Abstract
Objectives
To explore the epidemiological and clinical factors predictive of the case fatality rate (CFR) of Middle East respiratory syndrome-coronavirus (MERS-CoV) infection in an outbreak in Daejeon, the Republic of Korea.
Methods
We reviewed the outbreak investigation reports and medical records of 1 index case and 25 additional MERS cases in hospitals A (14 cases) and B (11 cases), and conducted an in-depth interview with the index case.
Results
The CFR in hospital B was higher than that in hospital A (63.6% vs. 28.6%, respectively). Higher MERS-CoV exposure conditions were also found in hospital B, including aggravated pneumonia in the index case and nebulizer use in a six-bed admission room. The host factors associated with high CFR were pre-existing pneumonia, smoking history, an incubation period of less than 5 days, leukocytosis, abnormal renal function at diagnosis, and respiratory symptoms such as sputum and dyspnea.
Conclusions
The conditions surrounding MERS-CoV exposure and the underlying poor pulmonary function due to a smoking history or pre-existing pneumonia may explain the high CFR in hospital B. The clinical features described above may enable prediction of the prognosis of MERS cases.
Keywords: Middle East respiratory syndrome coronavirus, Hospital, Outbreak, Mortality, Korea
Introduction
The Middle East respiratory syndrome-coronavirus (MERS-CoV) outbreak in the Republic of Korea (Korea) was a global issue in 2015. Through hospital-to-hospital transmission in 17 hospitals, a total of 186 patients were infected by MERS-CoV,1, 2 of whom 38 died. The 20.4% total case fatality rate (CFR) in the Korean MERS outbreak was lower than that in the Arabian Peninsula.3 However, the CFR differed among clusters in Korea. For example, the CFR of St. Mary’s hospital in Pyeongtaek (Hospital P) was 16.7%,4 whereas that of the Daejeon cluster was 44.0%. From May 2015 to June 2015, Daejeon, a metropolitan city in Korea, experienced an outbreak of MERS based around two hospitals, hospital A and hospital B, and recorded a higher CFR than the overall CFR in Korea.
Information on the risk factors for MERS mortality is limited,3 particularly outside of the Middle East. Previous reports on MERS mortality in the Arabian Peninsula focused on host factors, including demographic, pre-existing medical conditions, and laboratory results.3
Herein, we explored the viral exposure conditions and host factors associated with the CFR of MERS to explain the higher rates in the Daejeon cluster.
Methods
MERS case definition
A MERS case was defined as an individual diagnosed with MERS by a real-time reverse-transcription polymerase chain reaction (rRT-PCR) assay of nucleic acid extracted from sputum specimens. The rRT-PCR targets were the upstream envelope (upE) and the open reading frame 1a (ORF 1a) genes. Cycle threshold (Ct) values were used to indicate virus load.5 When sputum sampling was not possible, a nasopharyngeal or oropharyngeal swab sample was obtained. For each confirmed case, a case number was assigned by the Korea Centers for Disease Control and Prevention (KCDC) in the order in which cases were confirmed during the outbreak. For example, the index case in Daejeon was case #16. Among the 25 incident cases in Daejeon, 14 were infected in hospital A, and 11 in hospital B.
Data collection
We reviewed the outbreak investigation reports for one index case (case #16) and 25 incident cases of MERS in Daejeon. The investigation report provided by KCDC was based on interviews with hospital staff and patients during the outbreak, and included case-specific information such as demographic factors, current underlying diseases, history of MERS-CoV exposure, onset date and presenting symptoms of MERS, and contact history as a MERS infector. Closed-circuit television (CCTV) data were used to supplement the contact history of each MERS case.
The medical records of cases were reviewed to generate or supplement the symptom, smoking history, underlying disease, laboratory finding, and clinical outcome variables. The included underlying diseases were diabetes mellitus, chronic heart diseases (ischemic heart disease, congestive heart failure, valvular heart disease, and arrhythmias requiring monitoring), stroke (cerebral infarct and hemorrhage), chronic lung diseases (asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF)), malignancy, immunocompromising conditions (Cushing’s syndrome, human immunodeficiency virus (HIV), and immunosuppressive chemotherapy), and pre-existing pneumonia. Pre-existing pneumonia was defined as underlying pneumonia within 2 weeks before the onset of MERS.
We conducted an in-depth interview with the index case (case #16) to estimate his level of contact with the cases in each hospital. A medical radiologist evaluated the radiologic evidence of pneumonia in the index case. A specialist in architectural engineering inspected the ventilation systems on the wards where the index case stayed.
Data description and statistical analysis
Based on the collected data, we identified the infector for each case. Both the KCDC investigation team and the Daejeon in-depth investigation team reviewed potential exposures independently. In cases of disagreement between the two teams regarding infected–infector pairs, the most probable infector and exposure period were determined by consensus. One expert, who participated in a previous MERS investigation team,4 reviewed these decisions. The process was repeated until a final consensus was obtained.
The incubation period was defined as the interval from exposure to the onset of MERS-associated symptoms, including nonspecific clinical symptoms such as fever, chills, cough, sore throat, sputum production, dyspnea, myalgia, headache, nausea, vomiting, diarrhea, and/or abdominal discomfort. If the exposure period was longer than 2 days, the midpoint of the period was used as the date of exposure. The incubation period was converted to a binomial scale (less than 5 days vs. 5 days or more), and the cut-off point (5 days) was determined using a receiver operating characteristic (ROC) curve.
The CFR was defined as the percentage of fatal cases among the total number of MERS cases. To identify risk factors for MERS mortality among the epidemiological and clinical factors, we compared the CFRs between the two groups defined by binomial independent factors. Using the MedCalc Statistical Software version 16.2.1 (MedCalc Software BVBA, Ostend, Belgium; https://www.medcalc.org; 2016), the relative risk (RR) of each factor for CFR and the corresponding 95% confidence intervals (CIs) were calculated. All statistical tests were two-tailed; a value of p < 0.05 was considered to indicate statistical significance.
Results
Index and associated cases in Daejeon
The index case (case #16) was identical in hospital A and hospital B. A 41-year-old ex-smoker, he was exposed to patient zero (case #1) in Pyeongtaek St. Mary’s Hospital from May 15 to May 17. After discharge, due to his experiencing symptoms such as a febrile sense, chilling, cough, general weakness, and diarrhea, the patient was admitted to hospital A during 22–28 May 2015. Since his illness worsened, including development of a severe productive cough, the patient wanted a discharge from hospital A to visit the emergency room (ER) of hospital B via his own transportation on May 28. Chest radiographs showed the progression of pneumonia from May 22 to 28. The chest image in hospital B on May 28 showed aggravation of pneumonia compared with those obtained in hospital A. On the second day of admission in hospital B, he received lidocaine inhalation in his admission room using a jet nebulizer and then underwent a bronchoscopy in a separate examination room. Medical staff in the bronchoscopy room wore surgical masks, gloves, and vinyl gowns. He received treatment under the impression of viral pneumonia before being suspected to have MERS and being isolated on May 30. Cycle threshold (Ct) values were 22.2 for upE and 23.2 for ORF 1a from his sputum on May 31.
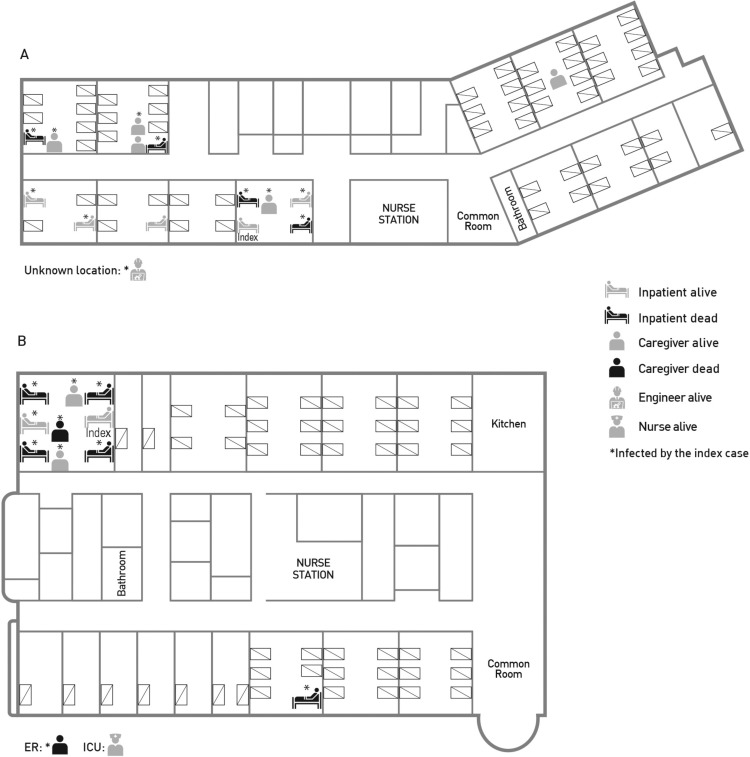
Among the 25 incident cases in Daejeon, 15 patients (60.0%) were ≥65 years of age, and 13 (52.0%) were males (Table 1 ). Fourteen (56.0%) cases were inpatients, 9 (36.0%) were commercial or family caregivers, and 2 (8.0%) were hospital employees (a nurse and engineer; Figure 1 ). There was no infected case among the healthcare workers involved in the bronchoscopy procedure. The median (IQR) incubation period was 8 days (IQR, 6.5-10.5 days) in hospital A and 4 days (IQR, 3-8 days) in hospital B. The cases whose infector was the index case had a higher CFR (52.4%) than other cases (0%). The cases who stayed in the same room as the index case had a higher CFR (58.3%; 50.0% in hospital A and 62.5% in hospital B) than other cases (30.8%), albeit not significantly so (Table 1) (Figure 1).
Table 1.
Relative risk of fatality according to epidemiologic characteristics among incident cases of Middle East respiratory syndrome in Daejeon, Korea.
| Case (N) | Death (n) | CFR (%)* | RR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Total | 25 | 11 | 44.0 | – | – |
| Hospital | |||||
| B | 11 | 7 | 63.6 | 2.23 (0.87–5.71) | 0.095 |
| A | 14 | 4 | 28.6 | Ref | |
| Age, years | |||||
| ≥65 | 15 | 8 | 53.3 | 1.78 (0.62–5.12) | 0.287 |
| 30-64 | 10 | 3 | 30.0 | Ref | |
| Sex | |||||
| Male | 13 | 9 | 69.2 | 4.15 (1.11–15.49) | 0.034 |
| Female | 12 | 2 | 16.7 | Ref | |
| Role | |||||
| Inpatient | 14 | 9 | 64.3 | 3.54 (0.95–13.14) | 0.059 |
| Others | 11 | 2 | 18.2 | Ref | |
| Infector | |||||
| Index case | 21 | 11 | 52.4 | 5.23 (0.37–74.71) | 0.223 |
| Others | 4 | 0 | 0 | Ref | |
| Stayed in the same room as the index case | |||||
| Yes | 12 | 7 | 58.3 | 1.90 (0.74–4.88) | 0.185 |
| No | 13 | 4 | 30.8 | Ref | |
| Smoking | |||||
| Current or ex-smoker | 8 | 6 | 75.0 | 2.55 (1.10–5.90) | 0.029 |
| Never-smoker | 17 | 5 | 29.4 | Ref | |
| Pre-existing pneumonia | |||||
| Yes | 4 | 4 | 100.0 | 3.00 (1.64–5.49) | <0.001 |
| No | 21 | 7 | 33.3 | Ref | |
| Chronic lung disease† | |||||
| Yes | 4 | 3 | 75.0 | 1.97 (0.90–4.32) | 0.091 |
| No | 21 | 8 | 38.1 | Ref | |
CFR (%) = case fatality rate (%) = (n/ N) × 100.
Asthma, chronic obstructive pulmonary disease, and idiopathic pulmonary fibrosis. RR = relative risk. CI = confidence interval. Ref = reference group.
Figure 1.
Location of the index case and subsequent fatal and non-fatal cases in hospitals A and B. The index case stayed in hospital A during 22–28 May 2015 and then in hospital B during 28–30 May 2015. (A) In hospital A, an engineer was presumed to be infected by the index case on a different floor; the location of his exposure is unclear. He survived. (B) In hospital B, one case (a family caregiver) was presumed to be infected by the index case in the emergency room (ER). He did not survive. Another case (a nurse) was infected by the fatal inpatient case in the isolation room of the intensive care unit (ICU). She survived.
Environmental conditions in hospitals A and B
The CFR in hospital B was higher than that of hospital A (63.6% vs. 28.6%, respectively) (Table 1). The differences between the two hospitals are described below.
First, the ward (ward 51) of hospital A in which the outbreak occurred was for patients who required chronic or long-term care and patients undergoing rehabilitation therapy. In contrast, hospital B was an acute-care university hospital. Moreover, the ward (ward 101) of hospital B in which the outbreak occurred was focused on pulmonary diseases.
Second, the ventilation systems of the two hospitals were different. Hospital A had a central ventilation system that circulated air through all wards, including the room occupied by the index case, via a duct system. The system underwent at least 6 air changes per hour (ACH) in the inpatient rooms of ward 51. In contrast, hospital B had no mechanical ventilation system in inpatient rooms; the central ventilation system of hospital B circulated air in the corridor and common room of ward 101, but it was not connected to the inpatient rooms. The bronchoscopy room in hospital B had two local ventilation machines to emit the room air through the ducts to the outside of the window: one delivered 5.67 ACH, and the other delivered 7.05 ACH.
Third, the density of inpatients in the room occupied by the index case was different between the two hospitals. The index case room in hospital A had four beds in an area of 35.3 m2 (or 8.8 m2 per bed), with a distance of ∼1.5 m between adjacent beds. However, the room occupied by the index case in hospital B had six beds in an area of 34.2 m2 (5.7 m2 per bed), with a distance of ∼0.6 m between adjacent beds.
Case fatality rates by host factors
CFR was compared according to sex, age, role in the hospital, comorbidities, pre-existing pneumonia, smoking history, symptoms and laboratory results.
First, the male MERS cases had a higher CFR than the female cases did (RR 4.15 [95% CI 1.11–15.49], p = 0.034), and inpatients who had been infected with MERS during hospitalization had a higher CFR than the MERS cases who were hospital workers, such as caregivers, nurses, and engineers, although the significance was marginal (RR 3.54 [95% CI 0.95–13.14], p = 0.059) (Table 1).
Second, smoking history (current or ex-smoker) was associated with the CFR (RR 2.55 [95% CI 1.10–5.90], p = 0.029). Pre-existing pneumonia was also significantly associated with the CFR (RR 3.0 [95% CI 1.64–5.49], p < 0.001) (Table 1).
Third, cases with incubation periods < 5 days had higher CFRs (RR 3.03 [95% CI 1.04–8.86], p = 0.043). Patients with a peak body temperatures >37.5 °C also had elevated CFRs, although this finding was not statistically significant (RR 6.57 [95% CI 0.45–96.05], p = 0.169). Cases with dyspnea or sputum had a higher CFR than those without either (RR 2.29 [95% CI 1.09–4.79], p = 0.029; RR 3.33 [95% CI 1.71–6.51], p < 0.001, respectively) (Table 2 ).
Table 2.
Relative risk of fatality according to incubation period, body temperature and symptoms among incident cases of Middle East respiratory syndrome in Daejeon, Korea.
| Case (N) | Death (n) | CFR* | RR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Incubation period | |||||
| <5 days | 10 | 7 | 70.0 | 3.03 (1.04–8.86) | 0.043 |
| ≥5 days | 13 | 3 | 23.1 | Ref | |
| Peak body temperature | |||||
| >37.5 °C | 20 | 11 | 55.0 | 6.57 (0.45–96.05) | 0.169 |
| ≤37.5 °C | 5 | 0 | 0 | Ref | |
| Fever/chills | |||||
| yes | 25 | 11 | 44.0 | 0.88 (0.12–6.58) | 0.905 |
| no | 0 | 0 | – | Ref | |
| Cough | |||||
| yes | 7 | 4 | 57.1 | 1.47 (0.62–3.49) | 0.383 |
| no | 18 | 7 | 38.9 | Ref | |
| Sputum | |||||
| yes | 5 | 5 | 100.0 | 3.33 (1.71–6.51) | <0.001 |
| no | 20 | 6 | 30.0 | Ref | |
| Dyspnea | |||||
| yes | 5 | 4 | 80.0 | 2.29 (1.09–4.79) | 0.029 |
| no | 20 | 7 | 35.0 | Ref | |
| Myalgia | |||||
| Yes | 9 | 3 | 33.3 | 0.67 (0.23–1.90) | 0.447 |
| no | 16 | 8 | 50.0 | Ref | |
| Headache | |||||
| yes | 5 | 1 | 20.0 | 0.40 (0.07–2.44) | 0.320 |
| no | 20 | 10 | 50.0 | Ref | |
| GI symptoms† | |||||
| yes | 8 | 3 | 37.5 | 0.80 (0.29–2.23) | 0.665 |
| no | 17 | 8 | 47.1 | Ref | |
| Sore throat | |||||
| yes | 3 | 0 | 0 | 0.25 (0.02–3.45) | 0.301 |
| no | 22 | 11 | 50.0 | Ref | |
CFR (%) = case fatality rate (%) = (n/ N) × 100.
Nausea, vomiting, diarrhea, gastric discomfort, and loss of appetite. RR = relative risk. CI = confidence interval. Ref = reference group.
Fourth, in terms of laboratory examinations, when leukocytosis was defined as a WBC level >10,000/mm3, four patients had leukocytosis and all died (RR 2.67 [95% CI 1.42–5.02], p = 0.002). However, lymphopenia (lymphocyte < 1,000/mm3) was not associated with increased CFR. Elevated blood urea nitrogen (BUN) and creatinine (Cr) levels were also associated with increased CFR (RR 2.25 [95% CI 1.34–3.77], p = 0.002; RR 2.11 [95% CI 1.31–3.39], p = 0.002, respectively) (Table 3 ). Cases with abnormal renal function had no history of chronic kidney disease. No difference in CFR was observed between higher (Ct ≤ 26) and lower virus load groups (Supplementary Table 1).
Table 3.
Relative risk of fatality by the initial laboratory results among incident cases of Middle East respiratory syndrome in Daejeon, Korea.
| Laboratory results | Case (N) | Death (n) | CFR* | RR (95% CI) | p-value |
|---|---|---|---|---|---|
| White blood cell count | |||||
| Leukocytosis (>10,000 cells/mm^3) | 4 | 4 | 100.0 | 2.67 (1.42–5.02) | 0.002 |
| Others | 16 | 6 | 37.5 | Ref | |
| Missing | 5 | ||||
| Lymphocyte count | |||||
| Lymphopenia (<1,000 cells/mm^3) | 9 | 5 | 55.6 | 1.22 (0.51–2.92) | 0.652 |
| Others | 11 | 5 | 45.5 | Ref | |
| Missing | 5 | 1 | |||
| Hemoglobin | |||||
| Decreased (male: <13 g/dL, female: <12 g/dL) | 10 | 7 | 70.0 | 2.33 (0.83–6.54) | 0.107 |
| Others | 10 | 3 | 30.0 | Ref | |
| Missing | 5 | ||||
| Platelet | |||||
| Decreased (<130 × 106 cells/mm^3) | 3 | 2 | 66.7 | 1.42 (0.55–3.65) | 0.470 |
| Others | 17 | 8 | 47.1 | Ref | |
| Missing | 5 | ||||
| Albumin | |||||
| Decreased (<3.3 g/dL) | 7 | 5 | 71.4 | 1.71 (0.76–3.88) | 0.196 |
| Others | 12 | 5 | 41.7 | Ref | |
| Missing | 6 | ||||
| Blood urea nitrogen | |||||
| Elevated (>26 mg/dL) | 2 | 2 | 100.0 | 2.25 (1.34–3.77) | 0.002 |
| Others | 18 | 8 | 44.4 | Ref | |
| Missing | 5 | ||||
| Creatinine | |||||
| Elevated (>1.4 mg/dL) | 1 | 1 | 100.0 | 2.11 (1.31–3.39) | 0.002 |
| Others | 19 | 9 | 47.4 | Ref | |
| Missing | 5 | ||||
| C-reactive protein | |||||
| Elevated (≥0.5 mg/dL) | 12 | 6 | 50.0 | 0.83 (0.33–2.08) | 0.695 |
| Others | 5 | 3 | 60.0 | Ref | |
| Missing | 8 | ||||
CFR(%) = case fatality rate (%) = (n/ N) × 100. RR = relative risk. CI = confidence interval. Ref = reference group.
Discussion
Korea was first affected by MERS-CoV in 2015. Therefore, the Korean population had no immunity against MERS-CoV. Fortunately, the CFR of the outbreak in Korea was lower than the total CFR globally (20.4% vs. 35.4%).1, 6 However, the CFR of hospital B was the highest in Korea and was similar to that of a nosocomial outbreak in Al hasa, Saudi Arabia, in 2013.7 The CFR of hospital A was about half that of hospital B, although the index case was the same. This suggests that the CFR can vary markedly by the outbreak hospital.
The MERS outbreak in Daejeon was nosocomial, which may suggest that hospital factors may have played a role in the high-level fatality rate in hospital B. Hospital factors such as a high prevalence of comorbid conditions among inpatients and the specific hospital environment may increase the CFR.8 The underlying diseases among inpatients influenced CFR, as the CFR was higher in the acute care pulmonary ward in hospital B compared to the chronic care patients in hospital A. Additionally, certain aspects of the hospital environment may affect the CFR. Compared to hospital A, the higher density of inpatients and lower air ventilation in the room occupied by the index case in hospital B could have increased exposure to MERS-CoV, which may also explain in part the higher CFR in hospital B.
The index case may be another important factor to consider since the patient had aggravated pneumonia and visited hospital B without communication between the two hospitals. This form of “doctor shopping” causes inpatients and caregivers to be unexpectedly exposed to high viral loads. The Ct values of the index case in hospital B indicated a high viral load status. Additionally, the jet nebulizer used in the admission room of hospital B may have increased virus transmission to surrounding patients, as was observed during the nosocomial outbreak of SARS.9 Cho et al.10showed a difference in incubation period according to the levels of MERS-CoV exposure in a super-spreading event in an ER. Virlogeux et al. reported associations between shorter incubation periods and disease severity in cases of SARS11 and MERS,12 respectively. Thus, a higher infecting dose may have led to greater viral replication, a shorter incubation period, more aggressive inflammatory responses, and more severe outcomes.12 In fact, cases in hospital B had lower mean incubation periods and higher CFRs than those in hospital A.
Pre-existing pneumonia was associated with increased CFR in this study. This is consistent with the single-center experience in Saudi Arabia in which concomitant infection was an important risk factor for mortality.13 Bacterial co-infection is common in any viral pneumonia such as influenza pneumonia,14 and pneumonia increases pulmonary permeability.15 We presume that MERS-CoV can infect more readily an injured lung and accelerate progression to systemic infection, which leads to death. For the same reason, smoking history was significantly associated with the CFR of MERS in this study.16, 17 Male gender was also a significant factor in the present study, possibly due to confounding by smoking and co-morbid conditions: males had a higher frequency of smoking history than females, and 84.6% of male cases (25.0% of female cases) were inpatients.
Symptoms and laboratory findings obtained during admission were predictive of mortality in this study. Specifically, incubation period, dyspnea and sputum, leukocytosis, and elevated BUN and Cr levels were associated with elevated CFR. As discussed above, the shorter incubation period may be associated with higher viral exposures and more aggressive clinical progression.12, 18 This suggestion is supported by the association between leukocytosis and CFR. An activated host immune response to viral infection may also increase the CFR, similar to the observed neutrophilic response in SARS cases with poor prognoses.19, 20 Moreover, since aggravated pulmonary infiltration may induce dyspnea and sputum, these symptoms may be predictive of higher CFR. On the other hand, cases with abnormal renal function had no history of chronic kidney disease in our study. Acute renal impairment during the clinical progression of MERS could predict mortality in patients with MERS-CoV,21 which was also found in SARS patients.22
The Ct values were not associated with CFR in this study, which agrees with the study of Kim et al.23 where no association was found between viral load and survival in respiratory specimens from 21 MERS-CoV patients. However, Kim et al.23 showed that blood viral RNA positivity was related to survival in the early phase of infection.
This study has several limitations. First, the number of fatal events (11 fatal cases) was insufficient to provide adequate statistical power in the multiple logistic regression analysis to adjust for age. According to the analysis of the CFR, the age-adjusted odds ratio of smoking or pre-existing pneumonia was not significant. The number of events per independent variable (EPV) usually needs to be at least 10 in a logistic regression model.24 Moreover, statistical power is generally very low even at 20 EPV.25 Second, non-fatal cases could have been missed, because serological analyses were not performed to identify such cases. Thus, missing non-fatal cases may have led to lower CFRs than presented. Third, we could not compare the level of environmental contamination with MERS-CoV between the main outbreak wards in the two hospitals because the wards had been cleaned prior to investigation of the outbreak.
In summary, the risk factors for mortality of MERS cases in Daejeon were underlying poor pulmonary function such as pre-existing pneumonia and smoking history, and exhalation of a large quantity of virus by the index case using a nebulizer in a more-crowded room with inefficient air ventilation in hospital B. Furthermore, the shorter incubation period, leukocytosis, abnormal renal function at diagnosis, and respiratory symptoms such as dyspnea and sputum were predictive of a fatal prognosis. The risk factors and predictors described above may have played an important role in MERS mortality in Daejeon.
Conflicts of interest
None to declare.
Ethical Approval
This study was approved by the Chungnam National University Hospital Institutional Review Board. The index case provided informed written consent for the publication of chest images and clinical descriptions.
Acknowledgments
We thank the epidemiological investigators, the civil servants from the Korea Centers for Disease Control and Prevention for their efforts in preventing the spread of MERS. We also thank Yu Sung Yoon, a radiologist, who reviewed the radiologic chest images of the index case. Also, we express our gratitude to Minki Sung (Department of Architectural Engineering, Sejong University, Seoul, Korea) who evaluated the ventilation systems. This work was supported by a research fund from Chungnam National University (2015-1373-01). Chungnam National University had no involvement in the study.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijid.2017.02.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Korea Centers for Disease Control and Prevention Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6:269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37:e2015033. doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K.M., Ki M., Cho S.I., Sung M., Hong J.K., Cheong H.K. Epidemiologic features of the first MERS outbreak in Korea: focus on Pyeongtaek St. Mary's Hospital. Epidemiol Health. 2015;37:e2015041. doi: 10.4178/epih/e2015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feikin D.R., Alraddadi B., Qutub M., Shabouni O., Curns A., Oboho I.K. Association of Higher MERS-CoV Virus Load with Severe Disease and Death, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21:2029–2035. doi: 10.3201/eid2111.150764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shehata M.M., Gomaa M.R., Ali M.A., Kayali G. Middle East respiratory syndrome coronavirus: a comprehensive review. Front Med. 2016;10:120–136. doi: 10.1007/s11684-016-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maltezou H.C., Tsiodras S. Middle East respiratory syndrome coronavirus: implications for health care facilities. Am J Infect Control. 2014;42:1261–1265. doi: 10.1016/j.ajic.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong R.S., Hui D.S. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10:339–341. doi: 10.3201/eid1002.030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho S.Y., Kang J.M., Ha Y.E., Park G.E., Lee J.Y., Ko J.H. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virlogeux V., Fang V.J., Wu J.T., Ho L.M., Peiris J.S., Leung G.M. Brief Report: Incubation Period Duration and Severity of Clinical Disease Following Severe Acute Respiratory Syndrome Coronavirus Infection. Epidemiology. 2015;26:666–669. doi: 10.1097/EDE.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virlogeux V., Park M., Wu J.T., Cowling B.J. Association between Severity of MERS-CoV Infection and Incubation Period. Emerging Infectious Disease journal. 2016;22:526. doi: 10.3201/eid2203.151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cilloniz C., Ewig S., Menendez R., Ferrer M., Polverino E., Reyes S. Bacterial co-infection with H1N1 infection in patients admitted with community acquired pneumonia. J Infect. 2012;65:223–230. doi: 10.1016/j.jinf.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 16.Jones J.G., Minty B.D., Lawler P., Hulands G., Crawley J.C., Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1:66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 17.Kang M.J., Lee C.G., Lee J.Y., Dela Cruz C.S., Chen Z.J., Enelow R. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho M.S., Chen W.J., Chen H.Y., Lin S.F., Wang M.C., Di J. Neutralizing antibody response and SARS severity. Emerg Infect Dis. 2005;11:1730–1737. doi: 10.3201/eid1111.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsui P.T., Kwok M.L., Yuen H., Lai S.T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha R.H., Joh J.S., Jeong I., Lee J.Y., Shin H.S., Kim G. Renal Complications and Their Prognosis in Korean Patients with Middle East Respiratory Syndrome-Coronavirus from the Central MERS-CoV Designated Hospital. J Korean Med Sci. 2015;30:1807–1814. doi: 10.3346/jkms.2015.30.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S.Y., Park S.J., Cho S.Y., Cha R.H., Jee H.G., Kim G. Viral RNA in Blood as Indicator of Severe Outcome in Middle East Respiratory Syndrome Coronavirus Infection. Emerg Infect Dis. 2016;22:1813–1816. doi: 10.3201/eid2210.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 25.Courvoisier D.S., Combescure C., Agoritsas T., Gayet-Ageron A., Perneger T.V. Performance of logistic regression modeling: beyond the number of events per variable, the role of data structure. J Clin Epidemiol. 2011;64:993–1000. doi: 10.1016/j.jclinepi.2010.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.