Abstract
Objectives
The present study provides a comprehensive review of the recently published data on RSV epidemiology in adults and the elderly in Latin America.
Methods
A systematic literature search was carried out in Medline, Scielo, Lilacs, and Cochrane Library. The search strategy aimed at retrieving studies focusing on RSV prevalence, burden, risk factors, and the routine clinical practice in the prevention and management of RSV infections in Latin American countries. Only articles published between January 2011 and December 2017 were considered.
Results
Eighteen studies were included. Percentages of RSV detection varied highly across included studies for adult subjects with respiratory infections (0% to 77.9%), influenza-like illness (1.0% to 16.4%) and community-acquired pneumonia (1.3% to 13.5%). Considerable percentages of hospitalization were reported for RSV-infected adults with influenza-like illness (40.9% and 69.9%) and community-acquired pneumonia (91.7%).
Conclusions
Recent RSV data regarding adult populations in Latin America are scarce. RSV was documented as a cause of illness in adults and the elderly, being identified in patients with acute respiratory infections, influenza-like illness and community-acquired pneumonia. The studies suggest that RSV infections may be a significant cause of hospitalization in adult populations in Latin America, including younger adults.
Keywords: Respiratory Syncytial Virus, Burden, Adults, Latin America, Upper respiratory tract infections
Introduction
Respiratory Syncytial Virus (RSV) was first identified in chimpanzees in 1955 and, shortly after, recognized as a cause of viral bronchiolitis in human infants (Hall, 2001, Falsey et al., 2005, Schweitzer and Justice, 2018). Since then, RSV has been widely acknowledged as a major pathogen in newborn infants and young children (Moore et al., 2014, Piedimonte and Perez, 2014, Gökçe et al., 2017), being associated with severe forms of disease.
Though overshadowed by its preeminent importance among pediatric populations for several decades since its recognition as a human pathogen (Walsh and Falsey 2012), RSV has become an increasingly documented cause of illness in adults. In fact, several studies exploring and reporting the burden and clinical impact of RSV among adult populations across various geographical regions, in both outpatient and inpatient settings, have been published in the last two decades (Falsey et al., 2005, Lee et al., 2013, Volling et al., 2014, Mullooly et al., 2007, Thompson et al., 2003), including in recent years (Colosia et al., 2017, Ambrosch et al., 2018, Belongia et al., 2018, Kestler et al., 2018, Ackerson et al., 2019). RSV infections have been shown to be more severe among immunocompromised adults, those with cardiopulmonary conditions, and the elderly (Volling et al., 2014, Colosia et al., 2017, Belongia et al., 2018, Chatzis et al., 2018). Notably, various studies have suggested that the disease burden associated with RSV infections in the adult population is higher than that resulting from influenza virus infections, including in older adults (Kwon et al., 2017, Ambrosch et al., 2018, Kestler et al., 2018, Ackerson et al., 2019).
Notwithstanding the growing body of literature documenting the impact of RSV among adults, barriers to the identification of RSV in this population may result in an underappreciation of the real virus burden. In adults, the clinical features of RSV infection are non-specific, making its distinction from other frequent respiratory viral infections challenging (Walsh et al., 2007, Walsh and Falsey, 2012). As a result, laboratory confirmation is needed to confidently establish an RSV infection diagnosis (Walsh and Falsey 2012). Even still, compared to infants and young children, virus titers in respiratory secretions are lower and the shedding duration is shorter, further increasing the difficulty of RSV identification in adults (Hall, 1978, Falsey and Walsh, 2000, Walsh and Falsey, 2012). Optimal diagnosis requires Reverse Transcription Polymerase Chain Reaction (RT-PCR) assays or serology, while antigen detection with immunofluorescent tests, enzyme immunoassays and cell culture are considered to be of low sensitivity in adults (Walsh and Falsey 2012).
A systematic review and meta-analysis on the burden of RSV infection in Latin America was published in 2014 (Bardach et al., 2014). The results of this study clearly show the burden of RSV among infants and young children, as a high pooled percentage (41.5%) of RSV in lower respiratory tract infections was found in those aged 0–11 months. In the elderly (≥60 years), a considerable percentage of 12.6% has also been reported (Bardach et al., 2014).
Nonetheless, only a small number of included articles in this review reported data exclusively for adult patients, with the majority being conducted in pediatric populations or presenting data for all age groups combined. Thus, the present study aimed at providing a comprehensive review of the available published data on RSV epidemiology in Latin America, focusing exclusively on the adult and elderly populations. Additionally, we also aimed at evaluating the burden and healthcare resources utilization associated with RSV, as well as the risk factors and routine clinical practice in the management of the RSV infection.
Methods
Literature search
A systematic literature search was carried out in Medline, Scielo, Lilacs, and Cochrane Library, in January 2018. Search terms aimed at retrieving studies focusing on RSV prevalence, burden, risk factors, and the routine clinical practice in the prevention and management of RSV infections in Latin America. Though we intended to focus solely on adult populations (≥18 years), we opted not to limit the search strategy to retrieve studies with a reference to “adults” or similar terms only. The identification of the articles reporting data for adult populations was performed during the full-text review. This was intended to retrieve the maximum number of articles possible and to avoid the unintentional exclusion of articles reporting data for subsets of adult subjects, but with no reference to the term “adults”. The reference lists of selected articles were searched for additional studies.
Criteria to select studies for this review
The criteria considered to select studies for the review were: 1) studies reporting any pre-defined outcome of interest for RSV (prevalence, burden, risk factors, and routine clinical practice); 2) studies reporting outcome data for at least a subset of adult subjects (≥18 years); 3) studies reporting data for any Latin American countries. No restriction was made regarding study type. The systematic review and meta-analysis on RSV burden and epidemiology in Latin America published in 2014 (Bardach et al., 2014) considered studies published or reported until December 2010. To avoid overlap, and to focus on recent studies only, our search was limited to studies published since January 1st, 2011. Lastly, only articles in English, Spanish, or Portuguese were included.
Two independent reviewers evaluated the title and abstract of studies identified during the literature search. Full-text versions were obtained for all articles complying with pre-specified criteria, which were in turn assessed for eligibility. Articles not complying with selection criteria were excluded. Two reviewers discussed the results of the selection process, with impasses being resolved by a third reviewer.
Outcome measures
The following RSV-related outcomes were considered: 1) prevalence, considered as the primary outcome; 2) burden (incidence, mortality, hospital admissions, length of stay, use of healthcare resources, absenteeism, presenteeism); 3) risk factors; 4) RSV management (prevention and treatments used in routine clinical practice).
Data extraction process
Data from selected studies were extracted into an electronic data extraction form. These included the primary author, year of publication, study period, study type, country, number and characteristics (when available) of adult subjects included, means of RSV diagnosis, presenting condition, and outcomes of interest.
Risk of bias of individual studies
The quality of included studies was assessed based on four predefined criteria: a) means of RSV diagnosis; b) study duration; c) clear description of selection criteria; and d) statement of conflicts of interest presented. The first two criteria (a and b) are particularly likely to influence the prevalence of RSV reported by included studies, which was considered as the primary outcome of this review. Thus, these were considered as major criteria for the assessment of the studies’ risk of bias.
Distinct diagnostic methods are associated with different sensitivities for RSV detection. RT-PCR assays or serology have been considered necessary for an optimal RSV diagnosis, while antigen detection with an immunofluorescent test, enzyme immunoassays and cell culture have been considered insensitive in adults (due to the low levels of virus shed and instability of viable virus) (Walsh and Falsey 2012). On the other hand, short studies conducted during the RSV epidemic season in a particular country have the potential to introduce bias, given that they will likely report a considerably higher RSV detection than studies lasting at least a year. The same is true for short studies conducted during non-epidemic seasons, with a considerably lower percentage of RSV detection expected.
Thus, studies not using RT-PCR or serology to detect RSV and those with durations of less than one year were considered as having a high risk of bias. Studies using RT-PCR or serology and lasting at least one year were considered as having an intermediate risk of bias if they did not clearly describe the selection criteria or if no conflicts of interest statement was presented. Otherwise, a low risk of bias was assumed.
Results
Study selection
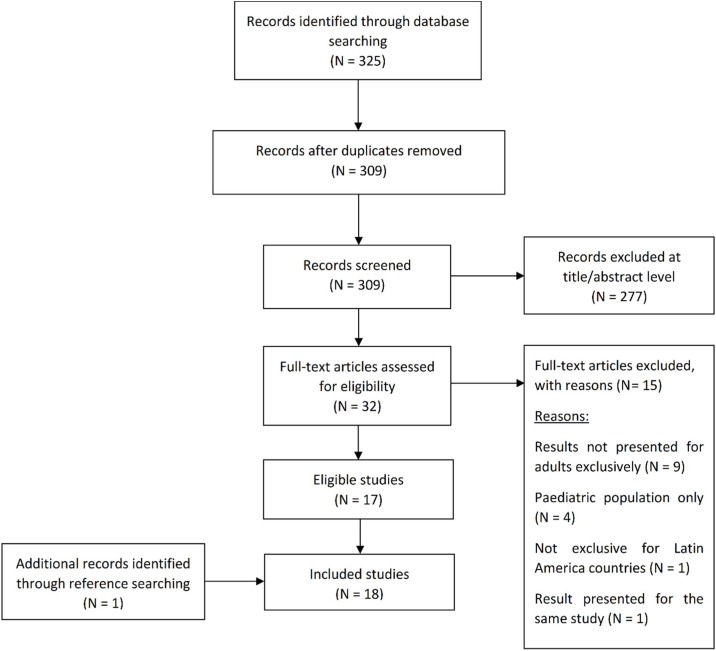
A total of 325 studies were retrieved from the literature search. Sixteen records were duplicates and were removed. Following the initial assessment based on the title and abstract of retrieved studies, 32 articles potentially met eligibility criteria. Review of full-text versions resulted in the exclusion of 15 articles (reasons for exclusion are presented in Figure 1 ). The hand-searching of the reference lists of included articles yielded one additional study, resulting in 18 included studies.
Figure 1.
Flow diagram of literature search: Modified from the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement flow diagram (N being the number of studies).
Figure 1 Flow diagram of literature search: Modified from the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement flow diagram (Moher et al., 2009). N, Number of studies.
Overall, the studies reported data for six Latin American countries: Mexico (five studies), Brazil (four studies), Chile (four studies), Venezuela (three studies), Colombia (one study) and Guatemala (one study).
Risk of bias
Of the included studies, five carry a high-risk of bias, four an intermediate risk of bias, and nine a low risk of bias (Table 1 ).
Table 1.
Risk of bias of included studies.
| Study | RT-PCR or serology used for RSV detection | Study duration of at least 1 year | Clear description of selection criteria | Conflicts of interest statement | Risk of bias |
|---|---|---|---|---|---|
| Fernandes-Matano et al. (2017) | Yes | No | Yes | No | High |
| Gamiño-Arroyo et al. (2017) | Yes | Yes | Yes | Yes | Low |
| Peñafiel et al. (2016) | Yes | Yes | Yes | No | Intermediate |
| Monteiro et al. (2016) | Yes | Yes | Yes | No | Intermediate |
| Falsey et al. (2014) | Yes | Yes | Yes | Yes | Low |
| de Lima et al. (2014) | Yes | Yes | Yes | Yes | Low |
| Bermúdez et al. (2014) | No | Yes | Yes | No | High |
| Ramirez et al. (2014) | Yes | Yes | Yes | Yes | Low |
| Riquelme et al., 2014 | No | No | Yes | No | High |
| Freitas (2013) | No | Yes | Yes | Yes | High |
| McCracken et al. (2013) | Yes | Yes | Yes | Yes | Low |
| Galindo-Fraga et al. (2013) | Yes | Yes | Yes | Yes | Low |
| Comach et al. (2012) | Yes | Yes | Yes | No | Intermediate |
| Luchsinger et al. (2012) | Yes | Yes | Yes | Yes | Low |
| Rioseco et al. (2012) | No | No | Yes | No | High |
| Zambrano et al. 2012 | Yes | Yes | Yes | No | Intermediate |
| Watanabe et al. 2011 | Yes | Yes | Yes | Yes | Low |
| Comas-García et al. (2011) | NA | NA | Yes | Yes | Low |
RT-PCR, Reverse Transcription Polymerase Chain Reaction.
NA, not applicable.
Outcomes of interest
Seventeen out of the 18 included studies reported the percentage of RSV detection in adult patients in Latin America. The studies included patients presenting with different conditions: five studies included patients with respiratory infections, six included patients with Influenza-like Illness (ILI), three included patients with Community-acquired Pneumonia (CAP), and patients with Severe Acute Respiratory Syndrome (SARS), Severe Acute Respiratory Infections (SARI), and transplant patients with respiratory symptoms were recruited in the remaining three studies. The case definitions used by the included studies to define and select eligible patients with the conditions of interest are summarized in Table 2 (for respiratory infections), Table 3 (for ILI), Table 4 (for CAP) and Table 5 (for the remaining conditions). Data on RSV-associated hospitalization and/or mortality were found in three studies. Two studies reported on the treatments used by RSV-infected adult patients.
Table 2.
Case-definitions for respiratory infections across included studies.
| Study | Case-definition |
|---|---|
| Bermúdez et al. (2014) | Respiratory infections: patients with symptomatology compatible with respiratory infections. |
| Ramirez et al. (2014) | Acute respiratory infections: patients with respiratory symptoms such as cough, sore throat, rhinorrhea and general feeling of discomfort. |
| McCracken et al. (2013) | Acute respiratory infections: For hospital patients: 1) At least one sign of acute infection (≥38 °C, temperature <35.5 °C, abnormal white blood cell [WBC] count [age ≥5 years, WBC count of < 3000 or >11 000 cells/μL], or abnormal differential) and 2) at least 1 of the following respiratory signs or symptoms: rapid breathing, cough, sputum production, pleuritic chest pain, hemoptysis, dyspnea, sore throat. For clinic patients: 1) Documented fever >38 °C and (2) cough or sore throat. |
| Zambrano et al. (2012) | Respiratory infections: not available. |
| Watanabe et al. (2011) | Acute respiratory viral infections: patients presenting acute respiratory symptoms |
Table 3.
Case-definitions for ILI across included studies.
| Study | Case-definition |
|---|---|
| Fernandes-Matano et al. (2017) | Fever greater than or equal to 38 °C, a cough, and cephalalgia accompanied by one or more of the following symptoms: rhinorrhoea, coryza, arthralgias, myalgias, prostration, odynophagia, thoracic pain, abdominal pain, or nasal congestion. In patients older than 65 years, fever was not required as a cardinal symptom. |
| Gamiño-Arroyo et al. (2017) | Presence of at least one respiratory symptom (e.g., shortness of breath, nasal congestion, and cough) and one of the two following criteria: (i) fever ≥38 °C on examination, or self-reported fever, or feverishness in the past 24 hours; (ii) one or more non-respiratory symptoms (e.g., malaise, headache, myalgia, or chest pain). Patients who had been hospitalized for more than 48 hours at the time of symptom onset were excluded. |
| Falsey et al. (2014) | Simultaneous occurrence of at least 1 respiratory symptom (nasal congestion, sore throat, new/worsening cough, dyspnea, sputum production, or wheezing) and one systemic symptom (headache, fatigue, myalgia, feverishness [feeling hot or cold, having chills or rigors], fever [oral temperature of ≥37.5 °C]). |
| Riquelme et al. (2014) | Axillary temperature ≥38.5 °C, cough and other symptoms such as myalgia, odynophagia or headache. |
| Galindo-Fraga et al. (2013) | Presence of at least one respiratory symptom (e.g., shortness of breath, postnasal drip, and cough) and one of the two following criteria: (1) fever (≥38 °C by any method) on examination, or participant-reported fever (≥38 °C), or feverishness in the past 24 h; (2) one or more non-respiratory symptoms (e.g., malaise, headache, myalgia, and chest pain). |
| Freitas (2013) | A case of fever accompanied by cough or sore throat with no other diagnosis. |
| Comach et al. (2012) | Sudden onset of fever (≥38 °C) and either cough or sore throat for less than five days in duration, with or without general symptoms such as myalgias, prostration, headache, or malaise. |
Table 4.
Case-definitions for CAP across included studies.
| Study | Case-definition |
|---|---|
| Peñafiel et al. (2016) | Infiltrate in a chest X-ray taken on admission and presence of one or more major findings (cough, mucopurulent or hemoptic expectoration, axillary temperature over 37.8 °C), or at least two minor findings (pleuritic chest pain, dyspnea, decreased level of consciousness, lung tissue condensation observed in the physical examination, or a while blood count over 12 000/mL). |
| Luchsinger et al. (2012) | Presence of acute respiratory symptoms for 1 week and a chest radiograph displaying new pulmonary infiltrates. |
| Rioseco et al. (2012) | Infiltrate in a chest X-ray taken on admission and presence of one or more major findings (cough, mucopurulent or hemoptic expectoration, axillary temperature over 37.8 °C), or at least two minor findings (pleuritic chest pain, dyspnea, decreased level of consciousness, lung tissue condensation observed in the physical examination, or a while blood count over 12 000/mL). |
Table 5.
Case-definitions for other conditions across included studies
| Study | Case-definition |
|---|---|
| Pañafiel et al. (2016) | Severe acute respiratory infection: a person of any age who presents difficulty breathing accompanied by a fever greater than or equal to 38 °C and a cough with one or more of the following symptoms: poor general condition, thoracic pain, polypnoea or acute respiratory distress syndrome (ARDS) or any death associated with ILI or SARI. |
| Luchsinger et al. (2012) | Serious Acute Respiratory Syndrome: patients hospitalized with ILI* of any age group and with signs/symptoms of dyspnea, oxygen saturation below 95% or respiratory distress. *ILI: sudden fever, even if it is self-reported, and cough or sore throat and at least one of the following symptoms in the absence of specific diagnosis: headache, myalgia or arthralgia. |
| Rioseco et al. (2012) | Transplant patients with fever, cough, and headache, plus at least one of the following symptoms: rhinorrhea, coryza, arthralgia, myalgia, prostration, sore throat, chest pain, abdominal pain, or nasal congestion. |
The sections below describe the results found in the included studies, per outcome of interest (RSV detection, hospitalizations and mortality, and treatments used).
RSV detection
The main characteristics and the percentages of RSV detection reported for adult patients with respiratory infections, ILI and CAP are summarized in Table 6, Table 7, Table 8 respectively. Studies describing RSV identification in patients with other conditions are presented in Table 9 .
Table 6.
Summary of studies reporting RSV detection among patients presenting with respiratory infections.
| Author, Year | Condition | Country | Study period | Patient status at enrollment | Number and characteristics of included adult subjects | Means of RSV diagnosis | RSV detection |
|---|---|---|---|---|---|---|---|
| Bermúdez et al. (2014) | Respiratory Infections | Venezuela | January 2005–December 2010 | Ambulatory | 119 patients ≥20 years | IFI |
Total: 16.8% (20/119) Per age sub-group: 20–40 years: 7.6% (9/119) 21–64 years: 7.6% (9/119) ≥65 years: 1.7% (2/119) |
| Ramirez et al. (2014) | Acute Respiratory Infections | Colombia | 2000–2011 | Not availablea |
Total: 3275 patients ≥31 years Per age sub-group: 31–65 years: 2434 ≥65 years: 841 Gender (male): 31–65 years: 42.0% ≥65 years: 48.3% |
IFA and RT-PCR |
Total: 31.7% (1038/3275) Per age sub-group: 31–65 years: 30.2% (737/2434) ≥65 years: 35.8% (301/841) |
| McCracken et al. (2013) | Acute Respiratory Infections | Guatemala | November 2007–December 2012 | Hospitalized |
Total: 1748 patients ≥18 years Per age sub-group: 18–49 years: 660 50–64 years: 422 ≥65 years: 666 |
Real-time RT-PCR |
Total: 5.6% (98/1748) Per age sub-group: 18–49 years: 5.0% (33/660) 50–64 years: 6.4% (27/422) ≥65 years: 5.7% (38/666) |
| McCracken et al. (2013) | Acute Respiratory Infections | Guatemala | November 2007–December 2012 | Ambulatory |
Total: 331 patients ≥18 years Per age sub-group: 18–49 years: 289 50–64 years: 30 ≥65 years: 12 |
Real-time RT-PCR |
Total: 5.4% (18/331) Per age sub-group: 18–49 years: 5.5% (16/289) 50–64 years: 3.3% (1/30) ≥65 years: 8.3% (1/12) |
| Zambrano et al. (2012) | Respiratory Infections | Venezuela | June 2008– June 2010 |
Not available | 154 patients ≥20 years | IFI |
Total: 77.9% (120/154) Per age sub-group: 20–40 years: 32.5% (50/154) 41–64 years: 35.1% (54/154) ≥65 years: 10.4% (16/154) |
| Watanabe et al. (2011) | Acute Respiratory Viral Infections | Brazil | 2002–2003 | Community patients |
Total: 47 patients ≥60 years (49 samples assessed) Age: Median: 69 (60–85) years Mean: 70 (±7.16) years Gender: Male: 29.8% |
Viral isolation and RT-PCR |
0% (0/49) |
IFI, Indirect Immunofluorescence.
IFA, Indirect Fluorescence Assay.
RT-PCR, Reverse Transcription Polymerase Chain Reaction.
RSV, Respiratory Syncytial Virus.
Swabs were collected in local health centers and hospitals.
Table 7.
Summary of studies reporting RSV detection among patients presenting with community-acquired pneumonia.
| Author, Year | Country | Study period | Patient status at enrollment | Number and characteristics of included adult subjects | Means of RSV diagnosis | RSV detection |
|---|---|---|---|---|---|---|
| Peñafiel et al. (2016) (25) | Chile | July 2014–December 2015 | Hospitalized |
Total: 240 patients ≥18 years Mean age: 71.4 ± 18.0 years Gender: Male: 49.6% |
RT-PCR | 2.9% (7/240) |
| Luchsinger et al. (2012) | Chile | February 2005–December 2007 | Ambulatory |
Total: 356 patients ≥18 years Median age: RSV +: 59 (18–92) years RSV -: 64 (19–94) years Gender (male): RSV +: 24 (50%) RSV -: 142 (46.1%) |
VI, IFA, Real-time RT-PCR and Microneutralization Assay |
13.5% (48/356) |
| Rioseco et al. (2012) | Chile | April 2005– March 2006 |
Ambulatory patients admitted to the hospital |
Total: 159 adult patients Mean age: 62 ± 20 years Gender: Male: 57.9% |
DIF | 1.3% (2/159) |
RSV, Respiratory Syncytial Virus.
RT-PCR, Reverse Transcription Polymerase Chain Reaction.
VI, Viral Isolation.
IFA, Immunofluorescence Assay.
DIF, Direct Immunofluorescence.
Table 8.
Summary of studies reporting RSV detection among patients presenting with influenza-like illness.
| Author, Year | Country | Study period | Patient status at enrollment | Number and characteristics of included adult subjects | Means of RSV diagnosis | RSV detection |
|---|---|---|---|---|---|---|
| Gamiño-Arroyo et al. (2017) | México | 2010–2014 | Hospital patientsa |
Total: 3700 patients ≥18 years Per age sub-group: 18–49 years: 2680 >50 years: 1020 |
Multiplex RT-PCR |
Total: 4.6% (171/3700) Per age sub-group: 18–49 years: 4.0% (108/2680) >50 years: 6.2% (63/1020) |
| Falsey et al. (2014)b | Mexicoc | Samples collected during the 2008–2009 and 2009–2010 influenza seasons | Community-based or living in a retirement home | Influenza-vaccinated elderly patients (≥65 years) | Multiplex RT-PCR |
2% (1/50 samples) |
| Riquelme et al. (2014)d | Chile | May 2012–September 2012 | Hospitalized | 128 patients ≥65 years | DIF | 16.4% (21e/128) |
| Galindo-Fraga et al. (2013) | Mexico | April 2010– April 2011 |
Ambulatory and hospitalized |
Total: 803 patients ≥19 years Per age sub-group: 19–59 years: 614 ≥60 years: 189 Gender: Male: 38.2% Inpatient at baseline: 323 (40.2%) Outpatient at baseline: 480 (59.8%) |
Multiplex PCR |
Total: 2.9% (23/803) Per age sub-group: 18–59 years: 2.4% (15/614) ≥60 years: 4.2% (8/189) |
| Freitas (2013) | Brazil | 2000–2010 | Not availablef |
Total: 1893 samples positive for respiratory virus Per age sub-group: 25–59 years: 1665 ≥60 years: 228 |
IFI |
Total: 12.8% (242/1893) of respiratory viruses Per age sub-group: 25–59 years:12.0% (199/1665) ≥60 years:18.9% (43/228) |
| Comach et al. (2012) | Venezuela | October 2006–December 2010 | Ambulatory |
Total: 206 patients ≥30 years with respiratory febrile viral infections Per age-subgroup: 30–44 years: 156 45–59 years: 43 ≥60 years: 7 |
Viral isolation |
Total: 1.0% (2/206) Per age sub-group: 30–44 years: 1.3% (2/156) 45–59 years: 0.0% (0/43) ≥60 years: 0.0% (0/7) |
RSV, Respiratory Syncytial Virus.
PCR, Polymerase Chain Reaction.
RT-PCR, Reverse Transcription Polymerase Chain Reaction, Direct Immunofluorescence.
IFI, Indirect Immunofluorescence.
Including ambulatory and hospitalized patients for less than 48 hours at the time of symptom onset.
Only samples from episodes of moderate-to-severe ILI were selected for this study.
Study was conducted in various countries. Only data from Mexico are reported in this table.
In addition to patients with ILI, those with fever or exacerbation of any underlying disease during hospitalization without evident cause were also included.
Three cases were hospital-acquired.
Samples collected from outpatient clinics, emergency care departments and general hospitals.
Table 9.
Summary of studies reporting RSV detection among patients with other conditions.
| Author, Year | Condition | Country | Study period | Patient status at enrollment | Number and characteristics of included adult subjects | Means of RSV diagnosis | RSV detection |
|---|---|---|---|---|---|---|---|
| Fernandes-Matano et al. (2017) | ILI and SARI | Mexico | Week 40 (October) of 2014–week 39 (September) of 2015 | Hospitalized and ambulatory |
Total: 579 patients ≥20 years Per age sub-group: 20–59 years: 263 ≥60 years: 316 Gender (male): Total: 49.6% 20–59 years: 48.3% ≥60 years: 50.6% Hospitalized: Total: 92.4% 20–59 years: 86.3% ≥60 years: 97.5% Ambulatory Total: 7.6% 20–59 years: 13.7% ≥60 years: 2.5% |
RT-PCR |
Total: 2.1% (12a/579) Per age sub-group: 20–59 years: 1.5% (4/263) ≥60 years: 2.5% (8/316) |
| Monteiro et al. (2016) | SARS | Brazil | January 2011–December 2013 | Hospitalized and awaiting hospitalization |
Total: 2368 patients ≥20 years Per age sub-group: 20–39 years: 493 40–59 years: 644 >60 years: 1231 |
RT-PCR and IFI |
Total: 0.4% (10/2368) Per age sub-group: 20–39 years: 0.0% (0/493) 40–59: 0.2% (1/644) >60 years – 0.7% (9/1231) |
| de Lima et al. (2014) | Transplant patientsb | Brazil | August 2011–August 2012 | Ambulatory |
Total: 50 patients ≥21 years Median age; 50.5 (21–71) years Gender: Male: 62% |
RT-PCR |
RSV prevalence among the detected respiratory viruses: 20% |
IFI, Indirect Immunofluorescence.
RT-PCR, Reverse Transcription Polymerase Chain Reaction.
RSV, Respiratory Syncytial Virus.
ILI, Influenza-like Illness.
SARI, Severe Acute Respiratory Infection.
SARS, Severe Acute Respiratory Syndrome.
All 12 RSV infections corresponded to hospitalized patients.
Transplant patients presenting with fever, cough and headache, plus at least one of the following symptoms: rhinorrhea, coryza, arthralgia, myalgia, prostration, sore throat, chest pain, abdominal pain, or nasal congestion.
Respiratory infections
Marked differences were observed in the percentages of RSV detection among adult subjects with respiratory infections. Percentages ranged from 0% (Brazil) (Watanabe et al., 2011) to 77.9% (Venezuela) (Zambrano et al., 2012).
Influenza-like Illness
Five studies reported the percent of ILI episodes that were positive for RSV, while one study presented the proportion of RSV identification among the detected respiratory viruses. Percentages of detection ranged from 1.0% (Venezuela) to 16.4% (Mexico). Three studies found higher prevalences in older subjects (6.2% in subjects >50 years vs. 4.0% in subjects aged 18–49 years (Gamiño-Arroyo et al., 2017); 4.2% in ≥60 years vs. 2.4% in 18–59 years (Galindo-Fraga et al., 2013); and 18.9% in subjects ≥60 years vs. 12.0% in subjects aged 25–59 years) (Freitas 2013). Comach G. et al. found almost no RSV cases in the age groups between 30–44 years (1.0%), 45–59 years (0.0%) and ≥60 years (0.0%) (Comach et al., 2012).
Community-acquired pneumonia
All three studies reporting the percentage of RSV detection among subjects presenting with CAP were conducted in Chile. One study reported a considerable percentage of RSV identification (13.5%) among ambulatory patients (Luchsinger et al., 2012), while the remaining two (conducted in ambulatory patients admitted to the hospital and hospitalized patients) presented low percentages (1.3% and 2.9%, respectively) (Rioseco et al., 2012; Peñafiel et al., 2016).
Other conditions
Fernandes-Matano et al. detected RSV in 2.1% of adults aged ≥20 years with ILI or SARI, in Mexico. All RSV cases were diagnosed in hospitalized patients (Fernandes-Matano et al., 2017). Monteiro et al. reported very low percentages (0.4%) of RSV detection among patients aged ≥20 years with SARS, in Brazil (Monteiro et al., 2016). Finally, De Lima CRA et al. reported that 19 respiratory viruses were detected in transplant patients presenting with fever, cough, and headache, plus at least one of the following symptoms: rhinorrhea, coryza, arthralgia, myalgia, prostration, sore throat, chest pain, abdominal pain, or nasal congestion. RSV was the second most prevalent virus (20%), behind parainfluenza virus III (32%) (de Lima et al., 2014).
Hospitalizations and mortality
A study in Mexico with patients presenting ILI reported that 40.9% (70/171) of RSV infected adults aged ≥18 years were hospitalized. Of these, three required ICU admission. This study also showed that older patients, males, those with any underlying conditions and asthma were significantly associated with a higher hospitalization risk (Gamiño-Arroyo et al., 2017). Another study conducted in Mexico in ILI patients found that 69.9% (16/23) of adult patients ≥18 years with RSV were hospitalized, corresponding to 53.3% (8/15) of hospitalizations in those aged 18–59 years and 100% (8/8) in those ≥60 years. While no deaths were reported for the former patients, 25.0% (2/8) of the latter died (Galindo-Fraga et al., 2013). Luchsinger V. et al. reported that 91.7% and 31.3% of patients with RSV-related CAP in Chile required hospitalization and ICU admission, respectively. There were no statistically significant differences between subjects with RSV and non-RSV related CAP concerning hospitalization or ICU admission proportions. Death during or 30 days after hospitalization was reported for three subjects with RSV-related CAP (6.3%:3/48) (Luchsinger et al., 2012). A study in Mexico reported an estimated average annual RSV-associated cardiovascular and respiratory death rate of 2.37 and 108.26 per 100 000 person-years, in subjects aged 20–59 years and ≥60 years, respectively. For all cause death rate, values of 3.97 and 183.55 per 100 000 person-years were reported (Comas-García et al., 2011). In patients with ARI, a study in Guatemala reported percentages of severe outcomes among RSV-positive hospital patients of 6.2% (18–49 years), 3.1% (50–64 years) and 10.3% (≥65 years). Severe outcomes were defined as hospitalizations leading to intensive care unit admission or mechanical ventilation, discharge in moribund conditions, or death (McCracken et al., 2013).
Treatments
We found no studies describing the routine clinical practice in the prevention or treatment of RSV infections in the adult populations. However, two studies reported the treatments used by patients presenting with RSV-related ILI and CAP, before the identification of respiratory pathogens was performed.
Galindo-Fraga A. et al. reported that, before the identification of the etiological agent, 35% of RSV-infected adults presenting with ILI had used an antiviral, 65% antibiotics, 35% inhaled steroids, and 43% systemic steroids (Galindo-Fraga et al., 2013). Luchsinger V. et al. showed that 28.9% of subjects with RSV-related CAP used antibiotics before hospital admission (Luchsinger et al., 2012).
Discussion
The present study aimed at providing the current status of knowledge on RSV epidemiology in adults and the elderly in Latin America, based on recently published data. The literature search retrieved only 18 eligible studies, indicating that recent RSV data focusing exclusively on adult populations in Latin American countries are scarce.
Seventeen out of the 18 studies included in the review reported the percentage of RSV detection in adult patients presenting with various conditions (such as ARI, ILI, CAP, SARS and SARI) and in distinct settings (community, ambulatory and hospitalized). As expected, considerable variation was found in the percentages of RSV identification reported by the included studies, even when considering only patients presenting with the same condition. Several factors may have contributed to the variability observed. First, one should consider that the case definitions used to define and select eligible patients differed across the included studies (Table 2 to Table 5). As a result, some heterogeneity is to be expected, even across studies focusing on the same condition (e.g., ILI). Second, further differences between included studies should be considered, such as disparities in the characteristics of the adult populations assessed, the different periods during which the studies were conducted, and the distinct methods used for RSV detection. Third, the variability may reflect the different impact of RSV across Latin American countries. These factors should be considered when interpreting the study results.
Based on the criteria defined to assess study quality (Table 1), studies considered as having a high or intermediate risk of bias present an appreciable risk of having over or underestimated the RSV prevalence reported. This is mainly due to two factors: not having used RT-PCR or serology for RSV detection and/or having a duration lower than one year (short studies conducted within or outside RSV epidemic season will likely report a higher or lower RSV detection, respectively, than that they would have if lasting one or more years). Results from high and, to a lesser extent, intermediate risk of bias studies, should thus be interpreted with this in mind.
The included studies reported outcomes of interest for Latin American countries with distinct RSV seasons. In Chile, the RSV season generally starts in April, peaks between June and July, and drops in September (Bardach et al., 2014). In Mexico, a study conducted in patients aged 0–18 years reported that RSV infections occurred less commonly in the warmer months of the year (between March and June) and peaked in the cooler months (between August and March) (Rodríguez-Auad et al., 2012). The RSV seasonal pattern is variable across Brazilian regions, which has been attributed to differences in climate characteristics across the country. In São Paulo (Southeast region), RSV season usually peaks between autumn and winter. In the Northeast region, RSV peaks have been observed in the rainy and winter seasons Med Crave (2016). In Colombia, a pediatric study reported that most RSV infections (63.6%) occurred between March and May (Rodríguez-Martínez et al., 2015). In Guatemala, the PAHO weekly reports suggest the existence of a long RSV season, peaking at different times throughout the years. Still, based on data from 2015 to 2018, one may observe that the peak generally occurs between July and October (PAHO, 2019). This review included three studies with durations less than one year. Two of these studies (Fernandes-Matano et al., 2017; Rioseco et al., 2012) had study durations of approximately 11 months, which are not expected to have influenced the percentage of RSV detection reported. The study by Riquelme et al. (2014), however, was a five-month study conducted within the RSV season in Chile, which may have overestimated the prevalence of this virus.
Three studies reported the percent of RSV detection among subjects presenting with Acute Respiratory Infections (ARI) in Latin American countries. According to the WHO, ARI is defined as a sudden/acute onset of at least one of the following four respiratory symptoms: cough, sore throat, shortness of breath and coryza (WHO, 2017). Indeed, the presence of respiratory symptoms was part of the case-definitions for ARI of all three studies (Watanabe et al., 2011, McCracken et al., 2013, Ramirez et al., 2014). In addition to this criterion, patients also had to present fever (ambulatory setting) or at least one sign of acute infection (hospital setting) to be included in the study by McCracken et al (McCracken et al., 2013). ARIs are the leading cause of morbidity and mortality worldwide. In the adult population, these infections are the most typical cause of outpatient care. Several distinct microorganisms may cause these infections, with an estimate of up to 80% of cases being due to a viral etiological agent. Globally, influenza viruses are amongst the primary causative agents of ARI, though various other respiratory viruses (namely RSV) may cause these infections (Fernandes-Matano et al., 2017). In Colombia, RSV accounted for a considerable amount of ARI cases between 2000 and 2011, with 31.7% of infections in adults aged ≥31 years testing positive for this virus. In Guatemala, a much lower detection of RSV was reported, with similar percentages being found among hospital and clinic patients aged ≥18 years (5.6% and 5.4%, respectively). The lack of positive samples for RSV in Brazil may have resulted from the small number of samples assessed (49).
The WHO defines ILI as an ARI with measured fever of ≥38 °C, cough and an onset within the last ten days (WHO, 2014). Generally, the case-definitions for ILI of the included studies focusing on this condition differed from the one by WHO and between studies. Unlike the WHO definition, various studies did not consider cough and fever as necessary to define an ILI case. On one hand, patients could present other respiratory symptoms (namely shortness of breath, nasal congestion and sore throat) instead of cough. On the other, patients could present non-respiratory or other systemic symptoms (such as malaise, headache, myalgia and chest pain) in place of fever. Lastly, most studies did not specify a maximum time for the onset of the symptoms. Most studies focusing on ILI reported percentages of RSV detection not exceeding 5%. The highest percentage (16.4%) was found in hospitalized patients aged ≥65 years, in Chile (Riquelme et al., 2014). Still, it should be noted that this study had a short duration (May to September 2012) and was conducted during the RSV season (Bardach et al., 2014), which may explain the high percentage of RSV infections reported.
RSV has been described as one of the leading causes of CAP among adults, surpassed only by the Streptococcus pneumoniae and influenza viruses (Dowell et al., 1996). This is in agreement with one of the included studies, in which RSV infection was established in 13.5% of CAP adult patients, in Chile. Notably, RSV was the second most commonly isolated pathogen, behind Streptococcus pneumoniae (21.1%) (Luchsinger et al., 2012). Conversely, two other studies conducted in Chile have reported low percentages of RSV detection (1.3% and 2.9%) (Rioseco et al., 2012; Peñafiel et al., 2016). In one of these studies (Rioseco et al., 2012), the use of Direct Immunofluorescence (DIF) for the detection of respiratory viruses may have resulted in an underdiagnosis of RSV. Nonetheless, a similar percentage has been reported among hospitalized adults with CAP in Canada (2.6%) (Johnstone et al., 2008). Two of these three studies (Peñafiel et al., 2016; Rioseco et al., 2012) used the same CAP definition, based on the diagnosis criteria for CAP proposed by Fang et al. (Fang et al., 1990). These criteria are as follows: 1) infiltrate in a chest X-ray taken on admission and 2) presence of one or more major findings (cough, mucopurulent or hemoptic expectoration, axillary temperature over 37.8 °C), or at least two minor findings (pleuritic chest pain, dyspnea, decreased level of consciousness, lung tissue condensation observed in the physical examination, or a white blood count of over 12 000/mL). The remaining study (Luchsinger et al., 2012) used a different definition, consisting of the presence of acute respiratory symptoms for one week and a chest radiograph displaying new pulmonary radiographs.
Only five studies reported data on RSV-associated hospitalization and/or mortality in adult patients. Still, those that did show that a considerable proportion of RSV-infected adult patients (≥18 years) presenting with ILI required hospitalization in Mexico (from 40.9% to 69.9%) (Galindo-Fraga et al., 2013, Gamiño-Arroyo et al., 2017). Of note, in both studies, the majority of RSV-infected adults were aged ≤50 years (approximately 63.2%: 63/108) and <60 years (approximately 65.2%: 15/23), respectively. These results suggest that RSV infections may be a significant cause of hospitalization in the adult populations, including younger adults. Nonetheless, older patients were shown to be at an increased risk for hospitalization (Gamiño-Arroyo et al., 2017). One study reported the percentage of hospitalizations in patients with RSV-related CAP. Naturally, the percentages found were even higher (91.7%) than those reported for ILI patients (Luchsinger et al., 2012). Finally, a study in Guatemala showed a substantial burden among hospitalized adult patients with RSV, as 19.6% required intensive care admission or mechanical ventilation, were discharged in moribund conditions, or died. Once again, though the highest percentage was found in those aged ≥65 years (26.3%), a considerable percentage of severe outcomes (18.2%) was found in younger hospitalized patients (18 to 49 years) (McCracken et al., 2013).
Studies conducted in various regions, including United States of America, Canada and Hong Kong, have shown a considerable RSV burden among adult populations, particularly in high-risk individuals (Thompson et al., 2003, Falsey et al., 2005, Lee et al., 2013, Volling et al., 2014). The present review demonstrates that RSV is a cause of illness in adults and the elderly in Latin America and suggests that RSV infections may be a significant cause of hospitalization, including in younger adults. Still, the low number of included studies (reflecting the scarcity of recent RSV data on adult populations in Latin America), coupled with the high variability of RSV identification percentages across studies, make it difficult to infer the real RSV burden in this region. Thus, further studies focusing on adult populations at risk for RSV infections in Latin America, particularly conducted during RSV seasons, could prove valuable to provide a clearer and more accurate picture of the virus’ real burden.
Limitations
Our review had some limitations. First, the EMBASE database was not used in the literature search. As a result, we may have failed to identify other potentially eligible studies. Second, we excluded studies that reported outcomes of interest for RSV in Latin American countries, but that did not present them exclusively for adult populations. Third, because only a small number of included studies focused exclusively on adults, most did not characterize the adult populations assessed for RSV. Thus, we were not able to assess the differences between the adult populations evaluated in each study, which could be in part responsible for the variability observed in terms of RSV detection.
Conclusions
The present review summarizes the available data on RSV epidemiology in adults and the elderly in Latin America, published since the start of the decade. RSV was documented as a cause of illness in adults and the elderly in the included studies, being identified in patients presenting with ARI, ILI and CAP. The proportion of RSV infections among these patients was variable across included studies, even when comparing those including patients with the same condition and conducted in the same country. The included studies suggest that RSV infections may be a significant cause of hospitalization in the adult populations in Latin America, including in younger adults. In view of the scarcity of RSV data focusing exclusively on the adult population in Latin America, further studies on this matter could prove invaluable to provide a clearer understanding of this virus’ real burden.
Declaration of interest
Abraham Ali has no conflicts of interest to declare. Gustavo Lopardo participated in an Advisory Board regarding RSV promoted by Janssen. Bruno Scarpellini is a Janssen employee. Renato T. Stein participated in Advisory Boards regarding RSV promoted by Janssen, Astra Zeneca and Novavax. Diogo Ribeiro is an employee of CTI Clinical Trial & Consulting Services. This company received honoraria from the funder of this project for support in the planning, literature search, data extraction and draft of the manuscript.
Funding
This work was supported by Janssen. Janssen participated in the conduction of the systematic review, analysis and interpretation of data, writing the manuscript and in the decision to submit the article for publication.
Authorship
Bruno Scarpellini and Diogo Ribeiro contributed to the conception and design of the study and acquisition of data. All authors contributed to the analysis and interpretation of data and drafted the article and revised it critically for important intellectual content. All authors have read and approved the final manuscript to be submitted.
Ethical approval
Not required.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark.
References
- Ackerson B., Tseng H.F., Sy L.S., Solano Z., Slezak J., Luo Y., et al. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis. 2019;69(July 2):197–203. doi: 10.1093/cid/ciy991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosch A., Klinger A., Luber D., Arp C., Lepiorz M., Schroll S., et al. Clinical characteristics and course of infections by influenza a- and respiratory syncytial virus (RSV) in hospitalized adults. Dtsch Med Wochenschr 1946. 2018;143(May 9):e68–75. doi: 10.1055/s-0044-102004. [DOI] [PubMed] [Google Scholar]
- Bardach A., Rey-Ares L., Cafferata M.L., Cormick G., Romano M., Ruvinsky S., et al. Systematic review and meta-analysis of respiratory syncytial virus infection epidemiology in Latin America. Rev Med Virol. 2014;24(March 2):76–89. doi: 10.1002/rmv.1775. [DOI] [PubMed] [Google Scholar]
- Belongia E.A., King J.P., Kieke B.A., Pluta J., Al-Hilli A., Meece J.K., et al. Clinical features, severity, and incidence of RSV Illness During 12 consecutive seasons in a community cohort of adults ≥60 Years Old. Open Forum Infect Dis. 2018;5(November 12) doi: 10.1093/ofid/ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez F J.E., Gotera Z J.L., Mavares M A.R., Calles U A.D., Paredes L C.R., Pirela I D.P., et al. Detección de anticuerpos contra agentes virales y bacterias atípicas en el suero de pacientes con infección respiratoria, Estado Zulia- Venezuela, periodo 2005 – 2010. Kasmera. 2014:141–155. [Google Scholar]
- Chatzis O., Darbre S., Pasquier J., Meylan P., Manuel O., Aubert J.D., et al. Burden of severe RSV disease among immunocompromised children and adults: a 10 year retrospective study. BMC Infect Dis. 2018;18(1):111. doi: 10.1186/s12879-018-3002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosia A.D., Yang J., Hillson E., Mauskopf J., Copley-Merriman C., Shinde V., et al. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS One. 2017;12(August 8) doi: 10.1371/journal.pone.0182321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comach G., Teneza-Mora N., Kochel T.J., Espino C., Sierra G., Camacho D.E., et al. Sentinel surveillance of influenza-like illness in two hospitals in Maracay, Venezuela: 2006–2010. PLoS One. 2012;7(September 9):e44511. doi: 10.1371/journal.pone.0044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas-García A., García-Sepúlveda C.A., Méndez-de Lira J.J., Aranda-Romo S., Hernández-Salinas A.E., Noyola D.E. Mortality attributable to pandemic influenza A (H1N1) 2009 in San Luis Potosí, Mexico. Influenza Other Respir Viruses. 2011;5(March 2):76–82. doi: 10.1111/j.1750-2659.2010.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell S.F., Anderson L.J., Gary H.E., Erdman D.D., Plouffe J.F., File T.M., et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174(September 3):456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(April 17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., McElhaney J.E., Beran J., van Essen G.A., Duval X., Esen M., et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209(12):1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Walsh E.E. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(July 3):371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G.D., Fine M., Orloff J., Arisumi D., Yu V.L., Kapoor W., et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 1990;69(September 5):307–316. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Fernandes-Matano L., Monroy-Muñoz I.E., Angeles-Martínez J., Sarquiz-Martinez B., Palomec-Nava I.D., Pardavé-Alejandre H.D., et al. Prevalence of non-influenza respiratory viruses in acute respiratory infection cases in Mexico. PLoS One. 2017;12(May 5):e0176298. doi: 10.1371/journal.pone.0176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas F.T. de M. Sentinel surveillance of influenza and other respiratory viruses, Brazil, 2000-2010. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2013;17(February 1):62–68. doi: 10.1016/j.bjid.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Fraga A., Ortiz-Hernández A.A., Ramírez-Venegas A., Vázquez R.V., Moreno-Espinosa S., Llamosas-Gallardo B., et al. Clinical characteristics and outcomes of influenza and other influenza-like illnesses in Mexico City. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2013;17(July 7):e510-517. doi: 10.1016/j.ijid.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamiño-Arroyo A.E., Moreno-Espinosa S., Llamosas-Gallardo B., Ortiz-Hernández A.A., Guerrero M.L., Galindo-Fraga A., et al. Epidemiology and clinical characteristics of respiratory syncytial virus infections among children and adults in Mexico. Influenza Other Respir Viruses. 2017;11(1):48–56. doi: 10.1111/irv.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökçe Ş., Kurugöl Z., Koturoğlu G., Çiçek C., Aslan A. Etiology, seasonality, and clinical features of viral respiratory tract infections in children hospitalized with acute bronchiolitis: a single-center study. Glob Pediatr Health. 2017;4(June) doi: 10.1177/2333794X17714378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(June 25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Hall W.J. Respiratory syncytial virus infection in adults: clinical, virologic, and serial pulmonary function studies. Ann Intern Med. 1978;88(February 2):203. doi: 10.7326/0003-4819-88-2-203. [DOI] [PubMed] [Google Scholar]
- Johnstone J., Majumdar S.R., Fox J.D., Marrie T.J. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134(December 6):1141–1148. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler M., Muñoz P., Mateos M., Adrados D., Bouza E. Respiratory syncytial virus burden among adults during flu season: an underestimated pathology. J Hosp Infect. 2018;100(December 4):463–468. doi: 10.1016/j.jhin.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Kwon Y.S., Park S.H., Kim M.-A., Kim H.J., Park J.S., Lee M.Y., et al. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults. BMC Infect Dis. 2017;17(December) doi: 10.1186/s12879-017-2897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Lui G.C.Y., Wong K.T., Li T.C.M., Tse E.C.M., Chan J.Y.C., et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57(October 8):1069–1077. doi: 10.1093/cid/cit471. [DOI] [PubMed] [Google Scholar]
- de Lima C.R.A., Mirandolli T.B., Carneiro L.C., Tusset C., Romer C.M., Andreolla H.F., et al. Prolonged respiratory viral shedding in transplant patients. Transpl Infect Dis Off J Transplant Soc. 2014;16(February 1):165–169. doi: 10.1111/tid.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger V., Piedra P.A., Ruiz M., Zunino E., Martínez M.A., Machado C., et al. Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;54(April 7):905–912. doi: 10.1093/cid/cir955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken J.P., Prill M.M., Arvelo W., Lindblade K.A., López M.R., Estevez A., et al. Respiratory syncytial virus infection in Guatemala, 2007-2012. J Infect Dis. 2013;208(December Suppl 3):S197–206. doi: 10.1093/infdis/jit517. [DOI] [PubMed] [Google Scholar]
- Med Crave Seasonality of respiratory syncytial virus - lower respiratory tract infection (RSV-LRTI) in children in developing countries. J Hum Virol Retrovirology [Internet] 2016 http://medcraveonline.com/JHVRV/JHVRV-03-00076.pdf [cited 2019 Aug 9]; Vol 3 (January Issue 1). Available from: [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(July 7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro C.C., Dezanet L.N.C., França E.B., Monteiro C.C., Dezanet L.N.C., França E.B. Monitoramento de vírus respiratórios na região metropolitana de Belo Horizonte, 2011 a 2013. Epidemiol E Serviços Saúde. 2016;25(June 2):233–242. doi: 10.5123/S1679-49742016000200002. [DOI] [PubMed] [Google Scholar]
- Moore H.C., Jacoby P., Hogan A.B., Blyth C.C., Mercer G.N. Modelling the seasonal epidemics of respiratory syncytial virus in young children. PLoS One. 2014;9(June 6) doi: 10.1371/journal.pone.0100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullooly J.P., Bridges C.B., Thompson W.W., Chen J., Weintraub E., Jackson L.A., et al. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25(January 5):846–855. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Peñafiel S.F., Gutiérrez O.M., López F.G., Aránguiz E., Manuel J., Monasterio U.J., et al. Participación de los virus respiratorios en la neumonía del adulto inmunocompetente adquirida en la comunidad. Rev Médica Chile. 2016;144(December 12):1513–1522. doi: 10.4067/S0034-98872016001200002. [DOI] [PubMed] [Google Scholar]
- Piedimonte G., Perez M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35(December 12):519–530. doi: 10.1542/pir.35-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J., Pulido Dominguez P., Rey Benito G., Mendez Rico J., Castellanos J., Páez Martinez A. Human respiratory syncytial virus and metapneumovirus in patients with acute respiratory infection in Colombia, 2000 - 2011. Rev Panam Salud Publica Pan Am J Public Health. 2014;36(August 2):101–109. [PubMed] [Google Scholar]
- Rioseco M.L., Riquelme O.M., Inzunza P.C., Oyarzún G.P., Agüero O.Y., Ferrés G.M., et al. Etiología viral en la neumonía del adulto adquirida en la comunidad en un hospital del sur de Chile. Rev Médica Chile. 2012;140(August 8):984–989. doi: 10.4067/S0034-98872012000800003. [DOI] [PubMed] [Google Scholar]
- Riquelme R., Rioseco M.L., Agüero Y., Ubilla D., Mechsner P., Inzunza C., et al. Respiratory virus infections in adult patients hospitalized in an internal medicine unit. Rev Med Chil. 2014;142(June 6):696–701. doi: 10.4067/S0034-98872014000600002. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Auad J.P., Nava-Frías M., Casasola-Flores J., Johnson K.M., Nava-Ruiz A., Pérez-Robles V., et al. The epidemiology and clinical characteristics of respiratory syncytial virus infection in children at a public pediatric referral hospital in Mexico. Int J Infect Dis. 2012;16(July 7):e508–513. doi: 10.1016/j.ijid.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Martínez C.E., Rodríguez D.A., Nino G. Respiratory syncytial virus, adenoviruses, and mixed acute lower respiratory infections in children in a developing country. J Med Virol. 2015;87(May 5):774–781. doi: 10.1002/jmv.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J.W., Justice N.A. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2018. Respiratory Syncytial Virus Infection (RSV)http://www.ncbi.nlm.nih.gov/books/NBK459215/ Available from: [PubMed] [Google Scholar]
- Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J., et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(January 2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Volling C., Hassan K., Mazzulli T., Green K., Al-Den A., Hunter P., et al. Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infect Dis. 2014;14(December):665. doi: 10.1186/s12879-014-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Falsey A.R. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets. 2012;12(April 2):98–102. doi: 10.2174/187152612800100116. [DOI] [PubMed] [Google Scholar]
- Walsh E.E., Peterson D.R., Falsey A.R. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis. 2007;195(April 7):1046–1051. doi: 10.1086/511986. [DOI] [PubMed] [Google Scholar]
- Watanabe A.S.A., Carraro E., Candeias J.M.G., Donalísio M.R., Leal E., Granato C.F.H., et al. Viral etiology among the elderly presenting acute respiratory infection during the influenza season. Rev Soc Bras Med Trop. 2011;44(February 1):18–21. doi: 10.1590/s0037-86822011000100005. [DOI] [PubMed] [Google Scholar]
- Zambrano G., Lucila J., Montes M., Rosana A., Fereira B., Enmanuel J., et al. Seroprevalencia de virus respiratorios y bacterias atípicas en una población del estado Zulia, Venezuela. Rev Soc Venez Microbiol. 2012;32(December 2):148–152. [Google Scholar]
- PAHO . 2019. Influenza and RSV Percent Positivity / Influenza y VSR porcentaje de positividad. [Internet]http://ais.paho.org/phip/viz/ed_flu.asp [Cited 2019a 9 August]. Available from: [Google Scholar]
- WHO . 2014. WHO surveillance case definitions for ILI and SARI [Internet]http://www.who.int/influenza/surveillance_monitoring/ili_sari_surveillance_case_definition/en/ [Cited 2019c 12 August]. Available from: [Google Scholar]
- WHO . 2017. RSV surveillance case definitions [Internet]http://www.who.int/influenza/rsv/rsv_case_definition/en/ [Cited 2019b 12 August]. Available from: [Google Scholar]