Abstract
Proventricular dilatation disease (PDD) is a common infectious neurologic disease of birds comprising a dilatation of the proventriculus by ingested food as a result of defects in intestinal motility, which affects more than 50 species of psittacines, and is also known as Macaw wasting disease, neuropathic ganglioneuritis, or lymphoplasmacytic ganglioneuritis. Definitive diagnosis of PDD has been problematic due to the inconsistent distribution of lesions. Since its discovery, avian bornavirus (ABV) has been successfully cultured from the brains of psittacines diagnosed with PDD, providing a source of antigen for serologic assays and nucleic acid for molecular assays. This article provides evidence that ABV is the etiologic agent of PDD. Recent findings on the transmission, epidemiology, pathogenesis, diagnosis, and control of ABV infection and PDD are also reviewed.
Keywords: Bornavirus, Parrot, Proventriculus, Encephalitis
Proventricular dilatation disease (PDD) is a common infectious neurologic disease of birds, affecting more than 50 species of psittacines, and has been reported in toucans, honey creepers, weaver finches, water fowl, raptors, and passerines.1, 2, 3, 4, 5, 6, 7, 8, 9 PDD is also known as Macaw wasting disease, after the first birds observed to be affected, and neuropathic ganglioneuritis or lymphoplasmacytic ganglioneuritis, after the lesions that it causes.1, 10, 11 The name PDD is derived from the predominant feature of the disease in parrots, a dilatation of the proventriculus by ingested food as a result of defects in intestinal motility. This intestinal dysfunction results from virus-induced damage to the enteric nervous system. As a result of this damage to the gastrointestinal tract, birds are unable to empty their digestive tract or digest their food, and this leads to weight loss, crop stasis, proventricular and intestinal dilatation, regurgitation, maldigestion (passing of whole seeds), and eventually starvation and death.12 The disease is also associated with significant central nervous system damage, which may include the development of encephalitis and myelitis resulting in depression, seizures, ataxia, blindness, and tremors.13 Affected birds may show both neurologic and gastrointestinal signs. Definitive diagnosis has been based historically on crop biopsy or necropsy findings, the pathognomonic lesions being the presence of a lymphoplasmacytic infiltration in the ganglia and myenteric plexus of the gastrointestinal tract or central nervous system.14 Definitive diagnosis of PDD has been problematic due to the inconsistent distribution of lesions. Berhane and colleagues1 found lesions in the crop in 43% of cases, proventriculus 36%, ventriculus 93%, duodenum 21%, heart 79%, adrenal gland 50%, spinal cord 69%, brain 46%, sciatic nerve 58%, brachial nerve 46%, and vagus nerve 46%. Similar results have been reported by Shivaprasad and colleagues.15 Whereas necropsy has provided a clear and concise diagnosis, premortem diagnosis has proven to be more difficult (Fig. 1 ).
Fig. 1.
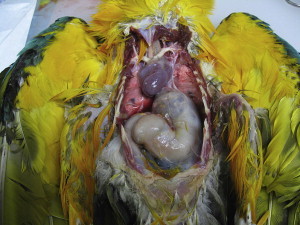
Dilated proventriculus in a blue and gold macaw, PCR-positive for ABV, with histopathological lesions of PDD.
PDD has long been considered an infectious disease, and multiple viruses have been suggested as its cause. For example, adenovirus-like particles were demonstrated within intranuclear inclusion bodies in the cells lining the kidneys of one affected bird,16 and a coronavirus was isolated from a bird with PDD by Gough and colleagues17 and was suggested to be the etiologic agent. A paramyxovirus related to Newcastle disease has also been considered a likely cause, because it was reported that this virus could be isolated in up to 60% of PDD cases.18 None of these agents, however, have been consistently present in affected birds.
In 2008, major advances were made in determining the etiology of PDD. Pyrosequencing of cDNA from the brains of parrots with PDD identified 2 strains of a novel bornavirus.19 Using real-time polymerase chain reaction (PCR), Honkavuori and colleagues19 confirmed the presence of this virus in brain, proventriculus, and adrenal gland in 3 birds with PDD but not in 4 unaffected birds. Kistler and colleagues20 used a microarray approach to identify a bornavirus hybridization signature in 5 of 8 PDD cases and none of 8 controls. Using high-throughput pyrosequencing in combination with conventional PCR cloning and sequencing, a complete viral genome sequence was recovered and named avian bornavirus (ABV). During this same time, Gray and colleagues21 succeeded in culturing ABV from the brains of 7 psittacines diagnosed with PDD, providing a source of antigen for serologic assays and nucleic acid for molecular assays.
Bornaviruses are negative-strand RNA viruses belonging to the family Bornaviridae. Their most unique characteristic is that they undergo transcription inside the nucleus. These viruses also undergo alternative splicing, and use different initiation and termination signals than other viruses.22 Two members of the family are known: Borna disease virus (BDV) and ABV. BDV causes neurologic disease in horses, cats, and sheep, and appears to be restricted to central Europe. While predominantly a disease of mammals, BDV has also been detected in the feces of wild mallards and corvids in Scandinavia. However, the significance of avian BDV infections and their epidemiologic significance are unclear.23 An outbreak of neurologic disease attributed to BDV has been reported in ostriches in Israel. This diagnosis was based on serology, and unfortunately the virus isolates were lost, so the significance and etiology of this outbreak remain unclear.24
Experimentally, BDV has been shown to cause infections in chickens and quail. The clinical signs of BDV infection can range from a fatal meningoencephalitis to minor behavioral problems and persistent asymptomatic infection.25 BDV appears to be only distantly related to ABV, although it is likely that one evolved from the other.
In this review the authors provide evidence that ABV is the etiologic agent of PDD. Recent findings on the transmission, epidemiology, pathogenesis, diagnosis, and control of ABV infection and PDD are also reviewed.
The isolation of avian bornavirus
Gray and colleagues21 inoculated primary mallard embryo fibroblasts with a fresh brain suspension from a PDD infected yellow-collared macaw (Primolius auricollis) and African gray parrot (Psittacus erithracus), and succeeded in growing viruses from each. These viruses caused no detectable cytopathic effect in the duck cells. However, serum from a PDD-affected green-winged macaw (Ara chloroptera) that had been shown to recognize ABV N-protein was available. By performing a Western blot on infected duck cell lysates, these investigators demonstrated a progressive increase in the quantity of this viral antigen when cultured over 5 days. Indirect immunofluorescence assays on these infected cells using this same antiserum showed foci of antigen-positive cells demonstrating characteristic speckled intranuclear fluorescence (see Fig. 1). This speckled pattern is similar to that considered diagnostic of mammalian bornavirus infection: the stained particles are called Joest-Degen bodies and are believed to be complexes of the viral N and P proteins.26, 27 PCR assays conducted on this and other infected tissue cultures confirmed the presence of ABV. Subsequently the authors have isolated ABV by culture in duck embryo fibroblasts using material from the brains of 5 additional birds with necropsy-confirmed PDD (Fig. 2 ).
Fig. 2.
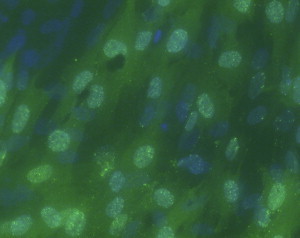
Immunofluorescence photomicrograph of cultured duck embryo fibroblasts infected with an ABV isolate from a yellow-collared macaw 3 days previously. The lack of apparent cytotoxicity and presence of speckled nuclear fluorescence is typical of bornavirus infection. (Immunoflorescense stain at 10X.)
When Kistler and colleagues20 first reported on the detection of ABV in PDD cases, they also reported several different genotypes. These genotypes differed by about 5% to 15% in their gene sequences. Given the huge diversity of species affected by PDD, the presence of diverse genotypes was unsurprising. However, Kistler's data show that specific genotypes do not favor certain avian species and appear to be of roughly equivalent pathogenicity. To date 7 different genotypes have been identified.28, 29 There is also significant genetic variation within these genotypes. Of the authors' 7 isolates, one is genotype 1 (ABV1) while the remainder belong to genotype 4 (ABV4). This predominance of genotype 4 has been observed by other groups.28, 29 This genotype could be an artifact resulting from increased pathogenicity of ABV4 and the fact that tissue from lethal PDD cases is being selectively cultured. It is more likely, however, that it is indeed the predominant circulating genotype. The authors have also found that ABV4 is the predominant genotype in isolates from subclinical carriers of ABV (see later discussion). It is noteworthy that BDV, in contrast to ABV, shows a remarkable lack of genotypic variation. Only 2 genotypes of this virus have been identified. The reasons for this major difference between the 2 bornaviruses are unknown.
The pathogenesis of avian bornavirus infection
Initial studies using immunoblots on tissues from 15 PDD-affected birds demonstrated that serum from PDD-affected birds consistently detected an antigen of 38 to 40 kDa present in their central nervous system.30 This antigen, now known to be ABV N-protein, could also be detected in myocardium but not in other organs. Ouyang and colleagues31 subsequently examined 24 stored avian brain samples, processed for histopathology and retained following their submission for necropsy or histopathology to the Schubot Exotic Bird Center diagnostic laboratory in 1992—a year selected at random. Thirteen of these samples were from PDD-infected birds. The remaining 11 were diagnosed with diseases other than PDD. Immunohistochemistry was performed using the macaw anti–N-protein serum and developed with a peroxidase-labeled anti-macaw serum. Cells containing ABV N-protein were found in the brain and spinal cord of all 13 PDD cases.31 One bird not previously diagnosed with PDD also had ABV N-protein–positive cells in its cerebrum. A review of this bird's necropsy report indicated that it was most probably also suffering from PDD. ABV antigen was located in the cerebrum, cerebellum, and spinal cord. In the cerebrum it was usually found in scattered neurons and glial cells. In the cerebellum viral antigen was expressed in the Purkinje layer of the cerebellum, although Purkinje cells were never observed to contain the antigen. The cells containing the viral antigen were located adjacent to the Purkinje cells. Similar lesions have been observed in mammalian bornavirus infections.32 All levels of affected spinal cord contained the ABV antigen in neurons and glia. Krähenbühl and colleagues33 have also detected ABV in tissues of birds with PDD using in situ hybridization. Ten birds submitted for necropsy after dying for reasons other than PDD had no detectable N-protein in their brains.
Lierz and colleagues34 have studied the distribution of ABV in infected cockatoos using real-time PCR on laser dissected tissues. These investigators found the virus in all organs of one bird with clinical PDD, implying viremia. In a second, apparently healthy bird, the virus was restricted to nerve ganglia. Rinder and colleagues,29 using immunohistochemistry employing reagents developed for BDV, also found the virus to be present in multiple avian organs of affected birds. By contrast, Western blot studies by Villanueva and colleagues,35 and the immunohistochemical studies reported by Gancz and colleagues,36 suggested that the virus was restricted to nervous tissue.
Avian bornavirus in the eye
Ouyang and colleagues31 noted that ABV was detectable in the optic lobe of many PDD cases. In a single separate case of PDD in an Eclectus parrot (Eclectus roratus), eye fluid (vitreous and aqueous fluid) was collected on necropsy. This fluid contained so much virus that the ABV N-protein band was visible on stained electrophoresis gels. This protein band was excised and sequenced by mass spectroscopy and was confirmed to be the ABV N-protein (see Fig. 2). In addition, particles with a morphology consistent with that of a bornavirus were observed by immunotransmission electron microscopy (Fig. 3 ). Subsequent analysis of multiple additional eye fluid samples from PDD cases, while demonstrating the presence of occasional virus-like particles on electron microscopy, did not show the numbers of particles observed in the first bird and some contained no detectable virus-like particles, so the phenomenon is inconsistent. It is pertinent that eye lesions are a feature of some cases of PDD; choroiditis and optic neuritis predominate. The single article describing blindness in a psittacine as a result of bilateral retinal degeneration suggests that the lesions in the optic lobe may well lead to local retinal destruction or degeneration (see Fig. 3, Fig. 4 ).13
Fig. 3.
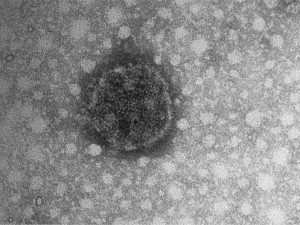
Negatively stained (with phosphotungstic acid [PTA]), virus-like particle (83 nm in diameter) from the eye fluid of an Eclectus parrot with confirmed PDD and ABV infection. The image was recorded with an FEI Morgagni 268 transmission electron microscope at a magnification setting of 180,000X.
(Courtesy of Dr Ross Payne.)
Fig. 4.

The amino acid sequence of a 438 kDa protein isolated from the eye fluid of an Eclectus parrot. The colored sequences are the peptides shown to be identical to ABV N-protein.
Autoimmunity
During the course of developing the Western blot assay, Villanueva and colleagues30 originally used an extract of brain tissue from PDD-affected birds as their antigen source. For control purposes they used an extract of brain tissue from a bird that had died of causes other than PDD and which was both seronegative and PCR-negative for ABV. During these studies, occasionally the serum of a seropositive bird would also react with antigens present in the normal bird brain. In one such case, serum from a “healthy” golden conure (Guarouba guarouba) was shown to react very strongly with a brain protein migrating in the 18- to 20-kDa region. Because this is the approximate molecular weight of myelin, this was investigated. This serum was also found to react with a similar-sized protein in normal chicken brain and with purified myelin basic protein (MBP) derived from that chicken brain. This ABV seropositive bird remains clinically healthy after 1 year. Serum evaluated 7 months later reacted weakly with MBP. It is proposed that the bird mounted a transient autoimmune response to myelin. Villanueva and colleagues30 also surveyed 12 ABV-positive parrot sera for reactions with normal macaw brain and found that 3 reacted with an uncharacterized 40-kDa protein. These findings are of significance because it has been suggested that PDD results from an autoimmune response to brain gangliosides following viral infection in a manner similar to the induction of Guillain-Barré syndrome.37 The authors do not believe that PDD is an autoimmune disease per se. However, the results described indicate that transient autoimmune responses do indeed occur in some PDD cases. The results are probably not clinically significant but may contribute to the complex pathogenesis of this disease.
Experimental ABV disease
There is now abundant evidence proving that infection with ABV is causally associated with clinical PDD. For example, Gancz and colleagues36 were able to induce PDD in 2 of 3 cockatiels after inoculation with ABV-infected brain homogenates. But Koch's postulates remains the standard by which an infectious agent is proven to cause a specific disease.38 When Gray and colleagues39 succeeded in culturing ABV4 in duck embryo fibroblasts, they also undertook a series of challenge experiments to establish Koch's postulates in 3 species of bird; mallards (Anas platyrhynchos), Patagonian conures (Cyanoliseus patagonus),21 and cockatiels (Nymphicus hollandicus).40
Mallards. Three groups of 5, 4-day-old, SPF mallard chicks were infected by the oral, intraocular, and intramuscular routes.39 Over a period of 6 weeks, no consistent clinical signs attributable to ABV infection occurred. However, the mallards were shown to be infected with ABV4 because their feces were positive by PCR when tested at weekly intervals. Likewise, these birds were seropositive for antibodies to ABV N-protein after 3 weeks. The mallards were euthanized at 3 to 8 months. On necropsy, no lesions compatible with PDD were observed. Histopathology of relevant organs showed no evidence of viral infection.
Patagonian conures. Three Patagonian conures, at least 15 years old, shedding psittacine herpesvirus but otherwise healthy, were used in a second challenge experiment.21 Two were placed in isolation and administered passage 6 cultured ABV4 by both the oral and intramuscular routes. The third, uninfected conure was housed in a separate aviary and inoculated with uninfected duck embryo fibroblasts (DEFs). The 2 infected birds became seropositive at 21 days and became fecal shedders by 35 days. Aviary workers reported that they were eating but losing weight. On day 64, one of these birds was found dead in its cage. Necropsy showed an emaciated bird with gross lesions typical of PDD. The next day, the second infected bird was examined; it too was very thin and was euthanized. Necropsy again revealed gross changes consistent with PDD. Tissues were examined histologically, revealing typical PDD lesions—lymphocytic ganglioneuritis throughout the intestine as well as a lymphoplasmacytic encephalitis and myocarditis. PCR assays on the brains of these birds were strongly positive for ABV. The RNA from these brains was sequenced and shown to be identical to the ABV4 challenge strain. The control bird was euthanized 77 days after receiving uninfected tissue culture. Necropsy and histopathological examination of this bird showed no evidence of PDD. PCR of 4 separate brain samples from the control bird was negative for ABV.
Cockatiels. Payne and colleagues40 have also challenged cockatiels with cultured ABV4 that had been passaged 6 times in DEFs. Four cockatiels were inoculated with ABV-M24 using the same routes and doses as the Patagonian conures described above. Two additional birds were inoculated with uninfected tissue culture and retained as negative controls. The inoculated birds remained in apparent good health until day 92 post infection. On that day, one bird was found dead in its cage. Necropsy revealed gross lesions typical of PDD. The remaining 3 infected birds were apparently healthy but their daily feed intake and activity levels declined. On day 110, 2 birds began showing neurologic signs, ataxia, and inability to walk. All 3 infected birds were euthanized humanely. Histopathology showed that 2 of these birds were suffering from PDD with lesions of unusual severity. The lesions were especially severe in the gastrointestinal tract and adrenal gland. The brain and spinal cord had mild to severe multifocal perivascular cuffing and gliosis randomly scattered throughout. The nerves had mild to moderate multifocal perivascular cuffing. The adrenal gland had a severe infiltration of lymphocytes and plasma cells in the medullary regions. The crop, proventriculus, ventriculus, and intestine had a severe infiltration of lymphocytes and a few plasma cells in the serosal and subserosal nerves and ganglia that also extended between muscle fibers. The bird with mild neurologic signs, but no gastrointestinal signs, had mild lesions compatible with PDD. The control, sham-infected birds remained healthy until euthanized on day 120. Their gross necropsy was unremarkable but on histopathology, they showed the presence of scattered lymphoid nodules throughout the spleen, kidney, and liver. It is important that in this study, the cockatiels were shown to be subclinical carriers of ABV4 prior to challenge. Their susceptibility indicates that they were not immune to the challenge virus despite prior exposure. This finding will be of significance in seeking to vaccinate against this virus and is discussed further below (Fig. 5 ).
Fig. 5.
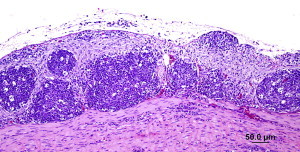
Severe PDD lesions in the ventriculus of experimentally infected cockatiels.
(Courtesy of H.L. Shivaprasad.)
Diagnosis of avian bornavirus infection
Animals mount a detectable antibody response to bornavirus infection. In mammals, immunofluorescence, enzyme-linked immunosorbent assay (ELISA), and Western blotting have been widely employed. In birds, Western blotting has been employed successfully to detect antibodies to ABV. For example, Villanueva and colleagues35 used a Western blot assay to test for antibodies to ABV in the serum of 117 psittacine birds. A lysate from ABV-infected DEFs served as the source of antigen. The predominant antigen recognized by these birds was the 38-kDa N-protein. Thirty of these birds were confirmed positive for PDD by biopsy or necropsy, whereas the remaining 87 birds were apparently healthy or were suffering from non-PDD related disease. Sera from 27 of the 30 PDD cases (90%) contained antibodies to ABV N-protein. Seventy-three (84%) of the 87 apparently “healthy” birds were seronegative. In addition, sera from 7 wild macaws and one mealy Amazon parrot trapped in the Peruvian Amazon rainforest were negative. Most of the positive sera recognized only the N-protein in infected DEF cells. These positive sera also reacted strongly with 2 different preparations of recombinant N-protein. One clone was generated in an Escherichia coli vector, the other was generated in Chinese hamster ovary (CHO-K1) cells. A small proportion of the positive sera could also recognize cloned bornaviral P-protein generated in an E coli vector, a finding also reported by Lierz and colleagues.34
Although the development of antibodies to ABV largely corresponded with the development of clinical PDD and provides a much superior diagnostic procedure than, for example, crop biopsy, it must be pointed out that 14 apparently healthy normal birds possessed detectable antibodies to ABV. It is believed that these birds are infected by ABV but have yet to develop clinical disease. As discussed later in this article, “healthy” or subclinical carriers of ABV are apparently common so the presence of seropositive healthy birds is not unexpected. It is unclear at this time whether these seropositive birds will eventually, inevitably, develop clinical PDD. Villanueva and colleagues35 also demonstrated that many seronegative birds were infected with ABV as detected by fecal PCR. Thus positive serology will be of limited usefulness in any disease eradication programs. The Western blot assay is also a slow and expensive test. A more practical and economic test would be an ELISA that employs purified, cloned ABV N-protein as the test antigen. Such a test is under development.
The transmission of avian bornavirus
Given the apparent sporadic nature of PDD cases and the apparent lack of explosive disease outbreaks, it has long been believed that the causal agent was slowly or inefficiently transmitted. New evidence indicates that this is by no means the case. The infection is indeed common and widespread among captive psittacines, at least in North America. That said, infection does not appear to inevitably or immediately cause PDD. Healthy or subclinical carriers of several species have been documented.
Fecal-Oral Route
The PCR using sequences from the N-gene as a primer readily detects ABV in fecal samples from infected birds. The authors have examined multiple samples from both feces, and cloacal swabs of large numbers of normal and PDD-affected birds. Any PCR products detected were confirmed by sequencing. For example, in the experimentally infected groups of birds, mallards began to shed virus in feces at week 14, yet showed no clinical disease.
A group of 15 “healthy” cockatiels from a single aviary, with no history of PDD or exposure to other birds, was screened for ABV by fecal PCR. Six were positive on first testing, 4 more on testing a week later, and 2 more on a third test. Thus, 12 of these birds were eventually ABV positive; only 2 were also positive by Western blot. The presence of so many PCR-positive birds in a “healthy” colony of cockatiels prompted the authors to screen other cockatiel colonies. A second colony of about 50 birds that practices no biosecurity and purchases birds at random from dealers was tested, and 2 of 10 fecal samples were PCR positive. The owner claimed that he had no significant health problems having lost only 5 birds in the last year (2009). The authors consider a 10% loss of some significance. A third cockatiel colony tested practiced fairly rigorous biosecurity and purchased very few birds from outside. From this colony, none of 15 fecal samples were PCR positive. This study is ongoing, but it is clear that there are “healthy” cockatiels shedding ABV. Lierz and colleagues34 have also shown that apparently healthy birds within an aviary where clinical cases were occurring were also shedding ABV as detected by fecal swabs.
In an unintentional study on the transmission of ABV, the 15 cockatiels were housed on arrival with the control, uninfected mallards. The cockatiels were in suspended cages while the mallards were housed on the floor of the isolation building. Within days, mallards were observed to be eating cockatiel droppings. The cockatiels were tested and found to be shedding ABV4 in their feces. Fourteen days later, fecal samples from the mallards were also positive for the presence of ABV4 by PCR.
Respiratory Route
A dry-filter unit (DFR 1000) was used to test air for the presence of ABV in the authors' infected aviary. A very high volume of air (700 L/min) was drawn through a filter for 24 hours. The filter was subsequently washed and the washings tested by PCR for the presence of ABV. The air was sampled at 4 separate sites ranging from 11 in (28 cm) to 150 in (381 cm) from a cage containing a known ABV-infected bird. Each site was tested 3 times. All filters within the aviary were PCR-positive, indicating the presence of ABV in the air. Three samples were taken from a room adjacent to the aviary where birds were handled, and 2 of these samples were positive. Of 3 samples taken within 4 ft (122 cm) of an external aviary door, 1 was weakly positive. Of 3 samples taken 15 ft (457 cm) from this door, none were positive. Thus ABV was present both in the aviary itself and in a nearby workroom. It is possible that this disease can be transmitted by the respiratory route. It is of interest that pulmonary lesions have been described in cases of PDD with lymphocytoplasmic infiltrates observed in nerve ganglia associated with the large pulmonary blood vessels.41
ABV Shedding
While the authors have chosen to focus on the presence of ABV in the feces of affected birds, the shedding of this virus from other sites has also been examined. For this purpose the authors have had available 3 healthy African gray parrots known to be persistent shedders of ABV. These birds were swabbed at weekly intervals for 7 weeks and at longer intervals thereafter. The virus was detected by PCR not only in feces and cloacal swabs but also in swabs from the nares, the choana, and from the feathers in the axilla. This shedding was variable both between individuals and locations. One bird (Table 1 ) shed virus from all test sites on every sampling. Another bird shed virus intermittently and at times only choanal swabs were positive. The third bird was also a frequent shedder but not as consistent as the first.
Table 1.
Avian bornavirus PCR shedding over a 5-month period
| 19-May-09 | 21-Jul-09 | 08-Sep-09 | 15-Sep-09 | 22-Sep-09 | 29-Sep-09 | 01-Oct-09 | ||
|---|---|---|---|---|---|---|---|---|
| AG1 = African gray “Juniper” | Nares | POS | POS | POS | POS | POS | POS | |
| Choana | POS | POS | POS | POS | POS | POS | ||
| Skin | POS | POS | POS | POS | POS | POS | ||
| Cloaca | POS | POS | POS | POS | POS | POS | ||
| AG2 = African gray “Quincy” | Nares | NEG | NEG | NEG | NEG | NEG | NEG | |
| Choana | POS | POS | POS | POS | POS | POS | ||
| Skin | NEG | NEG | NEG | NEG | NEG | NEG | ||
| Cloaca | POS | POS | POS | POS | NEG | NEG | ||
| AG3 = African gray “George” | Nares | POS | NEG | POS | NEG | POS | POS | |
| Choana | POS | POS | POS | POS | POS | POS | ||
| Skin | POS | POS | POS | POS | POS | POS | ||
| Cloaca | POS | POS | POS | POS | POS | POS | ||
| AG8 = African gray “Zelda” | Nares | NEG | NEG | NEG | NEG | NEG | NEG | |
| Choana | NEG | NEG | NEG | NEG | NEG | NEG | ||
| Skin | NEG | NEG | NEG | NEG | NEG | NEG | ||
| Cloaca | NEG | NEG | NEG | NEG | NEG | NEG |
Juniper, Quincy, and George were all crop biopsy positive and serologically positive for ABV. Zelda has been consistently negative on PCR, serology, and crop biopsy.
ABV in other avian species
PDD has largely been restricted to captive psittacines. However, there have been reports of a similar disease in Canadian geese (Branta canadensis),42 and several passerine and piciform species.41 Using fecal PCR the authors have tested feces from 102 ducks (Anatidae sp), a toco toucan (Ramphastos toco), 6 rock doves (Columba livia), 2 mourning doves (Zenaida macroura), 1 Carolina wren (Thryothorus ludovicianus), 2 eastern screech owls (Megascops asio), 2 barred owls (Strix varia), 2 barn owls (Tyto alba), 1 peregrine falcon (Falco peregrinus), 2 red-shouldered hawks (Buteo lineatus), and 1 red-tailed hawk (Buteo jamaicensis). All except the toucan were negative. The toco toucan was housed in a flight cage together with multiple macaws, some of which were shedding ABV4. The toucan was known to eat macaw droppings. The ABV detected in its feces was of genotype 4 and the bird was healthy at the time of sampling. Weissenböck and colleagues43 recently published an article describing PDD and ABV in a canary (Serinus canaria) in Hungary. The genotype of this virus was found to be intermediate between other ABV genotypes and BDV. The significance of this is unclear but it is entirely plausible, given the presence of BDV in European ducks,23 that ABV may have evolved from BDV. As described earlier, the authors succeeded in infecting mallards with ABV4 but no disease resulted within 8 months. The authors have tested serum from a domestic Muscovy duck (Cairina moschata) submitted to a veterinary clinic because of a tumor, whose serum was positive for antibodies to ABV N-protein by Western blotting. However, given the issues regarding the specificity and significance of Western blotting in humans, the significance of this single positive result is unclear at this time.
Control and prevention of ABV infection
The control of viral infections in many cases depends on appropriate management practices and infection control. No data are available to the authors regarding the survival of ABV in the environment. However, it is an enveloped RNA virus of similar size and structure to Newcastle disease virus (NDV); both are enveloped Mononegavirales. ABV would be expected therefore to show a sensitivity profile similar to that observed in NDV. The authors therefore suggest that NDV can be used as a surrogate and that disinfectants and cleansing agents that are effective for NDV will likely be effective for ABV. Control would include isolation, traffic control, sanitation and thorough cleaning, and the use of disinfectants such as phenols, formaldehyde, or hypochlorites such as bleach. With the availability of a fecal PCR, it should also be possible to control the admittance of ABV-infected birds to aviaries. It must be pointed out, however, that not all birds are constant ABV shedders. Thus, as with psittacine herpesviruses, repeated testing may be required to exclude any specific bird as an ABV carrier.
The authors have treated a group of clinically healthy, seropositive, ABV-shedding African gray parrots (Psittacus erithacus) with the antiviral drug amantadine for 6 weeks, with no apparent effect on fecal viral shedding. Amantidine was tested because there have been anecdotal reports of its therapeutic affect in parrots with PDD.44 The authors are currently looking at other antiviral compounds and are using virus shedding as a marker of efficacy. Treatment protocols will have to be developed, although experience with mammalian encephalitides suggests that it may be difficult to identify an effective antiviral therapy.
It is generally believed that Borna disease in mammals is in large part immunologically mediated.45, 46, 47 Immunosuppressive or anti-inflammatory treatments appear to reduce the severity of disease while immune stimulation may in some cases increase its severity. Thus Borna disease is believed to belong to that group of infections whereby immune responses increase severity and vaccination may be contraindicated.
It is certainly true that most birds suffering from PDD are strongly seropositive, implying that antibodies to the immunodominant N-protein are not protective. The authors' cockatiel study described herein also provided clear evidence that prior exposure to ABV is not protective. In addition, there are obvious economic issues involved in the commercial production of a vaccine for use in a small, specialized market.
Summary
Multiple investigators have now demonstrated that ABV is consistently present in cases of PDD. Likewise, Koch's postulates have been fulfilled in 2 species, cockatiels and Patagonian conures. ABV has been demonstrated in the lesions of PDD cases. All available evidence supports the contention that ABV is the etiologic agent of PDD. Diagnostic tests such as Western blots or fecal PCR can identify many, but not all ABV-infected birds, and should be employed to control the spread of this disease. Such tests may be very useful for diagnosis and in epidemiologic studies.
Acknowledgments
We would like to thank Dr Ross Payne who undertook the transmission electron microscopy of the virus.
References
- 1.Berhane Y., Binnington B., Hunter B. Peripheral neuritis in psittacine birds with proventricular dilation disease. Avian Pathol. 2001;73:563–570. doi: 10.1080/03079450120078770. [DOI] [PubMed] [Google Scholar]
- 2.Gregory C., Ritchie B. Advances in understanding of proventricular dilation disease (PDD): detection of virus and viral nucleic acid in infected birds. Proc Annu Conf Assoc Avian Vet. 1999:41–43. [Google Scholar]
- 3.Ritchie B. Epizootiology of proventricular dilatation disease in breeding cockatiels. Proc Annu Conf Assoc Avian Vet. 2004:41–45. [Google Scholar]
- 4.Boutette J.B., Taylor M. Proventricular dilation disease: a review of research, literature, species differences, diagnostics, prognosis, and treatment. Proc Annu Conf Assoc Avian Vet. 2004:175–181. [Google Scholar]
- 5.Shivaprasad H.L. Proventricular dilatation disease in a peregrine falcon. Proc Annu Conf Assoc Avian Vet. 2005:107–108. [Google Scholar]
- 6.Hadley T. Atypical presentation of proventricular dilatation disease in a yellow-headed Amazon. Proc Annu Conf Assoc Avian Vet. 2005:353–355. [Google Scholar]
- 7.Clark D. Proventricular dilation syndrome in large psittacine birds. Avian Dis. 1984;28:813–815. [PubMed] [Google Scholar]
- 8.Phalen D. An outbreak of psittacine proventricular dilation syndrome (PPDS) in a private collection of birds and an atypical form in a Nanday conure. Proc Annu Conf Assoc Avian Vet. 1989:27–34. [Google Scholar]
- 9.Gregory CR, Ritchie BW, Latimer KS, et al. Progress in understanding proventricular dilatation disease. Proceedings of the International Aviculture Society. Orlando (FL); 1998. p. 1–6.
- 10.Turner R. Macaw fading or wasting syndrome. Proc 33rd West Poult Dis Conf. 1984:87–88. [Google Scholar]
- 11.Suedmeyer W. Diagnosis and clinical progression of three cases of proventricular dilation syndrome. J Assoc Avian Vet. 1992;6:159–163. [Google Scholar]
- 12.Gregory C.R., Latimer K.S., Niagro F.D. A review of proventricular dilatation syndrome. J Assoc Avian Vet. 1994;8:69–75. [Google Scholar]
- 13.Steinmetz A., Pees M., Schmidt V. Blindness as a sign of proventricular dilatation disease in a grey parrot (Psittacus erithracus erithracus) J Small Anim Pract. 2008;49:660–662. doi: 10.1111/j.1748-5827.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt R.E., Reavill D.R., Phalen D.N. Iowa State Press; Iowa: 2003. Pathology of pet and aviary birds. p. 47–55. [Google Scholar]
- 15.Shivaprasad HL, Barr BC, Woods LW, et al. Spectrum of lesions (pathology) of proventricular dilation syndrome. Proceedings of the Association of Avian Veterinarians. Philadelphia; 1995. p. 507–08.
- 16.Heldstab A., Morgenstern D., Ruedi A. Pathologie einer endemieartig verlaufenden neuritis im magen/darmberich bei groBpapageien. Int Symp Zoo Animal. 1985:317–324. [in German] [Google Scholar]
- 17.Gough R.E., Drury S.E., Culver F. Isolation of a coronavirus from a green-cheeked Amazon parrot (Amazona viridigenalis Cassin) Avian Pathol. 2006;35:122–126. doi: 10.1080/03079450600597733. [DOI] [PubMed] [Google Scholar]
- 18.Grund C.H., Werner O., Gelderblom H.R. Avian paramyxovirus serotype I isolates from the spinal cord of parrots display a very low virulence. J Vet Med. 2002;49:445–451. doi: 10.1046/j.1439-0450.2002.00596.x. [DOI] [PubMed] [Google Scholar]
- 19.Honkavuori K.S., Shivaprasad H.L., Williams B.L. Novel bornavirus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis. 2008;14:1883–1886. doi: 10.3201/eid1412.080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kistler A.L., Gancz A., Clubb S. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J. 2008;5:88. doi: 10.1186/1743-422X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray P., Hoppes S., Suchodolski P. Use of avian bornavirus isolates to induce proventricular dilatation disease in conures. Emerg Infec Dis. 2010;16(3):473–479. doi: 10.3201/eid1603.091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briese T., de la Torre J.C., Lewis A. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci U S A. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg M., Johansson M., Montell H. Wild birds as a possible natural reservoir of Borna disease virus. Epidemiol Infect. 2001;127:173–178. doi: 10.1017/s0950268801005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malkinson M., Weismann Y., Ashash E. Borna disease in ostriches. Vet Rec. 1993;133:304. doi: 10.1136/vr.133.12.304-b. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig H., Kraft W., Kao M. Bornavirus infection (Borna disease) in naturally and experimentally infected animals: its significance for research and practice. Tierarztl Prax. 1985;13:421–453. [PubMed] [Google Scholar]
- 26.Joest E., Degen K. Über eigentümliche Kerneinschlüsse der Ganglienzellen bei der enzootischen Gehirn-Rückenmarksentzündung der Pferde. Z Infkrankh Haustiere. 1909;6:348–356. [in German] [Google Scholar]
- 27.Herzog S., Rott R. Replication of borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 28.Weissenböck H., Bakonyi T., Sekulin K. Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerg Infect Dis. 2009;15:1453–1459. doi: 10.3201/eid1509.090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinder M., Ackermann A., Kempf H. Broad tissue tropism of avian bornavirus in parrots with proventricular dilatation disease. J Virol. 2009;83:5401–5407. doi: 10.1128/JVI.00133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villanueva I., Gray P., Tizard I. Detection of an antigen specific for proventricular dilation disease in psittacine birds. Vet Rec. 2008;163:426. doi: 10.1136/vr.163.14.426. [DOI] [PubMed] [Google Scholar]
- 31.Ouyang N., Storts R., Tian Y. Detection of avian bornavirus in the nervous system of birds diagnosed with proventricular dilatation disease. Avian Pathol. 2009;38:393–401. doi: 10.1080/03079450903191036. [DOI] [PubMed] [Google Scholar]
- 32.Eisenman L.M., Brothers R., Tran M.H. Neonatal borna disease virus infection in the rat causes a loss of Purkinje cells in the cerebellum. J Neurovirol. 1999;5:181–189. doi: 10.3109/13550289909022000. [DOI] [PubMed] [Google Scholar]
- 33.Krähenbühl I., Lehner A., Hilbe M. Detection of avian bornavirus in parrots with proventricular dilatation disease (PDD) by in-situ hybridization. J Comp Path. 2009;141:294. [Google Scholar]
- 34.Lierz M., Hafez H.M., Honkavuori K.S. Anatomical distribution of avian bornavirus in parrots, its occurrence in clinically healthy birds and ABV-antibody detection. Avian Pathol. 2009;38:491–496. doi: 10.1080/03079450903349238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villanueva I., Gray P., Mirhosseini N. The diagnosis of proventricular dilatation disease: use of a western blot assay to detect antibodies against avian borna virus. Vet Micro. 2010;143:196–201. doi: 10.1016/j.vetmic.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 36.Gancz A.Y., Kistler A.L., Greninger A.L. Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4. Virol J. 2009;6:11. doi: 10.1186/1743-422X-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi G., Crosta L., Pesaro S. Parrot proventricular dilatation disease. Vet Rec. 2008;163:310. doi: 10.1136/vr.163.10.310-b. [DOI] [PubMed] [Google Scholar]
- 38.Koch R (1890). Ueber bakteriologische Forschung, Verhandl, des X. Internatl Med Congr, Berlin. August Hirschwald, Berlin: 1891. p. 35.
- 39.Gray P, Villaneuva I, Mirhosseini N, et al. Experimental infection of birds with avian bornavirus. Proceeding of the Association of Avian Veterinarians. Milwaukee; 2009. p. 7.
- 40.Payne S, Shivaprasad HL, Mirhosseini N, et al. Unususal and severe lesions of proventricular dilatation disease in Cockatiels (Nymphicus hollandicus) as healthy carriers of avian bornavirus and subsequently infected with a virulent strain of the same ABV genotype. Vet Micro, in press. [DOI] [PubMed]
- 41.Perpiñán D., Fernández-Bellon H., López C. Lymphocytic myenteric, subepicardial, and pulmonary ganglioneuritis in four nonpsittacine birds. J Avian Med Surg. 2007;21:210–214. doi: 10.1647/1082-6742(2007)21[210:LMSAPG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Daoust P.Y., Julian R.J., Yason C.V. Proventricular impaction associated with nonsuppurative encephalomyelitis and ganglioneuritis in two Canada geese. J Wildl Dis. 1991;27:513–517. doi: 10.7589/0090-3558-27.3.513. [DOI] [PubMed] [Google Scholar]
- 43.Weissenböck H., Sekulin K., Bakonyi T. Novel avian bornavirus in a nonpsittacine species (canary; Serinus canaria) with enteric ganglioneuritis and encephalitis. J Virol. 2009;83:11367–11371. doi: 10.1128/JVI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clubb S.L., Meyer M.J. Clinical management of psittacine birds affected with proventricular dilatation disease. Proc Annu Conf Assoc Avian Vet. 2006:85–90. [Google Scholar]
- 45.Stitz L., Dietzschold B., Carbone K.M. Immunopathogenesis of Borna disease. Curr topics Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto K., Hooper D.C., Bornhorst A. Intrinsic responses to Borna disease virus infection of the central nervous system. Proc Natl Acad Sci U S A. 1996;93:13345–13350. doi: 10.1073/pnas.93.23.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwemmie M., Lipkin W.I. Models and mechanisms of Bornavirus pathogenesis. Drug Discov Today. 2004;1:211–216. [Google Scholar]


