Abstract
Feline viral diseases are common and cats can be presented with a variety of clinical manifestations. Ocular disease associated with viral pathogens is not unusual, particularly with viruses causing upper respiratory tract disease in cats, such as feline herpesvirus type 1 and feline calicivirus. These agents mainly cause ocular surface disease. Other viruses, such as feline immunodeficiency virus and feline coronavirus, can cause uveitis, while feline leukemia virus can induce ocular lymphosarcoma. This review covers the most common viral pathogens of cats that cause ocular manifestations, the specific features of the ocular diseases caused by these viruses and therapeutic recommendations.
Keywords: Feline, Eye, Conjunctivitis, Keratitis, Uveitis, Virus
Introduction
Viral diseases in cats are a common occurrence, sometimes presenting both diagnostic and therapeutic challenges to veterinarians. Ocular manifestations of viral disease are many and varied, depending on the inciting pathogen. Familiarity with the clinical and ocular manifestations of the common viral pathogens of cats might help to direct diagnostic testing and therapy. This review describes the most common feline viral infections that could have an ocular manifestation, how to recognize which viral pathogen might be responsible, therapeutic options for treating ocular disease and prognosis.
Feline herpesvirus
Feline herpesvirus type 1 (FHV-1) is the most common viral pathogen of cats that causes ocular disease. It is a DNA virus that belongs to the subfamily Alphaherpesviridae and develops neuronal latency following primary infection. The trigeminal ganglion is a known site of latency for FHV-1, but the virus can persist in a quiescent form in ocular tissues, particularly the cornea (Townsend et al, 2004, Stiles, Pogranichniy, 2008).
Clinical signs
FHV-1 causes upper respiratory tract disease (URTD) and conjunctivitis or keratitis via its cytopathic effect on epithelial cells. Cats affected by both primary infection and viral recrudescence are likely to have ocular disease. Conjunctivitis is the most common ocular condition (Fig. 1 ), followed by corneal epithelial ulceration and keratitis, with or without ulceration (Fig. 2 ). Conjunctivitis is manifested by conjunctival hyperemia, with or without chemosis, tearing and discomfort. The nictitating membrane can sometimes be elevated due to pain or swelling. The early inflammatory response is neutrophilic and a purulent ocular discharge is common. Dendritic epithelial corneal ulceration is the classic herpetic lesion and, if evident, is helpful in making the diagnosis. However, many cats with FHV-1-related ocular disease are presented with geographic epithelial ulcers. Both of these types of epithelial ulcers can be seen after staining with fluorescein. Rose Bengal will highlight dendritic ulcers, but is irritating and a topical anesthetic should be used before this stain is applied. Keratitis without corneal ulceration can also occur in cats, manifesting as corneal vascularization, with or without inflammatory cell infiltrates (Fig. 2). Corneal disease from FHV-1 is almost always accompanied by conjunctivitis.
Fig. 1.
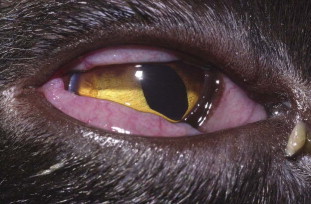
Conjunctivitis caused by feline herpesvirus type 1 in an adult cat. Note conjunctival hyperemia, chemosis and purulent ocular discharge. The diagnosis was made based on virus isolation from a conjunctival swab.
Fig. 2.
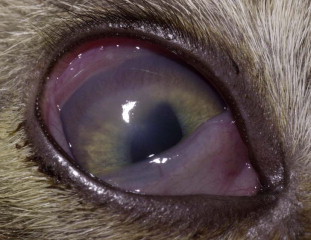
Keratitis without corneal ulceration caused by feline herpesvirus type 1 in a kitten. Note corneal vascularization and edema. Conjunctivitis is also present. The diagnosis was made based on virus isolation and positive PCR results from a conjunctival swab.
Other corneal conditions, such as sequestra and eosinophilic keratitis, have been associated with FHV-1 (Nasisse et al, 1998, Dean, Meunier, 2013). These conditions are more likely to occur with chronic FHV-1-associated ocular disease. Typically, primary infection with FHV-1 is self-limiting, lasting a few weeks, followed by clinical recovery. However, some cats develop chronic conjunctivitis that does not resolve spontaneously. Chronic conjunctivitis, or recurrent conjunctivitis, can occur bilaterally, but it is not uncommon for only one eye to be affected. This might lead the clinician to erroneously assume that FHV-1 could not be the underlying pathogen.
Diagnosis
The diagnosis of FHV-1 can sometimes be problematic, particularly in the adult cat. A history of recurrent episodes of conjunctivitis or corneal ulceration, especially if accompanied by sneezing, might allow for a presumptive diagnosis. Conjunctival cytology, if performed in the early phase of the disease, will show a neutrophilic inflammation. As the disease becomes more chronic, a mixed pattern of neutrophils, lymphocytes and plasma cells will be seen. Eosinophils and occasional mast cells might also be present. Intranuclear viral inclusions are generally not visible.
Definitive tests for the presence of FHV-1 include virus isolation, fluorescent antibody staining and PCR. PCR has become the most commonly utilized test. Samples from both the eye and oropharynx should be submitted. PCR is highly sensitive and specific, although variability amongst laboratories exists. However, a negative test does not rule out FHV-1 as the inciting agent, nor does a positive test prove that the virus is the cause of clinical signs. Several studies have demonstrated that cats with clinically normal eyes can have positive PCR results for FHV-1 from conjunctival samples (Stiles et al, 1997, Burgesser et al, 1999). However, a positive PCR result in the face of clinical disease consistent with FHV-1 should allow the clinician to proceed with reasonable certainty of a correct diagnosis. If diagnostic testing is not performed, or if results are negative in the face of clinical disease consistent with that caused by FHV-1, anti-viral therapy can be instituted and the response to treatment determined.
Treatment
Treatment of conjunctivitis, corneal ulceration or keratitis caused by FHV-1 should include specific anti-viral therapy. Corneal ulcers caused by FHV-1 alone should only involve the epithelium. If a stromal ulcer exists, a secondary bacterial pathogen is likely and would need more aggressive antibiotic therapy than would be instituted for a viral ulcer. Any loose epithelium should be removed by debridement with a cotton swab. Grid keratotomies should not be performed in cats, as this might worsen herpetic disease and lead to formation of a corneal sequestrum (La Croix et al., 2001). A broad-spectrum topical antibiotic, such as tobramycin, should be used if epithelial ulceration is present. Cleansing the eyes and removing discharge regularly is important.
Available anti-herpetic drugs are summarized in Table 1 . Many of the topical anti-viral compounds are not available commercially or are available only in certain countries. Cidofovir is an intravenous drug used in human medicine for treatment of cytomegalovirus retinitis (Ahmed, 2011) and is the author's preferred topical drug in cats. Compounded into a 0.5% ophthalmic solution, it is efficacious against FHV-1 and is stable when stored frozen or refrigerated for at least 6 months (Fontanelle et al, 2008, Stiles et al, 2010). Additional benefits include lack of ocular irritation and a long tissue half-life, so that twice daily administration is adequate (Sandmeyer et al, 2005, Van der Meulen et al, 2006). Acyclovir has a low efficacy against FHV-1 compared to its efficacy against human herpes simplex virus (HSV), and is not recommended for treatment of cats (Nasisse et al, 1989, Van der Meulen et al, 2006). Idoxuridine, trifluridine and ganciclovir are all effective (Nasisse et al, 1989, Maggs, Clarke, 2004), but need to be used 4–6 times daily and can cause ocular irritation. The use of oral famciclovir (prodrug of the active agent penciclovir), frequently used for treating HSV, has become more commonly used in cats. It has been shown to be both safe and effective for short periods of 2–3 weeks (Thomasy et al., 2011); however, the optimal dose remains uncertain. The most recent information indicates that 40 mg/kg three times daily is likely to be effective (Thomasy et al., 2012), although, anecdotally, lower doses and frequencies are reported to be effective by some veterinary clinicians. A recent study of high dose topical recombinant human α2b and feline ω interferon in a group of cats with naturally occurring viral keratoconjunctivitis found no beneficial effect of either interferon compared to placebo (Slack et al., 2013); thus, this treatment cannot be recommended.
Table 1.
Drugs used to treat feline herpesvirus type 1 infection in cats.
| Generic name | Concentration/dose | Route | Dosing interval (h) | Action |
|---|---|---|---|---|
| Cidofovir (compounded solution) | 0.5% | Ophthalmic | 12 | Acyclic nucleoside phosphonate, inhibits viral DNA synthesis |
| Trifluridine (solution) | 1% | Ophthalmic | 4–6 | Fluorinated pyrimidine nucleoside, inhibits viral DNA synthesis |
| Ganciclovir (ointment) | 0.15% | Ophthalmic | 4–6 | Guanosine analog, inhibits viral DNA synthesis |
| Idoxuridine (compounded solution) | 0.1% | Ophthalmic | 4–6 | Thymidine analog, inhibits viral DNA synthesis |
| Vidarabine (compounded ointment) | 3% | Ophthalmic | 4–6 | Adenosine analog, inhibits viral DNA synthesis |
| Acyclovir (ointment) | 3% | Ophthalmic | 4–6 | Purine analog, inhibits viral DNA synthesis |
| Famciclovir (tablet) | 40 mg/kg | Oral | 8 | Metabolized to penciclovir, acyclic nucleoside analog, inhibits viral polymerase and DNA synthesis |
The use of the amino acid l-lysine orally to treat FHV-1 in cats has met with mixed results. l-lysine is a competitive inhibitor of arginine, an amino acid necessary for synthesis of herpesviral proteins. In theory, excess ingestion of l-lysine will lead to decreased replication of FHV-1 through reduction in viral protein synthesis. In a placebo controlled experimental study, cats receiving 500 mg l-lysine every 12 h had less severe conjunctivitis than control cats (Stiles et al., 2002). In a study in which shelter cats were given 250 mg (kittens) or 500 mg (adults) l-lysine once daily in food, there was no difference in URTD in treated cats compared to untreated cats (Rees and Lubinski, 2008). In another study, shelter cats fed a diet high in l-lysine (5.7%) had no difference in the frequency of URTD signs compared to cats eating a diet with basal levels of l-lysine (1.7%) (Drazenovich et al., 2009). Anecdotally, it appears that some cats might benefit more than others from the administration of l-lysine. If this therapy is elected, a dose of 500 mg (adults) or 250 mg (kittens) twice daily with food should be used.
In some cats, particularly those with chronic FHV-1-related ocular disease, a topical anti-inflammatory drug might be indicated. Topical or systemic corticosteroids should be avoided, since the risk of exacerbating herpetic disease is high. The use of a topical non-steroidal anti-inflammatory drug (NSAID), such as 0.1% diclofenac, particularly in conjunction with an anti-viral medication, can be very helpful. Likewise, the use of topical 0.2% cyclosporine can have anti-inflammatory effects without significantly increasing the risk of worsening the herpetic disease (Stiles, 2013). In cats with chronic conjunctivitis, the improvement can be gradual and treatment periods of several months are often required.
Several factors can serve as triggers for recrudescent FHV-1 episodes in cats. These include the use of systemic or ocular corticosteroids or other immunosuppressive drugs, stress (psychological or physiological), pregnancy and lactation, surgery and other illnesses. In some cats, the use of modified live FHV-1 vaccines will cause clinical disease. When possible, clinicians and cat owners should attempt to minimize risk factors in cats known to have herpetic flare ups.
Prognosis
The prognosis for resolving FHV-1 related ocular surface disease is generally fair to good with appropriate therapy and adequate treatment time, although recrudescent disease can be expected in some cats.
Feline calicivirus
Feline calicivirus (FCV) is a single stranded RNA virus with the ability to undergo rapid mutation, resulting in strain diversity and widely varying antigenicity. Infection with FCV appears to be especially common in young cats housed in shelter environments (Bannasch and Foley, 2005). Although FCV does not develop latency like FHV-1, cats might harbor FCV long term and be chronic or intermittent shedders (Wardley et al., 1974).
Clinical signs
Feline calicivirus causes URTD and oral mucosal ulceration, and can cause ocular surface disease, primarily conjunctivitis. In some outbreaks of virulent systemic FCV, large percentages of cats have died (Hurley et al, 2004, Coyne et al, 2006, Reynolds et al, 2009) Feline calicivirus has been cited as causing only mild conjunctivitis (Ramsey, 2000). However, a recent study of 99 cats with URTD, as well as ocular surface disease, found that ocular samples analyzed using PCR were positive for FCV alone in 11 (11.1%) cats; in 19 (19.2%) cats, FCV was present with other infectious agents, including FHV-1 (Gerriets et al., 2012). Moderate to severe conjunctivitis with conjunctival epithelial erosions, as well as oral mucosal ulceration, were noted in FCV-positive cats. No corneal ulcers were noted in cats infected with only FCV. In the author's experience, some cats with FCV infection have severe conjunctivitis that often precludes visualization of the cornea (Fig. 3 ). It is likely that different strains of FCV and the host immune response play roles in the severity of ocular surface disease.
Fig. 3.
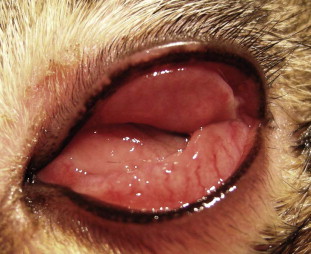
Severe conjunctivitis caused by feline calicivirus in a kitten. Note marked conjunctival hyperemia and chemosis that obscures the cornea. The diagnosis was made based on virus isolation from a conjunctival swab.
Diagnosis
The diagnosis of FCV ocular disease can be made based on virus isolation or PCR. Samples from both the eye and oropharynx should be submitted. The presence of oral mucosal ulceration should trigger a high suspicion of FCV infection, whereas the presence of corneal ulceration is more suggestive of infection with FHV-1.
Treatment
Therapy for cats infected with FCV, an RNA virus, is more difficult than for FHV-1, since the virus is not susceptible to drugs that inhibit DNA synthesis. The use of high dose topical ophthalmic interferon was not successful in reducing the severity of FCV-related ocular surface disease (Slack et al., 2013). Specific anti-viral treatment with phosphorodiamidate morpholino oligomers has been used in FCV outbreaks, reducing viral shedding and hastening clinical recovery (Smith et al., 2008). A topical ophthalmic anti-viral agent should be used when it is unknown whether FCV or FHV-1 (or both) are present. A topical broad spectrum ophthalmic antibiotic, such as tobramycin, should also be used, since erosions of the conjunctival epithelium are vulnerable to bacterial infection. Cleansing the eyes and removing discharge regularly is important. The most severe ocular manifestations of FCV tend to recede in a few weeks, although topical anti-inflammatory therapy might be needed, as described for infection with FHV-1.
Prognosis
The prognosis for resolution of FCV related conjunctivitis is generally good.
Feline immunodeficiency virus
Feline immunodeficiency virus (FIV) is a lentivirus with a worldwide distribution. Transmission of the virus occurs via saliva and blood (bite wounds), as well as in utero or post-partum via milk (Addie et al., 2009). Many cats infected with FIV remain asymptomatic.
Clinical signs
The ocular diseases associated with FIV are primarily chronic anterior uveitis and conjunctivitis. In one epidemiologic study, 35/318 (11%) FIV-positive cats had chronic conjunctivitis, although the relationship to FIV was not defined and other pathogens, such as FHV-1 or Chlamydia felis, might have played a role (Yamamoto et al., 1989). In 12 cats experimentally infected with FIV, virus was recovered from the aqueous humor of three animals, and a perivascular lymphoplasmacytic uveitis was noted in all nine animals euthanased ≥4 weeks post-infection (Ryan et al., 2003). The magnitude and distribution of lesions in the eyes and brain did not correlate with viral loads. In a study of 15 naturally infected cats that had been euthanased, anterior uveitis was documented in 13 (Loesenbeck et al., 1996). The presence of extravascular IgG and complement component C3 suggested that immune complexes might play a role in anterior uveitis in FIV-positive cats. Nine cats with naturally occurring FIV had ocular lesions, including anterior uveitis, secondary glaucoma and inflammation of the pars plana of the ciliary body (English et al., 1990). In an experimental study on the effect of FIV on the central nervous system, neurologic abnormalities related to the visual system included anisocoria, delayed pupillary light reflex and delayed visual evoked potentials (Phillips et al., 1994).
Infection with FIV is associated with lymphosarcoma in cats (Gabor et al, 2001, Magden et al, 2013), with the potential for ocular involvement (Fig. 4 ). Ocular lymphosarcoma can involve the uveal tract, conjunctiva or orbit.
Fig. 4.
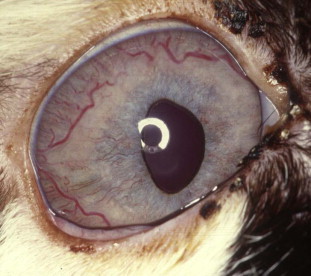
Uveal lymphosarcoma in a cat positive for feline immunodeficiency virus (FIV). Note the cellular infiltrate in the iris and iridal vascular congestion. The diagnosis was made based on finding neoplastic lymphocytes on an aqueous humor sample and a positive serum antibody test for FIV.
Diagnosis
For many years, FIV infection has been determined by serology. However, ELISAs (used for screening) and Western blot analysis (used for confirmation), do not distinguish antibodies due to FIV infection from antibodies due to vaccination, posing a diagnostic dilemma for veterinarians in countries where the vaccine is utilized and when the vaccination history is unknown. Quantitative PCR for identification of viral genes might be useful for distinguishing infected cats from vaccinated cats that test positive by ELISA and Western blot analysis (Ammersbach et al., 2013).
Treatment
Uveitis caused by FIV is likely to be chronic and to require longterm therapy. Treatment options are discussed under the section on therapy for uveitis. Inflammation of the pars plana of the ciliary body responds poorly to topical or systemic therapy. Chronic conjunctivitis in an FIV-positive cat should be investigated to determine if other pathogens, such as FHV-1 or Chlamydia felis, are present. If a specific agent cannot be detected, topical treatment with anti-inflammatory drugs is indicated.
Prognosis
The prognosis for controlling FIV related uveitis is thought to be fair at best, although there is no published evidence to support this opinion. Glaucoma is a common sequela to chronic anterior uveitis and intraocular pressure should be measured at each examination.
Feline leukemia virus
Feline leukemia virus (FeLV), an RNA retrovirus, occurs worldwide. The incidence of FeLV infection has decreased in recent decades as a result of vaccination and quarantine and removal programs.
Clinical signs
FeLV primarily causes ocular disease through the induction of lymphosarcoma. Possible sites of ocular lymphosarcoma include the uveal tract, conjunctiva (including the nictitating membrane) and orbit. The early stages of uveal lymphosarcoma can be manifested as uveitis, although the iris and ciliary body generally become thickened and irregular as the disease progresses, and glaucoma is not uncommon. With the overall reduction in the number of FeLV infected cats, the number of FeLV related lymphosarcoma cases has decreased (Louwerens et al, 2005, Weiss et al, 2010). However, in one histopathologic study of 50 cases of lymphosarcoma, a high percentage of tumors still contained FeLV proviral DNA, suggesting that regressively infected cats (those that are infected but have become aviremic) might have proviral DNA that can cause generalized lymphosarcoma (Weiss et al., 2010).
Other ocular abnormalities associated with FeLV infection include retinal hemorrhages associated with severe anemia and pupillary motility abnormalities (Brightman et al., 1991). On evaluation of corneal tissue by PCR and immunohistochemistry (IHC) in 17 naturally infected cats, 11 corneas were positive for FeLV by PCR and eight were positive by IHC (Herring et al., 2001). This study concluded that corneal donor tissue should not be used without screening cats for FeLV.
The role of FeLV, or the replication-defective feline sarcoma virus (FeSV), in spontaneously occurring ocular tumors in cats remains unclear. When FeLV was injected systemically or intravitreally into kittens, tumors of apparent retinal origin developed (Albert et al., 1977). When FeSV was injected into the anterior chamber of kittens, a pattern of iridal melanotic lesions occurred that progressed from distinct flat areas to raised infiltrative melanomas, very much like the naturally occurring anterior uveal melanomas of cats (Albert, 1980). In a study of 36 enucleated globes of cats with a diagnosis of diffuse anterior uveal melanoma, PCR identified three samples that were positive for FeLV–FeSV proviral DNA sequences (Stiles et al., 1999). In a subsequent study of 10 enucleated globes, FeLV–FeSV was not detected using IHC or PCR (Cullen et al., 2002).
Diagnosis
FeLV infection is diagnosed by detection of serum antigen (Lutz et al., 2009); any cat with uveitis or what appears to be ocular lymphosarcoma should be tested. If a diagnosis of intraocular lymphosarcoma is suspected but cannot be confirmed by other means, examination of a cytospin preparation of aspirated aqueous humor often reveals the presence of malignant lymphocytes.
Treatment
Treatment of uveal lymphosarcoma should include systemic chemotherapeutic agents and a topical corticosteroid such as 1% prednisolone acetate. If glaucoma is present, topical medications, such as dorzolamide and timolol, can be used. Enucleation should be considered for blind and painful eyes with uncontrollable glaucoma. Orbital lymphosarcoma can be treated with systemic chemotherapy and/or external beam radiation therapy.
Prognosis
The prognosis for FeLV positive cats with ocular lymphosarcoma is generally guarded to poor.
Feline infectious peritonitis
Feline infectious peritonitis (FIP) is caused by a feline coronavirus (FCoV), an RNA virus. Hypotheses to explain viral pathogenesis include de novo FCoV mutation giving rise to virulence and distinct circulating avirulent and virulent strains (Brown, 2011). Young animals from multi-cat environments are the most likely group to develop FIP.
Clinical signs
Since vasculitis is a feature of FIP, the eye is a common target organ. Ocular manifestations include pyogranulomatous anterior uveitis, often with fibrin in the anterior chamber and keratic precipitates (Fig. 5 ), choroiditis with retinal detachment and retinal vasculitis, with perivascular cuffing by inflammatory cells (Fig. 6 ; Doherty, 1971, Slauson, Finn, 1972, Campbell, Reed, 1975, August, 1984). Ocular manifestations of FIP are more common in the non-effusive (dry) form of the disease than the effusive (wet) form. Cats can be presented initially with ocular lesions and no (or vague) systemic signs.
Fig. 5.
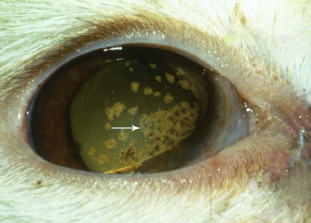
Anterior uveitis caused by feline coronavirus in a cat. Note the large keratic precipitates on the corneal endothelial surface (arrow). The diagnosis was made based on a rising serum corona virus titer and eventual postmortem results that supported a diagnosis of feline infectious peritonitis.
Fig. 6.
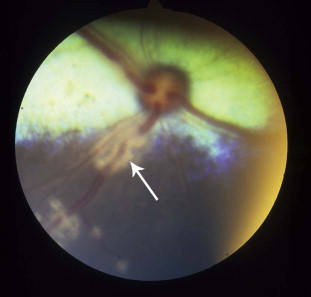
Retinal vasculitis caused by feline coronavirus in a cat. Note the inflammatory cell cuffing around retinal blood vessels (arrow). The diagnosis was made based on postmortem results that supported a diagnosis of feline infectious peritonitis.
In a study of naturally infected barrier-reared cats and their offspring, recurring bouts of URTD and conjunctivitis occurred at intervals of about 4 months, beginning at 4–5 weeks of age (Hok, 1993a). The cats were negative for FHV-1, FCV and FeLV on laboratory testing. FCoV antigen was detected in the conjunctiva of the nictitating membrane in 90% of cats and persisted throughout the investigation. Virus isolation was also positive from swabs of the nictitating membrane, suggesting that ocular exudates from these cats were potentially infectious. When kittens serologically negative for FCoV were placed in catteries with a history of FIP and observed for 100 days, there was 100% morbidity and 90% mortality, with affected kittens developing recurrent bouts of conjunctivitis, URTD and gastrointestinal signs (Hok, 1993b).
Diagnosis
The antemortem diagnosis of FIP remains difficult. Physical examination and laboratory tests, including a complete blood count and a serum chemistry panel, as well as analysis of abdominal or thoracic fluid samples, can be helpful in making a presumptive diagnosis of FIP. A rising serum FCoV titer in the face of clinical disease consistent with FIP is suggestive, but not diagnostic. The most definitive diagnosis is achieved through the histopathologic examination of tissues obtained through biopsy or necropsy (Addie et al., 2009).
Treatment
General treatment for uveitis associated with FIP is discussed below. In cats with large fibrin clots in the anterior chamber, an intra-cameral injection of 25 μg tissue plasminogen activator can be performed if the fibrin has not been present longer than about 10 days. However, it should be expected that the fibrin could reform given the underlying infection with FCoV.
Prognosis
The prognosis for FIP in general is poor; however, cats with mild uveitis can improve with topical therapy.
Feline panleukopenia
Feline panleukopenia is caused by feline parvovirus (FPV), a single stranded DNA virus. The virus can persist long term in the environment; thus, cats are easily exposed, mostly by 1 year of age. In utero transmission also occurs (Truyen et al., 2009).
Clinical signs
In most cats infected with FPV, there are no apparent clinical signs (Greene, 2012). Of those that become ill, systemic signs include acute onset fever, depression, vomiting, diarrhea, severe dehydration and thickened bowel loops. Animals are usually markedly leukopenic. Queens infected or vaccinated just before or during pregnancy can show infertility or abortion, but with no clinical illness detected in the queen. Kittens born to infected queens might have cerebellar hypoplasia, with intention tremors, ataxia and a hypermetric gait.
The ocular manifestations of FPV have primarily been reported as retinal dysplasia and degeneration in kittens infected in utero or in the neonatal period. Retinal dysplasia might have different appearances on fundic examination, depending on whether the lesions are geographic or focal, and whether they are located within the tapetal or non-tapetal fundus. Within the tapetal fundus, areas that have altered reflectivity (hyperreflectivity or hyporeflectivity) can be visualized. Rosettes (microscopic foldings of the retina into circular lesions) appear as tiny grey round foci of varying numbers (Fig. 7 ). Geographic dysplasia will appear as larger, irregular areas of altered reflectivity. Within the non-tapetal fundus, lesions will appear pale or de-pigmented as the retinal pigment epithelium becomes disrupted (Greene, 2012).
Fig. 7.
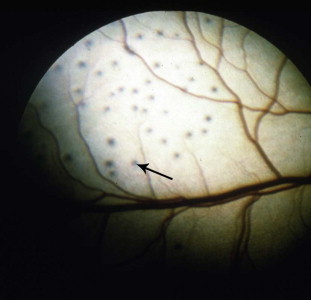
Retinal dysplasia in a kitten. Note the multifocal hyporeflective foci within the tapetal fundus (arrow) that represent rosettes. No specific cause of retinal dysplasia was found in this otherwise healthy kitten.
Conjunctivitis, along with URTD, has been reported in two experimentally infected kittens (Csiza et al., 1972). A 6-week old stray kitten with partial blindness, ataxia and incoordination had cerebellar hypoplasia and FPV was isolated from the cerebellum (Percy et al., 1975). On histopathological examination, the retinas were thin, with rosette formation and loss of normal architecture.
Diagnosis
The diagnosis of FPV in cases with ocular manifestations is usually through serum virus neutralization assays (Greene, 2012). The first assay should be run as soon as possible after clinical signs develop and a second titer run 2 weeks later. A fourfold rise in titer is indicative of acute infection. A presumptive diagnosis can be made on the basis of clinical signs and leukopenia.
Treatment
Therapy for feline panleukopenia is supportive. There is no treatment for retinal dysplasia or degeneration.
Prognosis
The prognosis for recovery from FPV depends on the severity of clinical signs, timeliness of and response to therapy. Visual deficits secondary to retinal dysplasia or degeneration can be expected to remain static once active infection has subsided (Greene, 2012).
General treatment for uveitis
Specific therapy for feline virus-induced uveitis is not available, but cats should be treated symptomatically with anti-inflammatory therapy. If anterior uveitis is mild, treatment with a topical NSAID such as 0.1% diclofenac 2–3 times daily might be adequate. The use of a NSAID will not have the same risk of exacerbating FHV-1 infection as corticosteroid treatment. However, if the uveitis is moderate to severe, the use of a topical potent corticosteroid, such as 1% prednisolone acetate or 0.1% dexamethasone, four times daily is indicated. When the uveitis has improved, it might be possible to switch to a topical NSAID for maintenance therapy. Combination therapy with a topical corticosteroid and a topical NSAID is safe and helpful in many cases by allowing the steroid to be used less frequently, and by treating the inflammation with two classes of drugs, each that alters the inflammatory cascade by a different mechanism (Holmberg, Maggs, 2004, Giuliano, 2004).
The use of topical atropine for mydriasis and iridocycloplegia, and prevention of synechiae, is helpful in cases with pain, miosis and low intraocular pressure. If intraocular pressure is normal or high in the face of uveitis, atropine should be avoided, since it can exacerbate the development of glaucoma. The ointment formulation of atropine, rather than solution, should be used in cats due to the bitter taste of the compound and because the solution runs down the nasolacrimal duct and onto the tongue quickly.
Oral anti-inflammatory drugs should be considered in cases of severe anterior uveitis or posterior segment inflammation that cannot be treated by topical drug application. Corticosteroids, such as prednisolone, provide the most potent anti-inflammatory activity, but have no analgesic effect. The use of NSAIDs should be considered on a case-by-case basis, giving full consideration to safety and duration of treatment. Oral corticosteroids and NSAIDs should never be used together.
Conclusions
Viral disease should always be on the differential diagnosis list for cats that are presented with ocular inflammation. FHV-1 is the most common virus associated with ocular manifestations, the only virus to cause corneal ulceration and the virus that can be specifically targeted with anti-viral drug therapy. Cats that present with uveitis should be tested for viral agents that have the ability to cause ocular inflammation (FeLV, FIV and FIP). Appropriate treatment instituted as early as possible in the course of ocular disease will result in the best prognosis.
Conflict of interest statement
The author has no financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper.
References
- Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A., Lutz H. Feline infectious peritonitis. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:594–604. doi: 10.1016/j.jfms.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. Antiviral treatment of cytomegalovirus infection. Infectious Disorders – Drug Targets. 2011;11:475–503. doi: 10.2174/187152611797636640. [DOI] [PubMed] [Google Scholar]
- Albert D.M. The role of viruses in the development of ocular tumors. Ophthalmology. 1980;87:1219–1225. doi: 10.1016/s0161-6420(80)35104-x. [DOI] [PubMed] [Google Scholar]
- Albert D.M., Lahay M., Colby E.D., Shadduck J.A., Sang D.N. Retinal neoplasia and dysplasia. I. Induction by feline leukemia virus. Investigative Ophthalmology and Visual Science. 1977;16:325–337. [PubMed] [Google Scholar]
- Ammersbach M., Little S., Bienzle D. Preliminary evaluation of a quantitative polymerase chain reaction assay for diagnosis of feline immunodeficiency virus infection. Journal of Feline Medicine and Surgery. 2013;15:725–729. doi: 10.1177/1098612X13475465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August J.R. Feline infectious peritonitis, an immune-mediated coronaviral vasculitis. Veterinary Clinics of North America: Small Animal Practice. 1984;14:971–984. doi: 10.1016/S0195-5616(84)50102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannasch M.J., Foley J.E. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. Journal of Feline Medicine and Surgery. 2005;7:109–119. doi: 10.1016/j.jfms.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman A.H., Ogilvie G.K., Tompkins M. Ocular disease in FeLV-positive cats: 11 cases (1981–1986) Journal of the American Veterinary Medical Association. 1991;198:1049–1051. [PubMed] [Google Scholar]
- Brown M.A. Genetic determinants of pathogenesis by feline infectious peritonitis virus. Veterinary Immunology and Immunopathology. 2011;143:265–268. doi: 10.1016/j.vetimm.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgesser K.M., Hotaling S., Schiebel A., Ashbaugh S.E., Roberts S.M., Collins J.K. Comparison of PCR, virus isolation, and indirect fluorescent antibody staining in the detection of naturally occurring feline herpesvirus infections. Journal of Veterinary Diagnostic Investigation. 1999;11:122–126. doi: 10.1177/104063879901100203. [DOI] [PubMed] [Google Scholar]
- Campbell L.H., Reed C. Ocular signs associated with feline infectious peritonitis in two cats. Feline Practice. 1975;5:32–35. [Google Scholar]
- Coyne K.P., Jones B.R., Kipar A., Chantrey J., Porter C.J., Barber P.J., Dawson S., Gaskell R.M., Radford A.D. Lethal outbreak of disease associated with feline calicivirus infection in cats. Veterinary Record. 2006;158:544–550. doi: 10.1136/vr.158.16.544. [DOI] [PubMed] [Google Scholar]
- Csiza C.K., Scott F.W., De Lahunta A., Gillespie J.H. Respiratory signs and central nervous system lesions in cats infected with panleukopenia virus. A case report. Cornell Veterinarian. 1972;62:192–195. [PubMed] [Google Scholar]
- Cullen C.L., Haines D.M., Jackson M.L., Grahn B.H. Lack of detection of feline leukemia and feline sarcoma viruses in diffuse iris melanomas of cats by immunohistochemistry and polymerase chain reaction. Journal of Veterinary Diagnostic Investigation. 2002;14:340–343. doi: 10.1177/104063870201400414. [DOI] [PubMed] [Google Scholar]
- Dean E., Meunier V. Feline eosinophilic keratoconjunctivitis: A retrospective study of 45 cases (56 eyes) Journal of Feline Medicine and Surgery. 2013;15:661–666. doi: 10.1177/1098612X12472181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M.J. Ocular manifestations of feline infectious peritonitis. Journal of the American Veterinary Medical Association. 1971;159:417–424. [PubMed] [Google Scholar]
- Drazenovich T.L., Fascetti A.J., Westermeyer H.D., Sykes J.E., Bannasch M.J., Kass P.H., Hurley K.F., Maggs D.J. Effects of dietary supplementation on upper respiratory and ocular disease and detection of infectious organisms in cats within an animal shelter. American Journal of Veterinary Research. 2009;70:1391–1400. doi: 10.2460/ajvr.70.11.1391. [DOI] [PubMed] [Google Scholar]
- English R.V., Davidson M.G., Nasisse M.P., Jamieson V.E., Lappin M.R. Intraocular disease associated with feline immunodeficiency virus infection in cats. Journal of the American Veterinary Medical Association. 1990;196:1116–1119. [PubMed] [Google Scholar]
- Fontanelle J.P., Powell C.C., Veir J.K., Radecki S.V., Lappin M.R. Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesvirus-1 infection in cats. American Journal of Veterinary Research. 2008;69:289–293. doi: 10.2460/ajvr.69.2.289. [DOI] [PubMed] [Google Scholar]
- Gabor L.J., Love D.N., Malik R., Canfield P.J. Feline immunodeficiency virus status of Australian cats with lymphosarcoma. Australian Veterinary Journal. 2001;79:540–545. doi: 10.1111/j.1751-0813.2001.tb10742.x. [DOI] [PubMed] [Google Scholar]
- Gerriets W., Joy N., Heubner-Guthardt J., Eule J.C. Feline calicivirus: A neglected cause of feline ocular surface infections? Veterinary Ophthalmology. 2012;15:172–179. doi: 10.1111/j.1463-5224.2011.00957.x. [DOI] [PubMed] [Google Scholar]
- Giuliano E.A. Nonsteroidal anti-inflammatory drugs in veterinary ophthalmology. Veterinary Clinics of North America: Small Animal Practice. 2004;34:707–723. doi: 10.1016/j.cvsm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Greene C.E. Feline enteric viral infections. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. Fourth Ed. Elsevier; St. Louis, Missouri, USA: 2012. pp. 80–91. [Google Scholar]
- Herring I.P., Troy G.C., Toth T.E., Champagne E.S., Pickett J.P., Haines D.M. Feline leukemia virus detection in corneal tissues of cats by polymerase chain reaction and immunohistochemistry. Veterinary Ophthalmology. 2001;4:119–126. doi: 10.1046/j.1463-5224.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- Hok K. Development of clinical signs and occurrence of feline corona virus antigen in naturally infected barrier cats and their offspring. Acta Veterinaria Scandinavica. 1993;34:345–356. doi: 10.1186/BF03548177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok K. Morbidity, mortality and coronavirus antigen in previously coronavirus free kittens placed in two catteries with feline infectious peritonitis. Acta Veterinaria Scandinavica. 1993;34:203–210. doi: 10.1186/BF03548211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg B.J., Maggs D.J. The use of corticosteroids to treat ocular inflammation. Veterinary Clinics of North America: Small Animal Practice. 2004;34:693–705. doi: 10.1016/j.cvsm.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Hurley K.E., Pesavento P.A., Pedersen N.C., Poland A.M., Wilson E., Foley J.E. An outbreak of virulent systemic feline calicivirus disease. Journal of the American Veterinary Medical Association. 2004;224:241–249. doi: 10.2460/javma.2004.224.241. [DOI] [PubMed] [Google Scholar]
- La Croix N.C., van der Woerdt A., Olivero D.K. Nonhealing corneal ulcers in cats: 29 cases (1991–1999) Journal of the American Veterinary Medical Association. 2001;218:733–735. doi: 10.2460/javma.2001.218.733. [DOI] [PubMed] [Google Scholar]
- Loesenbeck G., Drommer W., Egberink H.F., Heider H.J. Immunohistochemical findings in eyes of cats serologically positive for immunodeficiency virus (FIV) Zentralblatt für Veterinärmedizin. Reihe B. 1996;43:305–311. doi: 10.1111/j.1439-0450.1996.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Louwerens M., London C.A., Pedersen N.C., Lyons L.A. Feline lymphoma in the post-feline leukemia virus era. Journal of Veterinary Internal Medicine. 2005;19:329–335. doi: 10.1892/0891-6640(2005)19[329:flitpl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lutz H., Addie D., Belák S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A. Feline leukaemia. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:565–574. doi: 10.1016/j.jfms.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magden E., Miller C., MacMillan M., Bielefeldt-Ohmann H., Avery A., Quackenbush S.L., Vandewoude S. Acute virulent infection with feline immunodeficiency virus (FIV) results in lymphomagenesis via an indirect mechanism. Virology. 2013;436:284–294. doi: 10.1016/j.virol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Maggs D.J., Clarke H.E. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine, and acyclovir against feline herpesvirus type-1. American Journal of Veterinary Research. 2004;65:399–403. doi: 10.2460/ajvr.2004.65.399. [DOI] [PubMed] [Google Scholar]
- Nasisse M.P., Guy J.S., Davidson M.G., Sussman W., De Clercq E. In vitro susceptibility of feline herpesvirus-1 to vidarabine, idoxuridine, trifluridine, acyclovir, or bromovinyldeoxyuridine. American Journal of Veterinary Research. 1989;50:158–160. [PubMed] [Google Scholar]
- Nasisse M.P., Glover T.L., Moore C.P., Weigler B.J. Detection of feline herpesvirus 1 DNA in corneas of cats with eosinophilic keratitis or corneal sequestration. American Journal of Veterinary Research. 1998;59:856–858. [PubMed] [Google Scholar]
- Percy D.H., Scott F.W., Albert D.M. Retinal dysplasia due to feline panleukopenia virus infection. Journal of the American Veterinary Medical Association. 1975;167:935–937. [PubMed] [Google Scholar]
- Phillips T.R., Prospero-Garcia O., Puaoi D.L., Lerner D.L., Fox H.S., Olmsted R.A., Bloom F.E., Henriksen S.J., Elder J.H. Neurological abnormalities associated with feline immunodeficiency virus infection. Journal of General Virology. 1994;75:979–987. doi: 10.1099/0022-1317-75-5-979. [DOI] [PubMed] [Google Scholar]
- Ramsey D.T. Feline chlamydia and calicivirus infections. Veterinary Clinics of North America: Small Animal Practice. 2000;30:1015–1028. doi: 10.1016/s0195-5616(00)05004-x. [DOI] [PubMed] [Google Scholar]
- Rees T.M., Lubinski J.L. Oral supplementation with l-lysine did not prevent upper respiratory infection in a shelter population of cats. Journal of Feline Medicine and Surgery. 2008;10:510–513. doi: 10.1016/j.jfms.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B.S., Poulet H., Pingret J.L., Jas D., Brunet S., Lemeter C., Etievant M., Boucraut-Baralon C. A nosocomial outbreak of feline calicivirus associated virulent systemic disease in France. Journal of Feline Medicine and Surgery. 2009;11:633–644. doi: 10.1016/j.jfms.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G., Klein D., Knapp E., Hosie M.J., Grimes T., Mabruk M.J.E.M.F., Jarrett O., Callanan J.J. Dynamics of viral and proviral loads of feline immunodeficiency virus with the feline central nervous system during the acute phase following intravenous infection. Journal of Virology. 2003;77:7477–7485. doi: 10.1128/JVI.77.13.7477-7485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer L.S., Keller C.B., Bienzle D. Effects of cidofovir on cell death and replication of feline herpesvirus-1 in cultured feline corneal epithelial cells. American Journal of Veterinary Research. 2005;66:217–222. doi: 10.2460/ajvr.2005.66.217. [DOI] [PubMed] [Google Scholar]
- Slack J.M., Stiles J., Leutenegger C.M., Moore G.E., Pogranichniy R.M. Effects of topical ocular administration of high doses of human recombinant interferon alpha-2b and feline recombinant interferon omega on naturally occurring viral keratoconjunctivitis in cats. American Journal of Veterinary Research. 2013;74:281–289. doi: 10.2460/ajvr.74.2.281. [DOI] [PubMed] [Google Scholar]
- Slauson D.O., Finn J.P. Meningoencephalitis and panophthalmitis in feline infectious peritonitis. Journal of the American Veterinary Medical Association. 1972;160:729–734. [PubMed] [Google Scholar]
- Smith A.W., Iversen P.L., O'Hanley P.D., Skilling D.E., Christensen J.R., Weaver S.S., Longley K., Stone M.A., Poet S.E., Matson D.O. Virus-specific antiviral treatment for controlling severe and fatal outbreaks of feline calicivirus infection. American Journal of Veterinary Research. 2008;69:23–32. doi: 10.2460/ajvr.69.1.23. [DOI] [PubMed] [Google Scholar]
- Stiles J. Feline ophthalmology. In: Gelatt K.N., Gilger B.C., Kern T.J., editors. Veterinary Ophthalmology. Fifth Ed. John Wiley & Sons; Ames, Iowa, USA: 2013. pp. 1477–1559. [Google Scholar]
- Stiles J., Pogranichniy R.M. Detection of virulent feline herpesvirus-1 in the corneas of clinically normal cats. Journal of Feline Medicine and Surgery. 2008;10:154–159. doi: 10.1016/j.jfms.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J., McDermott M., Willis M., Roberts W., Greene C. Comparison of nested polymerase chain reaction, virus isolation, and fluorescent antibody testing for identifying feline herpesvirus in cats with conjunctivitis. American Journal of Veterinary Research. 1997;58:804–807. [PubMed] [Google Scholar]
- Stiles J., Bienzle D., Render J.A., Buyukmihci N.C., Johnson E.C. Use of nested polymerase chain reaction (PCR) for detection of retroviruses from formalin-fixed, paraffin-embedded uveal melanomas in cats. Veterinary Ophthalmology. 1999;2:113–116. doi: 10.1046/j.1463-5224.1999.00066.x. [DOI] [PubMed] [Google Scholar]
- Stiles J., Townsend W.M., Rogers Q.R., Krohne S.G. Effect of oral administration of l-lysine on conjunctivitis caused by feline herpesvirus in cats. American Journal of Veterinary Research. 2002;63:99–103. doi: 10.2460/ajvr.2002.63.99. [DOI] [PubMed] [Google Scholar]
- Stiles J., Gwin W., Pogranichniy R. Stability of 0.5% cidofovir stored under various conditions for up to 6 months. Veterinary Ophthalmology. 2010;13:275–277. doi: 10.1111/j.1463-5224.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- Thomasy S.M., Lim C.C., Reilly C.M., Kass P.H., Lappin M.R., Maggs D.L. Evaluation of orally administered famciclovir in cats experimentally infected with feline herpesvirus type-1. American Journal of Veterinary Research. 2011;72:85–95. doi: 10.2460/ajvr.72.1.85. [DOI] [PubMed] [Google Scholar]
- Thomasy S.M., Covert J.C., Stanley S.D., Maggs D.J. Pharmacokinetics of famciclovir and penciclovir in tears following oral administration of famciclovir to cats: A pilot study. Veterinary Ophthalmology. 2012;15:299–306. doi: 10.1111/j.1463-5224.2011.00984.x. [DOI] [PubMed] [Google Scholar]
- Townsend W.T., Stiles J., Guptill-Yoran L., Krohne S.G. Development of a reverse transcriptase-polymerase chain reaction assay to detect feline herpesvirus-1 latency-associated transcripts in the trigeminal ganglia and corneas of cats that did not have clinical signs of ocular disease. American Journal of Veterinary Research. 2004;65:314–319. doi: 10.2460/ajvr.2004.65.314. [DOI] [PubMed] [Google Scholar]
- Truyen U., Addie D., Belák S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A. Feline panleukopenia. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:538–546. doi: 10.1016/j.jfms.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meulen K., Garre B., Croubels S., Nauwynck H. In vitro comparison of antiviral drugs against feline herpesvirus-1. BMC Veterinary Research. 2006;2:13. doi: 10.1186/1746-6148-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardley R.C., Gaskell R.M., Povey R.C. Feline respiratory viruses – Their prevalence in clinically healthy cats. Journal of Small Animal Practice. 1974;10:579–586. doi: 10.1111/j.1748-5827.1974.tb06538.x. [DOI] [PubMed] [Google Scholar]
- Weiss A.T., Klopfleisch R., Gruber A.D. Prevalence of feline leukemia provirus DNA in feline lymphomas. Journal of Feline Medicine and Surgery. 2010;12:929–935. doi: 10.1016/j.jfms.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J.K., Hansen H., Ho E.W., Morishita T.Y., Okuda T., Sawa T.R., Nakamura R.M., Pedersen N.C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. Journal of the American Veterinary Medical Association. 1989;194:213–220. [PubMed] [Google Scholar]


