Abstract
The nitric oxide synthase (NOS) activity in the haemocytes of shrimps Fenneropenaeus chinensis (Osbeck) and Marsupenaeus japonicus (Bate) was studied after white spot syndrome virus (WSSV) infection to determine its characteristics in response to virus infection. First, the NOS activity in haemocytes of shrimps was determined by the means of NBT reduction and changes in cell conformation. And the variations of NOS activity in shrimps after challenge with WSSV intramuscularly were evaluated through the analysis of l-citrulline and total nitrite/nitrate (both as NO derivates) concentrations. The result showed that NOS activity in the haemocytes of F. chinensis increased slightly from 0 to 12 h postchallenge, indicated by the variations of l-citrulline (from 11.15 ± 0.10 to 12.08 ± 0.64 μM) and total nitrite/nitrate concentrations (from 10.45 ± 0.65 to 12.67 ± 0.52 μM). Then it decreased sharply till the end of the experiment (84 h postchallenge), the concentrations of l-citrulline and total nitrite/nitrate at 84 h were 1.58 ± 0.24 and 2.69 ± 0.70 μM, respectively. The LPS-stimulated NOS activity kept constant during the experiment. However, in M. japonicus, the NOS activity kept increasing during the first 72 h postchallenge, the concentrations of l-citrulline and total nitrite/nitrate increased from 7.82 ± 0.77 at 0 h to 10.79 ± 0.50 μM at 72 h, and from 8.98 ± 0.43 at 0 h to 11.20 ± 0.37 μM at 72 h, respectively. Then it decreased till the end of the experiment (216 h postchallenge), and the concentrations of l-citrulline and total nitrite/nitrate at 216 h were 5.66 ± 0.27 and 4.68 ± 0.16 μM, respectively. More importantly, an apparent increase of LPS-stimulated NOS activity was observed in M. japonicus at 48 h postchallenge, which was about 4 times higher than that in the control group of health shrimps. In correspondence with the difference of NOS activity between the two species of shrimps, the cumulative mortalities of the shrimps were also different. All shrimps of F. chinensis in the mortality experiment died in 66 h, much more quickly than M. japonicus, whose accumulative mortality reached 100% after 240 h. Data here reported let us hypothesize that NOS activity in the haemocytes of shrimps F. chinensis and M. japonicus responses to WSSV infection differently, and this might be one of the reasons for the different susceptibility of F. chinensis and M. japonicus to WSSV infection.
Keywords: Fenneropenaeus chinensis (Osbeck), Marsupenaeus japonicus (Bate), White spot syndrome virus, Nitric oxide synthase
Penaeid shrimp culture is a worldwide economic activity. However, the intensification and environment degradation of shrimp farming have been accompanied by the development of many infectious diseases, especially from viral origin, which cause a dramatic decrease in shrimp production [1], [2]. White spot syndrome virus (WSSV), a circular double-strand DNA virus [3], [4], [5], [6], has led to heavy crop loss and continues to seriously affect the cultured penaeids in the shrimp farming systems around the world [7]. In this context, control of disease is very important to insure the long-term survival of shrimp aquaculture. Therefore, it is necessary to study the immune factors of shrimp in response to the invasion of WSSV, which may be helpful in understanding the mechanism of shrimp immunity to WSSV infection and developing strategies for management of the disease.
Belonging to invertebrate, shrimp lack a true adaptive immune response system [8]. However, living in an aquatic environment rich in microorganisms, shrimps have developed effective systems for detecting and eliminating noxious microorganism depending on non-specific innate immune response based on the activity of haemocytes, including phagocytosis, phenoloxidase releasing, bactericidin synthesis, and so on [2]. Non-specific molecules like reactive oxygen intermediates (ROIs) and reactive nitrogen intermediates (RNIs) are also involved. Surprisingly, in shrimps, the function and immune status of nitric oxide synthase (NOS) and its catalyzing products—nitric oxide, have been seldom reported. Nitric oxide is a very important component of innate immune system, which has the anti-viral, anti-bacterial, and anti-parasite properties by damaging the DNA, enzyme and membrane of pathogen directly or indirectly through the action on the bases and strands of DNA, protein and lipid of membrane [9], [10], [11], [12]. Therefore, the role of NO and NOS in disease resistance of shrimps is worth studying. In this paper, we have tried different methods to determine NOS activity in the haemocytes of Fenneropenaeus chinensis and Marsupenaeus japonicus qualitatively and quantitatively, and the role of the NOS in haemocytes of shrimps is also discussed in response to virus infection.
Experimental procedures
Animals
Marsupenaeus japonicus and F. chinensis were obtained from Ocean Shrimp Farm in Qingdao, China, with average length of 14.43 ± 0.86 cm (mean ± SD, N = 80) and 16.70 ± 1.40 cm (mean ± SD, N = 80), and average weight of 19.84 ± 3.71 g (mean ± SD, N = 80) and 25.90 ± 7.05 g (mean ± SD, N = 80), respectively. They were fed with artificial diet twice a day and acclimated in aerated flat-bed tanks containing sand filtrated sea water (20 ± 1 °C; salinity, 30‰) for 7 days prior to experiment. Every day the unconsumed food and faeces were removed carefully with a siphon. All shrimps used in experiment were intermoult males.
Chemicals
All chemicals used in the experiment were A.R (Analytical reagent) grade or better. The glassware was pyrogen-free to avoid the enzymatic interruption by endotoxin.
Haemocytes sample preparation
Haemolymph of shrimp was collected from the ventral-sinus with a 26-gauge needle and 1-ml syringe containing pre-cooled (4 °C) anti-coagulant solution [13] (450 mM NaCl, 10 mM KCl, 10 mM EDTA-Na2, and 10 mM HEPES, pH 7.3, 850 mOsmol/kg, sterilized by filtration (0.2 μm) before using). The mixture was then injected into a 50 ml centrifuge tube containing anti-coagulant solution. The solution was mixed thoroughly and centrifuged at 600g for 10 min at 4 °C. The supernatant was removed and haemocytes were adjusted to a certain cell density and resuspended in l-arginine solution (0.1 M Bis–Tris buffer containing 5 mM l-arginine (Sigma) and 5 mM Ca2+) [14] to make haemocytes suspension for NOS activity analysis.
Identification of NOS activity in haemocytes of shrimps
NBT reduction method
The NOS activity in haemocytes of shrimps was determined with NBT reduction method, according to Weiske and Wiesner [15]. The procedure was introduced briefly as below: 500 μl LPS solution [0.1 M Bis–Tris buffer containing 200 μg/ml LPS (Sigma L2630)] [14] was mixed with 500 μl haemocytes suspension with cell density of 106 and incubated at 4 °C for 4 hours. The mixture was then centrifuged at 600g for 2 min at 4 °C, and the supernatant was discarded. The residue haemocytes were fixed with 4% formaldehyde (dissolved in 0.1 M Tris buffer with final pH of 7.5–8.0) for 20 min, and the cells were washed at least twice with 0.1 M Tris buffer [pH 7.8, containing 0.1% Triton X-100 (Serva 37240)]. The cells were then resuspended in the freshly prepared 0.01% NBT (Sigma N6876) solution [in 0.1 M Tris buffer containing 0.1% Triton X-100 and 0.01% NADPH (Sigma N1630)] and incubated for 10 min at room temperature. Subsequently the mixture was centrifuged at 600g for 5 min at 4 °C, and the supernatant was replaced with 0.7 ml of 70% methanol. After intensive mixing and a further centrifugation step at 600g for 10 min at room temperature, the methanol was replaced with 0.6 ml DMSO solution [54% (v/v) dimethyl sulfoxide (Sigma D8779) and 46% (v/v) 2 N KOH] to dissolve the formazan. After a final incubation for 30 min at room temperature with intensive mixing at 10 min intervals, the contents were centrifuged at 10,000g for 5 min at room temperature, and the absorbance of the resulting supernatants was determined at 670 nm (BECKMAN DU 650 spectrophotometer). The NADPH in the 0.01% NBT solution was replaced with NADH (Sigma N8129) was used as control.
Haemocyte conformation approach
The NOS activity in haemocytes of shrimps was also determined by haemocyte conformation approach, according to Ottaviani et al. [16]. Briefly, 25 μl mixture of anti-coagulant and haemolymph with cell density of 106 was placed on a dichromate cleaned glass slides and incubated in humidified chamber for 30 min at room temperature. The slides were then rinsed with 0.1 M Bis–Tris buffer to remove the anti-coagulant and the cells that did not adhere to the slide. Subsequently the slides were divided into four groups (A–D). Group A was added with 25 μl Escherichia coli suspension at density of 1 × 108 cells/ml, group B was added with 25 μl E. coli suspension and 25 μl LPS solution, group C was added with 25 μl E. coli suspension, 25 μl LPS solution, and 25 μl l-NMMA (N ω-monomethyl-l-arginine, Sigma M7033) solution with final concentration of 800 μM, and group D was added with 25 μl E. coli suspension and 25 μl SNP (sodium nitroprusside, Sigma) solution with final concentration of 10 mM. After incubation in humidified chamber for 4 h, samples were stained with toludine blue solution (0.5% toludine blue in 3 mM borate solution) for 5 min, and rinsed with water for 1 min. The samples were then observed under microscope (Zeiss, Jena, Germany) at a magnification of 400.
Analysis of NOS activity in haemocytes of shrimps
NOS activity was analyzed quantitatively by determination of l-citrulline and total nitrite/nitrate concentrations. Briefly, 500 μl haemocytes suspension with cell density of 105 cell/ml was mixed with 500 μl LPS solution, and incubated at 4 °C for 8 h [14]. Then, the haemocytes were lysed by adding 1% Triton X-100 and agitating on a vortex mixer. After centrifugation at 13,000g for 15 min at room temperature, the supernatants were removed and ultrafiltrated. The filtrate was used to determine the concentration of l-citrulline and total nitrite/nitrate. The haemocytes not incubated with LPS were used as control. The differences in NOS activity between the LPS treated haemocytes and the control was considered as LPS stimulated NOS activity.
Determination of l-citrulline
l-Citrulline was determined according to Marzinzig et al. (1997) [17] with modifications described in Weiske and Wiesner (1999) [15]. For each sample, 150 μl filtrate was mixed with 50 μl urease (Sigma U2125) solution (165 U/ml) and incubated at 37 °C for 30 min. Then, 50 μl of 2.45 M trichloroacetic acid was added and mixed thoroughly. After centrifugation at 13,000g for 15 min at room temperature, 150 μl supernatant was collected and mixed with 450 μl ADMS reagent [18% (v/v) 47.7 mM anti-pyrine (Sigma A5882) in distilled water + 40% (v/v) 79 mM diacetyl monoxime (Sigma B0753) in 83 mM acetic acid + 42% (v/v) 15 N H2SO4]. The mixture was incubated for 25 min in a boiling water bath. Samples were cooled to room temperature in the dark and then centrifuged at 13,000g for 5 min, and the absorbance of the supernatant was measured at 450 nm (BECKMAN DU 650 spectrophotometer). The concentration of l-citrulline (Sigma C7629) was calculated according to the standard curve of l-citrulline prepared.
Determination of total nitrite/nitrate
Nitrite/nitrate determination was carried out according to Marzinzig et al. [17] with improvements described in Weiske and Wiesner [15]. For each assay, 300 μl of ultrafiltrated supernatant was mixed with 20 μl Bis–Tris buffer containing FAD [flavin adenine dinucleotide (Sigma F6625), final concentration 5.5 μM], NADPH (final concentration 30.0 μM), and nitrate reductase (Sigma N7265, final concentration 0.1 U/ml). The mixture was incubated for 60 min at 37 °C under dark condition [18]. Then, 20 μl Bis–Tris buffer containing l-lactic dehydrogenase (Sigma L2500, final concentration 0.1 kU/ml) and sodium pyruvate (Sigma P2256, final concentration 0.3 mM) were added and incubated for another 30 min at 37 °C. After centrifugation at 10,000g for 15 min at 4 °C, 300 μl supernatant was mixed thoroughly with 150 μl dapsone solution (Sigma D2505, final concentration 4.7 μM), and 150 μl NEDA [N-(1-naphthyl)ethylenediamine, Sigma N9125] solution (final concentration 1 mM) was added 5 min later. The resulted solution was centrifuged at 10,000g for 5 min at 4 °C, and the absorbance of supernatant was measured at 550 nm (BECKMAN DU 650 spectrophotometer). The concentration of total nitrite/nitrate was calculated according to the standard curve of nitrite prepared.
Comparative study of NOS activity in F. chinensis and M. japonicus after WSSV-infection
Preparation of WSSV stock solution
The carapace, appendages, and hepatopancreas were discarded from frozen F. chinensis with severe WSSV-infections. The residual tissues were then ground into pieces and 1 g tissue was taken to mix with 4 ml of PPB [19] (NaCl, 23.0 g/L; K2SO4, 1.1 g/L; CaCl2, 1.6 g/L; MgSO4 · 7H2O, 1.6 g/L; NaH2PO4 · 2H2O, 0.35 g/L; and NaHCO3, 0.05 g/L; pH 6.5; osmolarity, 867.9 mOsmol/L, sterilized by filtration (0.2 μm) before using). The mixture was homogenized at 3000 rpm for 5 min and then centrifuged at 5520g for 30 min at 4 °C. The supernatant was centrifuged at 9820g for 30 min at 4 °C for 2 times. Then, the supernatant was centrifuged at 15,300g for 60 min at 4 °C, the precipitate diluted by PPB was used as a virus stock solution.
Experimental treatments
After acclimation, 80 shrimps were injected intramuscularly with 0.1 ml diluted virus stock solution (100×) in the lateral area of the fourth abdominal segment. The infected shrimps were then subdivided randomly into two groups and placed in two aquaria (200 L) with flow-through seawater supply. One group of shrimps was used for determination of NOS activity, and the other group was used for mortality observation. Another 80 shrimps injected with 0.1 ml PPB solution were used as control. The shrimps were fed with artificial diet twice a day, the unconsumed food and faeces were removed carefully with a siphon once a day.
Haemolymph sample collection and analysis of NOS activity
For both infected and health shrimps, haemolymph sample was collected from three shrimps each time and mixed together, as described in the paragraph of Haemocytes sample preparation. The haemocytes were adjusted to a cell density of 105 cells/ml and resuspended in l-arginine solution. The mixture was divided into six Eppendorf micro-centrifuge tubes, three of them were incubated with LPS, the other three were used as control, as described in the paragraph of Analysis of NOS activity in haemocytes of shrimps. For each tube, both l-citrulline and total nitrite/nitrate were analyzed.
The NOS activity of M. japonicus were assayed at 0, 24, 48, 72, 96, 120, 144, 168, 192, and 216 h postchallenge, and that of F. chinensis were assayed at 0, 12, 36, 60, and 84 h postchallenge, depending on the surviving time of the shrimps in the experiment.
Diagnosis of WSSV in M. japonicus and F. chinensis
Diagnosis of WSSV was performed by Digoxigenin Labeled DNA Probe Dot-blot hybridization Kit purchased from the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. Following blood sampling for analysis of NOS activity, gills of the same shrimps collected were used for WSSV detection [20].
Statistics
Data are reported as means ± SE for each treatment, respectively. The significance of variations between different treatments or groups was analyzed by Student’s t test or ANOVA in software Excel. The variation was considered to be significant if P < 0.01.
Results
Identification and analysis of NOS activity in haemocytes of shrimps
Both NBT method and haemocyte conformation approach confirmed the NOS activity in haemocytes of the shrimps. The results of NBT method showed that LPS stimulated the activity of NOS in both species of shrimps, after fixation with 4% formaldehyde, with the absorbance value significantly higher than the control (P < 0.001). For F. chinensis and M. japonicus, the absorbance value increased from 0.091 ± 0.006 to 0.160 ± 0.008 and from 0.106 ± 0.021 to 0.458 ± 0.048, respectively.
The results of haemocyte conformation approach also confirmed the NOS activity in the haemocytes of both species. Most of the haemocytes without any treatment were in amoeboid shape with sporadic bacteria congregated or clumped in their vicinity, as shown in Fig. 1, Fig. 2 A. However, most of the haemocytes incubated with LPS became round instead of amoeboid, with a lot of bacteria aggregated around the haemocytes (Figs. 1B and Fig. 2B). The phenomenon was the same as those haemocytes treated with SNP, a donor of NO, which suggested the production of NO stimulated by addition of LPS (Figs. 1D and Fig. 2D). This process could be effectively blocked by the addition of NOS inhibitor l-NMMA, which showed similar results with the haemocytes not treated with LPS (Fig. 1C, Fig. 2C). These results suggested the existence of NOS in haemocytes of shrimps, and the enzyme activity could be stimulated by the addition of LPS, and blocked by l-NMMA.
Fig. 1.
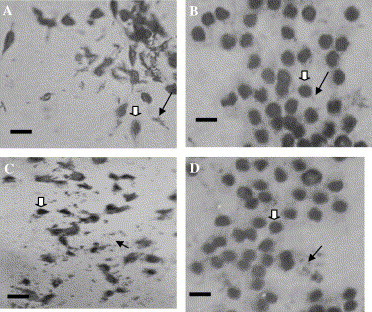
Haemocytes of F. chinensis under different treatments (scale bar: 10 μm, ( ) haemocyte, (
) haemocyte, ( ) E. coli.) (A) 4 h post-incubation with E. coli. (B) 4 h post-incubation with LPS and E. coli. (C) 4 h post-incubation with l-NMMA, LPS and E. coli. (D) 4 h post-incubation with SNP and E. coli.
) E. coli.) (A) 4 h post-incubation with E. coli. (B) 4 h post-incubation with LPS and E. coli. (C) 4 h post-incubation with l-NMMA, LPS and E. coli. (D) 4 h post-incubation with SNP and E. coli.
Fig. 2.
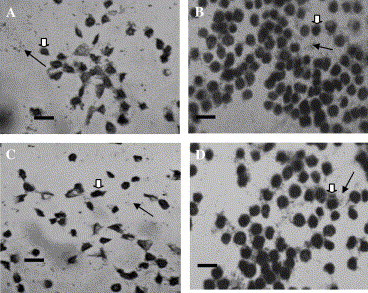
Haemocytes of M. japonicus under different treatments (scale bar: 10 μm, ( ) haemocyte, (
) haemocyte, ( ) E. coli.) (A) 4 h post-incubation with E. coli. (B) 4 h post-incubation with LPS and E. coli. (C) 4 h post-incubation with l-NMMA, LPS, and E. coli. (D) 4 h post-incubation with SNP and E. coli.
) E. coli.) (A) 4 h post-incubation with E. coli. (B) 4 h post-incubation with LPS and E. coli. (C) 4 h post-incubation with l-NMMA, LPS, and E. coli. (D) 4 h post-incubation with SNP and E. coli.
The two methods for the analysis of NOS activity are based on the principle that NOS can catalyze l-arginine to generate l-citrulline and NO quantitatively, therefore, concentrations of l-citrulline or total nitrite/nitrate (product of NO) determined could be used to evaluate the activity of NOS. Our results suggested that the two methods coincide well with each other, and either of the methods could be used for the analysis of NOS activity in haemocytes of shrimps.
Variations of NOS activity in F. chinensis and M. japonicus after WSSV-infection
In the group of F. chinensis infected by WSSV, the results showed that NOS activity in haemocytes increased slightly from 0 to 12 h postchallenge, indicated by the variations of l-citrulline (from 11.15 ± 0.10 to 12.08 ± 0.64 μM) and total nitrite/nitrate concentrations (from 10.45 ± 0.65 to 12.67 ± 0.52 μM). Then, the NOS activity decreased sharply till the end of the experiment, and the concentrations of l-citrulline and total nitrite/nitrate at 84 h were 1.58 ± 0.24 and 2.69 ± 0.70 μM, respectively (Fig. 3 ). The LPS-stimulated NOS activity kept constant during the experiment (Table 1 ). In contrast, both the NOS activity and LPS-stimulated NOS activity in the health shrimps used as control was relatively stable during the experiment, and the LPS-stimulated NOS activity in healthy shrimps is generally higher than that in the WSSV-infected shrimps (Fig. 3 and Table 1).
Fig. 3.
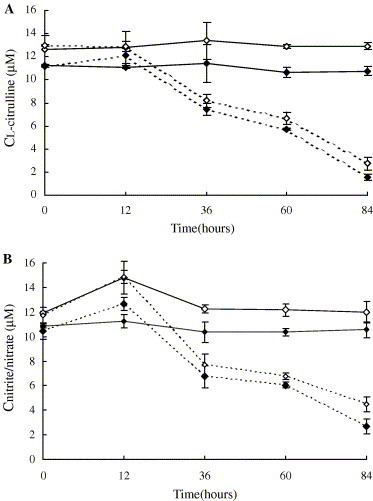
Variation of NOS activity in haemocytes of F. chinensis after WSSV infection. ((—♦—) NOS activity in haemocytes of healthy shrimps after incubation with l-arginine for 8 h, (—♢—) NOS activity in haemocytes of healthy shrimps after incubation with l-arginine and LPS for 8 h, (- -♢- -) NOS activity in haemocytes of WSSV infected shrimps after incubation with l-arginine for 8 h, (- -♦- -) NOS activity in haemocytes of WSSV infected shrimps after incubation with l-arginine and LPS for 8 h.) (A) Variation of NOS activity indicated by the analytical results of l-citrulline. (B) Variation of NOS activity indicated by the analytical results of total nitrite/nitrate.
Table 1.
Comparison of LPS stimulated NOS activity (indicated by l-citrulline and total nitrite/nitrate concentrations) between the healthy and WSSV-infected shrimps of F. chinensis
| Time (h) |
||||||
|---|---|---|---|---|---|---|
| 0 | 12 | 36 | 60 | 84 | ||
| Concentration (μM) | ||||||
| l-citrulline | Healthy shrimps | 1.37 ± 1.16 | 1.70 ± 1.29 | 1.96 ± 0.01 | 2.19 ± 0.26 | 2.14 ± 0.12 |
| Infected shrimps | 1.81 ± 0.01 | 0.80 ± 0.20 | 0.75 ± 0.01 | 0.96 ± 0.41 | 1.16 ± 0.35 | |
| Total | Healthy shrimps | 1.09 ± 0.01 | 3.54 ± 0.80 | 1.88 ± 0.50 | 1.82 ± 0.19 | 1.40 ± 0.20 |
| Nitrite/nitrate | Infected shrimps | 1.27 ± 0.12 | 2.12 ± 0.13 | 0.93 ± 0.01 | 0.72 ± 0.04 | 1.78 ± 0.08 |
In the group of M. japonicus infected by WSSV, the NOS activity in haemocytes kept increasing during the first 72 h postchallenge, the concentrations of l-citrulline and total nitrite/nitrate increased from 7.82 ± 0.77 at 0 h to 10.79 ± 0.50 μM at 72 h, and from 8.98 ± 0.43 at 0 h to 11.20 ± 0.37 μM at 72 h, respectively. The NOS activity in the haemocytes of infected shrimps kept higher than the control from 0 to 120 h postchallenge. Then it decreased till the end of the experiment, and the concentrations of l-citrulline and total nitrite/nitrate at 216 h were 5.66 ± 0.27 and 4.68 ± 0.16 μM, respectively (Fig. 4 ). For LPS-stimulated NOS activity, an apparent increase was observed during the first 48 h, and the highest value at 48 h postchallenge was about 4 times higher than that in the control group of health shrimps (Table 2 ). The LPS-stimulated NOS activity after 48 h then decreased dramatically and became lower than the control. For the health shrimps used as control, the NOS activity and LPS-stimulated NOS activity were relatively stable during the experiment (Fig. 4, Table 2). Compared to F. chinensis, the NOS activity in the haemocytes of M. japonicus was more potential to be stimulated by LPS at the early stage of infection (P < 0.01). The value of LPS-stimulated NOS activity was much higher than that of F. chinensis (P < 0.01, Fig. 3, Fig. 4).
Fig. 4.
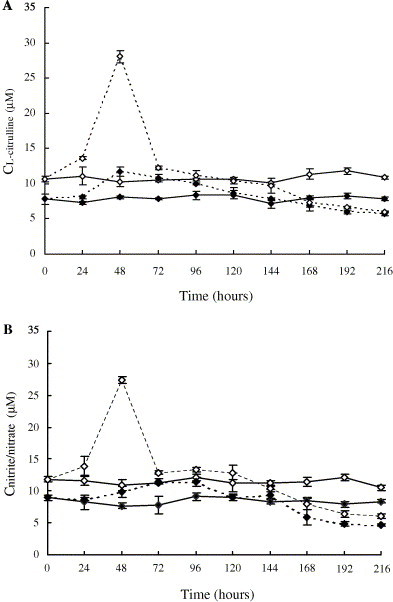
Variation of NOS activity in haemocytes of M. japonicus after WSSV infection. ((—♦—) NOS activity in haemocytes of healthy shrimps after incubation with l-arginine for 8 h, (—♢—) NOS activity in haemocytes of healthy shrimps after incubation with l-arginine and LPS for 8 h, (- -♢- -) NOS activity in haemocytes of WSSV infected shrimps after incubation with l-arginine for 8 h, (- -♦- -) NOS activity in haemocytes of WSSV infected shrimps after incubation with l-arginine and LPS for 8 h.) (A) Variation of NOS activity indicated by the analytical results of l-citrulline. (B) Variation of NOS activity indicated by the analytical results of total nitrite/nitrate.
Table 2.
Comparison of LPS stimulated NOS activity (indicated by l-citrulline and total nitrite/nitrate concentrations) between the healthy and WSSV-infected shrimps of M. japonicus
| Time (h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | 120 | 144 | 168 | 192 | 216 | ||
| Concentration (μM) | |||||||||||
| l-citrulline | Healthy shrimps | 2.83 ± 0.31 | 3.81 ± 1.08 | 2.23 ± 0.56 | 2.69 ± 0.11 | 2.21 ± 0.41 | 2.28 ± 0.36 | 2.90 ± 0.42 | 3.28 ± 0.48 | 3.50 ± 0.01 | 3.00 ± 0.06 |
| Infected shrimps | 2.83 ± 0.31 | 5.58 ± 0.06 | 16.31 ± 0.26 | 1.43 ± 0.25 | 1.18 ± 0.61 | 1.72 ± 0.30 | 1.90 ± 0.87 | 0.40 ± 0.13 | 0.70 ± 0.11 | 0.36 ± 0.13 | |
| Total | Healthy shrimps | 2.86 ± 0.04 | 3.27 ± 0.44 | 3.27 ± 0.43 | 3.39 ± 1.24 | 3.03 ± 0.07 | 2.40 ± 1.07 | 2.88 ± 0.01 | 3.03 ± 0.10 | 4.20 ± 0.08 | 2.16 ± 0.09 |
| Nitrite/nitrate | Infected shrimps | 2.86 ± 0.04 | 5.21 ± 1.26 | 17.45 ± 0.44 | 1.39 ± 0.06 | 1.82 ± 0.34 | 3.80 ± 0.63 | 1.10 ± 0.25 | 2.08 ± 0.39 | 1.52 ± 0.14 | 1.40 ± 0.25 |
Diagnosis of WSSV
The results of Dot-blot hybridization test showed that both species of shrimp were free of WSSV infection prior to experiment, and the shrimps used as control in the experiment were also free of WSSV infection. However, the infection of WSSV in both species after WSSV injection could be confirmed by the method. The virus was detected at 72 h in M. japonicus and at 36 h in F. chinensis after virus injection, which was in correspondence with the difference of NOS activities in the two species.
Cumulative mortalities of WSSV infected M. japonicus and F. chinensis
In correspondence with the difference of NOS activity between the two species, the cumulative mortalities were also different. All shrimps of F. chinensis in the mortality experiment died in 66 h, much more quickly than M. japonicus, whose accumulative mortality reached 100% after 240 h. These results of mortality suggested that F. chinensis was more sensitive to WSSV infection than M. japonicus.
Discussion
In the present study, we examined the NOS activity in the haemocytes of shrimps after LPS stimulation, and the variation of NOS activity after WSSV infection were also studied and compared between two species of shrimps, F. chinensis and M. japonicus.
NOS has been extensively studied among vertebrates and invertebrates. Some of the recent researches have confirmed the presence of NOS activity in haemocytes of invertebrates [15], [17], [21], [22], [29], [30]. Among these researches, techniques of analytical chemistry, immunohistochemistry, and cell and molecular biology have been used to identify NOS [15], [17], [23], [24], [25], [26], [27], [28]. In this experiment, the NBT reduction and cellular conformation approach were employed to identify NOS in the haemocytes of M. japonicus and F. chinensis, under the stimulation of LPS. Both of the methods could confirm the presence of NOS activity in the two species of shrimps, as reported previously in haemocytes of other invertebrates, like Mytilius edulis and Viviparus ater [15], [22]. However, the expression of NOS in haemocytes still needs to be further confirmed with techniques like immunochemistry or polymerase chain reaction (PCR).
In vertebrates, three isoforms of NOS, which can be divided into two functional classes, have been found so far. The Ca2+/calmodulin-dependent neuronal NOS (nNOS) and endothelial NOS (eNOS) belong to the constitutive class. They produce NO in short bursts in low concentrations for physiological purposes. The other class, which is mainly composed of Ca2+-independent inducible NOS (iNOS), is related to the diseases resistance of the organisms. iNOS found in innate immune cells and non-immune cells, such as macrophages and epithelial cells, can produce NO in high concentrations for as long as the enzyme is activated. The NO produced has anti-microbial actions against various intruding microbes, mediated by oxygen radicals, and active oxygen species through the formation of reactive nitrogen oxides like peroxynitrite (ONOO−), via a variety of mechanisms. However, in invertebrates, only Ca2+/calmodulin dependent form was found so far [28], [31], [32], [33], [34], [35], [36], [37]. And in crustaceans, NOS was reported mainly in the nervous system, regulating neuronal activity [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]. Recently, Kim [28] reported that NOS could also be expressed in the Y-organ, gill, testis and ovary of the land crab, which suggested that NO was also involved in other physiological processes except for neuromodulation. It was also suggested by Kim that the localization of NOS in connective tissue and epithelia in Y-organ and gill might function in an immune response to pathogens. The expression of NOS caused by bacterial infection in haemocytes of insects, suggesting the potential role of NOS related to disease resistance, have also been reported previously [15], [50]. Here in our experiment, only a slight increase in NOS activity could be observed in the two species of shrimps shortly after WSSV infection, and then it decreased till the end of the experiment, probably due to the destruction of haemocytes caused by WSSV infection at the late stage of experiment [51]. However, the NOS activity in M. japonicus kept at a high level for a longer time than that of F. chinensis after WSSV infection.More interestingly, there was an apparent increase of LPS-stimulated NOS activity in M. japonicus 48 h after WSSV infection, in contrast to the relatively stable level of LPS-stimulated NOS activity in F.chinensis. Referred to the different sensitivity of the two species to WSSV infection, as indicated in the mortality experiment, the results on NOS activity analysis let us hypothesize that the NOS activity in haemocytes of the shrimps might have a relation to the disease resistance ability of the shrimps to WSSV infection.
Although the importance of NO and iNOS in host defense reactions against bacteria have been extensively studied in vertebrates [11], their roles in resisting virus infections is not very well defined due to the very complicated situation in virus resistance of organisms. From some previous in vitro studies, it has been demonstrated that NO could inhibit the replication of a diverse range of viruses, especially for DNA viruses, such as murine poxvirus and herpes viruses, and some RNA viruses, such as Coxsackievirus[52]. However, for virus like coronavirus, lymphocytic choriomeningitis virus and murine vaccinia virus, etc., the anti-viral effect of NO remains unclear or uncertain [52]. Besides, the overproduction of NO will affect the host defense through its effects on the balance between the type 1 helper T cell (Th1) and type 2 helper T cell (Th2), and the pathological processes through the oxidative stress it caused. Therefore, its intriguing to understand the role of NO in resistance of virus infection. For iNOS activity, it has been reported that it could be induced by many different virus in vertebrates [53], [54], [55], through the direct up-regulation of virus or the indirect effect mediated by pro-inflammatory cytokines such as interferon-γ. However, the inhibition of iNOS activity and NO production was also reported recently. It’s hard to make a straightforward remark for the role of NO and iNOS in virus resistance among different organisms to the infection caused by a variety of virus. And the situation is even worse for invertebrates, although there are some reports about the invertebrate NO synthesis [56], [57], [58], [59], [60], [61], [62], [63], [64], none of them gave information either on NOS or NO in virus resistance. In our present study, we found that NOS activity in the haemocytes of the two species of shrimp’s response differently to WSSV infection, which is in correspondence with the different sensitivity of the two species. WSSV, reported as a large dsDNA virus belonging to the virus family Nimaviridae, genus Whispovirus [65], might be a target of NO. And the observed increase of NOS activity and LPS-induced activity in M. japonicus shortly after WSSV infection might play a role in resistance to virus infection, which lead to a relatively longer surviving time of the species after WSSV infection. However, all these hypotheses need to be further studied to elucidate the role of NO and NOS in host defense and pathological process in WSSV infected shrimps.
LPS have been successfully used in many studies to induce the NOS activity in haemocytes of invertebrates [17], [66], [29], [21]. In the present study, the addition of LPS could also induce the NOS activity in haemocytes of shrimps, and the LPS-stimulated NOS activity in M. japonicus was much higher than that without LPS stimulation after WSSV infection. However, it still needs to be further examined that the induction of NOS activity is good or bad for the mitigation of WSSV infection. The only thing we observed was that M. japonicacus, with higher LPS-stimulated NOS activity after WSSV infection, could survive longer than F. chinensis. If the mitigation effect of increased NOS activity on WSSV infection could be confirmed, it would be helpful for the development of proper immunostimulants, and for the understanding of the mechanisms of certain immunostimulants in host defense ability against the virus infection.
In summary, our results show that the haemocytes of both species of shrimps present NOS activity after LPS stimulation. The comparison of NOS activity in the two species of shrimps after WSSV infection shows that M. japonicus, with higher LPS–stimulated NOS activity after WSSV infection, could survive longer than F. chinensis. The results suggest that NOS in haemocytes of shrimps might be involved in the non-specific defense of shrimps to WSSV infection. However, further experiments are still needed to confirm the expression of NOS in haemocytes of shrimps, and to elucidate the role of NOS in resisting the infection of WSSV virus.
Acknowledgments
The research is supported by “973” project from Ministry of Science and Technology of PR China (G1999012011). Authors deeply appreciate the advice from Prof. Enzo Ottaviani (Department of Animal Biology University of Modena and Reggio Emilia, Italy) in editing the paper.
References
- 1.Destoumieux-Garzon D., Saulnier D., Garnier J., Jouffrey C., Bulet P., Bachére E. Crustacean immunity: antifungal peptides are generated from the C terminus of shrimp hemocyanin in response to microbial challenge. J. Biol. Chem. 2001;276:47070–47077. doi: 10.1074/jbc.M103817200. [DOI] [PubMed] [Google Scholar]
- 2.Bachère E. Shrimp immunity and disease control-introduction. Aquaculture. 2000;191:3–11. [Google Scholar]
- 3.Wang C.H., Lo C.F., Leu J.H., Chou C.M., Yeh P.Y., Chou H.Y., Tung M.C., Chang C.F., Su M.S., Kou G.H. Purification and genomic analysis of baculovirus associated with white spot syndrome WSBV of Penaeus monodon. Dis. Aquat. Org. 1995;23:239–242. [Google Scholar]
- 4.Wongteerasupaya C., Vickers J.E., Sriurairatana S., Nash G.L., Akarajamorn A., Boonsaeng V., Panyim S., Tassanakajon A., Withyachumnarnkul B., Flegel T.W. A non-occluded systemic baculovirus that occurs in cells of ectodermal and mesodermal origin and causes high mortality in the black tiger prawn Penaeus monodon. Dis. Aquat. Org. 1995;21:69–77. [Google Scholar]
- 5.Van Hulten M.C., Wittevel P.S., Kloosterboer N., Tarchini R., Fiers M., Sandbrink H., Lankhorst R.K., Vlak J.M. The white spot syndrome virus DNA genome sequence. Virology. 2001;286:7–22. doi: 10.1006/viro.2001.1002. [DOI] [PubMed] [Google Scholar]
- 6.Yang F., He J., Lin X., Li Q., Pan D., Zhang X., Xu X. Complete genome sequence of the shrimp white spot bacilliform virus. J. Virol. 2001;75:11811–11820. doi: 10.1128/JVI.75.23.11811-11820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lightner D.V. World Aquaculture Society; Baton Rouge LA.: 1996. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases World Aquaculture Society. pp. 305. [Google Scholar]
- 8.Hoffmann J.A., Kafatos F.C., Jr., Janeway C.A., Ezekowitz R.A.B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 9.De Groote M.A., Testerman T., Xu Y., Stauffer G., Fang F.C. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science. 1996;272:414–417. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- 10.Incze K., Farkas J., Mihalyi V., Zukal E. Antibacterial effect of cysteine-nitrosothiol and possible percursors thereof. Appl. Microbiol. 1974;27:202–205. doi: 10.1128/am.27.1.202-205.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakravortty D., Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003;5:621–627. doi: 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 12.Kröncke K-D., Fehsel K., Kolb-Bachofen V. Nitric Oxide: cytotoxicity versus cytoprotection—how, why, when, and where? Nitric Oxide. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- 13.Vargas-Albores F., Guzman M.A., Ochoa J.L. An anticoagulant solution for haemolymph collection and prophenolaxidase studies of penaeid shrimp (Penaeus californiensis) Comp. Biochem. Physiol. 1993;106A:299–303. [Google Scholar]
- 14.Jiang G., Yu R., Wang Y., Yan T., Zhou M. Studies on the methods of identification and activity analysis of inducible nitric oxide synthase in haemocytes of shrimp. J. Fish. Sci. China. 2004;11:1–9. (in Chinese) [Google Scholar]
- 15.Weiske J., Wiesner A. Stimulation of NO synthase activity in the immune–competent lepidopteran Estigmene acraea hemocyte line. Nitric Oxide. 1999;3:123–131. doi: 10.1006/niox.1999.0215. [DOI] [PubMed] [Google Scholar]
- 16.Ottaviani E., Paeman L.R., Cadet P., Stefano G.B. Evidence for nitric oxide production and utilization as a bacteriocidal agent by invertebrate immunocytes. Eur. J. Pharmacol. 1993;248:319–324. doi: 10.1016/0926-6917(93)90006-c. [DOI] [PubMed] [Google Scholar]
- 17.Marzinzig M., Nussler A.K., Stadler J., Marzinzig E., Barthlen W., Nussler N.C., Beger H.G., Morris J.S.M., Bruckner U.B. Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and Snitrosothiols. Nitric Oxide. 1997;1:177–189. doi: 10.1006/niox.1997.0116. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt H.H.H.W., Kelm M. Determination of nitrite and nitrate by the Griess reaction. In: Feelisch M., Stamler J.S., editors. Methods in Nitric Oxide Research. Wiley; Winchester: 1996. pp. 491–497. [Google Scholar]
- 19.Huang J., Song X.L., Yu J., Zhang L.J. The components of an inorganic physiological buffer for Penaeus chinesis. Methods Cell Sci. 1999;21:225–230. doi: 10.1023/a:1009876528852. [DOI] [PubMed] [Google Scholar]
- 20.Shi C.Y., Song X.L., Huang J., Yang C.H. Detection of Hypodermal and Hematopoietic Necrosis Baculovirus (HHNBV) By DIG-DNA PROBE DOT-BLOT Hybridization. Ocenol. Limnol. Sin. 1999;30:486–490. in Chinese. [Google Scholar]
- 21.Conte A., Ottaviani E. Nitric oxide synthase activity in molluscan hemocytes. FEBS Lett. 1995;365:120–124. doi: 10.1016/0014-5793(95)00439-g. [DOI] [PubMed] [Google Scholar]
- 22.Franchini A., Fontanili P., Ottaviani E. Invertebrate immunocytes—relationship between phagocytosis and nitric oxide production. Comp. Biochem. Physiol. 1995;110B:403–407. [Google Scholar]
- 23.Ottaviani E., Barbieri D., Malagoli D., Franchini A. Nitric oxide induces apoptosis in the fat body cell line IPLB-LdFB from the insect Lymantria dispar. Comp. Biochem. Physiol. 2001;128B:247–254. doi: 10.1016/s1096-4959(00)00311-0. [DOI] [PubMed] [Google Scholar]
- 24.Pfarr K.M., Qazi S., Fuhrman J.A. Nitric oxide synthase in Filariae: demonstration of nitric oxide production by embryos in Brugia malayi and Acanthocheilonema viteae. Exp. Parasitol. 2001;97:205–214. doi: 10.1006/expr.2001.4613. [DOI] [PubMed] [Google Scholar]
- 25.Kitano T., Matsumura S., Seki T., Hikida T., Sakimura K., Naganoe T., Mishina M., Nakanishi S., Ito S. Characterization of N-methyl-sc d-aspartate receptor subunits involved in acute ammonia toxicity. Neurochem. Int. 2004;44:83–90. doi: 10.1016/s0197-0186(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 26.Mabuchi T., Matsumura S., Okuda-Ashitaka E., Kitano T., Kojima T., Nagano T., Minami T., Ito S. Attenuation of neuropathic pain by nociceptin/orphanin FQ antagonist is mediated by inhibition of nitric oxide production. Eur. J. Neurosci. 2003;17:1384–1392. doi: 10.1046/j.1460-9568.2003.02575.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakatsubo N., Kojim H., Kikuchia K., Nagoshib H., Hirata Y., Maeda D., Imai Y., Nagano I.T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.W., Batista L.A., Hoppes J.L., Lee K.J., Mykles D.L. A crustacean nitric oxide synthase expressed in nerve ganglia, Y-organ, gill and gonad of the tropical land crab, Gecarcinus lateralis. J. Exp. Biol. 2004;207:2845–2857. doi: 10.1242/jeb.01117. [DOI] [PubMed] [Google Scholar]
- 29.Tafalla C., Gómez-León J., Novoa B., Figueras A. Nitric oxide production by carpet shell clam (Ruditapes decussatus) hemocytes. Dev. Comp. Immunol. 2003;27:197–205. doi: 10.1016/s0145-305x(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 30.Smith V.J., Brown J.H., Hauton C. Immunostimulation in crustaceans: does it really protect against infection? Fish Shellfish Immunol. 2003;15:71–90. doi: 10.1016/s1050-4648(02)00140-7. [DOI] [PubMed] [Google Scholar]
- 31.Davies S.A. Nitric oxide signalling in insects. Insect Biochem. Mol. Biol. 2000;30:1123–1138. doi: 10.1016/s0965-1748(00)00118-1. [DOI] [PubMed] [Google Scholar]
- 32.Korneev S.A., Pipe M.R., Picot J., Phillips R., Korneeva E.I., O’Shea M. Molecular characterization of NOS in a mollusc: expression in a giant modulatory neuron. J. Neurobiol. 1998;35:65–76. doi: 10.1002/(sici)1097-4695(199804)35:1<65::aid-neu6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Luckhart S., Rosenberg R. Gene structure and polymorphism of an invertebrate nitric oxide synthase gene. Gene. 1999;232:25–34. doi: 10.1016/s0378-1119(99)00121-3. [DOI] [PubMed] [Google Scholar]
- 34.Nighorn A., Gibson N.J., Rivers D.M., Hildebrand J.G., Morton D.B. The nitric oxide-cGMP pathway may mediate communication between sensory afferents and projection neurons in the antennal lobe of Manduca Sexta. J. Neurosci. 1998;18:7244–7255. doi: 10.1523/JNEUROSCI.18-18-07244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regulski M., Tully T. Molecular and biochemical characterization of dNOS—a Drosophila Ca2+ calmodulin-dependent nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1995;92:9072–9076. doi: 10.1073/pnas.92.20.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribeiro J.M.C., Nussenzveig R.H. Nitric oxide synthase activity from a hematophagous insect salivary gland. FEBS Lett. 1993;330:165–168. doi: 10.1016/0014-5793(93)80265-v. [DOI] [PubMed] [Google Scholar]
- 37.Stasiv Y., Regulski M., Kuzin B., Tully T., Enikolopov G. The Drosophila nitric oxide synthase gene (dNOS) encodes a family of proteins that can modulate NOS activity by acting as dominant negative regulators. J. Biol. Chem. 2001;276:42241–42251. doi: 10.1074/jbc.M105066200. [DOI] [PubMed] [Google Scholar]
- 38.Aonuma H., Newland P.L. Synaptic inputs onto spiking local interneurons in crayfish are depressed by nitric oxide. J. Neurobiol. 2002;52:144–155. doi: 10.1002/neu.10081. [DOI] [PubMed] [Google Scholar]
- 39.Aonuma H., Nagayama T., Takahata M. Modulatory effects of nitric oxide on synaptic depression in the crayfish neuromuscular system. J. Exp. Biol. 2000;203:3595–3602. doi: 10.1242/jeb.203.23.3595. [DOI] [PubMed] [Google Scholar]
- 40.Johansson K.U.I., Mellon D. Nitric oxide as a putative messenger molecule in the crayfish olfactory midbrain. Brain Res. 1998;807:237–242. doi: 10.1016/s0006-8993(98)00826-9. [DOI] [PubMed] [Google Scholar]
- 41.Lee C.Y., Zou H.S., Yau S.M., Ju Y.R., Liau C.S. Nitric oxide synthase activity and immunoreactivity in the crayfish Procambarus clarkii. Neuroreport. 2000;11:1273–1276. doi: 10.1097/00001756-200004270-00026. [DOI] [PubMed] [Google Scholar]
- 42.Scholz N.L. NO/cGMP signaling and the flexible organization of motor behavior in crustaceans. Am. Zool. 2001;41:292–303. [Google Scholar]
- 43.Scholz N.L., Chang E.S., Graubard K., Truman J.W. The NO/cGMP pathway and the development of neural networks in ostembryonic lobsters. J. Neurobiol. 1998;34:208–226. doi: 10.1002/(sici)1097-4695(19980215)34:3<208::aid-neu2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Scholz N.L., Labenia J.S., De Vente J., Graubard K., Goy M.F. Expression of nitric oxide synthase and nitric oxide-sensitive guanylate cyclase in the crustacean cardiac ganglion. J. Comp. Neurol. 2002;454:158–167. doi: 10.1002/cne.10442. [DOI] [PubMed] [Google Scholar]
- 45.Schuppe H., Aonuma H., Newland P.L. Distribution of NADPH-diaphorase-positive ascending interneurones in the crayfish terminal abdominal ganglion. Cell Tissue Res. 2001;305:135–146. doi: 10.1007/s004410100406. [DOI] [PubMed] [Google Scholar]
- 46.Schuppe H., Aonuma H., Newland P.L. NADPH-diaphorase histochemistry in the terminal abdominal ganglion of the crayfish. Cell Tissue Res. 2001;303:289–299. doi: 10.1007/s004410000319. [DOI] [PubMed] [Google Scholar]
- 47.Schuppe H., Cuttle M., Chad J.E., Newland P.L. 4,5-Diaminofluorosceimaging imaging of nitric oxide synthesis in crayfish terminal ganglia. J. Neurobiol. 2002;53:361–369. doi: 10.1002/neu.10117. [DOI] [PubMed] [Google Scholar]
- 48.Talavera E., Martinezlorenzana G., Leonolea M., Sanchezalvarez M., Sanchezislas E., Pellicer F. Histochemical distribution of NADPH-diaphorase in the cerebral ganglion of the crayfish Cambarellus montezumae. Neurosci. Lett. 1995;187:177–180. doi: 10.1016/0304-3940(95)11368-7. [DOI] [PubMed] [Google Scholar]
- 49.Zou H.S., Chang Y.Z., Chen S.C., Yau S.M., Shen Y.L., Lee C.Y. Localization of NADPH-diaphorase and nitric oxide synthase activity in the eyestalk of the crayfish, Procambarus clarkii. Zool. Stud. 2002;41:244–250. [Google Scholar]
- 50.Luckhart S., Vodovotz Y., Cui L.W., Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wongprasert K., Khanobdee K., Glunukarn S.S., Meeratana P., Withyachumnarnkul B. Time-course and levels of apoptosis in various tissues of black tiger shrimp Penaeus monodon infected with white-spot syndrome virus. Dis. Aquat. Org. 2003;55:3–10. doi: 10.3354/dao055003. [DOI] [PubMed] [Google Scholar]
- 52.Akaike T., Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tafalla C., Figueras A., Novoa B. Role of nitric oxide on the replication of haemorrhagic septicemia virus (VHSV), a fish rhabdovirus. Vet. Immunol. Immunopathol. 1999;72:249–256. doi: 10.1016/s0165-2427(99)00109-9. [DOI] [PubMed] [Google Scholar]
- 54.López-Guerrero J.A., Carrasco L. Effect of nitric oxide on poliovirus infection of two human cell lines. J. Virol. 1998;72:2538–2540. doi: 10.1128/jvi.72.3.2538-2540.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Majano P.L., García-Monzón C., López-Carrera M., Lara-Pezzi E., Fernandez-Ruiz E., García-Iglesias C., Borque M.J., Moreno-Otero R. Inducible nitric oxide synthase expression in chronic viral hepatitis. Evidence for a viral induced up-regulation. J. Clin. Invest. 1998;101:1343–1352. doi: 10.1172/JCI774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elphick M.R., Green I.C., O’Shea M. Nitric oxide synthesis and action in an invertebrate brain. Brain Res. 1993;619:344–346. doi: 10.1016/0006-8993(93)91632-3. [DOI] [PubMed] [Google Scholar]
- 57.Moroz L.L., Dahlgren R.L., Boudko D., Sweedler J.V., Lovell P. Direct single cell determination of nitric oxide synthase related metabolites in identified nitrergic neurons. J. Inorg. Biochem. 2005;99:929–939. doi: 10.1016/j.jinorgbio.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Stefano G.B., Mantione K., Jones D., Zhu W., Casares F., Cadet P. Immunocytes modulate ganglionic nitric oxide release which later affects their activity level. Neuro. Endocrinol. Lett. 2004;25:57–61. [PubMed] [Google Scholar]
- 59.Thavaradhara K., Leise E.M. Localization of nitric oxide synthase-like immunoreactivity in the developing nervous system of the snail llyanassa obsolete. J. Neurocytol. 2001;30:449–456. doi: 10.1023/a:1015669112986. [DOI] [PubMed] [Google Scholar]
- 60.Xie M., Hermann A., Kerschbaum H.H. Complementary distribution of NADPH-diaphorase and l-arginine in the snail nervous system. Cell Tissue Res. 2002;307:393–400. doi: 10.1007/s00441-001-0493-8. [DOI] [PubMed] [Google Scholar]
- 61.Rösze T., Tóth É.K., Szentmiklós A.J., Bánfalvi G. Seasonal periodicity of enteric nitric oxide synthesis and its regulation in the snail, Helix lucorum. Invertebr. Biol. 2005;124:18–24. [Google Scholar]
- 62.Stefano G.B., Liu Y., Golligorsky M.S. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human. J. Biol. Chem. 1996;271:19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- 63.Novas A., Cao A., Barcia R. Nitric oxide release by hemocytes of the mussel Mytilus galloprovincialis Lmk was provoked by interleukin-2 but not by lipopolysaccharide. Int. J. Biochem. Cell Biol. 2004;36:390–394. doi: 10.1016/s1357-2725(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 64.Bicker G. Sources and targets of nitric oxide signalling in insect nervous systems. Cell Tissue Res. 2001;303:137–146. doi: 10.1007/s004410000321. [DOI] [PubMed] [Google Scholar]
- 65.Mayo M.A. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 2002;147:1655–1663. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 66.Gourdon I., Guérin M.C., Torreilles J., Roch P. Nitric oxide generation by hemocytes of the mussel Mytilus galloprovincialis. Nitric Oxide. 2001;5:1–6. doi: 10.1006/niox.2000.0327. [DOI] [PubMed] [Google Scholar]


