Abstract
Follicular CXCR5+CD8+ T cells have antiviral effects in chronic virus infection, but the roles of these cells during dengue virus 2 (DENV2) infection remain poorly understood.
Objective
This study was conducted to analyzed in detail the dynamic changes and functional properties of circulating follicular CXCR5+CD8+ T cells to explore their effects on DENV2 infection.
Methods
Circulating follicular CXCR5+CD8+ T cells and cytokines were analyzed by flow cytometry in DENV2 patients at difference days after DENV2 infection. CD8+ T cells were isolated and purified from DENV2 patients, then were stimulated with NS1 peptides and TCR stimulant. After cultivation, multiple parameters were tested.
Results
(1) CXCR5+CD8+ T cells emerged after DENV2 infection, with high PD-1 expression, and were correlated with the reduction in DENV2 RNA viral loads. (2) PD-1+CXCR5+CD8+ T cells were negatively associated with disease progression. (3) Serum IFN-γ, IL-6 and IL-10 levels were increased late in the course of DENV2 infection. (4) CXCR5+CD8+ T cells from DENV2 patients exhibited increased cytotoxicity and IFN-γ and IL-10 secretion.
Conclusion
CXCR5+CD8+ T cells could play a protective role in dengue pathogenesis and may be a novel strategy for controlling DENV2 infection and vaccine development.
Keywords: Follicular CXCR5+CD8+T cells, Dengue virus 2 infection, Disease progression, Flow cytometry
Introduction
Dengue fever is the most prevalent mosquito-borne viral disease and is caused by four closely related serotypes of dengue viruses (DENV), which affect humans in tropical and subtropical regions worldwide (Bhatt et al., 2013). The presentation of the infection ranges from asymptomatic infection to the severe forms of the disease, such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (Guo et al., 2017). However, there is no satisfactory vaccine and no means of predicting disease severity at an early stage in DENV patients (Collins and Barrett, 2017). Both viral and host factors, including the host’s immune function, seem to be related to disease severity (Duangchinda et al., 2010, Rothman, 2011, Weiskopf and Sette, 2014). However, the immunological processes during DENV infection are not yet completely defined. Understanding the mechanisms that regulate the balance between immune-mediated protection and pathogenesis during DENV infections is a critical step toward the development of safe and effective DENV therapeutics and vaccines.
It is well known that CD8+ T cells play a role in antiviral immunity in human viral infections (de Alwis et al., 2016, Elong Ngono et al., 2016, Yauch et al., 2009, Zellweger et al., 2014, Zellweger et al., 2015). In response to infection, antigen-specific CD8+ T cells are primed in the T cell zone of secondary lymphoid organs and differentiate into effector cytotoxic T cells to eliminate infected cells (Mueller et al., 2013). Recently, a subset of CD8+ T cells with high C-X-C chemokine receptor type 5 (CXCR5) expression have been shown to possess antiviral properties in chronic virus infections, during which CD8+ T cells generally display exhausted phenotypes (Cross et al., 2015, He et al., 2016, Leong et al., 2016, Quigley et al., 2007). In addition, CXCR5+CD8+ T cells exhibit a more potent pro-inflammatory function than CXCR5−CD8+ T cells during chronic SIV infections (Mylvaganam et al., 2017). Recent studies have reported CXCR5+CD8+ T cells from chronic LCMV and HIV infections selectively entered into B-cell follicles eradicating both infected follicular helper T cells (Tfh) and B cells, exhibited more potent cytotoxicity, and demonstrated synergistic therapeutic potential when combined with PD-L1 neutralization (He et al., 2016, Quigley et al., 2007). Upon adoptive transfer, mice receiving CXCR5+CD8+ T cells presented significantly lower viral load than mice receiving CXCR5−CD8+ T cells.
CD8+ T cell dysfunction in chronic viral infections is associated with the expression of PD-1 inhibitory receptor (Trautmann et al., 2006, Trautmann et al., 2007, Velu et al., 2015, Zhang et al., 2007). At present, the expression of PD-1 on CXCR5+CD8+ T cells during viral infections remains controversial. Some studies (Im et al., 2016, Petrovas et al., 2017) reported high PD-1 expression on CXCR5+CD8+ T cells compared to CXCR5-CD8+ T cells during chronic viral infections, whereas He et al. (He et al., 2016) reported that CXCR5-CD8+ T cells expressed significantly lower levels of inhibitory molecules Tim-3 and PD-1 but higher levels of proinflammatory cytokines TNF-α and IFN-γ.
However, studies on CXCR5+CD8+ T cells in DENV2 patients are still lacking. Moreover, whether CXCR5+CD8+ T cells contribute to antiviral immunity during DENV2 infection remains unknown. To better understand the CXCR5+CD8+ T cell response and its role in protection and immunopathology, we examined the dynamic changes and functional properties of CXCR5+CD8+ T cells during DENV2 infection.
Materials and methods
Patient characteristics
Blood from patients suspected of having dengue fever was obtained when they presented at Zhejiang Province People's Hospital due to an outbreak of DENV2 in Zhejiang Hangzhou, China. The diagnosis was confirmed if samples tested positive for DENV2 RNA by reverse transcriptase-polymerase chain reaction (RT-PCR). All these samples were screened by the serological tests which include herpes simplex virus 2, rubella virus, cytomegalovirus, toxoplasma, rotavirus, coxsackie virus, mycoplasma, chlamydia, and hepatitis A, B, C, D to exclude other virus or bacteria infection. We also enrolled 21 healthy individuals (HI) who were asymptomatic and negative for DENV IgM and RNA. The characteristics of the DENV2 patients and HI are depicted in Table 1 . Heparinized peripheral blood samples were collected at diagnosis (D0) and subsequently, at 7 days (D7), 15 days (D15)) after diagnosis for the longitudinal study. Patients gave informed consent for their sample analysis in accordance with the Declaration of Helsinki. The study was approved by the Zhejiang Province People’s Hospital Review Board.
Table 1.
Clinicopathologic characteristics of the two groups of patients.
| Dengue | HI | P | |
|---|---|---|---|
| Sex, n | 36 | 21 | |
| Male | 19 | 11 | |
| Female | 17 | 10 | |
| Age | 48.5 ± 31.5 | 46.5 ± 13.5 | 0.8096 |
| WBC (/μL) | 3.88 ± 2.79 | 6.75 ± 2.69 | <0.001 |
| LYM (/μL) | 1.64 ± 1.40 | 2.15 ± 1.05 | 0.046 |
| PLT (103/μL) | 174.0 ± 146.2 | 194.16 ± 86.7 | 0.035 |
| ≥ 146 | 14 | ||
| <146 | 22 | ||
| HCT (%) | 32.56 ± 2.04 | 30.5 ± 1.79 | 0.0417 |
| LDH (U/L) | 281.57 ± 104.48 | 165.52 ± 40.17 | <0.001 |
| 109–244 | 12 | 21 | |
| ≥245 | 24 | 0 | |
| CK (U/L) | 206.06 ± 55.04 | 71.45 ± 21.5 | <0.001 |
| < 250 | 30 | 21 | |
| ≥ 250 | 7 | 0 | |
| Urea (mmol/L) | 4.67 ± 2.28 | 4.54 ± 1.98 | 0.459 |
| <7.5 | 27 | 21 | |
| ≥7.5 | 9 | 0 | |
| Urine protein | |||
| Negative | 27 | 21 | |
| Positive | 9 | ||
| Cr (μmol/L) | 84.38 ± 50.72 | 72.5 ± 22.7 | 0.0414 |
| <123 | 27 | 21 | |
| ≥123 | 9 | 0 | |
| AST (U/L) | 123.5 ± 107.5 | 23.5 ± 8.42 | <0.001 |
| <40 | 25 | 21 | |
| ≥40 | 11 | 0 | |
| AST (U/L) | 198.5 ± 175.5 | 24.0 ± 5.62 | <0.001 |
| <35 | 25 | 21 | |
| ≥ 35 | 11 | 0 |
Routine clinical indexes including white blood cell (WBC), lymphocyte (LYM), and platelet (PLT) counts and hematocrit (HCT) were determined from peripheral whole blood. Glutamic-pyruvic transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), creatinine (Cr), and urea were detected from serum samples that were isolated from clotted blood by centrifugation.
Flow cytometric analysis
The following monoclonal antibodies were used for the surface immunophenotyping assays: CD8-phycoerythrin-cyanine 7 (PE-Cy7, clone B911, BD Biosciences, USA), CXCR5-Alexa 488 (clone RF8B2, BD Biosciences, USA), PD-1-PerCP-Cy5.5 (clone H12.1, BD Biosciences, USA), and CD3-APC (clone UCHT1, Beckman Coulter, USA); After cells were incubated with antibodies for 30 min at 4 °C in the dark, and washed with PBS. Isotype antibody controls were used in all procedures. The stained cells were then analyzed using a FACSCantoII flow cytometer and FACSDiva software (Becton Dickinson, Sparks, MD). The strategy is shown in Figure S1.
Cytokine concentrations in serum were measured with the BD cytometric bead array (CBA) Human Th1/Th2/Th17 Cytokine Kit (BD Biosciences, San Jose, USA), following the manufacturer’s instructions.
Function analyses of CXCR5+CD8+ T cells
CD8+ T cells were isolated from the peripheral blood by Ficoll-Hypaque density gradient centrifugation in combination with the Human CD8+ T cell Enrichment kit (Thermo Scientific) according to manufacturer's protocols. The efficacy of CD8+ isolation was routinely ≥94% and these were plated at 2 × 106 cells/well in 96-well U-bottom plates. Cells were stimulated by 5 μg/mL DENV2 NS1 peptides (Santa, USA) with TCR stimulant (1 μg/mL anti-CD3 plus 1 μg/mL anti-CD28), 10 μg/mL Brefeldin A and CD107a-PE-cy5 (clone H4A3, eBioscience, Waltham, MA, USA) during the last 6 h. Cells stimulated with PIB (PMA + ionomycin + Brefeldin A) were used as a positive control whereas unstimulated cells were used as a negative control. Cells were collected, washed and labeled with CXCR5-Alexa 488 (clone RF8B2, BD Biosciences, USA) and CD8-PerCP (clone SK1, BD Biosciences, USA). Then, cells were fixed and permeabilized using an IntrePrep Permeabilization Reagent kit (Beckman Coulter, Miami, FL, USA) and incubated for 30 min at 4 °C with specific antibodies: TNF-α-PE-Cy7 (clone MP6-XT22, eBioscience, Waltham, MA, USA), IFN-γ-APC (clone 4S.B3, eBioscience, Waltham, MA, USA), IL-6-PE (clone MP5-20F3, eBioscience, Waltham, MA, USA), and IL-10-PE (clone JES5-19F1, BD Biosciences, Franklin Lakes, NJ, USA); or perforin- Alexa Flour647 (clone dG9, eBioscience, Waltham, MA, USA). Flow cytometric acquisition was performed on a FACSCanto II instrument with FACSDiva software (BD Biosciences, USA).
DENV2 RNA quantification
DENV2 infection was confirmed by RT-PCR as previously described (Lai et al., 2007, Pang et al., 2007). Total cellular RNA was isolated from 250 μL of whole blood obtained from DENV2 patients using Trizol LS reagent according to manufacturer’s protocol (Invitrogen). 200 ng RNA was used to estimate DENV viremia by Taqman one step RT-PCR as described previously. Peripheral blood DENV RNA viral load was determined using RT-PCR crossover values (Ct). The detection limit of this assay was 100 copies/mL.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5.01 software. Statistical tests for data analysis included the Mann Whitney U-test. Correlations between variables were determined using Spearman’s correlation coefficient. The quantitative data are presented as the mean ± standard deviation (SD). P < 0.05, P < 0.01, and P < 0.001 were considered to be statistically significant.
Results
Circulating follicular CXCR5+CD8+ T cells increased late in the course of DENV2 infection
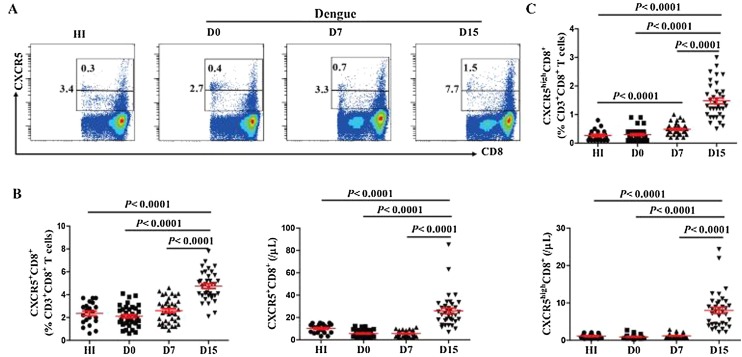
We first evaluated the level of circulating follicular CXCR5+CD8+ T cells in DENV2 patients. The frequency and number of CXCR5+CD8+ T cells was very low in DENV2 patients on D0 (Figure 1 A, B), which was comparable with that in HI, remained constant up to D7. However, this frequency and number increased on D15 and were higher than that observed in HI (Figure 1 B). We further investigate the level of CXCR5highCD8+ T cells in DENV2 patients, and observed a higher frequency of CXCR5highCD8+ T cells on D7 and a higher frequency and number of CXCR5highCD8+ T cells on D15 than those in HI (Figure 1 C).
Figure 1.
Circulating follicular CXCR5+CD8+ T cells increased late in the course of DENV2 infection. (A) Representative flow cytometric dot plots of CXCR5+CD8+ on CD3+CD8+ T cells. The values in the histogram represent the frequency of CD3+CD8+ T cells; (B) Frequency (left) and number (right) of CXCR5+CD8+ T cells from the peripheral blood of HI (n = 21) and DENV2 patients at different days after diagnosis (n = 36). Each data point represents an individual subject; (C) Frequency (top) and number (bottom) of CXCR5highCD8+ T cells from the peripheral blood of HI and DENV2 patients at different days after diagnosis. HI: healthy individuals; D0: at diagnosis; D7: days 7 after diagnosis; D15: days 15 after diagnosis.
CXCR5+CD8+ T cells were negatively association with DENV2 RNA viral load in DENV2 patients
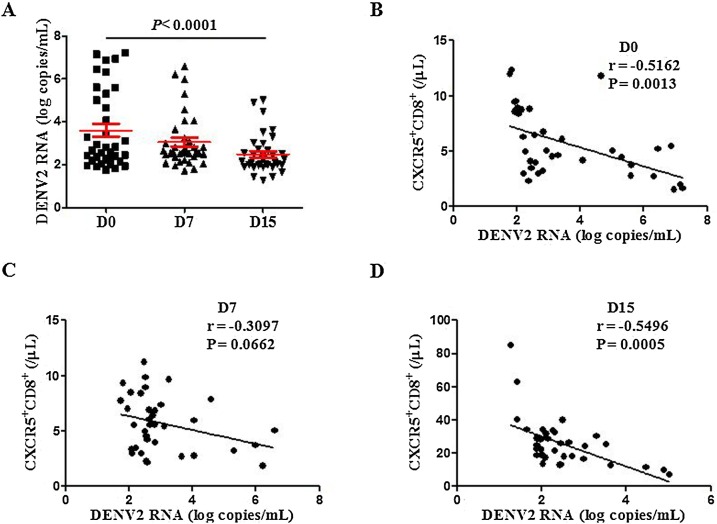
Since CXCR5+CD8+ T cells were increased in the late course of DENV2 infection, this phenomenon lead us to question whether CXCR5+CD8+ T cells are associated with DENV2 RNA viral load in DENV2 patients. DENV2 RNA viral load was evaluated at different days after DENV2 infection. As expected, higher viral loads were observed on D0 and D7, as shown in Figure 2 A, decreasing thereafter. Remarkably, DENV2 RNA viral load was negatively correlated with the number of circulating CXCR5+CD8+ T cells on D0 and D15 ( Figure 2 B–D). These results imply that CXCR5+CD8+ T cells may play a role in the control of DENV2 replication during the acute phase of dengue.
Figure 2.
CXCR5+CD8+ T cells were significantly negatively correlated with DENV2 RNA viral load in DENV2 patients. (A) DENV2 RNA viral load at different days after diagnosis; (B–D) A correlation between the number of CXCR5+CD8+ T cells and DENV RNA viral load at different days after diagnosis.
PD-1 was highly expressed on CXCR5+CD8+ T cells and negatively associated with disease progression
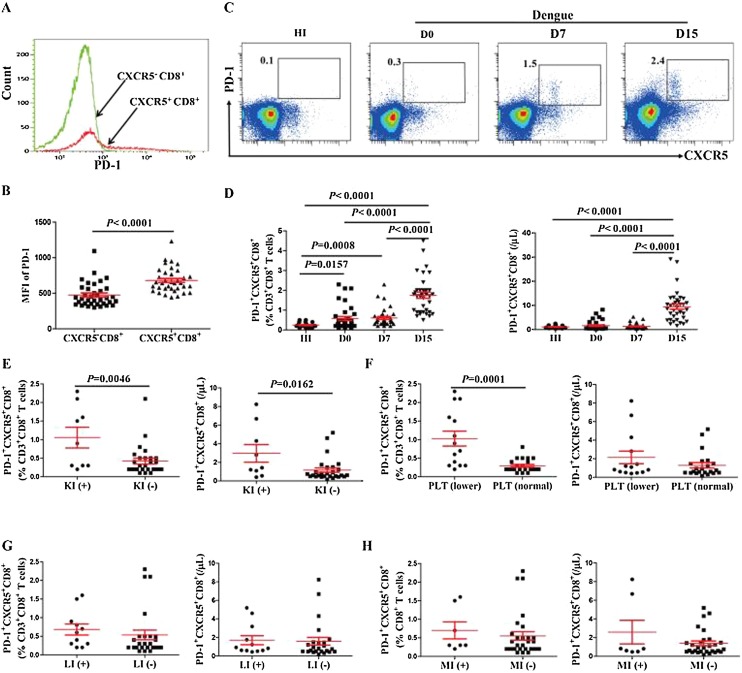
PD-1 is an important functional marker highly expressed on CD4+ follicular T helper cells (Banga et al., 2016, Xu et al., 2014); however, the function of PD-1 on follicular CXCR5+CD8+ T cells remains controversial. Thus, we analyzed the expression of PD-1 on CXCR5+CD8+ T cells. Compared with CXCR5-CD8+ T cells, CXCR5+CD8+ T cells exhibited enhanced PD-1 expression (Figure 3 A, B). Herein, we further investigated the frequency and number of PD-1+CXCR5+CD8+ cells in DENV2 patients. Statistical analysis demonstrated higher frequency of PD-1+CXCR5+CD8+ T cells at different days after diagnosis and higher number of PD-1+CXCR5+CD8+ T cells on D15 in DENV2 patients than HI (Figure 3 C, D).
Figure 3.
PD-1 was highly expressed on CXCR5+CD8+ T cells and negatively associated with disease progression. (A) Representative expression of PD-1 on CXCR5-CD8+ and CXCR5+CD8+ T cells from one DENV2 patient; (B) MFI of PD-1 in CXCR5+CD8+ and CXCR5-CD8+ T cells in DENV2 patients; (C) Representative flow cytometric dot plot of PD-1+CXCR5+ gated on CD3+CD8+ T cells from one HI and one DENV2 patient at different days after diagnosis; (D) The frequency (left) and number (right) of PD-1+CXCR5+CD8+ T cells in DENV2 patients at different days after diagnosis; (E) Comparison of the frequency (left) and number (right) of PD-1+CXCR5+CD8+ T cells in DENV2 patients with or without kidney injury; (F) Comparison of the frequency (left) and number (right) of PD-1+CXCR5+CD8+ T cells in two groups categorized by platelet counts; (G) Comparison of the frequency (left) and number (right) of PD-1+CXCR5+CD8+ T cells in DENV2 patients with or without liver injury; (H) Comparison of the frequency (left) and number (right) of PD-1+CXCR5+CD8+ T cells in DENV2 patients with or without myocardial injury. KI: kidney injury; LI: liver injury; MI: myocardial injury; PLT: platelets.
These data lead us to question whether PD-1+CXCR5+CD8+ T cells are associated with disease progression in DENV2 patients. In the clinical setting, some DENV2 patients will develop kidney injury and thrombocytopenia. Subsequently, when comparing DENV2 patients grouped according to kidney injury, the level of PD-1+CXCR5+CD8+ T cells on D0 in DENV2 patients with kidney injury was significantly higher than in those without kidney injury (Figure 3 E). The frequency of PD-1+CXCR5+CD8+ T cells on D0 were higher in DENV2 patients with lower platelet counts (≤146 × 103/μL) than in those with normal platelet counts (>146 × 103/μL) (Figure 3F). Moreover, when we compared the PD-1+CXCR5+CD8+ T cells on D0 with the clinical characteristics including liver injury and myocardial damage (Figure 3 G, H), we found no significant correlation between the PD-1+CXCR5+CD8+ T cells with these clinical characteristics. This finding suggested that PD-1+CXCR5+CD8+ T cells on D0 could be negatively associated with disease progression.
Serum IFN-γ, IL-6 and IL-10 are increased late in the course of DENV2 infection
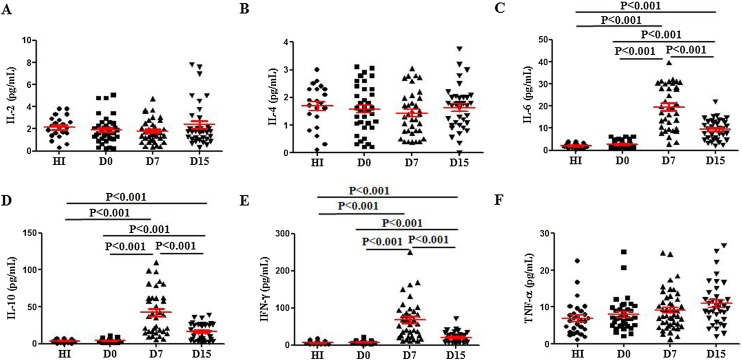
We know that cytokines play a crucial role in the pathogenesis of dengue, which leads to severe dengue disease (Oliveira et al., 2017, Pandey et al., 2015). To further understand the kinetics of the immune response to DENV2 with respect to secretion of cytokines, we detected the serum cytokine levels in DENV2 patient at different days after diagnosis. Serum levels of IL-2, IL-4, and TNF-α were low in DENV2 patients from D0 to D15, which were comparable with those in HI (Figure 4 A, B, F). IL-6, IL-10 and IFN-γ levels increased on D7 and D15 in DENV2 patients, which were higher than those in HI (Figure 4 C–E). IL-17 was also evaluated, but the results were below the limit of detection for this assay (20 pg/ml).
Figure 4.
Serum IL-6, IL-10 and IFN-γ levels increased late in the course of DENV2 infection. Cytokines were determined by CBA in the blood serum of the HI and DENV2 patients; (A–F) Serum IL-2, IL-4, IL-6, IL-10, IFN-γ and TNF-α levels.
CXCR5+CD8+ T cells exhibited increased cytotoxicity and IFN-γ and IL-10 secretion in DENV2 patients
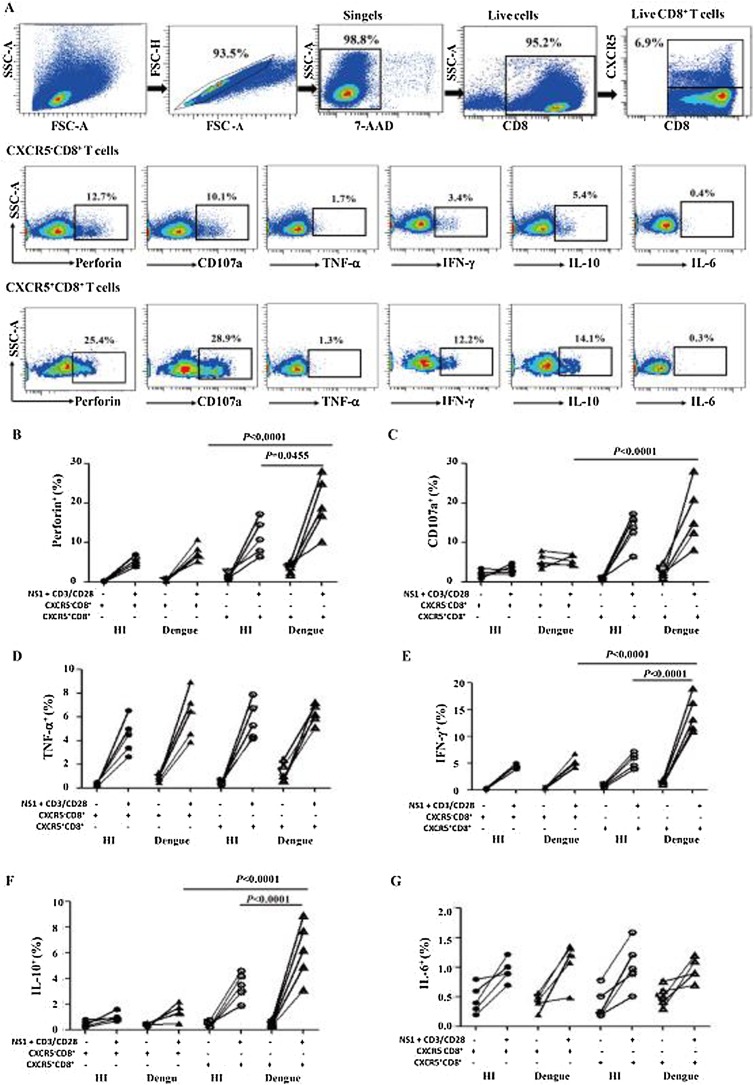
Some studies have demonstrated that primary DENV infection elicits poly-functional effector CD8+ T cells that express IFN-γ, TNF-α, CD107a and perforin and thus contribute to viral clearance (Alter et al., 2004). To perform mechanistic studies of CXCR5+CD8+ T cell response to DENV2 in vitro, we first examined whether CXCR5+CD8+ T cells were capable of mediating cytotoxic effects. The CD8+ T cells from five DENV2 patients and five HI were harvested and stimulated by NS1 peptides and TCR stimulant. In all five DENV2 patients examined, CXCR5+CD8+ T cells presented higher perforin- and CD107a- releasing T cells than CXCR5-CD8+ T cells. DENV2 patients also had higher perforin-releasing CXCR5+CD8+ T cells than HI (Figure 5 A–C).
Figure 5.
CXCR5+CD8+ T cells from DENV2 patients possessed increased cytotoxicity and high capacity for secreting IFN-γ and IL-10. CXCR5+CD8+ T cells and CXCR5-CD8+ T cells from DENV2 patients (n = 5) and HI (n = 5) were stimulated for 8 h by NS1 peptides with TCR stimulant. After live cell gating with 7-AAD staining, the perforin, CD107a, TNF-α, IFN-γ, IL-10 and IL-6 expressions on CXCR5− and CXCR5+CD8+ T cells were then examined by intracellular staining. (A) The flow cytometric plots of perforin, CD107a, TNF-α, IFN-γ, IL-10 and IL-6 expression on CXCR5− and CXCR5+CD8+ T cells from one DENV2 patient. Statistical analysis of perforin (B), CD107a (C), TNF-α (D), IFN-γ (E), IL-10 (F) and IL-6 (G) expression on CXCR5− and CXCR5+CD8+ T cells.
Next, we further examined the function of CXCR5+CD8+ T cells to secrete TNF-α, IFN-γ, IL-10 and IL-6. We found that immediately following isolation, the CXCR5+CD8+ and CXCR5-CD8+ T cells were demonstrated significantly lower levels of TNF-α, IFN-γ, IL-10 and IL-6 secretion, and after stimulation by NS1 peptides with TCR stimulant, the levels of TNF-α and IL-6 were comparable between DENV2 patients and HI (Figure 5 D, G). However, CXCR5+CD8+ T cells from DENV2 patients after stimulation produced higher levels of IFN-γ and IL-10 compared to CXCR5-CD8+ T cells (Figure 5 E, F). CXCR5+CD8+ T cells from DENV2 patients were significantly more potent in IFN-γ and IL-10 secretion than those from HI (Figure 5 E, F). These results suggested that CXCR5+CD8+ T cells had the potential of degranulation and cytokine secretion after DENV2 infection.
Discussion
Follicular cytotoxic CXCR5+CD8+ T cells are shown to have antiviral properties in chronic virus infections (Cross et al., 2015, He et al., 2016, Leong et al., 2016, Quigley et al., 2007, Liu et al., 2002). In this study, we found that circulating CXCR5+CD8+ T cells, especially CXCR5highCD8+ T cells, emerged later in the course of DENV2 infection, which may be related to the gradual migration of CXCR5+CD8+ T cells from the lymph nodes to the periphery during DENV2 infection. Importantly, the peak of CXCR5+CD8+ T cells was correlated with DENV2 RNA reduction, suggesting a protective role for CXCR5+CD8+ T cells in controlling DENV2 replication.
Abnormal expression of PD-1 on CD8+ T cells play a role in chronic viral infections (Day et al., 2006, Sharpe et al., 2007). There are different opinions regarding the expression of PD-1 on CXCR5+CD8+ T cells during chronic viral infections. We found high PD-1 expression on CXCR5+CD8+ T cells compared to CXCR5-CD8+ T cells, consistent with other findings (Im et al., 2016, Petrovas et al., 2017). Additionally, higher levels of PD-1+CXCR5+CD8+ T cells were found in DENV2 patients. Interestingly, higher levels of PD-1+CXCR5+CD8+ T cells were found in DENV2 patients with kidney injury or those with lower platelets numbers. These findings suggested that PD-1+CXCR5+CD8+ T cells could be negatively associated with disease progression.
Further functional experiments demonstrated that CXCR5+CD8+ T cells had the potential of degranulation and cytokine secretion after DENV2 infection, which suggested CXCR5+CD8+ T cells may protect against DENV2 infection in large part via their cytotoxic effector function. These observations were in partial agreement with studies performed in chronic LCMV-infected mice and follicular lymphoma, which showed that CXCR5+CD8+ T cells expressed more proinflammatory cytokines than CXCR5−CD8+ T cells (He et al., 2016, Eppy et al., 2016). Further investigation into early immune signatures will help to address what mechanisms influence the in vivo generation of these antiviral CXCR5+CD8+ T cells, but our data in combination with these recently published studies highlight the importance of CXCR5+CD8+ T cells and underscore the need to develop immune-based strategies capable of generating these cells in vivo as a means of targeting and eliminating DENV2-infected cells.
We know that cytokines are associated with severe dengue disease (Oliveira et al., 2017, Pandey et al., 2015, Eppy et al., 2016, Tsai et al., 2014). Studies have shown high levels of IL-6 in dengue infected liver sections and elevated IL-10 levels in patients with severe dengue (Oliveira et al., 2017, Pandey et al., 2015). Consistent with other findings (Oliveira et al., 2017, Pandey et al., 2015, Flores-Mendoza et al., 2017), we observed high levels of IFN-γ, IL-6 and IL-10 late in the course of DENV2 infection. Although IL-10 was generally regarded as an anti-inflammatory cytokine, several studies suggested that IL-10 contributed to antitumor immunity by assisting the expansion of tumor-resident CD8+ T cells (Emmerich et al., 2012, Mumm et al., 2011). In mice with coronavirus-induced acute encephalitis, IL-10+CD8+ T cells demonstrated more potent activation and cytolytic activity than IL-10-CD8+ T cells, which suggest that IL-10 might improve CD8+ T cell survival and activation during acute inflammation (Trandem et al., 2011). In this study, we also observed that CXCR5+CD8+ T cells in DENV2 patients exhibited high IL-10 secretion, while at the same time, the level of IFN-γ, a canonical CD8+ T cell activation marker, increased as would be expected under high IL-10 levels (Inoue et al., 2006, Jarnicki et al., 2006). Combining these results, CXCR5+CD8+ T cells might have contributed to controlling DENV2 infection through IL-10-mediated stimulation rather than suppression.
In conclusion, we demonstrated that CXCR5+CD8+ T cells emerge after DENV2 infection, with high PD-1 expression and functionally cytotoxic response, and were correlated with DENV2 RNA reduction. These results show, for the first time, a protective role for CXCR5+CD8+ T cells in dengue pathogenesis and indicate that CXCR5+CD8+ T cells represent a novel strategy for controlling DENV2 infection and vaccine development.
Funding
This study was partly supported by the Zhejiang Provincial Natural Science Fund (No: Y18H200014).
Conflicts of interest
The authors declare no conflicts of interest.
Clinical assessment
Written informed consent was obtained from all enrolled patients and healthy individuals.
Acknowledgments
Q.L.N. designed the experiments and wrote the manuscript. Y.Q.H. and C.S.F. conducted flow cytometry and the CBA technique. W.H. identified the patients and obtained the samples. L.J.J. assessed the functional assay. Z.Z. conducted RT-PCR and IgM test. All authors read and approved the final manuscript.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2019.03.024.
Appendix A. Supplementary data
The following are Supplementary data to this article:
Gating strategy applied to identify the CXCR5+CD8+, CXCR5highCD8+ and the PD-1+CXCR5+CD8+ T cells.
References
- Alter G., Malenfant J.M., Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Banga R., Procopio F.A., Noto A., Pollakis G., Cavassini M., Ohmiti K., et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. 2016;22:754–761. doi: 10.1038/nm.4113. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N.D., Barrett A.D. Live attenuated yellow fever 17D vaccine: a legacy vaccine still controlling outbreaks in modern day. Curr Infect Dis Rep. 2017;19:14. doi: 10.1007/s11908-017-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E., Edwards L., Kedl R. 2015. Follicular CD8+ T cells: a novel subset in immune protection and antibody responses. (VAC3P.1062) [Google Scholar]
- Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Duangchinda T., Dejnirattisai W., Vasanawathana S., Limpitikul W., Tangthawornchaikul N., Malasit P., et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci U S A. 2010;107:16922–16927. doi: 10.1073/pnas.1010867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alwis R., Bangs D.J., Angelo M.A., Cerpas C., Fernando A., Sidney J., et al. Immunodominant dengue virus-specific CD8+ T cell responses are associated with a memory PD-1+ phenotype. J Virol. 2016;90:4771–4779. doi: 10.1128/JVI.02892-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A., Chen H.W., Tang W.W., Joo Y., King K., Weiskopf D., et al. protective role of cross-reactive CD8 T cells against dengue virus infection. EBioMedicine. 2016;13:284–293. doi: 10.1016/j.ebiom.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich J., Mumm J.B., Chan I.H., LaFace D., Truong H., McClanahan T., et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012;72:3570–3581. doi: 10.1158/0008-5472.CAN-12-0721. [DOI] [PubMed] [Google Scholar]
- Eppy Suhendro, Nainggolan L., Rumende C.M. The differences between interleukin-6 and C-reactive protein levels among adult patients of dengue infection with and without plasma leakage. Acta Medica Indonesiana. 2016;48:3–9. [PubMed] [Google Scholar]
- Flores-Mendoza L.K., Estrada-Jimenez T., Sedeno-Monge V., Moreno M., Manjarrez M.D.C., Gonzalez-Ochoa G., et al. IL-10 and socs3 are predictive biomarkers of dengue hemorrhagic fever. Mediat Inflamm. 2017;2017 doi: 10.1155/2017/5197592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Zhou Z., Wen Z., Liu Y., Zeng C., Xiao D., et al. Global epidemiology of dengue outbreaks in 1990-2015: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2017;7:317. doi: 10.3389/fcimb.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R., Hou S., Liu C., Zhang A., Bai Q., Han M., et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- Im S.J., Hashimoto M., Gerner M.Y., Lee J., Kissick H.T., Burger M.C., et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Leitner W.W., Golding B., Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- Jarnicki A.G., Lysaght J., Todryk S., Mills K.H. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- Lai Y.L., Chung Y.K., Tan H.C., Yap H.F., Yap G., Ooi E.E., et al. Cost-effective real-time reverse transcriptase PCR (RT-PCR) to screen for Dengue virus followed by rapid single-tube multiplex RT-PCR for serotyping of the virus. J Clin Microbiol. 2007;45:935–941. doi: 10.1128/JCM.01258-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong Y.A., Chen Y., Ong H.S., Wu D., Man K., Deleage C., et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17:1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Huang K.J., Lin Y.S., Yeh T.M., Liu H.S., Lei H.Y. Transient CD4/CD8 ratio inversion and aberrant immune activation during dengue virus infection. J Med Virol. 2002;68:241–252. doi: 10.1002/jmv.10198. [DOI] [PubMed] [Google Scholar]
- Mueller S.N., Gebhardt T., Carbone F.R., Heath W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Mylvaganam G.H., Rios D., Abdelaal H.M., Iyer S., Tharp G., Mavigner M., et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A. 2017;114:1976–1981. doi: 10.1073/pnas.1621418114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm J.B., Emmerich J., Zhang X., Chan I., Wu L., Mauze S., et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Oliveira R., Cordeiro M.T., Moura P., Baptista Filho P.N.B., Braga-Neto U.M., Marques E.T.A.J., et al. Serum cytokine/chemokine profiles in patients with dengue fever (DF) and dengue hemorrhagic fever (FHD) by using protein array. J Clin Virol. 2017;89:39–45. doi: 10.1016/j.jcv.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Pandey N., Jain A., Garg R.K., Kumar R., Agrawal O.P., Lakshmana Rao P.V. Serum levels of IL-8, IFNgamma, IL-10, and TGF beta and their gene expression levels in severe and non-severe cases of dengue virus infection. Arch Virol. 2015;160:1463–1475. doi: 10.1007/s00705-015-2410-6. [DOI] [PubMed] [Google Scholar]
- Pang T., Cardosa M.J., Guzman M.G. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS) Immunol Cell Biol. 2007;85:43–45. doi: 10.1038/sj.icb.7100008. [DOI] [PubMed] [Google Scholar]
- Petrovas C., Ferrando-Martinez S., Gerner M.Y., Casazza J.P., Pegu A., Deleage C., et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M.F., Gonzalez V.D., Granath A., Andersson J., Sandberg J.K. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol. 2007;37:3352–3362. doi: 10.1002/eji.200636746. [DOI] [PubMed] [Google Scholar]
- Rothman A.L. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- Sharpe A.H., Wherry E.J., Ahmed R., Freeman G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- Trautmann L., Chomont N., Sekaly R.P. Inhibition of the PD-1 pathway restores the effector function of HIV-specific T cells. Med Sci. 2007;23:24–25. doi: 10.1051/medsci/200723124. [DOI] [PubMed] [Google Scholar]
- Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Trandem K., Zhao J., Fleming E., Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J Immunol. 2011;186:3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.T., Chuang Y.J., Lin Y.S., Chang C.P., Wan S.W., Lin S.H., et al. Antibody-dependent enhancement infection facilitates dengue virus-regulated signaling of IL-10 production in monocytes. PLoS Negl Trop Dis. 2014;8:e3320. doi: 10.1371/journal.pntd.0003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V., Shetty R.D., Larsson M., Shankar E.M. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology. 2015;12:14. doi: 10.1186/s12977-015-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Sette A. T-cell immunity to infection with dengue virus in humans. Front Immunol. 2014;5:93. doi: 10.3389/fimmu.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wang X., Lackner A.A., Veazey R.S. PD-1(HIGH) follicular CD4 T helper cell subsets residing in lymph node germinal centers correlate with B cell maturation and IgG production in Rhesus Macaques. Front Immunol. 2014;5:85. doi: 10.3389/fimmu.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch L.E., Zellweger R.M., Kotturi M.F., Qutubuddin A., Sidney J., Peters B., et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Eddy W.E., Tang W.W., Miller R., Shresta S. CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J Immunol. 2014;193:4117–4124. doi: 10.4049/jimmunol.1401597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Tang W.W., Eddy W.E., King K., Sanchez M.C., Shresta S. CD8+ T cells can mediate short-term protection against heterotypic dengue virus reinfection in mice. J Virol. 2015;89:6494–6505. doi: 10.1128/JVI.00036-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y., Zhang Z., Wang X., Fu J.L., Yao J., Jiao Y., et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy applied to identify the CXCR5+CD8+, CXCR5highCD8+ and the PD-1+CXCR5+CD8+ T cells.