Abstract
The purpose of this article is to provide for practitioners a comprehensive overview of respiratory diseases, both infectious and noninfectious, in the mouse, rat, hamster, and gerbil. The information presented will also be useful for veterinarians pursuing board certification. Anatomy and physiology are briefly addressed, as those two facets alone could encompass an entire article for these species.
Keywords: Rodent, Chronic, Respiratory, Disease, Mycoplasmosis, Sendai virus, Cilia-associated respiratory bacillus
The purpose of this article is to provide for practitioners a comprehensive overview of respiratory diseases1, 2 in the mouse, rat, hamster, and gerbil, whether they treat family-owned pets, classroom pets, animals housed in nature/wildlife centers, pet stores, zoos, commercial breeding operations, or laboratory animal facilities. The information presented will also be useful for veterinarians pursuing board certification. Anatomy and physiology are briefly addressed, as those two facets alone could encompass an entire article for these species.
Print and electronic resources have vastly increased in recent years, and our knowledge of exotic animal medicine continues to develop. Therefore, practitioners must stay on the cutting edge of this information.
Basic principles of a sound workup should be followed when dealing with respiratory disease in these species, including anamnesis, husbandry evaluation, physical examination, differential diagnoses, diagnostics, treatments, disease control (prevention and quarantine), research complication assessment, and zoonotic potential. Multiple animal outbreaks should employ the same systematic approach as individual animal situations. Standard criteria are discussed here, and specifics as they apply to individual diseases are addressed in later sections.
Genetically engineered mice (GEMs), immunodeficient (ID), and immunosuppressed animals are at increased risk for disease expression than their immunocompentent (IC) counterparts. Lack of genetic diversity within a commercial breeding operation also predisposes animals to infection because of their weakened immune systems.
When an individual or group of animals is presented for respiratory disease, it is crucial to obtain a thorough and accurate history. Significant information includes:
-
•
Date of birth or approximate age
-
•
Sex, breed, and/or strain of the animal
-
•
Time of acquisition
-
•
Acquisition source (if acquired from a store/vendor, where did the store/vendor obtain the animal)
-
•
Husbandry practices including cage size/construction, substrate material(s), room temperature, humidity levels, lighting (intensity and cycle), ventilation, and sanitation/disinfection protocols (intervals, chemicals)
-
•
Diet, water supply, nutritional supplementation in the form of treats, vitamins, and/or minerals. How is the food stored/has it expired/is fresh food free of contamination
-
•
Are there other species of animals housed in the same room/facility and are any of these animals sick/recently deceased?
-
•
Have any new animals recently been added to the household/facility?
-
•
Has the animal been exposed to other animals recently such as a show/boarding/and so forth?
-
•
How long has the animal been ill?
-
•
What symptom(s) is the animal exhibiting?
-
•
Has the animal received treatment for a previous illness(es), when was it treated, and what treatment was provided?
-
•
Are any caretakers ill?
-
•
The country in which the animal resides can be another diagnostic clue with regard to disease syndromes known to occur in specific geographic locations.
Husbandry procedures and perceived stress cannot be overemphasized as to their role in respiratory disease in these rodent species.
These animals are normally comfortable in warm ambient temperatures ranging from 26° to 28°C (79°–82°F) with a relative humidity of 30% to 70%. The animals should be protected from drafts and because they do not have efficient cooling mechanisms, and should never be placed in direct sunlight. Temperature and humidity must be monitored closely, as extremes and variations can cause stress and significantly contribute to disease susceptibility. Ventilation is another important factor, taking into consideration the size of the room, strain and sex of the animals, number of animals present, number of animals per cage, and sanitization interval. Ten to 15 complete air changes per hour should occur in facilities housing large numbers of animals in high-density situations, with fewer air exchanges being adequate for small numbers of pet rodents in private homes. If recycled air is used within a housing system, it should be HEPA (high-efficiency particulate air) filtered. Closed systems should be avoided, as they result in poor air circulation and a buildup of potentially toxic levels of ammonia and carbon dioxide. Ammonia gas reduces the disease-resistance capabilities of the respiratory system. The metaplastic and ciliary inhibiting effects of ammonia can extend an innocuous upper respiratory infection into a bronchopneumonia. If an ammonia smell is detected with one’s nose down at the level of the animal(s) in the cage, the level is too high for the occupants (Angela M. Lennox, DVM, Indianapolis, IN, personal communication, January 2010). Controversy remains on the minimal concentration of ammonia that is deleterious, but levels as low as 25 ppm increase the severity of Mycoplasma-induced lesions.3 Other considerations of air quality might include factors such as dusts, fungal spores, disinfectant vapors, and environmental pollutants.
Strict sanitation and disinfection procedures should be followed on a regular basis. Dishwashers reaching temperatures of 82°C (180°F) can be used to disinfect food and water vessels once organic matter has been removed. Dishcloths or other utensils that could inadvertently be shared between humans and animals should never be used for cleaning. Knowledge of the biology and behavior of infectious organisms is necessary to select appropriate disinfectants that will be active against a particular agent in the environment.
Appropriate pest control must be implemented, as feral mice and rats are often a source of disease.
Other known stressors that affect these animals include transport to and from the veterinary hospital, long-distance shipping from vendors, concurrent disease(s), handling for a physical examination and/or diagnostic procedures, disruption of normal biorhythms, incorrect light intensity and cycles, overall room activity, overcrowding, excessive noise, and experimental manipulations. Stress can exacerbate symptoms, making a subclinical infection become apparent or worsening already existing signs.
Clinical signs are dependent on the aspect of the respiratory system that is affected and the severity of disease expression. However, many respiratory infections are subclinical, and signs may be absent altogether or animals may be found dead without premonitory symptoms. Symptoms are not always diagnostic, but coupled with an accurate history they can provide important clues. General signs associated with respiratory disease include:
-
•
Nasal discharge
-
•
Ocular discharge/chromodacyorrhea
-
•
Sneezing
-
•
Audible clicking, “chattering,” or “snuffling” when breathing
-
•
Dyspnea
-
•
Open-mouth breathing
-
•
Cyanosis
-
•
Head tilt or other vestibular involvement.
Signs of overall ill health such as decreased appetite, anorexia, lethargy, hunched posture, dehydration, and wasting commonly accompany respiratory disease. Respiratory signs can also occur as secondary manifestations of cardiac or other systemic illness.
Obtaining a correct diagnosis is essential, especially when dealing with multiple animal outbreaks; rare and costly research animals; and the pet that is a treasured member of the family. Many respiratory infections are multifactorial, necessitating a thorough investigation. Fundamental diagnostic procedures and methodology employed for traditional species are readily adaptable to rodents. Early and accurate diagnosis can lead to more successful treatment strategies and formulation of a prognosis. All options should be presented because owners can form very strong bonds with these animals, especially rats. The practitioner must be cognizant that performing an examination or stressful diagnostic procedures could be detrimental; therefore in some cases supportive care must be instituted without the aid of diagnostics. Preemptive sedation to relieve anxiety and distress when appropriate can be beneficial to some animals. Procedures requiring anesthesia in the severely compromised animal must be carefully considered, especially when using inhalant anesthetics. Clinical pathology, microbiology, serology, parasitology, environmental screening, and surveillance testing can furnish pieces of the diagnostic puzzle. Necropsy and histopathology should be considered standard elements of the diagnostic plan, especially when epizootics occur. Cases submitted to one diagnostic laboratory typically fall into two general categories: (1) young, recently shipped animals for the pet trade and (2) older animals kept as pets (Drury Reavill, DVM, West Sacramento, CA, personal communication, August 2010). It is important to use a diagnostic laboratory familiar with the specific testing needs of these species and their pathogens. Several independent, commercial, and university-based diagnostic laboratories now offer an array of testing services as outlined in Table 1 . Imaging studies using plain radiography, contrast computed tomography, and magnetic resonance imaging comprise yet another diagnostic and, in some cases, prognostic tool.
Table 1.
Diagnostic laboratories performing rodent health testing
| Diagnostic Laboratory | Mailing Address | Phone Number/Fax Number | Web Site Address | Email Address | Services Provided |
|---|---|---|---|---|---|
| Bioreliance Laboratory Animal Diagnostic Services (LADS) | 14920 Broschart Road, Rockville, MD 20850-3349 | (p) 800-804-3586 (f) 301-610-2587 |
www.bioreliance.com | lads@bioreliance.com | Clinical pathology, microbiology, parasitology, serology (ELISA, IFA, HAI, WIB), PCR, cell line testing, molecular antigen PCR identification test (MAP-IT), reagents for in-house testing, health assessment panels, necropsy, histopathology, environmental monitoring, custom profiles, consultations |
| Charles River | 251 Ballardvale Street, Wilmington, VA 01887 | (p) 800-338-9680 | www.crvier.com |
comments@crl.com askcharlesriver@crl.com |
Clinical pathology, microbiology, serology (MFIA, ELISA, IFA, WIB, HAI), PCR, prevalent rodent infectious agent (PRIA) panel (alternative to mouse and rat antibody production), necropsy, histopathology, environmental screening, custom testing, technical services/consultations |
| Comparative Pathology Laboratory, University of California, Davis | UCD Comparative Pathology Laboratory, 1000 Old Davis Road, Building R-1, Davis, CA 95616-8520 | (p) 530-752-2832 | www.vetmed.ucdavis.edu | cpl@ucdavis.edu | Clinical pathology, microbiology, parasitology, serology (MFIA, ELISA, IFA), PCR, necropsy, histopathology, environmental testing, custom testing, consultation |
| Molecular Diagnostic Services, Inc | 204 Sorrento Valley Blvd., Suite G, San Diego, CA 92121 | (p) 858-450-9990 (f) 858-450-0619 |
www.mds-usa.com | services@mds-usa.com | Clinical pathology, microbiology, serology (ELISA), necropsy, histopathology, environmental monitoring |
| Northwest Zoo Path | 654 W. Main Street, Monroe, WA 98272 | (p) 360-794-0630 (f) 360-794-4312 |
www.zoopath.com | zoopath@aol.com | Histopathology |
| Research Animal Diagnostic Laboratory | Discovery Ridge, Research Park, 4011 Discovery Drive, Columbia, MO 65201 | (p) 800-669-0825 (f) 573-882-5983 |
www.radil.missouri.edu | RADIL@missouri.edu | Clinical pathology, microbiology, parasitology, serology (MFI, IFA, WIB), PCR, necropsy, histopathology |
| Research Associates Laboratory | 14556 Midway Road, Dallas, TX 75244 | (p) 972-960-2221 (f) 972-960-1997 |
www.vetdna.com | Not available | DNA-based testing, microbiology |
| University of Georgia, Veterinary Diagnostic Laboratory, Georgia Laboratory Animal Diagnostic Services | AVDL, College of Veterinary Medicine, University of Georgia, Athens, GA 30602 | (p) 706-542-5568 (f) 706-542-5977 |
www.vet.uga.edu/dlab | Not available | Clinical pathology, microbiology, parasitology, serology (ELISA, confirmatory testing using MFIA and IFA), PCR, necropsy, histopathology, custom testing, consultation |
| University of Miami, Leonard M. Miller School of Medicine, Department of Pathology | 1611 NW 12th Avenue, Miami, FL 33136 | (p) 305-585-6303 (f) 305-326-9363 |
www.cpl.med.miami.edu | compathlab@med.miami.edu | Clinical pathology, microbiology, parasitology, serology (ELISA), histopathology, custom testing |
| Veterinary Molecular Diagnostics, Inc | 5989 Meijer Drive, Suite 5, Milford, OH 45150 | (p) 513-576-1080 (f) 513-576-6177 |
www.VMDLABS.com | Not available | DNA-based testing |
| Zoo/Exotic Pathology Service | 2825 KOVR Drive, West Sacramento, CA 95605 | (p) 916-725-5100 (f) 916-725-6155 |
www.zooexotic.com | mail@zooexotic.com | Nucleic acid-based diagnostics, toxicology, necropsy, histopathology, consultations |
| Zoologix | 9811 Owensmouth Avenue, Suite 4, Chatsworth, CA 91311-3800 | (p) 818-717-8880 (f) 818-717-8881 |
www.zoologix.com | info@zoologix.com | PCR |
This is not a complete listing of all laboratories providing diagnostic testing to rodents but laboratories with which the author has experience.
Abbreviations: ELISA, enzyme-linked-immunosorbent serologic assay; HAI, hemagglutination inhibition; IFA, immunofluorescent assay; MFIA, multiplexed fluorimetric immunoassay; PCR, polymerase chain reaction; WIB, Western immunoblot.
Treatment requires an intimate knowledge of adverse side effects of drugs used in rodents. Very few drugs used in these species are approved by the United States Food and Drug Administration, which presents not only legal but therapeutic considerations, and similar restrictions may exist in other countries. It must be conveyed to the owner that treatment is not always curative and is ameliorative at best for some diseases. The use of particular antimicrobials administered orally, parenterally, or via nebulization can result in dysbiosis with subsequent fatal enterotoxemia. This side effect is seen most commonly in hamsters and gerbils and infrequently in mice and rats. Drugs that fall into this category are β-lactams, macrolides, and lincosamides. Aminoglycosides can cause an ascending flaccid paralysis with respiratory arrest, coma, and death in addition to its ototoxic and nephrotoxic potential. Neuropathological lesions in rats have been associated with nitrofurantoin. Examples of “safe” antibiotics are enrofloxacin, ciprofloxacin, marbofloxacin, trimethoprim/sulfonamide combinations, tetracycline, doxycycline, azithromycin, erythromycin, clarithromycin, and chloramphenicol. However, even antibiotics considered safe can cause problems. Nebulization can be an adjunctive modality to administer with antimicrobials, bronchodilators (aminophylline), mucolytics (acetylcysteine), or mucokinetics (saline, F10) (Fig. 1 ). The nebulizer must be capable of producing particle sizes smaller than 3 μm to reach the alveolar space.4 Nonsteroidal anti-inflammatory drugs and analgesics can be administered if the animal seems to be in pain or discomfort. Corticosteroids are typically reserved for refractory cases. Numerous exotic animal formulary resources are available for specific drug dosages and contraindications.5, 6, 7, 8, 9, 10, 11, 12, 13 Chloramphenicol has been associated with aplastic anemia in humans; therefore, appropriate client education with documentation must occur when prescribing this drug.
Fig. 1.

Use of nebulization for treatment of rats with severe respiratory disease. Nebulizing formulas usually contain acetylcysteine, a bronchodilator, and an antibiotic with normal saline.
(Courtesy of Cathy A. Johnson-Delaney, DVM, Dipl ABVP (Avian), Edmonds, WA.)
For animals presenting with decreased appetite, anorexia, and/or dehydration, nutritional support is an important component of the therapeutic plan. Some animals may require oxygen supplementation and/or thermal support.
The situation must be evaluated as regards the risk to other rodents in the environment when dealing with diseases that are infectious and contagious. For commercial breeding or research situations, this often means depopulation or rederivation procedures by embryo transfer or cesarean section. Strict sanitation and decontamination procedures must be followed and appropriate quarantine measures implemented. Nonessential materials should be discarded and essential items cleaned with an appropriate disinfectant and/or autoclaved before new animals are introduced.
Consideration must be given to organisms carried by human caretakers and research investigators. Organisms such as Streptococcus spp and Klebsiella spp commonly colonize humans. Humans can also transmit viruses that may result in serologic cross-reactions, if not outright infection. In addition, veterinarians must be knowledgable regarding organisms transmissible from rodents to humans.
Anatomy and physiology
It is beyond the scope of this article to provide in-depth anatomy and physiology of these species; however, there are unique features, discussed here. Table 2 summarizes basic physiologic functions and lung lobation. Fig. 2 illustrates the anatomy of the lung in the rat.
Table 2.
Basic physiologic respiratory functions and lung lobation of the mouse, rat, hamster, and gerbil
| Parameter | Mouse | Rat | Hamster | Gerbil |
|---|---|---|---|---|
| Respiratory rate (breaths/min) | 60–230 | 70–115 | 100–250 | 90–160 |
| Tidal volume (mL) | 0.09–0.38 | 0.60–1.5 | 0.91–1.4 | NAa |
| Minute volume (mL/min) | 11–36 | 75–130 | 64 | NAa |
| Oxygen use per hour (mL O2/g body weight/h) | 1.63–3.5 | 0.68–1.10 | 0.6–1.4 | 1.4 |
| Left lung lobation | Single lobe | Single lobe | Single lobe | Single lobe |
| Right lung lobation | 4 lobes (cranial, middle, caudal, accessory) | 4 lobes (cranial, middle, caudal, accessory) | 5 lobes (cranial, middle, caudal, intermediate, accessory) | 4 lobes (cranial, middle, caudal, accessory) |
Data not available.
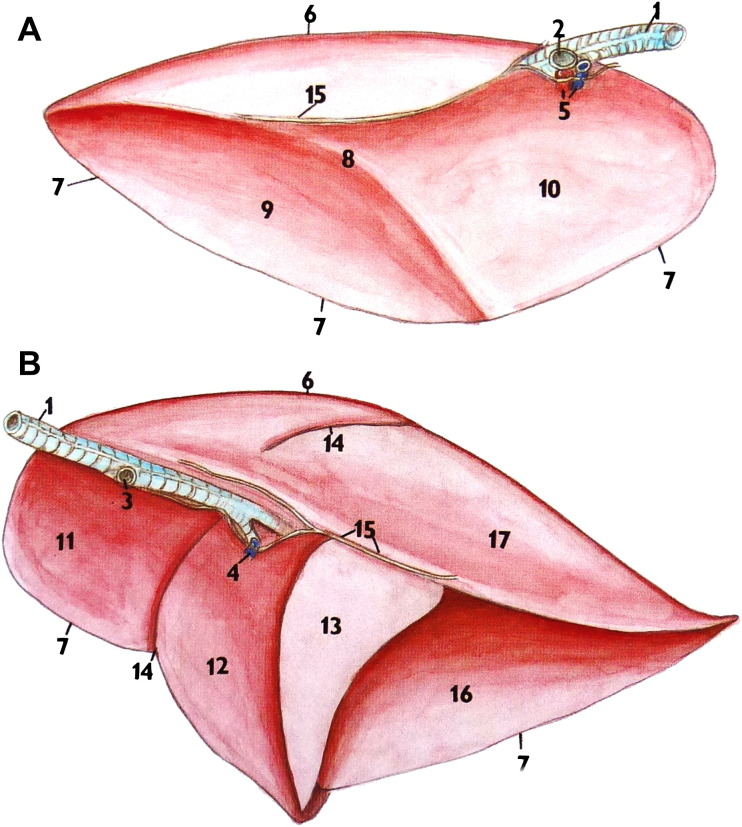
Fig. 2.
View of the mediastinal surfaces of left (A) and right (B) rat lungs. Key features include cranial (11), medial (12), accessory (13), and caudal (16, 17) lobes of the right lung. The left lung has only a single lobe.
(Reprinted from Popesko P, Rajtova V, Horak J. A colour atlas of anatomy of small laboratory animals, vol. 2: rabbit, guinea pig. Elsevier; 1992; with permission from Elsevier.)
Rodents are obligate nasal breathers; therefore, disease processes affecting the nasal cavity interfere with humidification and filtering of inspired air, respiration, and olfaction. Cartilage envelopes are present only in extrapulmonary airways in mice, rats, and hamsters. In rabbits and rodents, the lung volume increases with age, and the ratio of residual volume to vital capacity does not change. Mice, rats, guinea pigs, and rabbits have very high chest wall compliances and low functional residual capacities. Smaller rodents have proportionately wider airways than do larger animals. Rodents posses Clara cells in the bronchial epithelium, which are thought to provide the major component of the distal mucociliary escalator.
Of particular interest are the cardiac muscle fibers surrounding major branches of pulmonary veins that extend into the lung tissue in most rodents, making the pulmonary vein thicker. This route could allow infectious agents to spread from the heart, through the pulmonary veins, and into the lungs.
Mouse
In the mouse,14, 15, 16, 17, 18, 19 the nostrils open laterally at the tip of the snout and are guarded externally by folds of thickened skin. A vertical groove just below them forms a cleft in the upper lip, exposing the incisors. The nostrils communicate internally via vestibules with the anterior nasal cavities, which are separated by a median septum. The sinuses are also divided by the median septum, and are highly developed olfactory organs containing 7 rows of turbinal bones. The nasopharynx forms the posterior part of the pharyngeal duct, lying dorsal to the soft palate and communicating with both the oropharynx and the Eustachian tubes. The intermediate section of the respiratory tract consists of the larynx, trachea, and bronchi, all of which have cartilaginous support. The larynx is formed by 3 single (epiglottis, thyroid, and cricoid) and 3 paired (arytenoids, corniculate, and cuneiform) cartilages. Incomplete cartilaginous rings support the walls of the trachea by branching and fusing with one another dorsally. The trachea branches into the left and right bronchi dorsal to the aortic arch. Extrapulmonary bronchi have complete cartilaginous rings, whereas intrapulmonary bronchi have no cartilaginous rings. There are no muscle swellings in the pulmonary artery of the mouse as there are in the rabbit, guinea pig, and opossum. There are no bronchial artery-pulmonary artery precapillary anastomoses in the mouse as there are in humans. Nerve density in the lung is greater in the mouse than in the dog, cat, rabbit, or guinea pig. Respiratory bronchioles are short or nonexistent. Bronchus-associated lymphoid tissue is normally present only at the hilus of the lung. Lymphoid accumulations are present on the visceral pleura of mice, within interlobar clefts.
A mouse at rest can use up to 3.5 mL of oxygen per gram of body weight per hour, which is approximately 22 times that used by an elephant. To accommodate for this high metabolic rate, the mouse has a rapid respiratory rate, short air passage, high alveolar Po 2, moderately high erythrocyte concentration, high red blood cell hemoglobin and carbonic anhydrase concentrations, high capillary density, and high blood sugar concentration. The hemoglobin affinity for oxygen with changes in pH is more pronounced (Bohr effect). Mice also exhibit a slight shift in the oxygen-dissociation curve, enabling oxygen to be unloaded in the tissue capillaries at a high Po 2.
Rat
In the rat,20, 21, 22, 23, 24 the external nares are shaped like inverted commas, open on the lateral aspect of the nose, and can be closed under water. The rat has several well-developed nasal glands but the largest is the Steno's gland (glandula nasalis lateralis), which lies in the rostral maxillary sinus and its duct empties at the vestibule. This gland is homologous with the salt gland of marine birds. It produces a watery, nonviscous secretion at the nasal airway entrance where it may help to humidify inspired air and regulate mucus viscosity. Because of the large number of autonomic nerves that are found in close contact with its acinar cells, it is believed that this gland is regulated by the nervous system in such a way that rapid adjustment of the secretory activity to changes in the humidity of the inspired air or to airborne irritants is possible. Tracheal diameter is approximately 1.6 to 1.7 mm in the adult rat, and the shape is maintained by 18 to 24 rigid C-shaped cartilage structures that form the framework of the trachea. Tracheal length from the first cartilage to bifurcation is 33 mm and because of the cartilaginous rings, extension of the head of the rat can result in lengthening of the trachea by 50% with no decrease in lumen diameter. The lung in the newborn rat is immature and contains no alveoli or alveolar ducts; instead, gas exchange occurs in smooth walled channels and saccules, and the prospective alveolar structures. Once the rat reaches 4 days old, a rapid restructuring of lung parenchyma occurs so that by day 7, the lung is morphologically more mature. Respiratory bronchioles are also absent at birth but by day 10 are easily identified. Rats have the thinnest pulmonary artery and the thickest pulmonary vein of all rodent species. In the conscious resting rat, blood flow preferentially distributes to the central and hilar regions of the lung lobes, with less blood flow to the peripheral regions. Precapillary anastomoses between the bronchial and pulmonary arteries have been demonstrated in the rat, as they have been in man and guinea pig, and are limited to the hilar region in the rat. Innervation of the lung is complex, with high neuronal density similar to the calf, mouse, and guinea pig. The rat and rabbit do not have an adrenergic nerve supply to the bronchial musculature, and bronchoconstriction is controlled by vagal tone. At least 10 morphologically distinct cell types have been identified in the intrapulmonary airways. Rats possess serous cells in respiratory epithelium, which are unique to this species. These cells secrete a product that has less viscosity than the mucous cell, and is thought to be responsible for the low-viscosity pericilliary liquid layer found at all levels of the rat’s respiratory tract.
Total lung capacity of the rat is 11.3 ± 1.4 mL and vital capacity 8.4 ± 1.7 mL. Although surfactant is composed of mostly monounsaturated phospholipids in many mammals, rat surfactant has a high content of polyunsaturated phospholipids. Carotid bodies located in the bifurcation of the common carotid artery function as chemoreceptors and respond when the tissue partial pressure of oxygen decreases to below 100 mm of mercury. Similar chemoreceptors located in the aorta are called aortic bodies, whose afferents travel via the vagi to the brain. Regulation of respiration occurs through tissue CO2 exchange in the medullary respiratory center, with the carotid bodies playing a role. Rats have high serotonin activity and low histamine activity in the lungs.
Hamster
Hamsters25, 26, 27, 28 have several nasal serous glands that open into the internal ostium of the external nares. These glands include 1 infraseptal, 2 nasoturbinate, 5 maxilloturbinate, 1 ventromedial nasal, 4 or 5 dorsal medial nasal, and the lateral nasal gland (Steno’s gland). There are 4 endoturbinates and 3 ectoturbinates, unlike the rat which has 4 endoturbinates and 2 ectoturbinates. These very intricately folded turbinates project into the lumen of the nasal cavity and thus provide for an increased nasal mucosal surface. The trachea bifurcates at the height of the fourth rib pair into thicker right and thinner left main bronchi. Reisseisen’s membrane, a layer of smooth muscle and elastic tissue, lines the lobar bronchi. The diaphragm originates dorsally on the first lumbar vertebrae and is composed of a well-developed pars muscularis and a transparent centrum tendineum. The pleura forms a large right and left sac surrounding the lungs. There are no respiratory bronchioles as in the rat and guinea pig, although the guinea pig and hamster have a transition to alveolar airways within a single generation that could be classified as producing one order of respiratory bronchioles. The conductive airways contain a limited number of glandular structures, primarily in the proximal trachea. The histologic appearance of the hamster trachea closely resembles the human bronchus. The pulmonary vascular bed is similar to that of humans in many ways, and hamsters develop pulmonary lesions that resemble human centrilobular emphysema. This similarity makes the Syrian hamster a potential model for studies of chronic bronchitis. Bronchus-associated lymphoid tissue, normally present only at the hilus of the lung in rodents, is absent in hamsters. Spontaneous bronchiogenic and pulmonary cancers are rare; hence, the Syrian hamster is a good model in which to study chemical carcinogenesis in the respiratory tract.
Resting respiration rate is inversely proportional to the body weight, whereas tidal volume and mean minute volume are directly related. Arterial blood pH is 7.4 and Pco 2 is 45.3 mm Hg. Blood pH increases slightly during hibernation and Pco 2 decreases, indicating that hibernating animals are slightly acidotic. Hamsters are fairly resistant to pulmonary infection and are able to decompose nicotine, and therefore make good subjects for the study of effects of long-term smoke inhalation.
Gerbil
The gerbil29, 30 has not been studied as extensively as the mouse, rat, and hamster, but its respiratory anatomy and physiology are similar to those of other small rodent species.
Infectious diseases
Bacterial Agents
Bordetella bronchiseptica
Bordetella bronchiseptica 31, 32, 33, 34 is a gram-negative bacillus or coccobacillus belonging to the family Alcaligenaceae. Infection is more likely in pet rodents and rabbits, especially those exposed to other species such as cats and dogs. Because of the frequency of Bordetella in the laboratory guinea pig and rabbit, contact with these species should be avoided. Frequently there is an identifiable concurrent infection, such as coronavirus.
Transmission is by direct contact with clinically affected animals, carrier hosts, contaminated fomites, and respiratory aerosols. Although many surviving animals develop immunity and eliminate the infection, subclinical and carrier animals are common. The bacteria can form biofilms in vitro that may serve to protect it from host defenses.
Diagnosis is best achieved by isolation of the organism in large numbers from affected tissues. Enzyme-linked immunosorbent serologic assay (ELISA) is commercially available and the polymerase chain reaction (PCR) is possible. Treatment is usually not practical with the exception of small numbers of pets, and even then treatment of chronic infections is palliative at best. The organism is normally sensitive to trimethoprim-sulfonamide products, chloramphenicol, enrofloxacin, and marbofloxacin. If the animal is anorexic, nutritional support should also be provided.
The importance of infection of humans in minimal, although the organism is recovered occasionally from the human nasopharynx and could serve as a source of infection to animals. The organism could cause a whooping-cough syndrome and bronchopneumonia in young, elderly, or immunocompromised humans.
Mouse
Although no naturally occurring disease has been reported, mice are susceptible to experimental infection. Strains such as C3H/HeJ show increased susceptibility to clinical disease.
Rat
Infection is typically opportunistic, but aerosol exposure in laboratory rats has resulted in lesions characterized by suppurative rhinitis. The organism tends to colonize on the apices of the ciliated respiratory epithelial cells, resulting in impaired clearance. In spontaneous cases, there has been a suppurative bronchopneumonia with consolidation of affected anteroventral areas of the lung. Multifocal bronchopneumonia with polymorphonuclear cell and lymphocytic infiltration, and peribronchial lymphoid hyperplasia are seen microscopically.
In experimental trials, B bronchiseptica caused pneumonia and was more pathogenic for the respiratory system of weanling rats than Pasteurella pneumotropica. 35
Hamster
The hamster appears to be uniquely resistant to intranasal inoculation with this organism.
Gerbil
This organism is a potential problem for gerbils, but has not been reported as a natural disease. Young gerbils inoculated intranasally with B bronchiseptica developed a severe disease with high mortality, whereas older gerbils appeared to be more resistant. Both the Meriones unguiculatus and Meriones shawi species appear to be susceptible.
Chlamydophila spp
Chlamydophila spp36, 37 belong to the family Chlamydiaeceae, and are gram-negative obligate intracellular bacteria whose name remains in a state of constant flux. Mice are susceptible to natural infections with Chlamydophila muridarum, the mouse pneumonitis (MoPn) agent. Clara Nigg discovered the agent, so it has also been referred to as the “Nigg Agent.” Mice are experimentally susceptible to both Chlamydophila trachomatis and Chlamydophila psittaci of human origin. IC animals develop transient infections that are typically silent in natural infections. Natural infection of laboratory mice is rare, but infection with other chylamidiae, such as C psittaci or Chlamydophila pneumoniae, does occur, with increased incidence in ID animals. Experimental lung infections are more severe in BALB mice than in B6 mice. In addition, C psittaci can experimentally cause respiratory and septicemic disease in mice. These infections are more severe in C3H, BALB/c, or A/J strains than in resistant B6 mice. Immunity to the MoPn agent is dependent on functional CD4 T cells. B-cell–deficient mice (Igh6 null) recover from infection, but T-cell–deficient RAG, SCID, and MHC class II (CD4 null) (but not β2-microglobulin [CD8] null mice) develop severe disease. Mice of the C3H/HeN strain develop infections of longer duration than those of BALB/c or B6 strains.
Based on experimental infection, transmission is presumed to occur via respiratory aerosols and/or venereal transmission. Contact exposure rarely results in transmission. Both mouse and human agents are used in laboratory mice as models for respiratory and genital chlamydiosis, therefore serving as potential iatrogenic sources of infection for mouse colonies. Severe acute infections are characterized by ruffled fur, hunched posture, and labored respiration due to interstitial pneumonitis, followed by death within 24 hours. Mice dying more slowly may develop progressive emaciation and cyanosis of the ears and tail.
Intranasal inoculation results in nonsuppurative interstitial pneumonia with atelectasis and pulmonary perivascular/peribronchiolar lymphocytic infiltration. Lesions are manifested grossly as pinpoint, elevated gray foci on the pleural surfaces. Organisms grow within bronchiolar epithelium, type I alveolar cells, and macrophages, which can possess intracytoplasmic vesicles containing inclusions. The agent readily disseminates hematogenously and by lymphatics to multiple organs regardless of route of inoculation, due to its affinity for macrophages.
Diagnosis can be made with impression smears, growth in cell culture, or embryonated chicken eggs. Accurate speciation can be made via DNA sequencing.
Cilia-associated respiratory bacillus
The cilia-associated respiratory bacillus (CARB)38, 39, 40, 41, 42, 43, 44, 45, 46, 47 organism is an unclassified, gram-negative, motile, non–spore-forming bacterium. It is closely related genetically to Flexibacter spp and the Flavobacterium group of bacteria known as “gliding bacteria,” based on the fact they are motile but without visible means for such motility. These bacteria are widespread and noteworthy respiratory pathogens in rats, commonly infect rabbits, and probably infect mice at a higher rate than is currently recognized. Disease has also been reported in wild rats, hamsters, guinea pigs, dogs, cats, goats, swine, and cattle. No data exist for pet populations but infections are likely to be common. CARB can act as a primary pathogen or can exacerbate infections caused by other agents. Colonization with the organism leads to interference with the mucociliary apparatus, and secondary infections with other opportunistic invaders in chronic cases may occur.
CARB is transmitted via direct contact, and there is no evidence for transmission by fomites, vectors, or aerosols. With an infected population, CARB tends to spread slowly. Serology can be used to monitor healthy populations using multiplexed fluorimetric immunoassay (MFIA), ELISA, or immunofluorescent assay (IFA) along with PCR and/or histopathology to detect the organism in diseased animals. However, CARB serology has a higher rate of false positives because the reagents used are often bacterial lysates containing numerous antigens. PCR is the preferred confirmatory method for follow-up to positive serology, and is best performed on nasopharyngeal or tracheal swabs or lavages. Because transmission is by direct contact, screening of sentinel rats exposed to bedding may miss infections.
Culture and sensitivity testing have demonstrated that CARB is sensitive to sulfonamides, procaine penicillin G, ampicillin, chloramphenicol, neomycin, gentamicin, and streptomycin. The efficacy of antimicrobial therapy in eliminating CARB from enzootically infected colonies or in chronically infected pets remains unknown.
The primary consideration for exclusion of this agent from a facility or colony should be the avoidance of direct contact between infected and uninfected animals. Colony animals should be screened regularly for CARB, and incoming animals should be quarantined and screened. The appearance of this organism in an established facility, previously free of it, would indicate the entry of infected rodents, most likely feral or wild. Repopulation or rederivation are generally recommended. It is unlikely that survival of the organism in the environment should play a significant role in the transmission of CARB. Typical animal room sanitation and disinfection should serve to remove any CARB from the environment.
Mouse
In breeding populations, CARB is transmitted from infected dams to pups shortly after birth, and infection can be transmitted among adult mice by direct contact. Natural outbreaks of disease in mice seem to be associated with concurrent viral infections, including Sendai virus (SeV) and pneumonia virus of mice (PVM). Experimental, and probable natural, infections may be inapparent with no discernible lesions. Chronic disease and seroconversion have been produced in BALB/c mice inoculated intranasally with the CARB, but B6 mice developed less severe lesions and lower antibody responses. The organism has been associated with chronic respiratory disease in conventional B6 and B6 obese mutant mice dying of the disease. Microscopic changes include chronic suppurative cranioventral bronchopneumonia with marked peribronchiolar infiltration with lymphocytes and plasma cells, and luminal neutrophilic exudation.
Rat
CARB was first reported in association with respiratory disease in rats in 1980; however, the organisms have been found in archived tissues collected in the 1950s, and are seen on electron microscopy photographs published in the 1960s.48
Naturally occurring, uncomplicated disease has been observed in rats, and signs are similar to those seen with Mycoplasma pulmonis infection. Although infected rats are often asymptomatic, signs generally associated with respiratory disease can occur. Rats appear to have a more significant clinical presentation than mice. Lesions similar to those seen in confirmed cases of mycoplasmosis have been produced in Mycoplasma-free rats inoculated intranasally with CARB. Its pathogenic potential as a potentiator of M pulmonis respiratory disease has been demonstrated most clearly in the rat. Intranasal inoculation of young Wistar rats resulted in colonization of the upper respiratory tract and airways by 14 days. Necropsy signs are variable, and depend on the pathogenicity of the bacterial strain involved and the chronicity of the infection. CARB can be present anywhere there is ciliated respiratory epithelium, including the Eustachian tubes and middle ears. A multifocal to coalescing pyogranulomatous bronchopneumonia with bronchiectasis, enlarged mediastinal and bronchial lymph nodes, and dilated bronchi are seen microscopically. Chronic suppurative bronchitis and bronchiolitis, with peribronchiolar cuffing with lymphocytes and plasma cells, are typical microscopic findings.
Hamster
A multifocal to coalescing pyogranulomatous bronchopneumonia with bronchiectasis, enlarged mediastinal and bronchial lymph nodes, and dilated bronchi are seen in experimentally infected hamsters.
Gerbil
Gerbils are susceptible to experimentally induced CARB infections. Young gerbils inoculated intranasally with a rat isolate remained asymptomatic during the study. At necropsy, there was colonization of the apices of epithelial cells lining the trachea and airways, with marked peritracheal and peribronchial lymphocytic infiltration.49
Corynebacterium kutscheri
Corynebacterium kutscheri 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 is a gram-positive bacillus belonging to the family Corynebacteriaceae. It causes corynebacteriosis or pseudotuberculosis, and is considered an opportunistic pathogen in IC animals (Fig. 3 ). This infectious disease syndrome was one of the first to be recognized in mice and rats by Kutscher in 1894. It remains a significant pathogen that occasionally infects colonies of rats and mice, and infection is usually latent and subclinical. Infections only become overt after immunosuppression or other stressors such as nutritional deficiencies. Natural transmission is via the oral-fecal route, with prenatal transmission occurring experimentally. Infected animals may shed the bacterium into the environment for extended periods of time, as it has been detected in the feces of mice up to 5 months post infection. Pet rodents, and rats in particular, can transmit the bacterium to their human handlers.
Fig. 3.
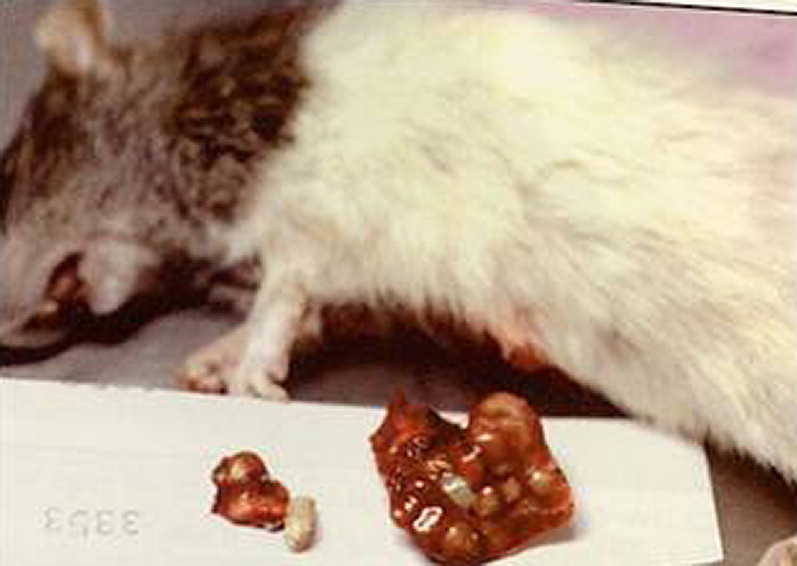
Gross necropsy of rat lung with abscesses due to Corynebacterium kutcheri.
(Courtesy of Cathy A. Johnson-Delaney, DVM, Dipl ABVP (Avian), Edmonds, WA.)
The acute clinical disease has high morbidity and low mortality, and infected animals exhibit signs associated with respiratory disease in addition to abnormal gait, with septic and swollen joints. Death usually occurs in 1 week. A chronic infection, with low morbidity and mortality, may be inapparent or produce nonspecific signs.
Hematogenous extension of the organism from the oral cavity via small abrasions or from regional lymph nodes results in focal embolic abscessation in a variety of organs, including the lungs. Histologically, lesions are chronic and consist of a pyogranulomatous infiltrate around the central necrotic core, surrounded by a mantle of infiltrating lymphocytes, plasma cells, and fibroblasts. Lung lesions eventually become granulomatous, giving rise to the name pseudotuberculosis.
Diagnosis is by examination of impression smears from affected tissues or tissues sections. Definitive diagnosis requires characterization of the cultured bacteria or serology. Positive ELISA should always be confirmed by culture. The isolation rate of this organism is most successful from specimens collected from the oral cavity and submaxillary lymph nodes. This agent is difficult to recover from animals latently infected in enzootically affected colonies, although oral swabs of the gingiva may be helpful. PCR is not widely available.
The bacterium is sensitive to a variety of antimicrobials, including ampicillin, chloramphenicol, and tetracyclines. Treatment of animals with antimicrobials may serve to treat illness, but would probably not resolve the carrier state nor eliminate the bacteria from the bedding or cage surfaces. Thus, treatment is only recommended to ameliorate clinical signs or for rederivation, if necessary.
Mouse
The usual sites of colonization in mice are the oral cavity, cecum, and colon. Clinical manifestations usually occur in conjunction with predisposing factors that compromise the immune system.
Susceptibility to this organism among various strains of mice is attributed to the effectiveness of the mononuclear phagocyte system. BALB/c-nude, A/J, CBA/N, MPS, and BALB/cCr mice are most susceptible, C3H/He mice intermediate, and C57BL/6Cr, B10.BR/SgSn, ddY, and ICR resistant to colonization and disease induction. Male mice harbor higher numbers of bacteria and a higher carrier rate. Strains of mice sensitive to C kutscheri infections tend to be resistant to Salmonella spp infections and vice versa.
Raised gray nodules may be present in the lungs along with other organ involvement. Lesions may contain material that varies from friable caseous exudate to liquefied pus. Microscopically, lesions feature coagulation to caseation necrosis, with peripheral aggregations of leukocytes composed primarily of neutrophils. Suppurative thrombosis and embolization involving the pulmonary or mesenteric and portal vessels may be evident.
Rat
Rats are more resistant to acute spontaneous disease than mice. Rats infected with sialodacryoadenitis virus (SDAV), SeV, or parvovirus do not transform preexisting subclinical C kutscheri into clinically apparent disease. Clinical symptoms are those typically seen with respiratory disease. Gross lesions include raised pale foci of suppuration of variable size with a characteristic hyperemic peripheral zone in the lung. Affected areas frequently coalesce with adjacent lesions. Fibrinous exudate may be present on the pleura and/or pericardial sac. Histologically, lesions occur most frequently in the lung. There are foci of coagulation to caseation necrosis, with leukocytic infiltration as in the mouse. Neutrophils are the predominant cellular infiltrates in the early stages. Subsequently there are mononuclear cells composed of macrophages, lymphocytes, and plasma cells. Lesions are usually not associated with airways and are interpreted to be hematogenous in origin. There is an associated pneumonia, with hypercellularity of alveolar septa, perivascular cuffing, and pulmonary edema. Some airways adjacent to affected areas may contain purulent exudate.
The presence of bacterial colonies is pathognomonic. Lymphoid hyperplasia is a frequent finding in chronic cases, and residual scars may be present in target tissues of recovered animals.
There is one report of a human C kutscheri infection in an infant after a bite from an infected rat.60
Hamster
Both C kutscheri and Corynebacterium paulometabulum have been isolated from the respiratory tracts of hamsters. Although the hamster can serve as a host, it appears to be relatively resistant to systemic infection.
Haemophilus spp
During routine quality control of a laboratory rodent colony, 16.8% of the rats were found to be infected with this organism, which was characterized as a member of the family Pasteurellaceae.61 The organism was cultured from the nasal cavity, trachea, lung, and the female genital tract. Investigation of rats immediately on receipt from the breeder showed that they were culturally and serologically positive for Haemophilus spp. Histologic examination of the lungs in rats infected with Haemophilus spp demonstrated a mild inflammatory cell infiltration and diffuse hyperemia. The prevalence of this organism is unknown. In view of the sites of colonization and the presence of lesions in the respiratory tract, this represents a possible complicating factor in the laboratory rat under experiment.
Klebsiella pneumoniae
Klebsiella pneumoniae 62, 63, 64, 65 is a gram-negative anaerobic rod belonging to the family Enterobacteriaceae that can be a normal component of the intestinal flora in mice and rats. The bacterium may be also common in the environment. It is considered an opportunistic pathogen in these species, but is not a significant cause of naturally occurring disease.
K pneumoniae can be readily transmitted from one species to another, including humans to animals and vice versa. Transmission is probably fecal-oral or via direct contact. Colonization of animals may be from human caretakers or from exposure to infected soil.
Clinical signs and lesions are very rare in IC animals. These organisms are low-level opportunists; therefore, ID animals are more susceptible to disease. Infection may also be seen after antibiotic treatment. There is no pattern of infection or characteristic lesions, but it has been associated with mild suppurative rhinitis in otherwise pathogen-free rats. It has been associated with bacteremic disease in mice with cervical lymphadenopathy, liver and kidney abscesses, emphysema, pneumonia, ventricular endocarditis and myocarditis, and thrombosis.
Diagnosis is by culture and biochemical identification to differentiate the species. Treatment with antimicrobials may serve to treat illness, but rarely, if ever, resolves the carrier state; nor will therapy eliminate bacteria from the bedding or cage surfaces. This organism is an important cause of human nosocomial infection, and human isolates of are often multidrug resistant.
Mycobacterium avium-intracellulare
Mycobacterium avium-intracellulare 66, 67 is a gram-positive, acid-fast, obligate intracellular bacterium belonging to the family Mycobacteriaceae that can be found in soil, water, and bedding materials. Naturally occurring infections are rare but mice are susceptible to experimental infections. A naturally occurring outbreak of infection in C57BL/6N mice within a B6C3F1 hybrid production colony has been documented. B6C3F1 hybrid mice did not develop lesions of mycobacterial infection when intratracheally inoculated. Adult mice were more susceptible to infection than 8-week-old animals. Grossly, subpleural 1- to 5-mm diameter tan-colored masses were present in the lungs. Microscopic findings consisted of focal accumulations of epithelioid cells, foamy macrophages, and lymphocytes in alveolar spaces and septa, with variable amounts of necrosis and neutrophilic leukocyte infiltration.
Mycoplasma spp
Mycoplasma pulmonis 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 is a gram-negative, small bacterium devoid of cell walls and is a member of the family Mycoplasmataceae. Infection and disease are common in pets, nonbarrier-housed rats and mice, and wild rodents. The organism can be carried in the in the upper respiratory passages in the absence of disease.
Mycoplasmosis is exacerbated by viral infections, particularly SeV; by other bacteria including P pneumotropica, Actinobacillus spp, Streptococcus pneumoniae, B bronchiseptica, CARB, and C kutscheri; and by environmental ammonia levels. These cofactors play a significant role in causing subclinical infections to manifest into outright disease. The most important aspect for clinicians is that respiratory mycoplasmosis varies greatly in disease expression because of environmental, host, and pathogen factors that influence the host-pathogen relationship. M pulmonis colonizes the apical cell membranes of respiratory epithelium, interferes with mucociliary clearance, and is mitogenic for B cells, which contributes to the pathology observed in the lungs. The acquired immune response is important in limiting hematogenous dissemination but does little to eliminate infection or resolution of disease.
Diagnosis is based on history, clinical findings, gross and microscopic lesions, and isolation of the organism from tissues. PCR offers a rapid way to screen cell lines, biologic agents, and other tissues. Colony surveillance can employ serology (MFIA, ELISA, or IFA), as the organisms persist despite the presence of antibodies. However, animals may be infected for months before antibodies develop against these surface-dwelling organisms, yielding false-negative results in early stages of the disease. Therefore, culture and PCR are recommended to detect early infections. CARB is frequently a copathogen with M pulmonis, and diagnostic investigations should also include screening for this organism.
Despite developing high antibody titers to Mycoplasma and high antibiotic tissue levels, affected animals typically have persistent M pulmonis infection; therefore, antimicrobial therapy may alleviate clinical signs but does not eliminate the infection. For many years the standard of treatment for laboratory rats was to add tetracycline to sweetened drinking water; however, this treatment is ineffective because blood antibiotic concentrations are below minimum inhibitory concentration (MIC) and pulmonary tissue concentration of tetracycline is not inhibitory. Tetracyclines in water can cause a reduction in water consumption, and those at high concentrations in tap water form a scale that can block sipper tubes. Although scientific studies of effectiveness have not been conducted, tylosin administered in drinking water has been shown to reach concentrations in serum and lung well above MIC concentrations. Enrofloxacin (10 mg/kg) in combination with doxycycline (5 mg/kg) administered per os every12 hours for 7 to 10 days appears to be an effective regimen to control symptomatic animals. Sulfamethazine at 0.02% in the drinking water or 1 mg/4 g feed, tylosin at 66 mg/L (2.5 g/gallon) for 21 days, and chloramphenicol at 30 mg/kg for 5 days are other treatment protocols used in colony situations. Nebulization, anti-inflammatory and analgesic medication, and nutritional support are indicated in chronic cases of infection. Environmental factors contributing to the severity of the disease must also be corrected.
Effective control and prevention depend primarily on maintenance of Mycoplasma-free colonies under barrier conditions supported by careful surveillance for infection. Progress has been made in developing DNA-based vaccines against M pulmonis, but these have not achieved clinical application. Prevention of this organism in a facility should focus on the entry of animals and biologic materials. Animals should be obtained from reputable vendors or quarantined and screened before entry. Vigorous pest control should be in place. Pet rats and mice commonly harbor this organism, and caretakers in laboratory facilities should not keep pet rodents or have secondary employment that may expose them to pets or wild rodents. Mycoplasma spp are common contaminants of animal and human tumor cell lines, but M pulmonis is rarely confirmed in these materials. Nonetheless, these materials should be screened via PCR or antibody production.
Elimination of M pulmonis from large populations of rats and mice, for all practical purposes, is impossible without rederivation or depopulation. The organism can be found in both male and female reproductive tissues, so the pretreatment of donor animals with antibiotics may be helpful in decreasing the chance of vertical transmission.
In general, these organisms are not considered to be viable for long periods of time outside of a host. Some mycoplasmas are able to form biofilms, which may afford them better resistance to heat and desiccation than previously thought. Decontamination appropriate for more robust non–spore-forming bacteria should be sufficient for decontamination after an outbreak.
Research protocols involving inhalation toxicology and pulmonary carcinogenesis can be compromised by chronic, progressive infection. One of the most important complications is contamination of cell lines and transplantable tumors. There is evidence that M pulmonis may depress humoral and cellular mediated responses. Animals with M pulmonis infection have decreased delayed hypersensitivity responses, T-cell subset changes, and increased total lymphocyte and neutrophil counts.
Although the organism can be carried in the nasal passage, it does not normally affect humans. M pulmonis has been detected, isolated, and sequenced in animal facility workers exposed to infected rats. The mode of transmission is unknown.78
Mouse
M pulmonis, Mycoplasma arthritidis, and Mycoplasma neurolyticum inhabit the upper respiratory tract of mice. M arthritidis may cause respiratory disease following intranasal inoculation, but under natural conditions it is generally nonpathogenic. However, it is problematic because it can cause seroconversion to M pulmonis.
The organism referred to as the gray lung agent (GLA) has been characterized as a Mycoplasma spp. It appears to be closely related to Mycoplasma hominis and distantly related to M pulmonis. The name Candidatus Mycoplasma ravipulmonis has been proposed.79
Compared with rats, mice are relatively resistant to the disease caused by M pulmonis, and there has been a marked decrease in incidence of clinical disease in laboratory mice. Asymptomatic infection is more common. Exposure occurs by aerosol transmission, but venereal transmission may also occur. Although not documented, transplacental transmission is likely in ID mice with disseminated infections. Disease severity in experimental infection is closely linked to inoculum dose, and disease susceptibility depends on the strain or isolate of M pulmonis and the strain of mouse. Genetic resistance is complex and does not appear to be H-2 linked. Mice of the C57BR, B6, and B10 strains are resistant, whereas C57L, SJL, BALB, A/J, C3H/HeJ, C3H/HeN, C3HeB, SWR, AKR, CBA/N, C58, and DBA/2 have varying susceptibility. Experimental studies compared infections between susceptible C3H and resistant B6 mice, and found that female mice develop more severe disease. Athymic nude, thymectomized, CBA/N (X-linked ID), and SCID mice inoculated intranasally with M pulmonis develop significantly less severe respiratory disease than IC mice, but have disseminated infection with severe polyarthritis.
When clinical signs occur they reflect a suppurative rhinitis, otitis media, and chronic pneumonia. Affected mice may display inactivity, weight loss, and ruffled hair coat, but the most prominent signs are “chattering” and dyspnea, due to rhinitis and purulent exudate in the nasal passages. Otitis media may cause head tilt, circling, and other vestibular signs. Suppurative inflammation in the brain and spinal cord, although rare, can cause flaccid paralysis. Survivors develop chronic bronchopneumonia, bronchiectasis, and occasionally pulmonary abscesses.
Rat
M pulmonis infection is common in rats and should be considered essentially ubiquitous in rats other than specific pathogen-free laboratory rats. It is by far the most common cause of clinical respiratory disease in pet rats. Chronic respiratory disease (CRD) in rats has experienced an interesting evolution, as it was initially believed to be multifactorial, but later it became apparent that M pulmonis was the primary pathogen of the disease. Hence, the term murine respiratory mycoplasmosis (MRM) became the preferred nomenclature over CRD. Although other pathogens of the respiratory tract can play a role in the development of the disease, M pulmonis remains the major pathogen in cases of CRD in rats. Some seropositive animals may be cross-reacting because of exposure to M arthritidis. Reports of naturally occurring infections with clinical disease due to M arthritidis are rare, but this organism has been isolated from the respiratory tract and middle ear.
Transmission of M pulmonis among cage-mates and to adjacent cages occurs primarily through aerosols. It may require several months to establish an infection in contact animals, and clinical disease may not occur for up to 6 months. Intrauterine transmission also occurs, although newborn pups appear to be frequently infected by exposure to the dam during the postnatal period. Placentitis and fetal bronchopneumonia have been produced in pregnant rats inoculated intravaginally with M pulmonis prior to breeding.
As with mice, the incidence and intensity of the disease are influenced by a variety of factors, such as strain of rat, concurrent infection, and environmental conditions. LEW rats develop a more severe disease than do F344 rats. Concurrent infections with organisms such as SeV, rat coronavirus, CARB, or P pneumotropica have an additive effect on the disease.
Clinical signs include mild to severe respiratory distress, sniffling, torticollis, and infertility (Fig. 4 ). Dyspnea, ruffled hair coat, and weight loss may occur. Porphyrin-containing dark red encrustations may be present around the eyes and nares. Infections frequently extend from the Eustachian tube to the middle ear and then to the inner ear, causing labyrinthitis. Rats with labyrinthitis will spin, rotating their bodies rapidly when they are held in a vertical position by the tail. Unless the respiratory infections are complicated by bacterial infections, the terminal clinical stages of MRM may last weeks or months, which is common in the geriatric pet rat.
Fig. 4.
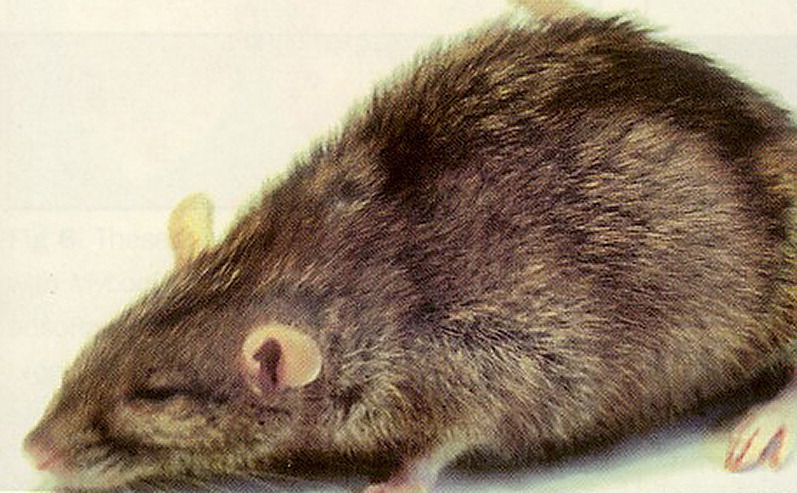
Rat with chronic murine respiratory disease and typical hunched postural presentation.
(Courtesy of Cathy A. Johnson-Delaney, DVM, Dipl ABVP (Avian), Edmonds, WA.)
The organism has an affinity for the epithelial cells of the respiratory tract, middle ear, and endometrium. Invasion of the middle ear occurs via the Eustachian tube and usually results in a chronic infection because the Eustachian tube opens into the tympanic bulla on the dorsal aspect, affording poor drainage to the nasopharnyx. Rats have cartilaginous rings only around primary bronchi. Damage to respiratory epithelium with ciliostasis and resultant accumulation of lysozyme-rich inflammatory exudate in the airways frequently results in weakening of bronchiolar walls and ensuing bronchiolectasis. Mycoplasma-associated host cell damage may occur by a variety of means, including uptake of essential cell metabolites and release of cytotoxic substances. Both the intact organisms and the cell membranes are nonspecifically mitogenic for lymphocytes. Thus, the marked lymphocytic infiltration seen in response to mycoplasmal infections does not appear to be due only to a response to a specific antigenic stimulus. The extensive lesions seen in some rat strains after exposure to M pulmonis may be attributable to an exaggerated and misdirected cellular immune response. The organism usually persists in infected rats, even in the presence of relatively high antibody titers. Microscopic changes in the affected tympanic bullae, turbinates, and major airways are characterized by a leukocytic infiltrate in the submucosa consisting of neutrophils, lymphocytes, and plasma cells. Peribronchial, peribronchiolar, and perivascular infiltration with lymphocytes and plasma cells is a prominent feature in all stages of the disease. Chronic bronchitis and bronchiolitis frequently progress to bronchiectasis and bronchiolectasis, which are characterized by dilation of airways and peribronchiolar cuffing with lymphocytes, with varying degrees of hyperplasia and metaplasia of respiratory epithelium.
At necropsy, serous to catarrhal exudate may be present in nasal passages, trachea, and major airways. In animals with copious viscous exudate in the airways, there may be patchy vesicular to bullous emphysema in the lungs. In affected lobes, lesions are unilateral or bilateral, and usually cranioventral in distribution. In advanced cases there are scattered areas of abscessation involving one or both lungs and in some animals the normal architecture may completely obliterated by the chronic suppurative process. One or both tympanic bullae may contain serous to inspissated purulent material, with thickening of the tympanic membrane.
Hamster
M pulmonis has been isolated from hamsters, but its pathogenic potential in these animals is not known.
Gerbil
M pulmonis has been isolated from gerbils, but disease due to natural infection or experimental inoculation is rare.
Pasteurella pneumotropica
P pneumotropica 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 is a very common commensal gram-negative coccobacillus belonging to the family Pasteurellaceae. Rats and mice are the main carriers although guinea pigs, hamsters, and gerbils may also be infected. P pneumotropica represents an important secondary bacterial invader and opportunistic infection in primary M pulmonis or SeV infections.
P pneumotropica is shed from upper respiratory secretions and feces, and is transmitted through direct contact. The organism has been found to be associated with conjunctivitis, rhinitis, otitis, and cervical lymphadenitis in rats and mice. The uterus and vagina are often colonized without disease, and thus transmission can occur from dam to pups during or shortly after birth. In enzootically infected colonies, nasopharyngeal colonization of laboratory rodents occurs around the time of weaning. Transmission from rodents to humans is rarely reported, but humans may be inadvertent sources of infection for barrier-sustained animals.
Culture of the organism with subsequent identification is required for diagnosis. Screening with serology is not recommended, as animals with subclinical infections are often negative and animals with other Pasteurellaceae may show cross-reactivity. In live animals, oral swabs or fecal culture appear to be the sites of choice for collection. PCR assay and DNA extraction are other techniques used to identify the organism.
Therapy with enrofloxacin may be beneficial in controlling clinical manifestations of infection, but will not eliminate the carrier state.
Prevention is best achieved by exclusion of carriers from the facility. Embryo transfer, rather than hysterectomy rederivation, may be the best choice for an infected colony. Fetuses may also be infected in utero, which may explain why this organism is the most frequent agent in failure of cesarean rederivation. Exclusion of wild or feral animals from facilities is also important. Sentinel monitoring programs for this organism are unreliable. Once a colony is free of the agent, there is relatively little risk of reinfection except through the introduction of infected animals.
Because of its fragility in the environment, stringent environmental decontamination is not necessary, and regular cleaning and use of a high-level disinfectant should suffice to rid the environment of the organism.
Mouse
P pneumotropica is ubiquitous in almost all wild mice and is common among laboratory mouse populations. Most infections in mice are asymptomatic; however, because of growing use of GEMs and ID mice, the incidence of clinical disease is also increasing. As an opportunistic invader it is associated with several lesions, but its true nature as a primary pathogen is questionable. Elimination of this organism from a mouse population allows other gram-negative bacteria, such as Klebsiella, to fill its opportunistic niche. Seroconversion normally occurs only in mice with overt disease.
Clinical signs are varied and include conjunctivitis, panophthalmitis, dacryoadenitis, periorbital abscessation, rhinitis, otitis (externa, media, interna), and cervical lymphadenitis. Lesions are also seen in the dermatologic, urinary, and reproductive organs. Severe suppurative bronchopneumonia has been documented in B-cell–deficient mice coinfected with Pneumocystis murina.
Prophylactic administration of trimethoprim/sulfamethoxazole (50–60 mg/kg) in the drinking water has been shown to prevent infection in immunodeficient mice. Enrofloxacin (25.5–85 mL/kg) in the drinking water for 2 weeks may be effective in eliminating infection in mice.90
Rat
In rats P pneumotropica readily colonizes in the intestine, where it may be carried for long periods of time. It can also be carried as an inapparent infection in the nasopharynx, conjunctiva, lower respiratory tract, and uterus. Transmission is most likely primarily by direct contact or fecal contamination in the rat, rather than by aerosols. Lesions associated with pasteurellosis include rhinitis, sinusitis, conjunctivitis, otitis media, suppurative bronchopneumonia, and interstitial pneumonia with polymorphonuclear cell infiltration has been observed. Pasteurella may cause a severe, multifocal to coalescing, acute to subacute, necrotizing to fibrinous bronchopneumonia, which must be differentiated from Streptococcus and Corynebacterium. Subcutaneous abscessation, suppurative or chronic necrotizing mastitis, and pyometra have been reported. The organism can be recovered from various tissues in the absence of lesions.
Hamster
In hamsters P pneumotropica can cause acute or chronic respiratory infections or be present in the carrier state. Lesions seen are associated with upper respiratory disease, otitis, and bronchopneumonia.
Gerbil
P pneumotropica has been isolated from gerbils, but disease due to natural infection or experimental inoculation is rare.
Proteus mirabilis
Proteus mirabilis 91, 92, 93 is a gram-negative facultative anaerobe and a member of the family Enterobacteriaceae. Ubiquitous in the environment, it can be isolated from the upper respiratory tract and feces of normal mice. Opportunistic infections have been observed in both IC and ID laboratory mice.
Disease is often septicemic, with suppurative lesions in various organs, including pneumonia, hepatitis, splenitis, pyelonephritis, and peritonitis. Pulmonary lesions, when present, are typified by serous flooding of alveoli and mobilization of alveolar macrophages. Lung infection has also been found in reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-deficient B6.129S6-Cybbtm1Din/J mice.
Streptobacillus moniliformis
Streptobacillus moniliformis 94, 95, 96 is a gram-negative pleomorphic bacillus. This zoonotic agent is virtually nonexistent in modern laboratory animals but can lead to infection in humans, with potentially serious consequences. In humans it is the cause of rat bite fever. A similar syndrome, called Haverhill fever, has been associated with ingestion of rat-contaminated foodstuffs, particularly milk. Rats may act as zoonotic reservoirs for mice.
The organism inhabits the nasopharynx, middle ear, and respiratory tract. It is present in the blood and urine of infected rats and is transmitted to humans by bite wounds, aerosols, and fomites. Clinical signs in humans follow a 3- to 10-day incubation period and include fever, vomiting, arthralgia, and rash.
S moniliformis can be associated with opportunistic respiratory infections in rats, and can cause wound infections and abscesses. It has been found in bronchiectatic abscesses or rats with CRD, in concert with Mycoplasma and CARB.
Colonies of laboratory rats should be monitored by culture of blood and nasopharyngeal swabs, and any animals with a positive diagnosis should be euthanized immediately. Because wild rats are the reservoir for S moniliformis, its detection in a laboratory rat colony would indicate exposure to wild rats.
Streptococcus pneumoniae
S pneumoniae 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 is a gram-positive α-hemolytic aerobic diplococcus belonging to the family Streptococcaceae. Numerous serotypes exist, and disease is predominantly associated with infection by the more pathogenic serotypes 2, 3, 8, 16, and 19. In rats serotypes 2, 3, and 19 are most common, but they may also have serotypes 8, 16, and 35.
Inapparent infections and carrier states are very common. Despite its periodic detection in large breeding colonies, no outbreaks have been reported in laboratory colonies for almost 35 years, raising the possibility that previous outbreaks were the result of S pneumoniae and other concurrent agents. When it occurs, disease is usually seen in young animals, especially after disruption of host defense mechanisms, such as concurrent infection, experimental manipulation, or a change in environment. Mortality is greater in the winter, after shipment, and in animals on marginal diets.
S pneumoniae can cause respiratory and meningeal disease in man, especially in immunocompromised individuals. Humans are a natural host of S pneumoniae, with both adults and children frequently colonized.
Depending on the season, 40% to 70% of human populations carry it in their respiratory passages and may be a source of animal infections. Human caretakers with pneumococcal pneumonia, otitis media, conjunctivitis, or other diagnosed or possible streptococcal infections should not work with animals until a course of antibiotics has been completed. Zoonotic transmission from rats or mice to humans has never been reported, but should be considered.
Transmission occurs primarily via aerosol or contact with nasal or lacrimal secretions of an infected animal. Carriers may have upper respiratory infection without clinical signs. Acute episodes or prolonged epizootics with variable morbidity and mortality may occur. Affected animals have signs apparent with generalized respiratory disease in addition to hematuria. Gross lesions include pleuritis, otitis media and interna, and bronchopneumonia.
Diagnosis is established by observation of the bacteria in inflammatory exudate. Samples for culture can be collected from the nasopharynx, tympanic bullae, and nasal passages by swab or lavage. Unlike for many bacterial diseases, large numbers of this organism can be seen in smears and tissue sections via histopathology. Serology using ELISA is also available.
Treatment of animals with antimicrobials may serve to abate clinical signs but does not resolve the carrier state, nor will antibiotic treatment eliminate bacteria from the bedding or cage surface. These organisms are generally sensitive to benzathine-based penicillins, methicillin, ampicillin, chloramphenicol, erythromycin, and lincomycin, Treatment must be aggressive, and the use of β-lactamase–resistant penicillins such as cloxacillin, oxacillin, and dicloxacillin is generally recommended.
Monitoring is conducted by nasopharyngeal culture onto blood agar. However, because of the occurrence of nonpathogenic isolates, isolation of S pneumoniae from rats, even if a respiratory problem is present in the colony, does not necessarily provide a diagnosis, nor does isolation of the organism from asymptomatic rats necessarily indicate a health threat to the colony. Action to eliminate is indicated in the presence of characteristic lesions or detection of known pathogenic serotypes.
Mouse
Bacterial pneumonia in mice is nearly always caused by this organism, but seldom develops in the absence of some combination involving M pulmonis, SeV, or CARB.
Rat
In clinically normal rats S pneumoniae is carried primarily in the nasoturbinates and tympanic bullae. Infection is rarely present in commercially obtained rats and is now considered to be a pathogen of low significance in laboratory animals. S pneumoniae in rats may cause acute primary disease with mortality, but more often it represents an important secondary invader, particularly in respiratory infections. Young rats are more severely affected than are older ones, and often the only sign they exhibit is sudden death.
Clinical signs can include serosanguinous to mucopurulent nasal discharge, rhinitis, sinusitis, conjunctivitis, vestibular signs consistent with middle ear infection, dyspnea, snuffling, and abdominal breathing. Infection in rats resembles that in both human and nonhuman primates, characterized by suppurative inflammation in the upper respiratory tract, which spreads to the lung to cause bronchopneumonia.
In the acute systemic form there are variable patterns of characteristic fibrinopurulent polyserositis, including pleuritis. Grossly, there is serous to mucopurulent exudate in the nasal passages, with variable involvement of the tympanic bullae. There may be consolidation of one or more lobes of the lung, and affected areas are dark red to dull tan and relatively firm and nonresilient. Pulmonary changes vary from localized suppurative bronchopneumonia to acute fibrinopurulent bronchopneumonia, with obliteration of the normal architecture in affected lobes. Suppurative rhinitis and otitis media may also occur. There is pericarditis in some cases. Fibrinopurulent peritonitis and pleuritis with minimal involvement of the lung parenchyma are not uncommon.
Hamster
S pneumoniae in hamsters is relatively uncommon and frequently associated with stress. Signs include depression, anorexia, nasal and ocular discharge, dehydration, and weight loss. The course of the disease is about 3 days.
Gerbil
S pneumoniae has been isolated from gerbils, but disease due to natural infection or experimental inoculation is rare.
Mycotic Agents
Pneumocystosis
The pneumocystosis organism107, 108, 109, 110, 111, 112, 113 was originally classified as a protozoan, but study of its nucleic acids and proteins places it among the fungi. The genus Pneumocystis has undergone sequence analysis of its genes, including the 18S rRNA gene, bringing about identification of distinct species that were once all classified as Pneumocystis carinii. These species include P murina in mice, P carinii and Pneumocystis wakefieldiae in rats, and Pneumocystis jirovecii in humans. Pneumocystis is an important respiratory pathogen in ID mice, rats, and guinea pigs but does not cause overt disease in IC animals. The organism is widespread, naturally acquired by airborne transmission of the respiratory tract, and establishes a persistent, quiescent infection in the lungs. Infection may also be transmitted through the atypical colonized and shedding IC animals. This shedding may occur after infection of an IC animal, but before the infection is eliminated by the immune system. Subclinical infections that resolve in 5 to 6 weeks are relatively common in IC mice and rats, whereas ID animals are unable to clear the organism from their respiratory tract and develop a chronic infection. Treatment with trimethoprim (40 mg/mL)/sulfamethoxazole (200 mg/mL) suspension at a rate of 15.6 mL per 500 mL water will control disease symptoms but will not extinguish the infection. Daily treatment or pulse therapy following a 3 days on/4 days off pattern are recommended protocols. The water bottle must be shaken at least daily to resuspend the agent. Antibiotic resistance due to mutations in the gene targeted by sulfa drugs has been reported in human isolates, so care should be taken with long-term administration of these drugs.
The organism’s widespread distribution strongly suggests that susceptible animals should be protected by microbarrier combined with macrobarrier housing. It does not cross the placenta, so cesarean section or embryo transfer rederivation will eliminate the organism. The strains of this organism appear to be species specific; therefore, interspecies transmission is unlikely and, although pneumocystosis occurs in humans, there has not been any confirmation of transmission between rodents and humans.
Mouse
Spontaneous enzootics pose a serious threat in colonies of ID mice, with high morbidity and mortality. Mice are typically infected as 3- to 4-week-old juveniles and because of the ubiquitous nature of the organism, virtually all mice in the 4- to 12-week-old age groups should be considered to be infected and shedding. Immunity to the organism develops rapidly, with young animals being protected initially by colostral antibodies. Infection in susceptible mice proceeds slowly, leading to clinical signs of suppurative bronchopneumonia within several months. Primary signs include dyspnea and a hunched posture, which may be accompanied by wasting and scaly skin. Severe cases, such as those that occur with advanced disease, may be fatal. At necropsy, lesions are especially severe in SCID or athymic nude mice. Nonfilamentous trophic forms attach to type I pneumocytes with clusters of developmental stages spreading into the alveolar lumen. The lungs collapse poorly and have a rubbery consistency, with pale, patchy areas of consolidation. The type of pneumonia can be variable depending on the host’s type of immune deficiency. Some ID mice may have very few visible cysts or alveolar exudation, with principally interstitial pneumonia. Histologic changes are characterized by interstitial alveolitis, with thickening of alveolar septa from proteinaceous exudate and infiltration with mononuclear cells. Immunocompromised mice subclinically infected with P murina that are inoculated with PVM develop severe respiratory tract lesions attributed to the dual infection. Superimposed viral infection and/or bacterial infections such as P pneumotropica can exacerbate pneumocystosis. Respiratory distress in ID mice should elicit suspicion of pneumocystosis. Pathologic examination of the lung with special staining is essential to confirm the presumptive diagnosis. Past infections may be detected through ELISA, and PCR is used to detect active infection.
Rat
The immunocompromised rat is a common animal model for P carinii pneumonitis that occurs in human AIDS patients. P carinii and P wakefieldiae can exist as coinfections in the same animal. Signs commonly seen in rats are weight loss, cyanosis, and dyspnea. There is diffuse to focal consolidation; lungs collapse poorly, and routinely have an opaque pale pink color. In athymic rats, pulmonary lesions vary from mild to severe interstitial pneumonia. Pulmonary lesions associated with this infection have been created in young laboratory rats from naturally infected colonies that were treated with immunosuppressants, such as cortisone, and fed a diet deficient in protein for several weeks. Diagnosis of infection in IC rats usually requires at least 6 weeks of treatment with corticosterioids or cyclophosphamide to elicit a histologically detectable level of infection. Special stains demonstrate the fungal cysts within the alveoli. PCR has been used to detect infection in rat lungs consistently after only 1 week of treatment. PCR can also be performed on oral swabs and bronchoalveolar lavage samples. Routine screening of asymptomatic animals, such as IC sentinels in colonies of ID animals, is best accomplished by PCR.
Miscellaneous mycotic agents
B6-p47 (phox) null mice, which are defective in NADPH oxidase, have been reported to develop pyogranulomatous lesions in the lung, liver, lymph nodes, salivary gland, and skin, from which Trichosporon beigelii was cultured. Another colony of B6-p47(phox) null mice with a concomitant γ-interferon mutation was found to develop granulomatous pneumonia in association with Aspergillus terreus. Mice that lack NADPH oxidase function through null mutation of gp91 (phox) developed pulmonary infections with Paecilomyces variotii. Chronic granulomatous disease in mice, especially in the lungs, was associated with Paecilomyces sp, Aspergillus fumigatus, Rhizopus sp, and Candida guilliermondii. 114
One report described almost one-fifth of Wistar rats in a 2-year carcinogenesis study as having chronic rhinitis associated with A fumigatus. The predisposing factor in these animals was thought to be subclinical SeV infection. Clinical signs included sniffling and nasal exudation. Yellowish, friable material was present either unilaterally or bilaterally in the nasal cavities, and in severe cases the nasal cavities were completely occluded. Lesions were, in most cases, limited to the nasoturbinates and maxilloturbinates. A bronchial abscess containing hyphae and multiple fruiting heads occurred in one rat.115
Tracheobronchial aspergillosis was reported in an aged F-344 rat with concurrent large granular cell leukemia. Immunodeficiency due to the leukemia was thought to be associated with the multifocal, transmural necrotic lesions of the trachea and bronchi.116
Two cases of Aspergillus spp rhinitis in rats that had no known immunosuppression were reported. Corncob and hardwood bedding from 2 sources were analyzed to determine if the source of the fungus was the substrate material. Six genera of fungi were isolated from corncob samples, whereas only negligible counts were isolated from hardwood samples. The investigators recommended that the use of either autoclaved or γ-irradiated corncob bedding should be considered as a method to eliminate fungal contamination of bedding.117
Parasitic Diseases
Primary respiratory tract parasitism in mice, rats, hamsters, or gerbils has not been confirmed in the United States; however, cases have been documented in other countries.
There is a report of Spleorodens clethrionomys observed in epizootic proportions in the nasal cavities of Syrian hamsters in Stockholm, Sweden. Mites were detected in the nasal cavities of the animals during preparations for an inhalation experiment. More than 90% of the adult animals and all offspring were infested within the colony. Examination of animals from the vendor revealed a pervasive infection. Surveillance of hamsters at 2 other research institutes in Stockholm, which obtained animals from the same source, revealed similar infection rates. The origin of nasal mites at the breeding facility was not determined. Affected animals did not show any clinical signs. This species of mite was originally reported from field voles in Holland, and has not been reported in other species of laboratory rodents.118
Trichosomoides sp, possibly Trichosomoides nasalis, was reported in England in parasagittal sections through the nose and in adjacent tissues in 5-week-old hamsters. Some animals had a distinctive pug-faced deformity, believed to be associated with the inoculation of sarcoma material as neonates. The parasitic ova were seen in the feces of 37 of 185 stock hamsters in the facility. The ova were infective for rats when given orally. T nasalis was later reported in the nasal cavities of hamsters in Switzerland. This nematode was originally described by Biocca and Azrizi in 1961 in the nasal cavities of Rattus norvegicus in Rome, Italy.119
Trichosomoides crassicauda, a nematode that affects the urinary tract of rats, has a migratory phase in its life cycle, which may produce lesions in the lung and other organs.120
Viral Agents
Hantavirus
Hantavirus (HV)121, 122, 123 is discussed from a zoonotic aspect, as it remains an agent of severe disease in humans, although humans are inadvertent hosts.
Hantaviruses are members of the family Bunyaviridae. Rodents are the primary reservoir hosts of the hantaviruses worldwide, with each virus in the genus being associated with a specific rodent species. Hantaviruses infect the majority of wild mouse and rat populations in the United States, with an antibody prevalence rate of up to 8%. In the laboratory animal facility, the rat is the primary animal responsible with the spread of these viruses. Rabbits, guinea pigs, cats, and dogs housed in the same room as infected rats can become seropositive.
Infected rodents shed the virus in saliva, urine, and feces for many weeks with lifetime persistence. Transmission occurs via contaminated fomites; by direct introduction into broken skin, mucous membranes, or conjunctiva; by ingestion of contaminated food/water; or by aerosols. Biting may be an important mode of transmission during conspecific aggression. Hantavirus infections in rodents are characterized by being chronic and subclinical, and although clinical signs and lesions have not been reported in laboratory mice or rats, they develop high antibody titers.
Transmission to humans occurs in a similar way as for rodents, and transmission between humans is unlikely. Two major lineages of hantaviruses are of zoonotic importance. One represents those associated with hemorrhagic fever and renal syndrome (HFRS) in humans, and the other represents viruses of the New World that are associated with hantavirus pulmonary syndrome (HPS). Hantaviruses that can cause HFRS include Hantann virus, Puumala virus, Dobrava virus, and Seoul virus. The deer mouse, Peromyscus maniculatus, is the reservoir for Sin Nombre virus (SNV), which is responsible for the great majority of human HPS cases in North America. However, HPS can be caused by infection from at least 11 other hantaviruses.
Mouse cytomegalovirus infection
Mouse
The official name for the agent responsible for mouse cytomegalovirus (MCMV) infection is murid herpesvirus 1 (MuHV-1), which is a member of the family Herpesviridae, genus Muromegalovirus.124, 125 The virus can be isolated from saliva or salivary glands in the majority of wild mice, and there are multiple MCMV strains within wild mouse populations. Outbreaks in laboratory mice are rare or nonexistent in most colonies. Lesions are primarily found in the salivary glands, but lung pathology can occur as well. Latency of MCMV has been documented to occur in the lung tissue and can persist for the life of the mouse.
The virus is transmitted oronasally by direct contact and is excreted through saliva, tears, urine, and semen. Although MCMV does not readily cross the placenta and in utero transmission does not appear to take place, latently infected dams have been documented to transmit low-level or latent infection to fetuses in utero. Experimental transmission by artificial insemination has been reported, making sexual transmission likely.
Experimental infection is greatly influenced by viral strain, host factors, dose, and route of inoculation. Neonates of all mouse strains are susceptible to disease, and severity of disease expression is significantly influenced by host strain genotype. Resistance begins to develop after weaning and increases until about 8 weeks of age. Resistant strains include B6, B10, CBA, and C3H mice. Susceptible strains include BALB/c and A strain mice. Resistance is associated with H-2k haplotype, but non-H2 associated factors exist, including one that is linked to loci on chromosome 6 within the natural killer (NK) cell complex.
Within 1 week of experimental infection, viremia with multisystemic dissemination occurs, including the lung and a variety of reproductive tissues. Monocytes are important for the viremic phase of infection, and macrophages are also targeted. Following dissemination, the virus is rapidly cleared from the host with exception of the salivary glands. Virus clearance is significantly prevented in beige mice or mice depleted of NK cells. Athymic or SCID mice fail to control active infection whereas B-cell deficient mice can recover. Of interest, MCMV can actively persist and replicate in the salivary glands of fully IC mice, due to genes of the virus that function to control immune response, determine cell tropism, and inhibit apoptosis. MCMV can persist in a nonreplicative state as a latent infection, but can be reactivated by stress or immunosuppression.
Overt clinical disease with disseminated lesions usually does not occur in naturally infected mice. Experimentally induced disease produces focal necrosis, cytomegaly, inclusions, and inflammation in many tissues, including the lung, during the acute phase. Diffuse interstitial pneumonitis has been described in BALB/c mice that were immunosuppressed by a variety of methods, and athymic nude mice develop progressive multifocal nodular pulmonary inflammation. A single case of naturally occurring MCMV disseminated infection has been reported in an aged laboratory mouse.126
Diagnosis of MCMV can be made via serology using MFIA, ELISA, IFA, PCR, and virus isolation.
Hamster
Inclusions have been identified in hamsters but overt clinical disease has not been reported.
Mouse hepatitis virus
The official name for the mouse hepatic virus is murine hepatitis virus (MHV),127, 128, 129 which is a member of the family Coronaviridae, genus Coronavirus. MHV encompasses several genetically and antigenically related strains that vary tremendously in their virulence and organotropism. MHV is generally separated into two biologically distinct groups, respiratory strains and enterotropic strains. Respiratory strains exhibit primary affinity for upper respiratory mucosa. As with all RNA viruses, MHV is capable of extreme mutation rates with resultant antigenic drift.
MHV continues to plague conventionally housed laboratory mouse populations in the United States and Europe as a major infectious agent. It is pervasive in wild mouse populations throughout the world.
Strains with respiratory affinity initially replicate in the nasal mucosa followed by dissemination to a variety of other organs, due to their polytropic nature. This disseminated pattern of infection is exhibited by highly virulent strains of the virus in mice younger than 2 weeks, in genetically susceptible strains, or in immunocompromised mice. Dissemination occurs from the nasal mucosa to the pulmonary vascular endothelium and then to draining lymph nodes. Secondary viremia further spreads the virus to multiple organs. Immune-mediated clearance of the virus begins after 5 to 7 days, with no persistence or carrier state detectable beyond 3 to 4 weeks. The majority of natural respiratory/polytropic MHV infections are subclinical, with mild or no gross lesions. The obvious exception is ID mice, which cannot clear the virus and develop progressively severe disease with chronic wasting, or die acutely. Fetal infection with MHV is fatal, as is infection in naïve nursing pups. In general, BALB/c mice are fairly susceptible whereas SJL mice are quite resistant. However, experimental infections clearly demonstrate that the biologic behavior of the wild-type MHV is unpredictable. Host immunity is decidedly virus strain dependent, which has given rise to the misconception that the virus has a latent phase. Recovery from one strain provides solid resistance to reexposure with the homotypic strain, but only partial to no resistance to infection with an antigenically heterotypic strain. Maternally derived passive immunity is also important in MHV epizootiology.
MHV is highly contagious, and is spread via respiratory aerosol and feces. Vertical transmission from infected dams to fetuses has been documented experimentally, but is highly unlikely in naturally occurring infections.
Pulmonary vascular endothelium syncytia are common in ID mice. Animals can also develop nasoencephalitis due to localized infection of olfactory mucosa, nerves, bulbs, and tracts of the brain. This pattern of infection occurs regularly after intranasal inoculation of many MHV strains, but is uncommon with natural exposure.
Active infection is confirmed by immunohistochemistry or virus isolation. During the acute phase, histologic diagnosis can be made by finding characteristic syncytial lesions in target tissues. Recovered mice may have perivascular lymphocytic infiltrates in the lung. Serology is the most useful means for detection of retrospective infection in a colony. Female mice usually have higher titers than do males, as they ingest the infected feces of nursing pups. C57BL/6 mice produce a high antibody titer and are commonly used in sentinel testing. PCR can detect the virus in feces or tissues of infected mice.
Research complications are attributable to the viruses’ polytropic, contagious, and persistent nature, making it the most probable virus to interfere with biologic responses in mice and to contaminate transplantable tumors and cell lines. MHV can also infect embryonic stem cells without cytopathic effects.
Elimination of MHV from a colony requires complete cessation of breeding of seropositive animals and no introduction of new animals for an 8-week period in conjunction with meticulous environmental decontamination. This action allows for “burn-out” of the infection within the colony. For extensive outbreaks, it may be necessary to rederive the populations by embryo transfer or cesarean section of pups to clean foster dams.
Maintenance of disease-free populations depends on the exclusion of infected animals, domestic or wild.
Murine norovirus
Murine norovirus (MNV-1) is a member of the family Caliciviridae, genus Norovirus, and mice are the only known host for this virus. MNV-1 is present in a relatively high percentage (≥30%) of laboratory animal facilities where viral monitoring has been initiated.130, 131, 132 Its prevalence in wild and pet populations is unknown.
The virus was first reported in 2003, in a study133 that described isolation of a calicivirus from a colony of ID mice experiencing unexpected mortality. The investigators later classified the virus as a norovirus, thus naming it murine norovirus 1 (MNV-1). In the outbreak, mice lacking both interferon-αβ receptors and interferon-γ receptors were very susceptible to lethal infection, demonstrating that interferons are necessary for resistance to MNV-1. STAT1-dependent innate immunity is also needed for resistance to MNV. Therefore, unlike human noroviruses, MNV-1 is remarkably nonpathogenic except under highly specific circumstances. There is no evidence for zoonotic transmission to date.
The virus is transmitted by the fecal-oral route. Fecal shedding following infection in both IC and ID mice can persist for months. STAT1 null mice with intact B and T cells, STAT1 null mice lacking B and T cells (RAG null), and STAT1 null mice lacking RNA-dependent protein kinase (PKR null) exhibit high mortality with encephalitis, pneumonia, and hepatitis. Other strains showed low mortality but became persistently infected. Infection of 129, B6, RAG1, RAG2, interferon-αβ receptor null, interferon-γ receptor null, inducible nitric oxide synthase null, or PKR null mice with functional STAT1 resulted in no clinical disease. IC mice appear to seroconvert and are only transiently infected, with no clinical signs. Most ID mice are asymptomatic and only those with severe deficiencies in innate immunity as described can have an infection leading to wasting and death.
Microscopic lesions in STAT1 null mice inoculated per os or intranasally include alveolitis, pulmonary edema, pneumonia, and multifocal areas of coagulation necrosis in the liver, with minimal or no inflammatory cell response. In another study, microscopic and gross lesions encountered were strain dependent.
MNV-1 can be diagnosed using serology via MFIA, ELISA, and IFA. Antibody titers may be slow to increase; therefore, 8 weeks of exposure is recommended for sentinel mice housed on soiled bedding. There are many field strains of MNV having antibodies that only faintly cross from strain to strain, so it is important that the diagnostic laboratory uses assays validated for the full spectrum of MNV strains. At least 6 strains of MNV have been identified to date. PCR can detect strains 2, 3, and 4 from tissues 8 weeks post infection, with jejunal and mesenteric lymph nodes being the preferred sites for sampling.
Caliciviruses are notoriously difficult to eradicate from the environment. Aggressive chemical decontamination with the help of detergents and disinfectants is recommended, with bleach being the only disinfectant known to kill this virus.
Murine pneumotropic virus or Kilham polyomavirus
Murine pneumotropic virus (MPTV) or Kilham polyomavirus (KPYV)134 is a member of the family Polyomaviridae, genus Polyomavirus, which is different from murine polyoma virus (MPyV). It is sometimes referred to as K-virus and should not be confused with Kilham rat virus.
This disease is of interest from a historical aspect, and rarely occurs in modern mouse colonies although it is found in wild mouse populations. It was discovered by Lawrence Kilham while performing investigations with the mammary tumor virus.
Adult nude mice are susceptible to experimental infection with resultant pathologic lesions. Other highly susceptible strains are AKR and CB, with C57BL/6 being resistant to disease. Transmission is via the oronasal route. Oral inoculation of neonatal mice causes initial replication in the intestinal capillary endothelium followed by dissemination to other organs, including the lung, where it replicates in the vascular endothelium. There is a rapid onset of dyspnea due to pulmonary vascular edema and hemorrhage, resulting in death 6 to 15 days post infection. MPTV may be suspected if 6- to 15-day-old mice or ID mice present with an interstitial pneumonia. Mice that are inoculated between 12 and 18 days of age do not develop pulmonary disease because of passive immunity from the dam, allowing them to mount an early and effective immune response and thereby preventing the viremic phase of infection. Regardless of age, mice remain persistently infected, and the site for virus persistence is renal tubular epithelium.
Gross lesions are restricted to lungs of neonatal or immunodeficient mice. Pulmonary lesions consist of hemorrhage, congestion, edema, atelectasis, and septal thickening with prominent basophilic nuclear inclusions in affected endothelial cells. Interstitial pneumonia with lymphocytic infiltrates develops in recovering mice.
Antibodies may be detected via MFIA, ELISA, or IFA. Because of the extreme rarity of this virus, positive serology results have a very low predictive value and are likely to be false positives. PCR can also detect the virus.
Pneumonia virus of mice
The PVM133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143 is a member of the family Paramyxoviridae, subfamily Pneumovirinae, and genus Pneumovirus. The official name of this virus is murine pneumovirus (MPV), which should not be confused with the other official MPV, mouse parvovirus, not to mention murine pneumotropic virus. PVM can cause natural infections in mice, rats, hamsters, gerbils, guinea pigs, and probably other rodents, and may be infectious for rabbits. Serologic data indicate that PVM is prevalent in mice, is infrequently reported in rats, and has a worldwide distribution. The possibility of interspecies transmission to other animals within the facility should be considered when dealing with this disease.
Mouse
PVM is relatively benign in IC mice, causing an acute and self-limiting infection, but causes significant disease in GEMs. As with many other viral infections, susceptibility is strain dependent and can be increased by a variety of local and systemic stressors. DBA/2, C3H/HeN, and 129Sv are very susceptible when inoculated with a pathogenic strain, followed by BALB/cBy and B6 exhibiting intermediate susceptibility, while SJL strains are highly resistant. Infection appears to persist in ID mice. MPV is transmitted via aerosol and contact exposure to the respiratory tract. Because the virus has a low degree of contagion and environmental inactivation occurs rapidly, close contact between mice is required for transmission.
Clinical signs of disease and gross lesions are typically absent in natural infections of IC mice. ID mice exhibit listlessness, cyanosis, and dyspnea with chronic wasting.
Experimental infection of immunocompetent BALB/c mice with an intranasal pathogenic PVM strain produced pulmonary lesions peaking within 2 weeks and commencing resolution within 3 weeks. Virus replication occurs in alveolar lining cells, alveolar macrophages and, to a lesser extent, bronchiolar epithelium. Natural isolates of PVM are nonpathogenic, with replication occurring primarily in the nasal mucosal epithelium with few pathologic lesions. The low pathogenicity of PVM allows ID mice to develop a progressive severe interstitial pneumonia with the wasting syndrome instead of acute death. In these mice, PVM antigen is confined to alveolar type II cells and occasionally bronchiolar epithelial cells. SCID mice naturally infected with P murina, then inoculated with a nonpathogenic isolate of PMV, developed more severe Pneumocystis pneumonia. PVM-associated pneumonia in ID mice is often complicated by pneumocystosis and vice versa, as both are common agents in mouse colonies.
Microscopic lesions of experimentally infected mice consist of mild necrotizing rhinitis, necrotizing bronchiolitis, and nonsuppurative interstitial pneumonia. Lungs are pale, firm, and do not collapse. Alveolar septa are thickened as a result of edema and infiltrating macrophages and leukocytes. Alveolar spaces are collapsed and filled with fibrin, blood, macrophages, and large mononuclear cells, representing desquamated alveolar type II cells. Although MPV lesions are similar to Sendai viral lesions microscopically, MPV tends not to cause proliferative bronchiolar lesions as does SeV.
Serologic diagnosis can be achieved via MFIA, ELISA, and IFA. Seroconversion confirmation is the most practical method to make a diagnosis; however, because PVM is not highly contagious, the number of seropositive animals within a colony can be small. Nude mice do not seroconvert to PVM. During active infections, PVM can be identified by virus isolation, PCR, or mouse antibody production testing of suspect tissues. Immunohistochemistry staining can demonstrate viral antigen within infected pneumocytes in tissue sections.
Rat
Based on serologic surveys, enzootics do occur in laboratory rats. Intranasal inoculation of F344 rats resulted in gross and microscopic lesions by the sixth day, although animals showed no overt disease. In experimental infections, complement-fixing antibodies to PVM peaked at 14 days and declined markedly by 19 days. Multifocal, nonsuppurative vasculitis and interstitial alveolitis with necrosis are typical lesions seen during the acute stages of the disease. There are prominent perivascular infiltrates, with hyperplasia of bronchus-associated lymphoid tissue, perivasculitis, and multifocal interstitial pneumonitis. These lesions tend to persist for weeks in the rat. The presence of microscopic lesions attributed to PVM requires confirmation by serologic conversion, as in mice. PVM may also be a copathogen in other respiratory diseases, such as mycoplasmal infections.
Hamster
PVM infection in this species normally goes undetected as a subclinical event. Animals inoculated with PVM exhibit sneezing, dyspnea, and weakness, with death ensuing 6 to 15 days post infection. Gross lesions include patchy, plum-colored lung consolidation involving 50% to 75% of the lung. Multifocal, nonsuppurative vasculitis and interstitial pneumonitis with necrosis are prominent lesions seen during the acute phase of the disease. Alveolar walls are thickened and contain a predominantly mononuclear cell infiltrate.
Poxvirus
This poxvirus is also known as Turkmenian rodent poxvirus, and infections have been reported to occur in laboratory rats from Europe and the former Soviet Union.144 The virus is closely related to cowpox virus, but distinctly different from ectromelia virus (ECTV), which causes mousepox. Clinical signs in affected rats resemble those seen in ectromelial infections in mice, with both dermal and respiratory lesions occurring. Microscopically, rats with respiratory signs have severe interstitial pneumonia with edema, hemorrhage, and pleural effusion. Focal inflammatory lesions occur in the upper respiratory tract.
Serologic surveys of wild rodents have identified animals that are seropositive for cowpox virus, and the reservoir hosts were usually asymptomatic. There have been documented reports of the transmission of cowpox from rats to humans, felids, and nonhuman primates. Whether the Turkmenian rodent poxvirus in previous reports was actually cowpox virus is not known.
Rat polyoma virus
The rat polyoma virus145 is serologically distinct from the MPTV/Kilham polyomavirus of mice, and its incidence is unknown. The virus was discovered in a colony of 32 athymic nude (rnu) rats. Clinically, affected animals developed a wasting disease with interstitial pneumonia and parotid sialoadenitis. The rats had bronchitis, bronchiolitis, and secondary bacterial pneumonia. Less commonly, rhinitis and Harderian gland adenitis were seen. Intranuclear inclusion bodies were seen in the ductal and acinar epithelial cells of the parotid salivary gland. Euthymic rats did not develop disease. Because this virus has not been isolated, serologic screening of populations is not performed.
Rat respiratory virus
Based on initial characterization studies, the rat respiratory virus (RRV)146 was classified as a member of the Hantavirus genus. Further investigations revealed that the reactions seen on IFA were nonspecific and false positive. Attempts to demonstrate a relationship with other hantaviruses using hantavirus-specific PCR primer sets have been consistently negative. Therefore, more studies are needed to classify the virus. Investigators at the Research Animal Diagnostic Laboratory in Columbia, Missouri have data strongly supporting P carinii as the agent responsible for lung lesions previously attributed to RRV in rats. This information was presented at the annual American Association for Laboratory Animal Science National Meeting in October 2010.
Sendai virus
The official name for this pathogen is Sendai virus (SeV), a member of the family Paramyxoviridae, genus Paramyxovirus.147, 148, 149, 150, 151, 152, 153, 154 It was first isolated in Sendai, Japan; hence, the name. SeV is antigenically related to parainfluenza virus (HPIV) serotype 1 of humans, and there has long been a debate as to the human or mouse origin, or if humans are naturally susceptible to SeV infection. Studies have demonstrated that both SeV and HPIV serotype 1 replicate equally well in the upper and lower respiratory tracts of African green monkeys and chimpanzees, suggesting that SeV lacks a significant host range restriction and could very well be an anthropozoonotic agent.
From a historical perspective, SeV infections have caused some of the most significant disease outbreaks among laboratory rodents. Mice, rats, hamsters, guinea pigs, and swine are natural hosts for infection whereas gerbils seem resistant to infection. As with many diseases, improvements in housing systems, production standards, and health monitoring have led to a drastic decline in the incidence of SeV over recent years. Although SeV was once very pervasive in commercial sources of mice and rats, it now rarely occurs in barrier-maintained commercial sources. However, conventionally maintained commercial and institutional colonies may still be sources for introduction of the virus to naïve populations.
The disease is highly contagious, with morbidity in infected colonies commonly reaching 100%, with mortality rates of 0% to 100%. Transmission occurs through aerosol exposure, direct contact, contaminated tissues, and fomites. In utero infections do not occur.
Introduction of SeV into a susceptible population can result in epizootic disease, the severity of which depends on age, genetic factors, and presence of other potential pathogens. This virus is one of the few that may cause overt disease in IC animals. SeV can predispose animals to secondary bacterial infections, affect the immune response, and delay wound healing. Although vertical transmission does not occur, SeV infections of dams is associated with fetal resorption, prolonged gestation, fetal death, and poor growth in surviving pups.
Humans commonly serve as a source of non-Sendai parainfluenza infections in laboratory rodents. Mouse and rat antibody production or PCR testing should be done on all transplantable tumors, cell lines, and other biologic materials to prevent transmission of SeV from infected materials to recipient animals.
In the past, a killed vaccine of duck embryo origin was available commercially. The vaccine was administered intraperitoneally and afforded approximately 7 months of protection. Rats were resistant to intranasal virus challenge after receiving 2 doses of the vaccine. The vaccine was equally effective when administered by intravenous, intramuscular, or subcutaneous routes, but not the intranasal route. Animals receiving intraperitoneal SeV vaccine were also protected from contact infection. Nursing pups born to immunized dams were resistant to challenge infection at 3 weeks of age, but the resistance was not demonstrated after weaning at 4 weeks of age.155
A temperature-sensitive mutant of the original vaccine was also used with some success. Experimental infections of mice with a SeV temperature-sensitive (ts) mutant (HVJ-pB) were studied. Infection with the mutant induced the priming effect of interferon production and both humoral and cellular immune responses, although the mutant virus neither propagated satisfactorily in the respiratory tracts of mice nor caused appreciable microscopic lesions. Inoculation with the mutant protected mice from subsequent challenge with a parental wild-type virus. The efficacy of this protection began as little as 1 day after vaccination and continued for a minimum of 12 weeks. The report also suggested that serum antibodies were efficacious in the nasal turbinates, whereas specific immune cells acted more protectively in the lungs.156
Mouse
SeV is the most likely pathogen to cause clinical respiratory disease in adult IC mice. Nursing and weanling mice are most commonly and most seriously affected. Neonatal mice, aged mice; strains 129/Re, DBA/2, C3H, and male BALB/c mice are highly susceptible to lethal disease. C57BL/6, SJL, female BALB/c, and random bred mice are moderately resistant whereas B6, AKR, SJL, and outbred Swiss mice are highly resistant. Susceptibility also is increased in protein-deprived mice.
Enzootic infection is commonly detected in post-weaned mice that seroconvert within 7 to 14 days, at which time the infection is terminated. Therefore, entrenched infection is perpetuated by the introduction of susceptible animals. There is no evidence of persistent infection in IC mice, but prolonged infection is common in ID mice. Maternally acquired immunity protects young mice from infection, and actively acquired immunity is thought to be long lived. Adults usually have mild respiratory signs including a characteristic “chattering,” and recover fully in a few days; whereas nursing, weanling, and aged mice can have more severe disease with respiratory distress. Acute outbreaks in breeding colonies can cause production to decrease, then return to normal in a few weeks, although enzootic subclinical infection can persist. Athymic and immunosuppressed animals develop illness later than their IC counterparts.
Gross lesions are often absent but when present, the lungs are plum-colored with sharply demarcated foci and consolidation of the anteroventral lung or entire lobes. These areas may turn gray in surviving mice. Mice develop a descending infection of respiratory epithelium, which is eliminated by a cell-mediated immune response that clears the infection but also provokes tissue pathology. The location to which the infection extends into the respiratory tract is determined by mouse strain, differences in mucociliary clearance, virus burden, and kinetics of the immune response. SeV infects nasal, tracheal, bronchial, bronchiolar, and middle ear epithelium, and spreads to type II alveolar cells. T-cell–immunodeficient mice develop progressive pulmonary consolidation with wasting. These mice develop severe, diffuse alveolitis, similar to PVM pneumonia. SeV and PVM lesions in nude and SCID mice are similar, although in SCID mice, bronchial and bronchiolar lesions are more extensive with PVM than with SeV infection. Proliferation with or without destruction of bronchiolar epithelium, increased cellularity of alveolar septa, proteinaceous exudation, and the presence of alveolar macrophages and neutrophils in bronchioles and alveoli are frequent findings during the acute phase of infection. Necrotizing bronchiolitis is a classic lesion. The sloughing of virus-infected bronchiolar epithelial cells corresponds with the appearance of measurable antibody. During the regenerative phase, there is hyperplasia of type II pneumocytes lining airways, with fibrosis, thickening, and mononuclear cell infiltration in alveolar septa. The squamous metaplasia found in recovering lungs has been misconstrued as neoplasia. Lesions are more pronounced in ID animals, and the terminal bronchioles can be severely damaged, with scarring, distortion, or polypoid outgrowths of the mucosa in survivors.
Clinical signs of respiratory distress along with gross and microscopic lesions are highly suggestive of SeV infection, especially among infant mice or adults of genetically susceptible strains. Antibody titers increase rapidly, and serologic diagnosis may be made 8 to 12 days after infection using MFIA, ELISA, or IFA. PCR is recommended on symptomatic animals. Sentinel animals can be added to seropositive colonies to detect active infection. Histopathology, immunohistochemistry and, where possible, virus isolation should be used to confirm infection. In rats, guinea pigs, and rodents other than mice, positive serology using SeV antigen can be due to exposure to PI-2 or PI-3 virus. Positive MFIA, ELISA, or IFA should be confirmed by strain-specific hemagglutination inhibition (HAI), which will discriminate between PI-1, PI-2, and PI-3.
In enzootically infected colonies there is a danger of transmission to other susceptible species. Control and eradication measures must eliminate exposure of susceptible animals, so that infection can “burn out” as previously described for MHV. Control is also aided by the fact that SV is highly labile; therefore, no special measures are required for disinfection. Barrier housing is preferred for prevention and control of transmission.
This virus may alter the incidence of pulmonary neoplasia in experimental carcinogenesis studies. This effect has been attributed to virus-induced modification of tumor cell surface membranes. Pulmonary changes during SeV pneumonia can compromise interpretation of experimentally induced lesions.
Rat
As in mice, susceptibility varies with age, genotype, and immune status. An asymptomatic self-limiting disease is usually induced in rats, unlike for SeV-induced disease in mice. Clinical respiratory signs infrequently occur. Based on experimental infection, lesion severity is more pronounced in Brown Norway and LEW rats than in F-344 rats. Reduced production and litter sizes, as well as delayed growth of young within breeding colonies, may be seen in rats as in mice. SeV in the rat is recognized to have an additive effect on respiratory infections with M pulmonis. Concurrent bacterial and other viral infections increase the severity of clinical disease and pulmonary lesions. In Lewis rats inoculated intranasally with SeV, the draining lymph nodes of the upper respiratory tract are the initial and major site of antibody production. Development of serum immunoglobulin G (IgG) antibodies coincides with clearance of respiratory tract infection and recovery from viral infection.
Following exposure, the virus replicates in the upper respiratory tract, then spreads down the trachea and smaller airways. Pathogenesis of SeV infection in the rat parallels SeV in genetically resistant strains of mice. Acute bronchitis and bronchiolitis are features of the disease. Multifocal, nonsuppurative vasculitis and interstitial alveolitis with necrosis are typical lesions seen during the acute phase of the disease. There are prominent perivascular infiltrates, with hyperplasia of bronchus-associated lymphoid tissue, perivasculitis, and multifocal interstitial pneumonia. These lesions tend to persist for several weeks to months in the rat.
MFIA or ELISA are the best serologic choices for diagnosis of Sendai in rats, due to their sensitivity in detecting early antibody and detecting small amounts of antibody, as compared with complement fixation and HAI. PCR testing is also very sensitive.
If the virus is introduced into a colony of IC animals, neutralizing antibody in infected rats renders the infection self-limiting. Allowing “burn-out” of the virus as in mice is an effective means to eliminate the virus from a colony. In addition to research complications associated with the respiratory tract affinities of the virus, it may modulate some immunologic responses, for example, reducing the severity of adjuvant arthritis and depressing T-cell and thymocytotoxic autoantibody.
Hamster
Infection is often clinically silent with low mortality, and there are very few reports of confirmed clinical disease due to SeV infections in this species. Seronegative animals introduced into a facility housing infected rodents may seroconvert, but it is unlikely that any clinical signs will be observed, although there are reports of mortality in newborn Syrian and Chinese hamsters. One colony of enzootically infected animals experienced occasional deaths in nursing pups as the only clinical sign.
Young adult Syrian hamsters inoculated intranasally with SeV remained asymptomatic during one study, and the animals seroconverted by day 7 post infection. There was a focal to segmental rhinitis progressing to necrotizing tracheitis and multifocal bronchoalveolitis. Immunohistochemistry can demonstrate viral antigen in respiratory epithelial cells during the acute phase of the disease. In animals examined at 3 to 9 days post inoculation, lesions were very similar to those present in mice. In the reparative stages of the disease, hyperplasia of epithelial cells lining affected airways and peribronchial lymphocytic infiltration were seen. Overall, most lesions had resolved by 12 days post infection.
Sialodacryoadenitis virus and Parker's rat coronavirus
Sialodacryoadenitis virus (SDAV) and Parker's rat coronavirus (PRC) belong to the family Coronaviridae, genus Coronavirus.157, 158, 159, 160, 161, 162, 163, 164 SDAV is a morphologic classification and represents all coronavirus isolates that produce sialodacryoadenitis. These viruses should be considered as part of a single biologic grouping (rat coronaviruses) but because of historical precedent, the separation and nomenclature continues. PRC was initially isolated from the lungs of rats after intranasal inoculation of newborn and weanling rats produced rhinitis, tracheitis, and interstitial pneumonia, with focal atelectasis and high mortality in infants. PRC also induced salivary and lacrimal gland lesions in early studies, but these were omitted in the original descriptions. SDAV produces lacrimal and salivary gland lesions in addition to pulmonary disease in young rats. Like MHV, the rat coronavirus groups likely contain many constantly changing strains that shift in virulence. Although the two viruses were once thought to cause distinctly different diseases, clinical signs or pathology cannot differentiate infection with either virus.
Rat coronavirus and mouse coronavirus share antigenic similarities, and antisera against SDAV cross-react with MHV strains. IFA and enzyme immunoassay were not able to differentiate antibodies to MHV or SDAV. Thus at present no diagnostic method is available to differentiate between the two. The hypervariable region identified in the SDAV S sequence may be used as a genetic marker to develop a reliable diagnostic PCR.165
Mouse
Mice may develop a transient interstitial pneumonia with seroconversion. Athymic nude mice are particularly susceptible to coronavirus infections, and develop chronic persistent disease and wasting.
Rat
SDAV is a highly infectious enzootic or epizootic disease of rats, and probably is the single most common viral infection in these animals. Mortality is usually low, but morbidity and subclinical infection commonly reach 100%. These viruses are common in both pets and conventionally housed rat populations. Natural infection can be epizootic, if newly introduced into a susceptible colony, or enzootic within a breeding colony. Age and genetic factors affect susceptibility. Pneumotropic strains of rat coronaviruses may cause an interstitial pneumonia in young rats, especially of the F-344 strain.
Immunity is not lifelong, and it has been shown that rats are susceptible to reinfection as early as 6 months after initial infection and that such rats are able to transfer infection to naïve rats by cage contact. However, the severity of lesions in reinfected rats is minimal compared with initial infection. Neutralizing antibodies to one virus prototype will not offer significant cross-protection from the other virus strain, thus allowing viral shedding and recurrence of clinical signs and lesions, although diminished.
The respiratory tract is the primary portal of entry with transmission occurring via aerosol or direct contact exposure with respiratory secretions. Passage among exposed rats is exceptionally rapid, with infected animals shedding the virus for about 7 days. The disease can become endemic but like MHV, SDAV does not exist in a latent carrier state in IC animals. Extension of the infection from the respiratory epithelium occurs via ducts of the salivary, lacrimal, and Harderian glands. The virus has not been detected in feces.
Clinical signs are seen in a colony for several weeks during epizootics, with individuals exhibiting signs for up to 1 week. Chromodacryorrhea, squinting, photophobia, blepharospasm, and eye rubbing are followed by sneezing and cervical swelling within 5 to 7 days. Keratoconjunctivitis may be the only clinical sign of infection in some outbreaks. Acute keratoconjunctivitis can resolve or progress to keratitis with opacities, ulceration, scarring, and even perforation, in which case there can be secondary bacterial anterior uveitis or panophthalmitis. Swelling under the neck is caused by cervical edema, enlarged cervical lymph nodes, and necrotic and inflamed salivary glands. In general, the swelling subsides in 10 to 14 days and the rat returns to normal. Unilateral or bilateral suborbital or periorbital swelling, prominent or bulging eyes, and keratitis sicca can develop secondary to decreased lacrimation. Self-mutilation may occur as a result of scratching at the eyes and other affected areas, and very young animals may enucleate the globe. During the infection rats usually remain active and continue to eat, although certain behavioral activities may be suppressed; for example, pain may reduce food intake and complicate feeding studies. Glaucoma or persistent megaglobus may be permanent side effects in recovered animals. Infection may be exacerbated by concurrent SeV infection or mycoplasmosis, resulting in death. There may be high mortality associated with general anesthesia during the pneumonic form of the disease. Behavioral changes and reproductive disorders, including irregularities of the estrous cycle and neonatal mortality, have also been associated with epizootics of the disease. Athymic rats develop chronic, active lesions that persist in target organs for months, with accompanying wasting.
The infection progresses rapidly from the respiratory epithelium to the lacrimal and serous or serous-mucous mixed salivary glands, regional lymph nodes, and adjacent tissues. Affected glands are enlarged, edematous, pale, and often reddened. The thymus becomes atrophic; however, this lesion and the chromodacryorrhea may be stress responses. The salivary, Harderian, and exorbital lacrimal glands may all be affected as a group or individually. Lesions are frequently unilateral, and paired salivary and lacrimal glands should be harvested for histopathologic examination. The glands will return to normal or become permanently scarred, depending on the severity of the infection and the degree of tissue damage, within 2 to 4 weeks of infection. Epithelial cells of the respiratory tract and ducts and acini of the glands undergo severe necrosis and inflammation. As the virus is eliminated from the lesion in about 1 week, the restoration process ensues. Harderian glands often have blotchy brown pigmentation with focal residual inflammatory lesions persisting for several weeks, resulting in prominent squamous metaplasia. During the reparative stages, cellular infiltrates of the affected glands are primarily lymphocytes, plasma cells, mast cells, and macrophages. In salivary glands, regeneration of acinar and ductal epithelial cells is usually completed within 3 to 4 weeks post exposure. In the respiratory tract, necrotizing rhinitis with mononuclear and polymorphonuclear cell infiltration occurs in the initial stages of the disease. Both respiratory and olfactory epithelium are affected, and although major repair is complete by 14 days post exposure, residual lesions may persist longer in specialized areas such as the vomeronasal organ. In the lower respiratory tract there is transient tracheitis, focal bronchitis and bronchiolitis with leukocytic infiltration, hyperplasia of respiratory epithelial cells, and flattening and loss of ciliated cells. Focal alveolitis, when present, is characterized by hypercellularity of alveolar walls and mobilization of alveolar macrophages. Lesions in the distal tract are transient, and usually dissipate by 8 to 10 days post exposure.
The presence of typical lesions in the salivary and lacrimal glands confirmed on histopathologic examination is sufficient to make the diagnosis. Viral antigen may be demonstrated in the respiratory tract and affected tissues (including the urinary bladder) by 4 to 6 days post exposure. In general, virus isolation is not a practical procedure in most circumstances. Serology (MFIA, ELISA, IFA) can detect enzootic infections. The combination of pathognomonic clinical signs and histopathology in animals will confirm the diagnosis in the first week of infection, and serology should be employed after 7 to 10 days of infection. PCR is also available for salivary or lacrimal tissue.
Treatment is not indicated unless ophthalmic lesions are present for which topical preparations are indicated. This disease process warrants supportive care in the form of a warm environment, comfortable quarters, and tasty food treats, especially for pet rats. Treatment with antibiotics during the rapid phase of the disease can alleviate the effects of secondary ophthalmic trauma and bacterial opportunistic invaders. Anti-inflammatory medications and analgesics are indicated for any animals exhibiting signs of pain and/or distress.
Preventing transfer of this highly contagious coronavirus to naïve colonies is predicated on preventing entry of infected rats into a facility through knowledge of the pathogen status of vendor colonies and an effective quarantine program. Control of an infection within a colony or facility is based on the fact that rats only shed the virus for about 1 week, after which they are immune and not latently infected. The virus is not transmitted vertically. Strict control of movement of animals, materials, and people into the animal house is useful in preventing contamination with SDAV. Elimination of the virus can be accomplished by the typical “burn-out” method previously described. Strict isolation and microbarrier caging are usually required to prevent an outbreak from infecting an entire facility.
As an enveloped virus, SDAV probably does not remain infectious in the environment for more than a few days and is susceptible to detergents, disinfectants, drying, and ethanol.
There may be significant effects on particular types of research in view of the confirmed effect of epidermal growth factor (EGF) on functions such as reproduction and carcinogenesis. There is a significant depletion of EGF in affected submandibular salivary glands during the convalescent stages of the disease. Active infection has been reported to precipitate graft-versus-host disease in the salivary and lacrimal glands of rats with allogenic bone marrow grafts.
Noninfectious diseases
Neoplasia
Neoplastic diseases have been studied in a variety of laboratory animals, including mice, rats, and hamsters.166, 167, 168, 169, 170
Mouse
The National Cancer Institute’s Mouse Models of Human Cancer Consortium (MMHCC) consensus has endeavored to classify murine pulmonary tumors with those that occur in humans. Therefore, the terms “bronchiolo-alveolar” or “alveolar/bronchiolar” are no longer used. Spontaneous or carcinogen-induced tumors are now simply classified as pulmonary adenomas or carcinomas with approximate qualifications (solid, papillary, or mixed). Other types of tumors included in the new designations are papilloma, squamous cell carcinoma (SCC), and adenosquamous carcinoma.
Primary pulmonary respiratory tumors of mice occur at a relatively high frequency. It has been estimated that more than 95% of these tumors are pulmonary adenomas that arise from either type II pneumocytes and/or Clara cells lining terminal bronchioles. The onset and prevalence of pulmonary tumors can be enhanced with chemical carcinogens or viral infections, such as SeV. The tumors invade pulmonary parenchyma and are prone to metastasize. The prevalence of spontaneous respiratory tumors is mouse strain–dependent and the number of tumors per lung is also higher in susceptible mice. “A” strain mice are uniquely susceptible because of their K-ras allele, with activation of K-ras in the tumors. Tumors can arise by 3 to 4 months of age and can reach 100% prevalence by 18 to 24 months. Less susceptible strains are outbred Swiss, inbred FVB, BALB/c, 129, B6, and C57BL.
Pulmonary tumors are often encountered as incidental findings on necropsy, but those that grow expansively can result in clinical signs. There may be evidence of pleural invasion, with seeding of the visceral and parietal pleura, and there may be occasional extension into the intercostal muscles. Malignant alveologenic tumors are infrequent and consist of adenocarcinomas and SCC. Neoplasms must be differentiated from focal alveolar hyperplasia of mucin-containing epithelial cells (pulmonary adenomatosis) and from multifocal inflammatory lesions. Mammary carcinoma and hepatocellular carcinoma should be considered in the differential diagnosis, as these commonly metastasize to the lungs.
Rat
Nasal cavity tumors, including SCC and rhabdomyosarcoma, occur in rats. SCC is reported to have a relationship with malocclusion syndrome in aging rats.
Primary lung tumors are uncommon, but when they do occur they are usually of alveolar type II pneumocyte and/or Clara cell origin, as in mice. An unusual component of pulmonary oncogenesis in the rat is the predilection of this species to develop primary pulmonary neoplasm when exposed to low-toxicity, insoluble particulates at extremely high concentrations. This process overwhelms the ability to maintain alveolar macrophage-mediated lung clearance, and is known as “pulmonary overload” tumorigenesis. Other reported pulmonary tumors include adenoma, hemangioma, SCC, carcinoma, and adenocarcinoma.
Hamster
Neoplasia is rare but when it does occur, benign tumors (nasal polyps) of the nasal cavity and proximal trachea are most common, with carcinoma of the nasoturbinates occurring infrequently. Clear-cell carcinomas of the larynx have been seen in several colonies without gross lesions, and are of unknown cellular origin. Malignant epithelial tumors of the lower bronchial tree, such as polyps of the trachea covered with mucus-producing tracheal epithelium, bronchogenic adenomas with mucus production, and rare bronchial carcinomas, are extremely uncommon. Because spontaneous bronchogenic and pulmonary cancers are rare, the hamster serves as a good animal model in which to study chemical carcinogenesis in the respiratory tract. Many pulmonary tumors have arisen elsewhere including carcinomas of the adrenal cortex, melanomas of the skin, lymphomas, and sarcomas from a variety of sites. Intratracheal instillation of polynuclear hydrocarbons results in benign and malignant squamous cell lesions of the tracheobronchial lumen and bronchioalveolar tumors, which are usually benign. The squamous lesions are accompanied by anaplastic carcinomas and adenocarcinomas of the bronchi.
Miscellaneous Conditions
A range of miscellaneous noninfectious conditions also cause respiratory illness in the mouse, rat, hamster, or gerbil.171, 172, 173, 174, 175, 176, 177, 178, 179
Acidophilic macrophage pneumonia/epithelial hyalinosis
Acidophilic macrophage pneumonia (AMP), or epithelial hyalinosis, is characterized by focal to diffuse aggregation of acidophilic crystals within macrophages, alveolar spaces, and airways, and is prevalent among many strains of mice. Strains such as B6, 129 (particularly 129S4/SvJae), and Swiss mice tend to have an increased incidence and earlier onset of this lesion. AMP can cause mortality in various types of GEMs on the B6 or 129 background, and is particularly severe in B6 (ptpn6me) moth-eaten mice. AMP tends to be most evident in older animals and can occur in wild mice as well. Any disease that impairs normal clearance (pulmonary tumors, pneumocystosis, or other chronic pneumonias) can predispose to AMP and will lead to dyspnea if very extensive. Grossly there is lobar to diffuse tan to red discoloration of the lungs, which do not collapse. Microscopically, macrophages have abundant cytoplasm filled with large numbers of needle-shaped to rhomboid-shaped eosinophilic crystals, present in alveolar spaces, alveolar ducts, terminal airways, and bronchiolar glands. The crystalline material is a conglomerate of substances primarily composed of Ym1 chitinase, but also contains iron, α-1 antitrypsin, immunoglobulin, and granulocyte breakdown products. Based on ultrastructural studies, the crystals resemble Charcot-Leyden crystals, which are unique to nonhuman primates and humans in association with eosinophil-related diseases.
Although AMP is the most overt manifestation of this condition, hyalinosis of multiple organs can occur, including olfactory, nasal, middle ear, trachea, lung, stomach, gall bladder, bile duct, and pancreatic duct epithelium as part of the syndrome. Neonatal mice with lesions in the olfactory and vomeronasal areas fail to nurse.
Agent-induced pulmonary edema
A report in 1991 documented that xylazine administration in rats resulted in pleural effusion and alveolar edema. The optimal “edemagenic” dose was approximately 43 mg/kg, which has been used to study the progression of increased pulmonary vascular permeability. Other agents causing varying degrees of pulmonary edema in rats are pentobarbital and carbon dioxide.
Allergic disease/allergic rhinitis
Animals may be allergic to components of their food, dusty hay, bedding, cigarette smoke, and a variety of aerosols. Allergies in hamsters may be hereditary.
Alveolar hemorrhage
Regardless of the cause of death, focal intra-alveolar hemorrhage is a uniform agonal finding in lungs of mice, which must be differentiated from congestive heart failure and other causes.
Pulmonary histiocytosis/alveolar histiocytosis/alveolar proteinosis/alveolar lipoproteinosis
Mouse
Focal accumulations of foamy lipid-laden macrophages are sporadically observed in the peripheral, particularly subpleural, regions of the lung in aging mice of all types. These lesions are rare in specific pathogen-free (SPF) mice. Some of the macrophages may contain cholesterol crystals. These changes can follow pulmonary hemorrhage, and hemoglobin crystals may also be present in the area. Alveolar lipoproteinosis is a more severe condition, in which there is hypertrophy and vacuolization of type II pneumocytes, mobilization of scattered macrophages, and progressive intra-alveolar accumulation of granular, pale, eosinophilic phospholipid. Experimental procedures (inhalation of toxic aerosols and so forth) are frequently used to produce this type of lesion. To add to the perplexity, there appears to be considerable overlap in the interpretation and nomenclature assigned to these changes, and they may overlap with AMP.
Rat
Aggregates of alveolar macrophages and pulmonary foam cells (PFC) are seen occasionally in the lungs of older rats. The cause is unknown and does not appear to be infectious, although there is usually a minimal concurrent inflammatory cell response. Factors such as excess surfactant production over breakdown and clearance or impaired mucociliary clearance have been implicated to explain the excess recruitment of alveolar macrophages. Lesions are grossly visible on the pleural surface as white to pale tan foci, usually about 1 mm diameter. Foci may extend slightly above the pleural surface in the uninflated lung. Microscopically, clusters of alveoli, often in a subpleural location or adjacent to a terminal bronchiole, contain increased numbers of large, pale, foamy-appearing macrophages. Occasionally cholesterol clefts may be visible in denser accumulations of macrophages, and a slight infiltration of lymphocytes may be present around adjacent vessels, probably as a response to proinflammatory mediators released by the macrophages. This condition should not be mistaken for any of the viral pneumonias of rats, because affected animals are seronegative, and any lymphoid infiltrate is slight and localized to the area of macrophage accumulation.
Hamster
Alveolar histiocytosis similar to that seen in rats also occurs in hamsters.
Amyloidosis
Mouse
Amyloidosis is a common event in many aging laboratory and wild mice, and can be difficult to distinguish between primary and secondary in spontaneous cases There are two types of amyloid in the mouse, AapoAII and AA. The prevalence of spontaneous amyloidosis is significantly affected by stress; ectoparasitism; and chronic inflammatory conditions, such as ulcerative dermatitis, preputial adenitis, cervical lymphadenitis, conjunctivitis, pyometra, and others. There does not appear to be a clear sex-related predisposition, although it can be more common in males that are prone to fighting due to stress. Individually housed SPF mice have lower prevalence of amyloidosis compared with group-housed mice. Amyloidosis tends to occur at high prevalence and early onset in A, SJL, and outbred Swiss mice, at high prevalence but late onset in C57BL, B6, and B10 mice, and is extremely rare in BALB/c, C3H, and DBA mice.
AapoAII is known as “primary” or “senile amyloid.” Although the precursor is produced by the liver, deposition of AapoAII tends to be less severe in spleen and liver (compared with AA), with more deposition in the lungs and other internal organs. Primary amyloidosis is common among aging mice but also may occur in young mice of highly susceptible strains. AA amyloid is associated with an increase in serum precursors apoSAA, which is induced in hepatocytes and elevated in response to cytokines produced during inflammatory and neoplastic disease. AA amyloidosis can be induced by repeated injections of casein and other inflammatory stimuli, thereby earning the name “secondary amyloidosis.” Localized amyloidosis can also be found. Tumor-associated amyloid can be found in pulmonary adenomas of A and BALB mice. A common site for amyloid-like deposition is in the nasal submucosa, particularly above the vomeronasal organs.
Hamster
Tracheal and lung amyloidosis has been reported in hamsters.
Aspiration pneumonia
Although rodents are obligate nasal breathers, aspiration pneumonia is a common sequela to accidental inhalation of foreign material, which occurs under several circumstances but especially when shipping containers using wood shavings are handled roughly during transportation. It can also occur secondary to megaesophagus or gastrointestinal impaction.
Eosinophilic granulomatous pneumonia in brown Norway rats
The brown Norway (BN) rat has been a model to study the pathogenesis of asthma because they readily develop increased bronchiolar responsiveness and elevated Immunoglobulin E (IgE) after exposure to allergens. However, they can develop a spontaneous eosinophil-rich granulomatous pneumonia in the absence of any experimental manipulations. The changes have been attributed to inadvertent exposure to the allergen, but because of the inflammatory nature of the lesions, they could be due to an unidentified infectious agent. Incidence can be up to 100% in both males and females at 3 to 4 months of age. BN rats from colonies worldwide are affected, including those maintained in isolators. Affected animals are seronegative for all known agents, and rats of other strains housed with affected animals do not develop lung lesions. The lesions are distributed throughout the parenchyma and are characterized by well-organized granulomas of Langerhans giant cells, macrophages, and eosinophils. No foreign material, fungi, or bacteria can be demonstrated microscopically. This syndrome remains an important complication for the researcher evaluating histopathological changes in the lung of the BN rat. Transient pulmonary eosinophilia and granulomatous vasculitis have been produced in laboratory rats following intravenous administration of Sephadex. However, the pulmonary lesions are markedly different to those of the spontaneous disease.
Freund adjuvant pulmonary granuloma
Focal histiocytic granulomas can be found in the lungs of mice immunized with Freund adjuvant, regardless of the site of immunization.
Hyperplasia of alveolar or bronchial epithelium
This condition occurs in old mice and must be differentiated from pulmonary tumors.
Inflammation of nasal mucosa
Common nasal lesions of aging mice include squamous epithelial hyperplasia in the nasal vestibule, intracytoplasmic hyaline inclusions, inflammation of the nasal mucosa and nasolacrimal duct, and olfactory degeneration with atrophy.
Lymphohistiocytic lung lesions
An unidentified agent has been associated with this condition in rats. Lesions occur in 8- to 18-week-old animals, with rats 8 to 12 weeks of age the most severely affected. Grossly, lesions appear as multiple small gray to tan foci on the pleural surface of the lung. Microscopically, mild to moderate multifocal histiocytic alveolitis and perivascular cuffing are present. Microbiological culturing and PCR assays suggest that the lesions are not bacterial in origin. A viral etiology is suggested, because inoculation of lung tissue homogenates passed through bacteriologic filters induces cytopathic effects in tissue culture, and lesions have been reported in barrier-maintained commercial breeding colonies.
Miscellaneous lesions reported in hamsters
Other lesions reported in hamsters include tracheal mucosal gland adenitis, ossification of tracheal rings, tracheal gland degeneration, lung mineralization, and heterotopic bone in the lung.
Perivascular lymphoid infiltrates
Mild to severe infiltrates can be seen in the adventitia of pulmonary vessels, with extension into adjacent alveolar septa in mice. This condition is consistently found in response to antigenic stimuli, such as viral infection. Perivascular lymphoid infiltrates also frequently appear in older mice with perivascular mononuclear cell infiltrates in salivary glands, kidneys, and other organs. The condition seems to precede lymphoproliferative disorders.
Tracheal cartilage degeneration
F-344 rats seem particularly susceptible to developing age-related tracheal cartilage degeneration and seromucinous adenitis. The use of rigid, metal gavage tubes and the irritant properties of the gavaged material may play a role, although spontaneous lesions did occur in untreated animals and may be seen as early as 6 weeks of age. These inflammatory lesions are thought to lead to impaired salivation. Food and bedding may become lodged in the oropharyngeal cavity, resulting in asphyxia.
Footnotes
The author has nothing to disclose.
References
- 1.Harkness J.E., Turner P.V., VandeWoude S. Introduction, general husbandry, and disease prevention. In: Greenacre C.B., editor. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. pp. 11–17. [Google Scholar]
- 2.Jenkins J.R. Rodent diagnostics. J Exot Pet Med. 2008;17:16–25. doi: 10.1053/j.jepm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King W.W., Russell S.P. Metabolic, traumatic, and miscellaneous diseases. In: Suckow M.A., Weisbroth S.H., Franklin C.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2005. p. 521. [Google Scholar]
- 4.Mayer J. Annoying respiratory tract diseases in rats. In: Proceedings from the North American Veterinary Conference. Orlando: 2008. p. 1859.
- 5.Carpenter J.W. Exotic animal formulary. 3rd edition. Elsevier Saunders; St Louis: 2005. Rodents; pp. 377–397. [Google Scholar]
- 6.Johnson-Delaney C.A. Exotic companion medicine handbook. Zoological Education Network; Lake Worth: 1996. Small rodents; pp. 3–9. [Google Scholar]
- 7.Sayers I., Smith S. Mice, rats, hamsters, and gerbils. In: Meredith A., Johnson-Delaney C.A., editors. BSAVA manual of exotic pets. 5th edition. BSAVA; Gloucester, England: 2010. pp. 26–27. [Google Scholar]
- 8.deMatos R. Rodents: therapeutics. In: Keeble E., Meredith A., editors. BSAVA manual of rodents and ferrets. BSAVA; Gloucester, England: 2009. pp. 52–62. [Google Scholar]
- 9.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; pp. 58–60. [Google Scholar]
- 10.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; pp. 95–96. [Google Scholar]
- 11.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Gerbils; pp. 121–123. [Google Scholar]
- 12.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Hamsters; pp. 140–143. [Google Scholar]
- 13.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Clinical procedures; pp. 141–146. [Google Scholar]
- 14.Feldman S.H., Gograge S.I., Kohn D.F. Laboratory mouse handbook. AALAS; Memphis: 2006. Biology; p. 14. [Google Scholar]
- 15.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; pp. 42–43. [Google Scholar]
- 16.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; pp. 7–11. [Google Scholar]
- 17.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 43–45. [Google Scholar]
- 18.Cook M.J. Anatomy. In: Foster H.L., Small J.D., Fox J.G., editors. vol. 3. Academic Press; New York: 1983. pp. 102–120. (The mouse in biomedical research). [Google Scholar]
- 19.Kaplan H.M., Brewer N.R., Blair W.H. Physiology. In: Foster H.L., Small J.D., Fox J.G., editors. vol. 3. Academic Press; New York: 1983. pp. 248–292. (The mouse in biomedical research). [Google Scholar]
- 20.Hofstetter J., Suckow M.A., Hickman D.L. Morphophysiology. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 106–108. [Google Scholar]
- 21.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; pp. 81–82. [Google Scholar]
- 22.O’Malley B. Clinical anatomy and physiology of exotic species. Elsevier Saunders; St Louis: 2005. Rats; p. 13. [Google Scholar]
- 23.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; pp. 125–126. [Google Scholar]
- 24.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 124–125. [Google Scholar]
- 25.Bivin W.S., Olsen G.H., Murray K.A. Morphophysiology. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando: 1987. pp. 19–24. [Google Scholar]
- 26.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Hamsters; pp. 131–133. [Google Scholar]
- 27.Hankensen C.F., vanHoosier G.L., Jr. Biology and diseases of hamsters. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 171–172. [Google Scholar]
- 28.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Hamsters; p. 180. [Google Scholar]
- 29.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Gerbils; pp. 113–114. [Google Scholar]
- 30.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Gerbils; p. 207. [Google Scholar]
- 31.Winsser J. A study of Bordetella bronchiseptica. Proc Anim Care Panel. 1960;10:87–104. [Google Scholar]
- 32.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 262–265. [Google Scholar]
- 33.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 141–142. [Google Scholar]
- 34.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 380–381. [Google Scholar]
- 35.Burek J.D., Jersey C.G., Whitehair C.K. The pathology and pathogenesis of Bordetella bronchiseptica and Pasteurella pneumotropica infection in conventional and germ-free rats. Lab Anim. 1993;27:342–349. [PubMed] [Google Scholar]
- 36.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 63–64. [Google Scholar]
- 37.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 96. [Google Scholar]
- 38.Schoeb T.R. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Pract. 2000;3(2):487–488. doi: 10.1016/S1094-9194(17)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 269–272. [Google Scholar]
- 40.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; p. 64. [Google Scholar]
- 41.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 83. [Google Scholar]
- 42.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; p. 61. [Google Scholar]
- 43.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 366–369. [Google Scholar]
- 44.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; p. 98. [Google Scholar]
- 45.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 140–142. [Google Scholar]
- 46.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 142–143. [Google Scholar]
- 47.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Gerbil; p. 211. [Google Scholar]
- 48.Schoeb T.R., Lindsey J.R. Cilia-associated respiratory bacillus infection, rat, mouse, and rabbit. In: Jones T.C., Mohr U., Hunt R.D., editors. International life sciences institute monographs on pathology of laboratory animals: respiratory system. Springer-Verlag; Berlin: 1996. p. 325. [Google Scholar]
- 49.St. Claire M.B., Besch-Williford C.L., Riley L.K. Experimentally induced infection of gerbils with cilia-associated respiratory bacillus. Lab Anim Sci. 1999;49(4):421–423. [PubMed] [Google Scholar]
- 50.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 283–287. [Google Scholar]
- 51.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; p. 72. [Google Scholar]
- 52.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 130–132. [Google Scholar]
- 53.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 135. [Google Scholar]
- 54.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; p. 99. [Google Scholar]
- 55.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 362–366. [Google Scholar]
- 56.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 92. [Google Scholar]
- 57.Hankensen C.F., vanHoosier G.L., Jr. Biology and diseases of hamsters. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 182. [Google Scholar]
- 58.Frisk C.S. Bacterial and mycotic infections. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando: 1987. p. 129. [Google Scholar]
- 59.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Hamster; p. 192. [Google Scholar]
- 60.Holmes N.E., Korman T.M. Corynebacterium kutscheri infection of skin and soft tissue folloing rat bite. J Clin Microbiol. 2007;45:3468–3469. doi: 10.1128/JCM.00607-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicklas W. Haemophilus infection in a colony of laboratory rats. J Clin Microbiol. 1989;27:1636–1639. doi: 10.1128/jcm.27.7.1636-1639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; p. 64. [Google Scholar]
- 63.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 95. [Google Scholar]
- 64.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; p. 152. [Google Scholar]
- 65.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. p. 380. [Google Scholar]
- 66.Waggie K.S., Wagner J.E., Lentsch R.H. A naturally occurring outbreak of Mycobacterium avium-intracellulare infections in C57BL/6N mice. Lab Animal Sci. 1983;33:249–253. [PubMed] [Google Scholar]
- 67.Waggie K.S., Wagner J.E., Lentsch R.H. Experimental murine infections with a Mycobacterium avium-intracellulare complex organism isolated from mice. Lab Anim Sci. 1983;33:254–257. [PubMed] [Google Scholar]
- 68.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 331–334. [Google Scholar]
- 69.Donnelly T. Small rodents: disease problems. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits, and rodents clinical medicine and surgery. 2nd edition. Elsevier Publishing; St Louis: 2004. pp. 304–307. [Google Scholar]
- 70.Goodman G. Rodents: respiratory and cardiovascular disorders. In: Keeble E., Meredith A., editors. BSAVA manual of rodents and ferrets. BSAVA; Gloucester, England: 2009. p. 147. [Google Scholar]
- 71.Schoeb T.R. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Pract. 2000;3(2):485–487. doi: 10.1016/S1094-9194(17)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 65–68. [Google Scholar]
- 73.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 80–83. [Google Scholar]
- 74.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 143–146. [Google Scholar]
- 75.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 142–143. [Google Scholar]
- 76.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; pp. 97–98. [Google Scholar]
- 77.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 389–393. [Google Scholar]
- 78.Ferreira J.B., Yamaguti M., Marques L.M. Detection of Mycoplasma pulmonis in laboratory rats and technicians. Zoonoses Public Health. 2008;55(5):229–234. doi: 10.1111/j.1863-2378.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 79.Neimark H., Mitchelmore D., Leach R.H. An approach to characterizing uncultivated prokaryotes: The grey lung agent and proposal of a candidatus taxon for the organism, “Candidatus Mycoplasma ravipulmonis”. Int J Syst Bacteriol. 1998;48:389–394. doi: 10.1099/00207713-48-2-389. [DOI] [PubMed] [Google Scholar]
- 80.Schoeb T.R. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Pract. 2000;3(2):491. doi: 10.1016/S1094-9194(17)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 235–236. 281–2. [Google Scholar]
- 82.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 66–68. [Google Scholar]
- 83.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 87–88. [Google Scholar]
- 84.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 146–147. [Google Scholar]
- 85.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 137–138. [Google Scholar]
- 86.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 374–378. [Google Scholar]
- 87.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Hamster; p. 192. [Google Scholar]
- 88.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Hamsters; p. 145. [Google Scholar]
- 89.Frisk C.S. Bacterial and mycotic infections. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando: 1987. p. 129. [Google Scholar]
- 90.Goelz M.R., Thigpen J.E., Mahler J. Efficacy of various therapeutic regimens in eliminating Pasteurella pneumotropica from the mouse. Lab Anim Sci. 1996;46:280–285. [PubMed] [Google Scholar]
- 91.Schoeb T.R. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Pract. 2000;3(2):491. doi: 10.1016/S1094-9194(17)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 95. [Google Scholar]
- 93.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; p. 69. [Google Scholar]
- 94.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 340–344. [Google Scholar]
- 95.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; p. 153. [Google Scholar]
- 96.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 139. [Google Scholar]
- 97.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 380–382. [Google Scholar]
- 98.Schoeb T.R. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Pract. 2000;3(2):489–490. doi: 10.1016/S1094-9194(17)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donnelly T. Small rodents: disease problems. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits, and rodents clinical medicine and surgery. 2nd edition. Elsevier Publishing; St Louis: 2004. pp. 304–307. [Google Scholar]
- 100.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; p. 98. [Google Scholar]
- 101.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 346–350. [Google Scholar]
- 102.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 149–150. [Google Scholar]
- 103.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 133–135. [Google Scholar]
- 104.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Hamsters; p. 145. [Google Scholar]
- 105.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Hamster; p. 192. [Google Scholar]
- 106.Frisk C.S. Bacterial and Mycotic Infections. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando: 1987. p. 129. [Google Scholar]
- 107.Schoeb T.R. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Pract. 2000;3(2):492–493. doi: 10.1016/S1094-9194(17)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 363–364. [Google Scholar]
- 109.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 83–84. [Google Scholar]
- 110.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 96–98. [Google Scholar]
- 111.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 157–158. [Google Scholar]
- 112.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. p. 152. [Google Scholar]
- 113.Weisbroth S.H., Kohn D.F., Boot R. Bacterial, mycoplasmal, and mycotic infections. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 384–389. [Google Scholar]
- 114.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; p. 82. [Google Scholar]
- 115.Rehm S., Waalkes M.P., Ward J.M. Aspergillus rhinitis in Wistar (Crl:(WI)BR) rats. Lab Anim Sci. 1998;38:162–166. [PubMed] [Google Scholar]
- 116.Hubbs A.F., Hahn F.F., Lundgren D.L. Invasive tracheobronchial aspergillosis in an F-344/N rat. Lab Anim Sci. 1991;41:521–523. [PubMed] [Google Scholar]
- 117.Royals M.A., Getzy D.M., Vandewoude S. High fungal spore load in corncob bedding associated with fungal rhinitis in two rats. Contemp Topics. 1999;38:64–66. [PubMed] [Google Scholar]
- 118.Bornstein S., Iwarsson K. Nasal mites in a colony of Syrian hamsters Lab Anim. 1980;14:31–33. doi: 10.1258/002367780780943105. [DOI] [PubMed] [Google Scholar]
- 119.Wagner J.E. Parasitic diseases. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando: 1987. p. 152. [Google Scholar]
- 120.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Clinical signs and differential diagnoses; p. 242. [Google Scholar]
- 121.Gerardo S., Gerardo C., Mills Serologic evidence of hantavirus infection in sigmodontine rodents in Mexico. J Wildl Dis. 2001;37(2):391–393. doi: 10.7589/0090-3558-37.2.391. [DOI] [PubMed] [Google Scholar]
- 122.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 1132–1133. [Google Scholar]
- 123.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 308–309. [Google Scholar]
- 124.Lussier G. Manual of microbiological monitoring of laboratory animals. 2nd edition. National Institutes of Health Publications; Bethesda: 1994. Murine cytomegalovirus; pp. 69–74. [Google Scholar]
- 125.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 19–21. [Google Scholar]
- 126.Chen H.C., Cover C.E. Spontaneous disseminated cytomegalic inclusion disease in an ageing laboratory mouse. J Comp Pathol. 1988;98:489–493. doi: 10.1016/0021-9975(88)90097-7. [DOI] [PubMed] [Google Scholar]
- 127.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 278–281. [Google Scholar]
- 128.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; p. 66. [Google Scholar]
- 129.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 31–36. [Google Scholar]
- 130.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 334–335. [Google Scholar]
- 131.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; p. 67. [Google Scholar]
- 132.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; p. 44. [Google Scholar]
- 133.Karst S.M., Wobus C.E., Lay M. STAT1-Dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 134.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 23–24. [Google Scholar]
- 135.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 334–335. [Google Scholar]
- 136.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; p. 67. [Google Scholar]
- 137.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 36–37. [Google Scholar]
- 138.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 71–72. [Google Scholar]
- 139.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; p. 134. [Google Scholar]
- 140.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 146–147. [Google Scholar]
- 141.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Hamster; p. 184. [Google Scholar]
- 142.Hankensen C.F., vanHoosier G.L., Jr. Biology and diseases of hamsters. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 184–185. [Google Scholar]
- 143.Parker J.C., Ganaway J.R., Gillett C.S. Viral diseases. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando: 1987. pp. 95–106. [Google Scholar]
- 144.Percy D.H., Barthold S.W. Pathology of laboratory rabbits and rodents. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 129–130. [Google Scholar]
- 145.Ward J.M., Lock A., Collins M.J. Papoviral sialoadenitis in athymic nude rats. Lab Anim. 1984;18:84–89. doi: 10.1258/002367784780864884. [DOI] [PubMed] [Google Scholar]
- 146.Livingston RS, Besch-Williford I, Myles MH, et al. Pneumocystis carinii infection causes lung lesions indistinguishable from those previously attributed to rat respiratory virus. In: Programs of the 61st AALAS National Meeting (abstract PS101). Atlanta, October 10–14, 2010.
- 147.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 373–375. [Google Scholar]
- 148.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 37–39. [Google Scholar]
- 149.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 59–72. [Google Scholar]
- 150.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Mice; pp. 65–66. [Google Scholar]
- 151.Jacoby G.A., Gaertner D.J. Viral diseases. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. p. 443. [Google Scholar]
- 152.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 134–135. [Google Scholar]
- 153.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 143–144. [Google Scholar]
- 154.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Hamster; pp. 184–185. [Google Scholar]
- 155.Tsukui M., Ito H., Tada M. Protective effect of inactivated virus vaccine on Sendai virus infection in rats. Lab Anim Sci. 1982;32:143–146. [PubMed] [Google Scholar]
- 156.Iwata H., Tagaya M., Matsumoto K. Aerosol vaccination with a Sendai virus temperature-sensitive mutant (HVJ-pb) derived from persistently infected cells. J Infect Dis. 1989;162:402–407. doi: 10.1093/infdis/162.2.402. [DOI] [PubMed] [Google Scholar]
- 157.Harkness J.E., Turner P.V., VandeWoude S. Harkness and Wagner’s biology and medicine of rabbits and rodents. 5th edition. Wiley Blackwell; Ames: 2010. Specific diseases and conditions; pp. 281–283. [Google Scholar]
- 158.Fallon M.T. Rats and Mice. In: Laber-Laird K., Swindle M.M., Flecknall P., editors. Handbook of rodent and rabbit medicine. Elsevier Science, Inc.; Tarrytown: 1996. pp. 16–17. [Google Scholar]
- 159.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 69–71. [Google Scholar]
- 160.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 130–132. [Google Scholar]
- 161.Jacoby G.A., Gaertner D.J. Viral diseaes. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 435–442. [Google Scholar]
- 162.Hrapkiewicz K., Medina L. Clinical laboratory animal medicine. 3rd edition. Blackwell Publishing; Ames: 2007. Rats; pp. 100–102. [Google Scholar]
- 163.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 144–146. [Google Scholar]
- 164.Hankensen C.F., vanHoosier G.L., Jr. Biology and diseases of hamsters. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 184–185. [Google Scholar]
- 165.Yoo D., Pei Y., Christie N. Primary structure of the sialodacryoadenitis virus genome: sequence of the structural-protein region and its application for differential diagnosis. Clin Diagn Lab Immunol. 2000;7(4):568–573. doi: 10.1128/cdli.7.4.568-573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schoeb T.R. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Prac. 2000;3(2):493. doi: 10.1016/S1094-9194(17)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Adacemic Press; San Diego: 2002. p. 113. [Google Scholar]
- 168.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 111–118. [Google Scholar]
- 169.Boorman G.A., Everitt J.I. Neoplastic diseases. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 487–488. [Google Scholar]
- 170.Stradberg J.D. Neoplastic diseases. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando (FL): 1987. pp. 158–159. [Google Scholar]
- 171.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Mouse; pp. 93–96. 105–6. [Google Scholar]
- 172.Jacoby R.O., Fox J.G., Davisson M. Biology and Diseases of Mice. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 105–109. [Google Scholar]
- 173.King W.W., Russell S.P. Metabolic, traumatic, and miscellaneous diseases. In: Suckow M.A., Weisbroth S.H., Franklin D.L., editors. The laboratory rat. 2nd edition. Academic Press; Orlando: 2002. pp. 520–522. [Google Scholar]
- 174.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Rat; pp. 155–156. 164. [Google Scholar]
- 175.Kohn D.F., Clifford C.B. Biology and diseases of rats. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 153–154. [Google Scholar]
- 176.Percy D.H., Barthold S.W. Pathology of laboratory rodents and rabbits. 3rd edition. Blackwell Publishing; Ames: 2007. Hamster; pp. 125–126. [Google Scholar]
- 177.Hankensen C.F., vanHoosier G.L., Jr. Biology and diseases of hamsters. In: Fox J.G., Anderson L.C., Loew F.M., editors. Laboratory animal medicine. 2nd edition. Academic Press; San Diego: 2002. pp. 188–189. [Google Scholar]
- 178.Richardson V.C.G. Diseases of small domestic rodents. 2nd edition. Blackwell Publishing; Ames: 2003. Hamsters; p. 161. [Google Scholar]
- 179.Hubbard G.B., Schmidt R.E. Noninfectious diseases. In: vanHoosier G.L. Jr., McPherson C.W., editors. Laboratory hamster. Academic Press; Orlando: 1987. p. 174. [Google Scholar]