Highlights
-
•
An indirect porcine epidemic diarrhea virus (PEDV) anti-immunoglobulin (Ig) G ELISA based on the S1 portion of the spike protein was validated and compared with an indirect immunofluorescence assay.
-
•
On field serum samples the diagnostic sensitivity of the S1 ELISA was 100%, the diagnostic specificity was 94% and the S1 ELISA showed no cross-reactivity with antibodies against other porcine coronaviruses.
-
•
On colostrum samples, the diagnostic sensitivity was 92% for IgG and 100% for IgA, and the diagnostic specificity was 90% for IgG and 99.4% for IgA.
-
•
The results suggest that the S1 ELISA is a sensitive and specific test that could also be used to evaluate PEDV colostral immunity.
Keywords: Porcine epidemic diarrhea virus, Serology, ELISA, S1 protein, Diagnosis
Abstract
An indirect porcine epidemic diarrhea virus (PEDV) anti-immunoglobulin (Ig) G ELISA based on the S1 portion of the spike protein was validated and compared with an indirect immunofluorescence assay. In serum samples from experimentally infected pigs (n = 35), anti-IgG PEDV antibodies were detected as early as 7 days post-infection. In field serum samples (n = 239), the diagnostic sensitivity of the S1 ELISA was 100% and the diagnostic specificity was 94%. The S1 ELISA showed no cross-reactivity with antibodies against other porcine coronaviruses. Colostrum samples (n = 133) were also tested for anti-PEDV IgG and IgA. The diagnostic sensitivity was 92% for IgG and 100% for IgA, and the diagnostic specificity was 90% for IgG and 99.4% for IgA. These data suggest that the S1 ELISA is a sensitive and specific test that could also be used to evaluate PEDV colostral immunity.
Introduction
Porcine epidemic diarrhea virus (PEDV) was initially recognized as a pathogen in Europe in 1971, followed by its introduction into Asia (Song and Park, 2012) and, in 2013, North America (Huang et al., 2013). Clinical signs include acute vomiting, anorexia and watery diarrhea in pigs of all ages, with up to 90–95% mortality in suckling pigs (Huang et al., 2013). PEDV belongs to the family Coronaviridae, which includes transmissible gastroenteritis virus (TGEV) and porcine respiratory coronavirus (PRCV).
Coronaviruses contain four major structural proteins: spike (S), envelope (E), membrane (M) and nucleocapsid (N) (Brian and Baric, 2005). The S protein is further divided into S1 (1–735 amino acids, aa) and S2 (736–1383 aa) domains, which include major neutralizing epitopes (Brian and Baric, 2005). PEDV strains can be grouped into genogroup 1, which contains most PEDV strains circulating prior to 2010, and genogroup 2, associated with recent outbreaks with high mortality in Asia and the USA (Huang et al., 2013).
A number of commercial and in-house ELISAs have been developed for detection of antigen or antibodies against PEDV (Song and Park, 2012). Most assays for antibody detection are based on whole virus (Hofmann, Wyler, 1990, Carvajal et al, 1995, Oh et al, 2005) or viral antigen preparations (Knuchel et al., 1992). The first aim of this study was to develop an indirect immunoglobulin (Ig) G ELISA based on the recombinant PEDV S1 protein and to compare its performance with an immunofluorescence assay (IFA).
High IgA levels in milk are correlated with protection of suckling pigs against PEDV infection, whereas neutralizing antibodies in serum do not correlate with protection (Ha et al., 2010). Tools to monitor PEDV colostral immunity could be useful for developing preventive programs on affected farms. Therefore, the second aim of this study was to test the ability of the S1 ELISA to detect anti-PEDV IgG and IgA antibodies in samples of colostrum.
Materials and methods
Immunofluorescence assay
The reference method to detect antibodies against PEDV in serum was IFA. Monolayers of Vero cells grown to 100% confluency in 96-well plates were inoculated with 1 × 102 plaque forming units (PFUs) per well of PEDV (GenBank KF650370) suspended in modified Eagle's medium (MEM) supplemented with 1 µg per well trypsin. After 24 h, monolayers were fixed with 80% acetone, then air-dried and incubated with two-fold dilutions of serum from 1:40 to 1:320 at 37 °C for 1 h, followed by incubation for 30 min with a fluorescein labeled goat anti-pig IgG diluted 1:50 (02–14-06, KPL). Cell staining was examined under a fluorescent microscope. A positive signal at a sample dilution of 1:40 or higher was considered to be positive.
Porcine epidemic diarrhea virus S1 ELISA
The region encoding the S1 domain (aa 1–781) of the PEDV IA1 strain (GenBank KF468753; Huang et al., 2013) was 3′ terminally fused with a thrombin cleavage sequence (encoding LVPRGS), followed by a human IgG Fc domain (available from a ICAM3-Fc construct; Huang et al., 2009). The fusion fragment was generated by overlapping PCR and cloned into pcDNA3.1- (Invitrogen) between the EcoRI and HindIII restriction sites to generate an expression construct designated pcDNA-PEDV-S1Fc (Fig. 1 ).
Fig. 1.
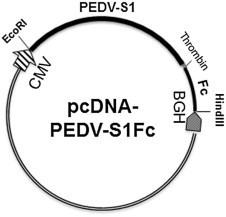
Porcine epidemic diarrhea virus-S1-Fc fusion expression plasmid.
Plasmid pcDNA-PEDV-S1Fc was transfected into Freestyle 293-F cells (Invitrogen; catalog number R790-07, <50 passages) using Lipofectamine LTX (Invitrogen) according to the manufacturer's protocol. The cells were grown in FreeStyle 293 Expression Medium (Invitrogen). At 4 days post-transfection, the cell culture supernatants were collected to verify the expression of S1-Fc fusion proteins by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The expression products were purified by using a HiTrap Protein A Sepharose High Performance column (GE Healthcare; catalog number 17–0402-01). The purified proteins were treated with thrombin (20 IU/mg) overnight at 30 °C and then purified again using the HiTrap protein A column. The concentration of endotoxin was <1000 endotoxin units (EU)/mg. The final protein products were quantified by NanoDrop spectrophotometry (A280 protein program) and frozen at −80 °C until use.
The optimal antigen concentration and the optimum serum and colostrum dilutions for the ELISA were determined using a checkerboard titration. Microtiter plates (Nunc) were coated with 0.44 ng per well of the S1 polypeptide diluted in coating buffer (50 mM carbonate buffer, pH 9.6) and incubated overnight at 4 °C. After three washes with phosphate buffered saline (PBS) containing 0.05% Tween 20 (PBST), the plates were blocked with 1% bovine serum albumin (Gibco, Life Technologies) for 2 h at 22 °C and then incubated with serum or colostrum diluted 1:100 in PBS containing 10% goat serum (Gibco, Life Technologies) for 30 min at 37 °C. After a washing step, a 1:20,000 diluted peroxidase-conjugated goat anti-porcine IgG (114-035-003, Jackson ImmunoResearch) or IgA (A100-102P, Bethyl Laboratories) was added and incubated at 37 °C for 30 min. The peroxidase reaction was visualized using tetramethylbenzidine–hydrogen peroxide as the substrate (KPL) for 10 min at room temperature and stopped by adding 50 µL 2.5 M sulfuric acid to each well. Optical densities (OD) were measured at 450 nm using an ELISA plate reader (BioTek). Positive, negative and blank (sterile water) samples were tested in duplicate and included on each plate.
Serum samples
A PEDV genogroup 1 and a TGEV antibody positive serum sample (Callebaut et al., 1982) were obtained from Dr Kristin van Reeth, Ghent University, Belgium. A 2013 PEDV genogroup 2 and a TGEV antibody positive serum sample were acquired from the VDL-ISU. Thirty-five serum samples were obtained from 4-week-old pigs experimentally infected with a 30% fecal suspension containing a 2013 USA PEDV isolate (GenBank KF804028). Fecal samples were collected prior to inoculation and at 3 and 7 days post-inoculation (dpi). Blood was collected prior to infection and at 7, 10, 14 and 21 dpi, centrifuged at 3220 g for 10 min at 4 °C and aliquots of serum were stored at −80 °C until testing. The experimental protocol for the animal study was approved by the Iowa State University Institutional Animal Care and Use Committee (approval number 1-11-7071S, date of approval November 2013).
Serum samples from 239 pigs from 19 commercial USA herds were selected from routine diagnostic cases submitted to the VDL-ISU for PEDV investigation due to the occurrence of diarrhea or to monitor PEDV infection status from September to December 2013. In addition, 133 colostrum samples from sows were selected, including 102 PEDV negative colostrum samples with unknown TGEV and PRCV status collected in 2010 (O'Neill et al., 2012). The remaining 31 samples were collected in November 2013 from a PEDV positive farm known to be PRCV positive and TGEV negative.
Testing strategy and farm status
All serum samples were tested for anti-PEDV IgG antibodies by IFA and S1 ELISA, and for anti-TGEV and anti-PRCV IgG antibodies by a commercial ELISA (Swinecheck TGEV/PRCV ELISA; Biovet). All colostrum samples were tested with the S1 ELISA for PEDV IgG and IgA antibodies. The PEDV, PRCV and TGEV infection status was determined based on a duplex real-time reverse-transcriptase (RT)-PCR for PEDV and TGEV in fecal samples using a commercially available assay test (Tetracore) and PRCV serology.
A farm was considered to be positive when antibodies and/or viral RNA were detected in 50% of the submitted samples in at least two consecutive submissions. A farm was considered to be negative when all tested samples of at least two consecutive submissions were antibody and/or viral RNA negative. Farms that could not be classified as negative and had <50% of positive samples for a given pathogen were excluded. This 50% cut-off was adopted arbitrarily to ensure the reliability of the classification considering that some farms had a submission size as low as three samples. Instead of establishing different parameters depending on the number of samples submitted, a unique conservative value of 50% was used for all cases.
ELISA cut-off values and assay performance
The cut-off value for the serum-based S1 ELISA was calculated by using the mean OD value of 40 PEDV negative serum samples plus three standard deviations (SDs). This value was further evaluated by receiver operator characteristic (ROC) analysis using 60 PEDV positive serum samples in addition to the cumulative data from all samples using the IFA as the reference method for sample classification. The cut-off value for the colostrum-based ELISA was calculated using ROC analysis on 102 PEDV negative samples and 31 samples from a positive PEDV farm.
Diagnostic sensitivity and specificity were estimated for different S1 ELISA cut-offs using a maximum likelihood method considering a sensitivity of 95.6% and a specificity of 98.1% for the IFA. McNemar's test for pairwise comparisons was used to determine whether the proportions of positive samples were significantly different by assay. Differences between groups were considered to be significant at P < 0.05. The Kappa (κ) index was calculated to determine the agreement between assays. Statistical analyses were performed using SAS version 9.2.
Results
ELISA cut-off values and assay performance
The mean OD value of the 40 negative serum samples was 0.08 (x), with a SD of 0.04. Therefore, samples with an OD value ≥0.20 (x + 3 SD) were considered to be positive. Based on the maximum likelihood method, the sensitivity was 100% and the specificity was 88.9% for a cut-off of 0.2, and the sensitivity was 100% and the specificity was 94.0% for cut-off of 0.3. Therefore, a final cut-off 0.3 was selected and samples with OD values between 0.2 and 0.3 were considered to be indeterminate (Fig. 2 ).
Fig. 2.
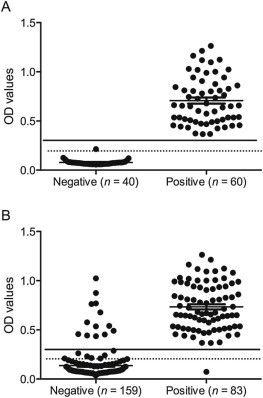
Distribution of anti-IgG porcine epidemic diarrhea virus (PEDV) ELISA results by sample classification (negative, positive) relative to the assay cut-off (full line; optical density, OD, value 0.3), considering field samples from farms with known positive or negative PEDV status (A) or using the immunofluorescence assay (IFA) as the reference method to classify positive and negative samples (B). Samples with OD values ranging from 0.2 (dotted line) to 0.3 were considered to be indeterminate.
In colostrum samples, the optimal cut-off for the PEDV IgG assay was a sample OD value of 0.61, for which the sensitivity was 92.3% and the specificity was 90.0%; for detection of PEDV IgA, the optimal cut-off was a sample OD value of 0.4, for which the sensitivity was 100% and the specificity was 99.4% (Fig. 3 ).
Fig. 3.
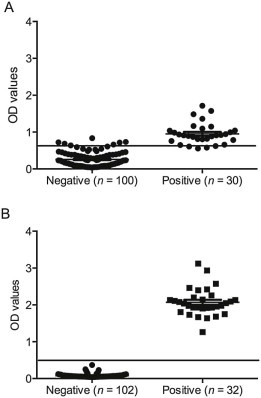
Distribution of anti-IgG (A) and IgA (B) porcine epidemic diarrhea virus (PEDV) ELISA results on colostrum samples by sample classification (negative, positive) relative to the assay cut-off (full line) considering field samples from farms with known positive or negative PEDV status.
The assay specificity was determined using two TGEV reference serum samples, and 20 TGEV and 10 PRCV ELISA positive field serum samples known to be PEDV negative. The S1 ELISA OD values ranged from 0.03 to 0.1 (x ± SD = 0.07 ± 0.03) indicating a lack of cross-reaction with TGEV and PRCV.
Experimentally infected pigs
In experimentally infected pigs, anti-PEDV IgG was detected as early as 7 dpi with the S1 ELISA and as early as 10 dpi with the IFA (Table 1 ). At 14 dpi, all seven pigs were seropositive (Fig. 4 , Table 1). There was no significant difference between detection rates among assays (P > 0.05).
Table 1.
Detection rates for antibodies against porcine epidemic diarrhea virus (PEDV) over time in serum samples from experimentally infected pigs as determined by the S1 ELISA and immunofluorescence assay (IFA) at different days post-inoculation.
| Number positive/total number of samples tested (%) | |||||
|---|---|---|---|---|---|
| 0 | 7 | 10 | 14 | 21 | |
| S1 ELISA | 0/7 (0) | 2/7 (28.6) | 6/7 (85.7) | 7/7 (100) | 7/7 (100) |
| IFA | 0/7 (0) | 0/7 (0) | 2/7 (28.6) | 6/7 (85.7) | 5/7 (71.4) |
Fig. 4.
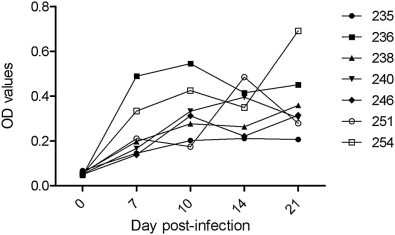
Kinetics of IgG antibody to porcine enteric diarrhea virus (PEDV) over time from individual experimentally infected pigs as determined by the S1 ELISA.
Field serum samples
The average OD values and detection rates of anti-PEDV antibodies in field serum samples are summarized in Table 2 . Seven farms were negative for PEDV, TGEV and PRCV. An additional seven farms tested positive for TGEV and PRCV, but were negative for PEDV. The average OD values obtained with the S1 ELISA were similar in these 14 farms, regardless of TGEV and PRCV status, and all samples obtained from these farms were correctly classified as negative using the S1 ELISA (Table 2).
Table 2.
Detection rate (number of positive/total number of samples tested) of anti-porcine epidemic diarrhea virus (PEDV) IgG antibodies in serum samples from farms classified as positive or negative for porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV) and porcine respiratory coronavirus (PRCV), as determined using the S1 ELISA.
| Number of farms | Number of samples | Farm infection statusa | Number of S1 ELISA positives (average OD values ± SD) | ||
|---|---|---|---|---|---|
| PEDV | TGEV | PRCV | |||
| 7 | 60 | − | − | − | 0 (0.09 ± 0.04) |
| 2 | 25 | − | − | + | 0 (0.08 ± 0.05) |
| 1 | 20 | − | + | − | 0 (0.08 ± 0.04) |
| 4 | 40 | − | + | + | 0 (0.08 ± 0.04) |
| 3 | 42 | + | − | − | 42 (0.77 ± 0.20) |
| 2 | 52 | + | − | + | 52 (0.71 ± 0.24) |
OD, optical density; SD, standard deviation.
Farm infection status determined by at least 50% IgG antibodies positive samples in each farm by immunofluorescence assay (PEDV) or a commercial ELISA (TGEV and PRCV).
Field colostrum samples
Among colostrum samples, 28/31 (90.3%) from the PEDV positive farm were positive for anti-IgG PEDV antibodies, while 31/31 (100%) were positive for anti-IgA PEDV antibodies. On PEDV negative farms, 9/102 (8.9%) colostrum samples were positive for anti-IgG PEDV antibodies and all samples were negative for anti-IgA PEDV antibodies.
Comparison of serological assays
Results of testing using the S1 ELISA and IFA had almost perfect agreement (κ = 0.88); however, the S1 ELISA detected a higher number of positive serum samples (P < 0.01; Table 3 ). On the five PEDV positive farms the S1 ELISA detected 13/94 (13.8%) additional positive samples compared to the IFA (Fig. 2).
Table 3.
Comparison between the S1 ELISA and immunofluorescence assay (IFA) for detection of antibodies against porcine epidemic diarrhea virus (PEDV) on 242 serum samples (experimental and field samples).
| S1 ELISAa | |||||
|---|---|---|---|---|---|
| Positive | Indeterminate | Negative | |||
| IFA | Positive | 82 (33.4%)b | 0 | 1 (0.4%) | 83 (34.3%) |
| Negative | 13 (5.4%) | 8 (3.3%) | 138 (57.0%) | 159 (65.7%) | |
| 95 (39.2%) | 8 (3.3%) | 139 (57.4%) | Total | ||
OD values: positive ≥0.30; indeterminate ≥0.20 and <0.30; negative <0.20.
Data presented as number of samples (%).
Discussion
A recombinant PEDV S1 polypeptide-based ELISA was evaluated for its ability to detect anti-PEDV IgG and IgA antibodies. Commercial ELISAs currently available in Asia and Europe appeared to have low sensitivity when applied for antibody detection in the PEDV infected pig population in the USA (R. Main, Iowa State University, personal communication). Most in-house assays for anti-PEDV antibody detection are based on cell cultivated virus, limiting large-scale testing. More recently, an in-house ELISA based on a recombinant N protein has been validated in China (Hou et al., 2007). However, this study did not assess the cross-reactivity of the ELISA with other coronaviruses. In addition, a higher sensitivity and specificity of an S protein-based ELISA compared to an N-protein based ELISA was demonstrated (Knuchel et al., 1992). No cross-reactivity with PRCV or TGEV was detected with the S1 ELISA developed in the present study.
Since there is no PEDV vaccine currently available in the USA, pregnant sows in many herds have been deliberately exposed to the intestinal contents of infected piglets on PEDV-infected farms, thus artificially stimulating lactogenic immunity. However, because intestinal contents may not have homogenous PEDV antigen levels, the induction of a solid and uniform immunity could be inconsistent. Therefore, monitoring IgA titers in colostrum and milk samples could be done to ensure that the piglets receive adequate passive immunity. The S1 ELISA for IgA antibodies developed in the present study provides a sensitive and specific tool for monitoring passive immunity against PEDV.
The S1 ELISA detected anti-PEDV antibodies in serum as early as 7 dpi, 3 days sooner than the PEDV IFA as also reported by Carvajal et al. (1995). Among negative farms, the agreement between tests was 100%. However, among samples from PEDV positive farms, the S1 ELISA detected 14% more positive samples, suggesting a higher sensitivity for the S1 ELISA.
Conclusions
The S1 ELISA using a recombinant S1 protein from PEDV is a useful, rapid, sensitive and specific tool for the detection of anti-PEDV IgG antibodies in serum and for detection of anti-PEDV IgA antibodies in colostrum.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper.
Acknowledgments
The authors thank Dr Kristin van Reeth, Ghent University, Belgium, for providing control serum samples, and Dr Darin Madson, Iowa State University, USA, for providing TGEV positive field serum samples.
References
- Brian D.A., Baric R.S. Coronavirus genome structure and replication. Current Topics in Microbiology and Immunology. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut P., Debouck P., Pensaert M. Enzyme-linked immunosorbent assay for the detection of the coronavirus-like agent and its antibodies in pigs with porcine epidemic diarrhea. Veterinary Microbiology. 1982;7:295–306. doi: 10.1016/0378-1135(82)90009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal A., Lanza I., Diego R., Rubio P., Carmenes P. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. Journal of Veterinary Diagnostic Investigation. 1995;7:60–64. doi: 10.1177/104063879500700109. [DOI] [PubMed] [Google Scholar]
- Ha G.W., Kang B.K., Park S.J., Lee C.S., Oh J.S., Chai Y.G., Moon H.J., Park B.K., Song D.S. The colostral IgA level, but not the serum neutralization titer induced by immunization against porcine epidemic diarrhea virus is correlated with protection of new born piglets against virulent PEDV challenge. Korean Journal of Veterinary Public Health. 2010;34:53–58. [Google Scholar]
- Hofmann M., Wyler R. Enzyme-linked immunosorbent assay for the detection of porcine epidemic diarrhea coronavirus antibodies in swine sera. Veterinary Microbiology. 1990;21:263–273. doi: 10.1016/0378-1135(90)90037-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X.L., Yu L.Y., Liu J. Development and evaluation of enzyme-linked immunosorbent assay based on recombinant nucleocapsid protein for detection of porcine epidemic diarrhea (PEDV) antibodies. Veterinary Microbiology. 2007;123:86–92. doi: 10.1016/j.vetmic.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Dryman B., Li W., Meng X.J. Porcine DC-SIGN: Molecular cloning, gene structure, tissue distribution and binding characteristics. Developmental and Comparative Immunology. 2009;33:464–480. doi: 10.1016/j.dci.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Dickerman A.W., Pineyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio. 2013;4:e00737-13. doi: 10.1128/mBio.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuchel M., Ackermann M., Muller H.K., Kihm U. An ELISA for detection of antibodies against porcine epidemic diarrhoea virus (PEDV) based on the specific solubility of the viral surface glycoprotein. Veterinary Microbiology. 1992;32:117–134. doi: 10.1016/0378-1135(92)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.S., Song D.S., Yang J.S., Song J.Y., Moon H.J., Kim T.Y., Park B.K. Comparison of an enzyme-linked immunosorbent assay with serum neutralization test for serodiagnosis of porcine epidemic diarrhea virus infection. Journal of Veterinary Science. 2005;6:349–352. [PubMed] [Google Scholar]
- O'Neill K.C., Hemann M., Giménez-Lirola L.G., Halbur P.G., Opriessnig T. Vaccination of sows reduces the prevalence of PCV-2 viraemia in their piglets under field conditions. Veterinary Record. 2012;171:425–431. doi: 10.1136/vr.100660. [DOI] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


