBV (corresponding to 5000 FFU) that was sacrificed at day 18 after inoculation, as control. Similar mean values of nitrite were detected in all groups, except in the group of suckling mice that died following DOBV infection ( Table 4). Significant differences between the mean levels of NO in the groups were found (Kruskal–Wallis test, P = 0.014). High levels of nitrite were only detected in the DOBV-infected mice that died following infection with 5000 FFU of DOBV, but not in those that died after infection with 50 or 500 FFU of DOBV ( Fig. 1), suggesting that the NO production was dose-dependent, or that the levels in the other lethally infected mice had returned to normal before they died.
Table 4.
Levels of nitrite in brains from suckling mice after DOBV and SAAV infection
| Inoculated with | n | Outcome | Nitrite, μM (mean ± sd) |
|---|---|---|---|
| DOBV | 10 | † (d. 18–26)a | 31.52 ± 17.01 |
| 7 | S, infectedb | 18.26 ± 9.05 | |
| 7 | S, uninfectedb | 18.02 ± 3.18 | |
| Inactivated DOBV | 8 | Sb | 17.52 ± 6.80 |
| 8 | Sc | 14.15 ± 0.63 | |
| SAAV | 23 | S, infectedb | 16.37 ± 2.34 |
| 8 | S, infectedc | 16.00 ± 1.10 | |
| Inactivated SAAV | 8 | Sb | 15.08 ± 2.62 |
| Control, no virus | 6 | Sb | 15.15 ± 0.86 |
Brain nitrite levels were determined after conversion of nitrate to nitrite.
S, survived.
† d. 18–26, died at days 18–26 after infection.
Sacrificed at day 34 after infection.
Sacrificed at day 18 after infection.
Fig. 1.
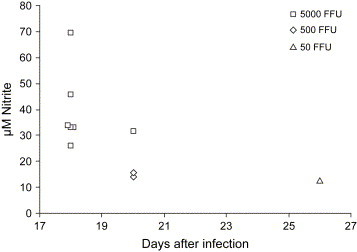
Levels of nitrite in brains from DOBV lethally infected suckling mice. Data shown are the level of nitrite detected in brain at the time of death.
3.5. DOBV, HTNV and SEOV, but not hantaviruses causing milder or no disease, are lethal for suckling mice
Since SAAV seems to cause a milder form of disease in humans compared to DOBV, we decided to test the lethality in suckling NMRI mice also for PUUV, TOPV and TULV, as those hantaviruses are believed to cause a mild form of HFRS, or even asymptomatic infections, in man [2]. All mice inoculated with 8000 FFU of DOBV died ( Fig. 2). To evaluate if a higher dose of SAAV would be lethal, undiluted SAAV (corresponding to 10,000 FFU) was inoculated into eight mice; however, none of the mice died or showed any symptoms (Fig. 2), clearly showing that SAAV was not lethal for suckling mice. PUUV esc-5A2 (20,000 FFU) and PUUV Kazan-wt (200 bank vole ID50) were inoculated into eight mice per group, and five mice were inoculated with PUUV Sot (5000 FFU). None of the mice inoculated with PUUV died, or showed any symptoms within 23–25 days after inoculation (Fig. 2). The same pattern was seen for TOPV and TULV: of eight mice inoculated with undiluted TOPV (900 FFU) or TULV (1,800 FFU) none died or showed any symptoms within 23 and 36 days after inoculation, respectively (Fig. 2). Infection of suckling mice with HTNV (100 FFU) was lethal for all eight inoculated mice (Fig. 2); all died within 15 days after infection. Infection with SEOV (18,000 FFU) was lethal for 25% of infected mice (n = 8) within 19 days of infection; the surviving mice showed no symptoms for the 29-day-long experiment (Fig. 2).
Fig. 2.
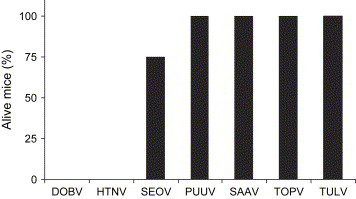
PUUV, SAAV, TOPV and TULV are not lethal for suckling mice. Survival was measured in suckling mice after intracerebral inoculation with 8000 FFU of DOBV (n = 16), 100 FFU of HTNV (n = 8), 18,000 FFU of SEOV (n = 8), 200 bank vole ID50 of PUUV Kazan-wt (n = 8), 5000 FFU of PUUV Sotkamo (n = 5), 20,000 FFU of PUUV esc-5A2 (n = 8), 10,000 FFU of SAAV (n = 8), 900 FFU of TOPV (n = 8) or 1800 FFU of TULV (n = 8).
3.6. Infection of adult NMRI mice with DOBV, SAAV, HTNV and PUUV
As most studies concerning hantavirus lethality in adult mice have been performed for short periods of time, we wanted to study if infection of adult mice with DOBV, SAAV, or HTNV for a longer time than 21 days would induce symptoms and/or lethality. We also included PUUV in this experiment, as this virus, to our knowledge, never before has been shown to be able to infect laboratory mice.
Female adult NMRI (n = 8) mice were intravenously and subcutaneously inoculated with totally 100,000 FFU of SAAV and followed for 49 days; during this time no symptoms were seen in any of the inoculated mice. However, all mice developed neutralizing antibodies (data not shown) and specific IgG responses against N ( Fig. 3A). IgG subclass ELISAs on pooled sera against viral N showed that all subclasses could be detected (Fig. 3B). The ratio between IgG2a and IgG1 titers was 2. Viral RNA was not detected by nested RT-PCR of SAAV S segment from lungs, indicating clearance of SAAV in the animals 49 days after infection (data not shown).
Fig. 3.
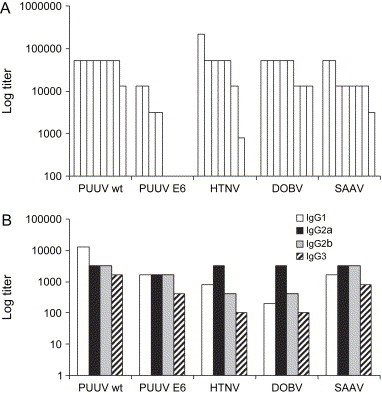
Total IgG and IgG subclass responses against N in sera from adult NMRI mice 60 days after DOBV, HTNV, PUUV Kazan-wt and PUUV Kazan-E6 p6 inoculation and 49 days after SAAV inoculation. (A) IgG anti-N responses. (B) IgG subclass anti-N responses in pooled sera from four mice inoculated intravenously with PUUV Kazan-wt (1000 bank vole ID50), four mice inoculated intravenously with PUUV Kazan-E6 (25,000 FFU), three mice inoculated intravenously with HTNV (90,000 FFU), four mice inoculated intravenously and subcutaneously with DOBV (80,000 FFU), and eight mice inoculated intravenously and subcutaneously with SAAV (100,000 FFU).
Potential symptoms or lethality after infection with DOBV and HTNV, and potential infectivity of Vero E6 cell line adapted and wild-type PUUV was then determined in adult female NMRI mice infected for 60 days.
All eight DOBV-inoculated mice, and seven out of eight HTNV inoculated mice, seroconverted, as did all eight PUUV Kazan-wt inoculated and all four PUUV Kazan-E6 inoculated mice (Fig. 3A), showing that both wild-type and cell culture adapted PUUV can infect laboratory mice. No symptoms or death due to the infection was recorded for any of the infected animals during the 60-day-long experiment, indicating that none of those hantaviruses are normally pathogenic in adult immunocompetent mice.
All subclasses were also present in the pooled sera from DOBV-infected mice (inoculated intravenously and subcutaneously with totally 80,000 FFU), with IgG2a being the dominant subclass (Fig. 3B), and an IgG2a/IgG1 ratio of 16. All subclasses were also detected in sera from intravenously HTNV and PUUV infected mice (Fig. 3B). The IgG2a/IgG1 ratio was 4 for HTNV, 0.25 for wild-type PUUV and 1 for cell line adapted PUUV.
3.7. Infection of adult BALB/c mice with DOBV, SAAV, HTNV and PUUV
As intraperitoneal inoculation of BALB/c mice with HTNV was previously shown to be lethal [35], we then tested if DOBV or SAAV would also be lethal for adult mice using this route. However, although all mice infected with DOBV (n = 6, 400,000 FFU/mice), SAAV (n = 6, 400,000 FFU/mice) and HTNV (n = 6, 800,000 FFU/mice) seroconverted (data not shown), no lethality or symptoms were observed during the 43-day-long study. Interestingly, in line with our previous results [28] mice inoculated with PUUV (n = 6, 800,000 FFU/mice) remained sero-negative, showing that they were not infected (data not shown).
As for NMRI mice, we observed a higher mean IgG2a/IgG1 ratio in the DOBV-infected mice as compared to the SAAV-infected ones ( Fig. 4), indicating that DOBV induce a stronger Th1-type of immune response as compared to SAAV [48], [49], [50].
Fig. 4.
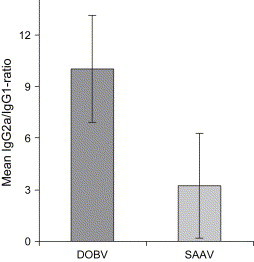
IgG2a/IgG1 ratio in intraperitoneally infected BALB/c mice 43 days after infection. Data shown represent mean ± SD of six mice/group.
3.8. HTNV does not induce systemically elevated levels of NO in C57/BL6 mice
Adult C57/BL6 mice were infected with 100,000 FFU of HTNV (n = 6), and the nitrate/nitrite concentration was determined in sera obtained before and 7, 14 and 20 days postinfection. No symptoms were observed in any of the mice during the experiment. The levels of nitrate/nitrite in serum during infection did not differ over time ( Fig. 5), showing that HTNV do not induce systemically detectable elevated levels of NO production in adult mice.
Fig. 5.
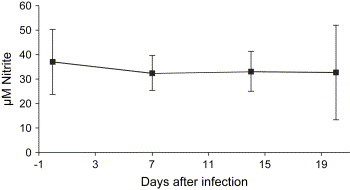
HTNV infection of C57/BL6 mice does not induce NO production. The total level of nitrate/nitrite in sera of HTNV-infected mice (n = 6) was measured by reduction of nitrate to nitrite and then the amount of nitrite was measured. The data represent mean ± SD.
3.9. HTNV infection of iNOS−/− mice is cleared despite a low neutralizing antibody response
Since mice clear HTNV within 28 days postinfection [12], we tested if iNOS is involved in viral clearance in mice. Adult C57/BL6 (n = 6) and iNOS−/− (n = 6) mice were intravenously infected with 100,000 FFU of HTNV. No symptoms were evident in any of the mice for the 21-day-long experiment. Replicating virus was not detected in lungs from the infected mutant or control mice. All mice were positive for anti-N IgG at day 21 postinfection (data not shown), showing that they were successfully infected. INOS−/− mice showed an almost ten-fold higher mean anti-N IgG2a/IgG1 ratio compared to C57/BL6 mice ( Fig. 6a), suggesting a Th1 shift in the absence of the iNOS enzyme, as reported earlier by other investigators [24], [51], [52], [53]. All mice, except two iNOS−/− mice, were positive for neutralizing antibodies against HTNV, and higher mean titers of neutralizing antibodies were detected in C57/BL6 mice as compared to iNOS−/− mice (Fig. 6b), indicating that some iNOS−/− mice cleared the viremia without the help of neutralizing antibodies. The individual FRNT-titers were 640 for all C57/BL6 mice, as compared to 0 for two, 40 for three, 160 for one, and 640 for one of the iNOS−/− mice.
Fig. 6.
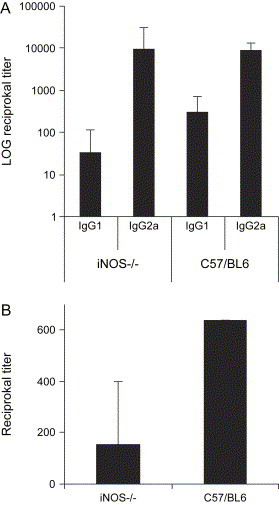
Antibody responses in C57/BL6 and iNOS−/− mice after HTNV infection. (A) IgG subclass specific response against N protein. (B) Neutralizing titers against HTNV. The data represent mean + SD.
4. Discussion
Hantaviruses are predominantly carried by one specific rodent species, and seem to have evolved together with the host [54], where a persistent and subclinical infection is established [55]. Although wild-trapped Mus musculus have been reported to be antibody positive against hantaviruses, no specific hantavirus has been proven to use feral mice as its primary host for replication, indicating that infection of feral mice is due to spillover events. Similar to the natural hosts, hantavirus infections of adult mice are normally asymptomatic, but in contrast to the natural host, and similar to findings in man, mice effectively clear the virus infection. It is currently not known why infection of the natural hosts is asymptomatic, or why infection of man results in severe disease, and although immune mediated mechanisms have been suggested to cause the diseases in man [14], this remains to be clearly shown.
This study was undertaken to investigate the potential differences in terms of lethality, viremia, and immune responses in mice after infection with two genetically similar, but distinct, hantaviruses, DOBV and SAAV. Adult laboratory mice have experimentally been infected with ANDV [33], SNV [33], HTNV [32], SEOV [56], SAAV [28], and DOBV [28]. In contrast to the general lack of symptoms and lethality observed in adult mice, inoculation of suckling mice with HTNV and SEOV has been reported to be lethal in several studies [30], [31], [47], [56], [57], [58], [59], [60], [61], although no symptoms resembling human infection have been reported. In suckling mice, neuropathological symptoms are prominent, and the disease pathogenesis mechanism has been suggested to be immune mediated [60]. However, in contrast to human patients, no evidence of dysfunction in any other organ, except the brain, has been described for suckling mice, suggesting that failure of other organs is not instrumental in the fatal outcome.
In this study, all but one of the mice that died following DOBV inoculation showed evidence of replicating virus or neutralizing antibodies, and all but one were negative for N-specific IgG responses. This indicates that they died rather early after a specific immune response was mounted. The level of lethality, as well as time to death, correlated with the amount of DOBV given: the more the virus, the higher the degree of lethality. However, although all mice in the group given 5000 FFU of DOBV were infected, the pattern observed for lethality was not observed for infection, as approximately half of the mice in both of the groups given 50 or 500 FFU became infected. For SAAV, all inoculated mice seroconverted, showing that all were infected, and replicating virus or viral RNA could also be detected in most of the mice at the time of sacrifice (day 34 after infection). When suckling mice inoculated with 5000 FFU of SAAV were sacrificed at day 18 after infection, higher virus titers were detected as compared to day 34; however, the titers were at least 50% lower than for the mice inoculated with DOBV that died between days 18 and 20. Thus, it seems that a low dose of SAAV can more readily infect suckling mice than DOBV, while DOBV replicates to higher titers after a successful infection. The data further indicate that some mice were not successfully infected by DOBV, as evident by the lack of replicating virus, viral RNA and specific antibody responses.
NO has been shown to be responsible for the pathogenesis during other viral infections of mice, particularly in those that like hantaviruses cause neuropathology [62]. We therefore measured the level of NO in the brains of SAAV and DOBV-inoculated mice. As NO is very short-lived, we measured the level of the stable end products of NO, nitrite and nitrate (nitrate was converted to nitrite by reductase). Interestingly, only mice that died following DOBV inoculation showed elevated levels of nitrite as compared to all other groups, including mice inoculated with 5000 FFU of SAAV and sacrificed at day 18 after infection. However, the highest level of nitrate was detected in mice given 5000 FFU of DOBV, the three mice that died following infection with 500 or 50 FFU of DOBV showed similar levels of nitrite as controls, suggesting that the peak of NO production occurs before or around day 18, or that NO production was not increased in those mice.
As suckling mice inoculated with SAAV showed no evidence of elevated NO production, but remained positive for replicating viruses at day 34 postinfection, while infection with DOBV could induce NO production and no replicating virus was observed in any of the mice that survived DOBV inoculation, we then tested if NO is involved in hantavirus clearance of adult mice. It was recently shown that SNV infection of the natural host (the deer mice), does not induce elevated levels of NO production [22]. In line with the results of Davis et al. we could not detect systemically elevated NO levels in adult mice asymptomatically infected with HTNV. Furthermore, no replicating virus was detected in iNOS−/− mice 3 weeks after HTNV infection, suggesting that virus clearance in mice is not dependent on NO. Moreover, as elevated levels of NO were only detected in suckling mice that succumbed to DOBV infection, and not in any asymptomatically infected suckling or adult mice, our results are in line with the reports of elevated levels of NO production in HFRS/HCPS-patients [20], [21], [22], indicating that NO may be involved in hantavirus pathogenesis and that the increased NO production is caused by the immune response, and not by the virus per se. However, the possible role of NO in hantavirus pathogenesis in man, and the mechanisms leading to elevated levels of NO, remain to be shown.
Our studies of adult mice showed higher IgG2a/IgG1 ratios for the DOBV-infected mice, as compared to SAAV-infected mice, suggesting that a stronger Th1-response is mounted against DOBV than SAAV. There is an intriguing connection between the Th-type of immune response and iNOS, the enzyme that produces the vast majority of NO during viral infection. During Th1-type immune responses IFN-γ is produced, which leads to the induction of iNOS, whereas a Th2-type of immune response down-regulates iNOS induction [62]. If suckling mice also mount a stronger Th1-type of immune response against DOBV than SAAV, this might explain why they showed elevated levels of NO production.
Interestingly, PUUV, TOPV and TULV, known to cause low to no lethality in man, were not lethal for suckling mice. Although additional studies need to be performed before any clear conclusions can be drawn, our, and other's, findings that infection of suckling mice with DOBV, HTNV and SEOV are lethal [30], [31], might indicate that there could be a correlation between lethality in suckling mice and the capacity to cause severe HFRS in man for the Eurasian hantaviruses.
To our knowledge, successful infection of laboratory mice with PUUV has not been reported earlier. Both cell line adapted, as well as wild-type, PUUV, induced antibody responses after intravenous inoculation, showing that both could successfully infect mice, although no symptoms or lethality was detected. However, when the intraperitoneal route was used, inoculated mice did not seroconvert. This finding is in line with our previous finding that subcutaneous inoculation of mice with PUUV is not infectious [28].
Neither lethality nor symptoms were observed after inoculation of adult mice with DOBV, SAAV, or HTNV, but strong antibody responses were mounted. HTNV infection of adult laboratory mice has been reported to be transient [34] and the finding that SAAV RNA could not be detected in the adult mice indicates that the infection was cleared within seven weeks. In contrast to our results as well as the results of other investigators [32], [34], [56], [61], Wichmann et al. recently reported that infection of adult laboratory mice with HTNV was lethal [35]. One explanation for this discrepancy might be the point mutations in the virus used by Wichmann et al.; this might explain the lethality observed, as has earlier been shown for HTNV infection of suckling mice [47].
In conclusion, we show that DOBV, but not SAAV, is lethal for suckling mice. The level of replicating virus and/or induction of NO production, probably in combination with the Th-type of immune response mounted, may in part explain why only DOBV is lethal. We believe that future studies addressing the effect of NO on the pathogenesis of hantavirus infections in suckling mice and HFRS/HCPS-patients, and why the NO production is increased in patients, could provide insight into some of the mechanisms behind hantavirus pathogenesis.
Acknowledgements
We thank Mats Spångberg, Christel Werner and Ulrika Edback for excellent animal care and sampling, and Sirkka Vene and Sara Åkerström for excellent technical help. This project was supported by grants from the Swedish Medical Research Council (Projects No. 12177 and 12642), the Swedish Society of Medicine, and by the European Community (Contract Nos. QLK2-CT-1999-01119, QLK2-CT-2002-01358). This publication has been partially funded under the EU 6th Framework Program (GOCE-CT-2003-010284 EDEN) and is officially catalogued by the EDEN Steering Committee as EDEN0014.
References
- 1.Linderholm M., Elgh F. Clinical characteristics of hantavirus infections on the Eurasian continent. Curr. Top. Microbiol. Immunol. 2001;256:135–151. doi: 10.1007/978-3-642-56753-7_8. [DOI] [PubMed] [Google Scholar]
- 2.Lundkvist Å., Plyusnin A. Molecular epidemiology of hantavirus infections. In: Leitner T., editor. Molecular Epidemiology of Human Viruses. Kluwer Academic Publishers; Boston: 2002. pp. 351–384. [Google Scholar]
- 3.Schmaljohn C., Hjelle B. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plyusnin A. Genetics of hantaviruses: implications to taxonomy. Arch. Virol. 2002;147:665–682. doi: 10.1007/s007050200017. [DOI] [PubMed] [Google Scholar]
- 5.Avsic-Zupanc T., Xiao S.Y., Stojanovic R., Gligic A., van der Groen G., LeDuc J.W. Characterization of Dobrava virus: a hantavirus from Slovenia, Yugoslavia. J. Med. Virol. 1992;38:132–137. doi: 10.1002/jmv.1890380211. [DOI] [PubMed] [Google Scholar]
- 6.Nemirov K., Vapalahti O., Lundkvist Å., Vasilenko V., Golovljova I., Plyusnina A., Niemimaa J., Laakkonen J., Henttonen H., Vaheri A., Plyusnin A. Isolation and characterization of Dobrava hantavirus carried by the striped field mouse (Apodemus agrarius) in Estonia. J. Gen. Virol. 1999;80:371–379. doi: 10.1099/0022-1317-80-2-371. [DOI] [PubMed] [Google Scholar]
- 7.Antoniadis A., Stylianakis A., Papa A., Alexiou-Daniel S., Lampropoulos A., Nichol S.T., Peters C.J., Spiropoulou C.F. Direct genetic detection of Dobrava virus in Greek and Albanian patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 1996;174:407–410. doi: 10.1093/infdis/174.2.407. [DOI] [PubMed] [Google Scholar]
- 8.Papa A., Johnson A.M., Stockton P.C., Bowen M.D., Spiropoulou C.F., Alexiou-Daniel S., Ksiazek T.G., Nichol S.T., Antoniadis A. Retrospective serological and genetic study of the distribution of hantaviruses in Greece. J. Med. Virol. 1998;55:321–327. doi: 10.1002/(sici)1096-9071(199808)55:4<321::aid-jmv11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Avsic-Zupanc T. Hantaviruses and hemorrhagic fever with renal syndrome in the Balkans. In: Saluzzo J.F., Dodet B., editors. Factors in the Emergence and Control of Rodent Borne Disease. Elsevier; Paris: 1999. pp. 93–98. [Google Scholar]
- 10.Lundkvist Å., Apekina N., Myasnikov Y., Vapalahti O., Vaheri A., Plyusnin A. Dobrava hantavirus outbreak in Russia. Lancet. 1997;350:781–782. doi: 10.1016/S0140-6736(05)62565-2. [DOI] [PubMed] [Google Scholar]
- 11.Lundkvist Å., Hukic M., Hörling J., Gilljam M., Nichol S., Niklasson B. Puumala and Dobrava viruses cause hemorrhagic fever with renal syndrome in Bosnia-Herzegovina: evidence of highly cross-neutralizing antibody responses in early patient sera. J. Med. Virol. 1997;53:51–59. [PubMed] [Google Scholar]
- 12.Lundkvist Å., Vasilenko V., Golovljova I., Plyusnin A., Vaheri A. Human Dobrava hantavirus infections in Estonia. Lancet. 1998;352:369. doi: 10.1016/S0140-6736(05)60467-9. [DOI] [PubMed] [Google Scholar]
- 13.Meisel H., Lundkvist Å., Gantzer K., Bar W., Sibold C., Krüger D.H. First case of infection with hantavirus Dobrava in Germany. Eur. J. Clin. Microbiol. Infect. Dis. 1998;17:884–885. doi: 10.1007/s100960050214. [DOI] [PubMed] [Google Scholar]
- 14.Khaiboullina S.F., St Jeor S.C. Hantavirus immunology. Viral Immunol. 2002;15:609–625. doi: 10.1089/088282402320914548. [DOI] [PubMed] [Google Scholar]
- 15.Pensiero M.N., Sharefkin J.B., Dieffenbach C.W., Hay J. Hantaan virus infection of human endothelial cells. J. Virol. 1992;66:5929–5936. doi: 10.1128/jvi.66.10.5929-5936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilpatrick E.D., Terajima M., Koster F.T., Catalina M.D., Cruz J., Ennis F.A. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J. Immunol. 2004;172:3297–3304. doi: 10.4049/jimmunol.172.5.3297. [DOI] [PubMed] [Google Scholar]
- 17.Nolte K.B., Feddersen R.M., Foucar K., Zaki S.R., Koster F.T., Madar D., Merlin T.L., McFeeley P.J., Umland E.T., Zumwalt R.E. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 1995;26:110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 18.Zaki S.R., Greer P.W., Coffield L.M., Goldsmith C.S., Nolte K.B., Foucar K., Feddersen R.M., Zumwalt R.E., Miller G.L., Khan A.S., Rollin P.E., Ksiazek T.G., Nichol S.T., Mahy B.W.J., Peters C.J. Hantavirus pulmonary syndrome, Pathogenesis of an emerging infectious disease. Am. J. Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 19.Temonen M., Mustonen J., Helin H., Pasternack A., Vaheri A., Holthofer H. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin. Immunol. Immunopathol. 1996;78:47–55. doi: 10.1006/clin.1996.0007. [DOI] [PubMed] [Google Scholar]
- 20.Groeneveld P., Colson P., Kwappenberg K., Clement J. Increased production of nitric oxide in patients infected with the European variant of Hantavirus. Scand. J. Infect. Dis. 1995;27:453–456. doi: 10.3109/00365549509047045. [DOI] [PubMed] [Google Scholar]
- 21.Linderholm M., Ahlm C., Settergren B., Waage A., Tärnvik A. Elevated levels of tumor necrosis factor (TNF)-α, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 1996;173:38–43. doi: 10.1093/infdis/173.1.38. [DOI] [PubMed] [Google Scholar]
- 22.Davis I.C., Zajac A.J., Nolte K.B., Botten J., Hjelle B., Matalon S. Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome. J. Virol. 2002;76:8347–8359. doi: 10.1128/JVI.76.16.8347-8359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klingström J., Plyusnin A., Vaheri A., Lundkvist Å. Wild-type Puumala hantavirus infection induces cytokines, c-reactive protein, creatinine, and nitric oxide in Cynomolgus macaques. J. Virol. 2002;76:444–449. doi: 10.1128/JVI.76.1.444-449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karupiah G., Chen J.H., Mahalingam S., Nathan C.F., MacMicking J.D. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akaike T., Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdan C., Rollinghoff M., Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 27.Åkerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist Å., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingström J., Heyman P., Escutenaire S., Brus Sjölander K., De Jaegere F., Henttonen H., Lundkvist Å. Rodent host specificity of European hantaviruses: evidence of Puumala virus interspecific spillover. J. Med. Virol. 2002;68:581–588. doi: 10.1002/jmv.10232. [DOI] [PubMed] [Google Scholar]
- 29.Klingström J., Maljkovic I., Zuber B., Rollman E., Kjerrström A., Lundkvist Å. Vaccination of C57/BL6 mice with Dobrava hantavirus nucleocapsid protein in Freund's adjuvant induced partial protection against challenge. Vaccine. 2004;22:4029–4034. doi: 10.1016/j.vaccine.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Tsai T.F., Bauer S., McCormick J.B., Kurata T. Intracerebral inoculations of suckling mice with Hantaan virus. Lancet. August 1982;28:503–504. doi: 10.1016/s0140-6736(82)90537-2. [DOI] [PubMed] [Google Scholar]
- 31.Yoo Y.C., Yoshimatsu K., Tamura M., Azuma I., Arikawa J. Comparison of virulence between Seoul virus strain and Hantaan virus strain 76-118 of hantaviruses in newborn mice. Microbiol. Immunol. 1993;37:557–562. doi: 10.1111/j.1348-0421.1993.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 32.Asada H., Tamura M., Kondo K., Okuno Y., Takahashi Y., Dohi Y., Nagai T., Kurata T., Yamanishi K. Role of T lymphocyte subsets in protection and recovery from Hantaan virus infection in mice. J. Gen. Virol. 1987;68:1961–1969. doi: 10.1099/0022-1317-68-7-1961. [DOI] [PubMed] [Google Scholar]
- 33.Hooper J.W., Larsen T., Custer D.M., Schmaljohn C.S. A lethal model for hantavirus pulmonary syndrome. Virology. 2001;289:6–14. doi: 10.1006/viro.2001.1133. [DOI] [PubMed] [Google Scholar]
- 34.Kariwa H., Kamimura M., Arikawa J., Yoshimatsu K., Takashima I., Hashimoto N. Characterization of the mode of Hantaan virus infection in adult mice using a nested reverse transcriptase polymerase chain reaction: transient virus replication in adult mice. Microbiol. Immunol. 1995;39:35–41. doi: 10.1111/j.1348-0421.1995.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 35.Wichmann D., Gröne H.J., Frese M., Pavlovic J., Anheier B., Haller O., Klenk H.D., Feldmann H. Hantaan virus infection causes an acute neurological disease that is fatal in adult laboratory mice. J. Virol. 2002;76:8890–8899. doi: 10.1128/JVI.76.17.8890-8899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laubach V.E., Shesely E.G., Smithies O., Sherman P.A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H.W., Lee P.W., Johnson K.M. Isolation of the etiological agent of Korean hemorrhagic fever. J. Infect. Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 38.Vapalahti O., Lundkvist Å., Kukkonen S.K.J., Cheng Y., Gilljam M., Kanerva M., Manni T., Pejcoch M., Niemimaa J., Kaikusalo A., Henttonen H., Vaheri A., Plyusnin A. Isolation and characterization of Tula virus, a distinct serotype in the genus Hantavirus, family Bunyaviridae. J. Gen. Virol. 1996;77:3063–3067. doi: 10.1099/0022-1317-77-12-3063. [DOI] [PubMed] [Google Scholar]
- 39.Vapalahti O., Lundkvist Å., Fedorov Å., Conroy C.J., Hirvonen S., Plyusnina A., Nemirov K., Fredga K., Cook J.A., Niemimaa J., Kaikusalo A., Henttonen H., Vaheri A., Plyusnin A. Isolation and characterization of a hantavirus from Lemmus sibiricus: evidence for host switch during hantavirus evolution. J. Virol. 1999;73:5586–5592. doi: 10.1128/jvi.73.7.5586-5592.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundkvist Å., Cheng Y., Brus Sjölander K., Niklasson B., Vaheri A., Plyusnin A. Cell culture adaption of Puumala hantavirus changes the infectivity for its natural reservoir, Clethrionomys glareolus, and leads to accumulation of mutants with altered genomic RNA S segment. J. Virol. 1997;71:9515–9523. doi: 10.1128/jvi.71.12.9515-9523.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vapalahti O., Kallio-Kokko H., Salonen E.M., Brummer-Korvenkontio M., Vaheri A. Cloning and sequencing of Puumala virus Sotkamo strain S and M RNA segments: evidence for strain variation in hantaviruses and expression of the nucleocapsid protein. J. Gen. Virol. 1992;73:829–838. doi: 10.1099/0022-1317-73-4-829. [DOI] [PubMed] [Google Scholar]
- 42.Hörling J., Lundkvist Å. Single amino acid substitutions in Puumala virus envelope glycoproteins G1 and G2 eliminate important neutralization epitopes. Virus Res. 1997;48:89–100. doi: 10.1016/s0168-1702(97)01436-6. [DOI] [PubMed] [Google Scholar]
- 43.Lundkvist Å., Fatouros A., Niklasson B. Antigenic variation of European haemorrhagic fever with renal syndrome virus strains characterized using bank vole monoclonal antibodies. J. Gen. Virol. 1991;72:2097–2103. doi: 10.1099/0022-1317-72-9-2097. [DOI] [PubMed] [Google Scholar]
- 44.de Carvalho Nicacio C., Gonzalez Della Valle M., Padula P., Björling E., Plyusnin A., Lundkvist Å. Cross-protection against challenge with Puumala virus after immunization with nucleocapsid proteins from different hantaviruses. J. Virol. 2002;76:6669–6677. doi: 10.1128/JVI.76.13.6669-6677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niklasson B., Jonsson M., Lundkvist Å., Hörling J., Tkachenko E. Comparison of European isolates of viruses causing hemorrhagic fever with renal syndrome by a neutralization test. Am. J. Trop. Med. Hyg. 1991;45:660–665. doi: 10.4269/ajtmh.1991.45.660. [DOI] [PubMed] [Google Scholar]
- 46.Plyusnin A., Hörling J., Kanerva M., Mustonen J., Cheng Y., Partanen J., Vapalahti O., Kukkonen S.K., Niemimaa J., Henttonen H., Niklasson B., Lundkvist Å., Vaheri A. Puumala hantavirus genome in patients with nephropathia epidemica: correlation of PCR positivity with HLA haplotype and link to viral sequences in local rodents. J. Clin. Microbiol. 1997;35:1090–1096. doi: 10.1128/jcm.35.5.1090-1096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebihara H., Yoshimatsu K., Ogino M., Araki K., Ami Y., Kariwa H., Takashima I., Li D., Arikawa J. Pathogenicity of Hantaan virus in newborn mice: genetic reassortant study demonstrating that a single amino acid change in glycoprotein G1 is related to virulence. J. Virol. 2000;74:9245–9255. doi: 10.1128/jvi.74.19.9245-9255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feltquate D.M., Heaney S., Webster R.G., Robinson H.L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 49.Finkelman F.D., Holmes J., Katona I.M., Urban J.F.J., Beckmann M.P., Park L.S., Schooley K.A., Coffman R.L., Mosmann T.R., Paul W.E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 50.Mosmann T.R., Coffman R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 51.MacLean A., Wei X.Q., Huang F.P., Al-Alem U.A.H., Chan W.L., Liew F.Y. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J. Gen. Virol. 1998;79:825–830. doi: 10.1099/0022-1317-79-4-825. [DOI] [PubMed] [Google Scholar]
- 52.Noda S., Tanaka K., Sawamura S.A., Sasaki M., Matsumoto T., Mikami K., Aiba Y., Hasegawa H., Kawabe N., Koga Y. Role of nitric oxide synthase type 2 in acute infection with murine cytomegalovirus. J. Immunol. 2001;166:3533–3541. doi: 10.4049/jimmunol.166.5.3533. [DOI] [PubMed] [Google Scholar]
- 53.Wei X.G., Charles I.G., Smith A., Ure J., Feng G.J., Huang F.P., Xu D., Muller W., Moncada S., Liew F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 54.Plyusnin A., Morzunov S.P. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr. Top. Microbiol. Immunol. 2001;256:47–75. doi: 10.1007/978-3-642-56753-7_4. [DOI] [PubMed] [Google Scholar]
- 55.Meyer B.J., Schmaljohn C.S. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 2000;8:61–67. doi: 10.1016/s0966-842x(99)01658-3. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimatsu K., Arikawa J., Ohbora S., Itakura C. Hantavirus infection in SCID mice. J. Vet. Med. Sci. 1997;59:863–868. doi: 10.1292/jvms.59.863. [DOI] [PubMed] [Google Scholar]
- 57.Huggins J.W., Kim G.R., Brand O.M., McKee K.T., Jr. Ribavirin therapy for Hantaan virus infection in suckling mice. J. Infect. Dis. 1986;153:489–497. doi: 10.1093/infdis/153.3.489. [DOI] [PubMed] [Google Scholar]
- 58.Kim G.R., McKee K.T., Jr. Pathogenesis of Hantaan virus infection in suckling mice: clinical, virological, and serological observations. Am. J. Trop. Med. Hyg. 1985;34:388–395. doi: 10.4269/ajtmh.1985.34.388. [DOI] [PubMed] [Google Scholar]
- 59.Kurata T., Tsai T.F., Bauer S.P., McCormick J.B. Immunofluorescence studies of disseminated Hantaan virus infection of suckling mice. Infect. Immun. 1983;41:391–398. doi: 10.1128/iai.41.1.391-398.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKee K.T., Kim G.R., Jr., Green D.E., Peters C.J. Hantaan virus infection in suckling mice: virological and pathological correlates. J. Med. Virol. 1985;17:107–117. doi: 10.1002/jmv.1890170203. [DOI] [PubMed] [Google Scholar]
- 61.Nakamura T., Yanagihara R., Gibbs C.J., Jr., Amyx H.L., Gajdusek D.D.C. Differential susceptibility and resistance of immunocompetent and immunodeficient mice to fatal Hantaan virus infection. Arch. Virol. 1985;86:109–120. doi: 10.1007/BF01314117. [DOI] [PubMed] [Google Scholar]
- 62.Akaike T. Role of free radicals in viral pathogenesis and mutation. Rev. Med. Virol. 2001;11:87–101. doi: 10.1002/rmv.303. [DOI] [PMC free article] [PubMed] [Google Scholar]


