Abstract
The dynamics of bovine respiratory syncytial virus (BRSV), bovine parainfluenza virus 3 (PIV-3), bovine corona virus (BCoV) and bovine viral diarrhoea virus (BVDV) infections were studied in 118 dairy herds in south western Sweden. By using serology on paired samples from three ∼7 vs. ∼15-month-old calves per herd, the propagation of infections was investigated over about a 1-year period. The results implied that at least 74% of calves had experienced one or more of the monitored infections at the age of ∼7 months (Sample 1, Spring); 30%, 48%, 34% and 8% were seropositive to BRSV, PIV-3, BCoV and BVDV, respectively. Seroconversions to BRSV, PIV-3, BCoV and BVDV occurred in 26%, 38%, 50% and 3% of seronegative animals and 63% had antibodies against two or more infections at ∼15 months (Sample 2). In total, 90–97% of animals that were seropositive in Sample 1 remained positive in Sample 2. A significant association was found between BVDV and BCoV (P = 0.01). Moreover, a significantly higher proportion of herds in which no calves had a recorded history of respiratory disease (n = 15) were classified as negative to all four infections monitored when compared to herds in which disease was observed (P = 0.0002). This study showed a high infection burden in young animals and effective spread of BRSV, PIV-3 and BCoV in one area of Sweden. BVDV infections were restricted to a few herds, reflecting the effect of a voluntary control program against BVDV in Sweden.
Keywords: BRSV, PIV-3, BCoV, BCV, BVDV
1. Introduction
Bovine respiratory syncytial virus (BRSV), bovine parainfluenza virus 3 (PIV-3), bovine corona virus (BCoV) and bovine viral diarrhoea virus (BVDV) are single-stranded RNA viruses frequently involved in the respiratory and enteric disease complexes of calves and adult cattle (Storz et al., 2000, Stott et al., 1980). In order to control these infections in a strategic manner, it is necessary to understand their prevalence and epidemiology in the cattle population. Although data exist indicating that BRSV and BCoV may persist within herds and that infections occur continuously (Collins et al., 1987, Heckert et al., 1991, Valarcher et al., 2001, Van der Poel et al., 1993), other studies have reported large intervals between infections, clearance of virus between outbreaks (Alenius et al., 1991, Elvander, 1996) and reintroduction of new strains (Larsen et al., 2000). The infections are most commonly detected during autumn and winter (Stott et al., 1980).
Control schemes based on increased herd bio-security will not be successful if latency and reactivation or continuous virus circulation within closed herds are common features. In these cases, vaccination of seropositive adult cattle (to prevent re-excretion) and young stock (to prevent both disease and excretion) might be more beneficial. Vaccination procedures must in any case be preceded by prevalence studies so that optimal target age groups can be identified for vaccination and so cost-benefit estimates can be made.
Bulk tank milk data, reflecting the antibody status of lactating cows, have revealed nation-wide high prevalences of BRSV, BCoV and BVDV in those European countries where vaccines are not used (Elvander, 1996, Paton et al., 1998, Tråvén et al., 1999), showing that these infections are wide-spread. However, since actively acquired antibodies may remain detectable for years without re-infection (Alenius et al., 1991, Elvander, 1996), these studies mirror infections over long time periods. A more immediate picture can be given by using serology in a few young animals (6–18 months old) per herd – a method that has been proved to be useful for herd screening for acute BVDV infections in Denmark (Houe, 1992). Knowing that the respiratory viruses spread effectively within herds (Alenius et al., 1991, Verhoeff and van Nieuwstadt, 1984), we extended the application of this method and used serology from young stock to monitor BRSV, PIV-3 and BCoV infections on a herd level. The selected animals thus served as sentinels for infections introduced or circulating in studied herds.
The purpose of our study was to investigate virus infection dynamics in dairy herds and the geographical spread of BRSV, PIV-3 and BCoV in an area of Sweden where these infections are considered endemic (Elvander, 1996, Tråvén et al., 1999). BVDV infections were also screened, although this virus is target for a national eradication program (Lindberg and Alenius, 1999) and is gradually being eliminated from the whole of Scandinavia (Sandvik, 2004).
2. Materials and methods
2.1. Animals and herds
Three heifer calves in each of 118 Swedish dairy herds were bled between March and May 1999 (Sample 1) and 1–11 months later (median 8, inter-quartile range (IQR) 2 months; Sample 2). The animals were 5–11 months of age at the first sampling (median 7, IQR 1 month) and were selected as having a recorded history of respiratory disease, as previously described in detail (Svensson et al., 2003). In herds where no heifers of this age fulfilled the criterion of disease (n = 15), animals were randomly selected. The second sample, obtained at the age of 7–21 months (median 15, IQR 2 months), was short of nine animals due to sale, slaughter or death unrelated to respiratory disease. The majority of animals were Swedish Holstein or Swedish Red and White breed with some cross-breds.
The herds were in south-western Sweden and participated in a long-term research project on the health and growth rate of dairy heifer calves. A detailed description of the selection process is given elsewhere (Svensson et al., 2003). Briefly, herds were included on the basis of size, rearing systems of heifer calves and the farmer’s will to participate in the investigation. The herds were a convenience sample representing Swedish dairy herds affiliated to the official eradication scheme for BVDV and having between 28 and 94 (median 48) cows (Svensson et al., 2003). During the winter season, cows were kept in tie-stalls and in cubicles in 78% and 22% of the herds, respectively. Visiting bulls were not used on any farm. The calves were kept in individual or group pens bedded with straw until weaning, and thereafter in litter pens or in slatted-floor pens. They were on pasture during the summer 1999. Bulk milk was collected from 115 of the 118 herds between March and August 2000.
2.2. Interviews
Information was collected through interviews with farmers in 112, 113, 113 and 113 of the herds, respectively. The questions asked for the following data: (i) estimated air-distance to the closest cattle-rearing herd, (ii) purchase of animals during 1998–2000 (YES/NO), (iii) co-pasturing with animals from other herds between samplings (YES/NO) and (iv) estimated number of visitors in the herd during an average month, including the routine visits of the milk tanker driver (>8, 5–8, 3–4, 1–2, one every second month or one more rarely). A visitor was defined as a person with probable contact with other herds within the previous 24 h.
2.3. Antibody detection and classification of infection status
Commercially available indirect enzyme-linked immunosorbent assays (ELISAs) were used for detection of IgG antibodies specific to BRSV, PIV-3, BCoV and BVDV (SVANOVA Biotech). Sera were diluted 1:25 and analyses performed according to the manufacturers’ instructions. Milk samples were not diluted and were tested for BRSV, BCoV and BVDV. Samples that generated a corrected optical density (COD) value of ⩾0.2 (sera) or ⩾0.05 (milk) at 450 nm were regarded as positive, whereas samples generating a value below these thresholds were regarded as negative (Niskanen, 1993, Niskanen et al., 1989). Seroconversion was defined as a negative COD value converting to a positive in paired sera. Tests for antibodies to bovine herpes virus 1 were not performed as Sweden has been declared free from this infection.
Animals were classified as infected with BRSV, PIV-3, BCoV or BVDV at first sampling if they were seropositive to the assessed infection in Sample 1 and remained seropositive in Sample 2. Virus-specific antibodies in Sample 1 that were not detected in Sample 2 were interpreted as of possible maternal origin (for n, see Table 1 ). Animals that were seropositive to BRSV, PIV-3, BCoV or BVDV in Sample 2 were classified as infected with respective virus at second sampling. Herds were classified as infected with BRSV, PIV-3, BCoV or BVDV when at least one animal was infected according to the described definition.
Table 1.
Seroprevalence for bovine respiratory syncytial virus (BRSV), bovine parainfluenza virus 3 (PIV-3), bovine corona virus (BCoV) and bovine virus diarrhea virus (BVDV) of calves in 118 Swedish dairy herds
| Virus | Sample 1, positive animalsa (%, n = 354) | Sample 2, positive animalsa(%, n = 345) | Negative to positiveb (%, n = 345) | Positive to negativec (%, n = 345) |
|---|---|---|---|---|
| BRSV | 106 (30) | 158 (46) | 64 (20) | 10 (3) |
| PIV-3 | 170 (48) | 227 (66) | 67 (19) | 9 (3) |
| BCoV | 120 (34) | 227 (66) | 113 (33) | 4 (1) |
| BVDV | 30 (8) | 38 (11) | 8 (2) | 2 (1) |
Number of seropositive animals detected in Sample 1 and Sample 2, respectively.
Number of animals that seroconverted between Samples 1 and 2.
Number of animals that converted from a positive COD value in Sample 1 to a negative COD value in Sample 2.
3. Results
3.1. Individual data
The serological status of sampled calves are shown in Table 1. In Sample 1, PIV-3 showed the highest seroprevalence, compared to BRSV and BCoV and to the much less frequent BVDV (Table 1). ELISA COD values in Sample 1 were high (⩾1) or low (<0.2) in most sera (Fig. 1 ), but COD values for animals that turned seronegative in Sample 2 were moderate (mean COD 0.4, 0.5, 0.3 and 0.3 for BRSV, PIV-3, BCoV and BVDV, respectively; for n, see Table 1). The age of these particular animals ranged between 6.1 and 8.6 months. In Sample 2, BCoV showed as high seroprevalence as PIV-3, whereas BRSV and BVDV remained lower (Table 1).
Fig. 1.
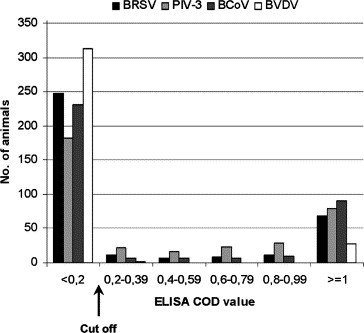
Distribution of ELISA results on serum analyses from 354 animals in 118 Swedish dairy herds (Sample 1). Values are given as corrected optical density (COD) values at 450 nm.
According to the classification described above, 8% of the animals were infected with three or four of the monitored viruses in Sample 1 and this percentage rose to 29 in Sample 2 (Table 2 ). Seroconversions to BRSV, PIV-3, BCoV and BVDV occurred in 64/242 (26%), 67/177 (38%), 113/228 (50%) and 8/318 (3%) of seronegative animals from which paired sera were available. Ninety to 97% of seropositive animals in Sample 1, from which paired sera were available, remained seropositive in Sample 2 (BRSV 93/103 (90%); PIV-3 159/168 (95%); BCoV 113/117 (97%); BVDV 25/27 (93%)).
Table 2.
Number of animals infected with combinations of 0–4 viruses (BRSV, PIV-3, BCoV and/or BVDV), based on paired serum samples from 345 calves in 118 Swedish dairy herds (2–3 calves per herd)
| Number of infectionsa | Sample 1 (%) | Sample 2 (%) |
|---|---|---|
| 4 | 1 (0.3) | 11 (3) |
| 3 | 25 (7) | 89 (26) |
| 2 | 83 (24) | 119 (35) |
| 1 | 148 (43) | 99 (29) |
| 0 | 88 (26) | 27 (8) |
Any combination of BRSV, PIV-3, BCoV and BVDV; for definition of infected animal, see Section 2.
3.2. Herd classifications and serological patterns
In agreement with the individual serological data, the number of herds that were classified as infected at first sampling was highest for PIV-3 and thereafter for BCoV, BRSV and BVDV (Table 3 ). In herds classified as negative for BRSV, PIV-3, BCoV and BVDV in Sample 1, seroconversions between samplings occurred in 26 (31%), 22 (39%), 37 (48%) and 4 (4%), respectively. The number of PIV-3 positive herds remained higher than that of BCoV in Sample 2 (Table 3). The distribution of herds with 1, 2 or 3 animals seropositive for the different infections is shown in Table 4 ; demonstrating that in as many as 68–97% of herds with one seropositive animal to BRSV, PIV-3 or BCoV, the remaining two sampled animals were also seropositive to respective virus. The data were least homogenous with respect to BVDV antibodies.
Table 3.
Number of herds classified as infected with BRSV, PIV-3, BCoV and BVDV, based on paired serum samples from 345 calves in 118 Swedish dairy herds (2–3 calves per herd)
| Sample 1a (%) | Sample 2a (%) | |
|---|---|---|
| BRSV | 34 (29) | 60 (51) |
| PIV-3 | 62 (53) | 84 (71) |
| BCoV | 41 (35) | 78 (66) |
| BVDV | 13 (11) | 17 (14) |
For classification of infection, see Section 2.
Table 4.
Distribution of herds according to number of seropositive animals, based on serum samples from three calves per herd in 118 (Sample 1) and 109 (Sample 2) Swedish dairy herds
| Virus | Number of herds (%b) with one, two and three seropositive out of three sampled animals |
|||
|---|---|---|---|---|
| 1/3 | 2/3 | 3/3 | ||
| Sample 1 | BRSV (n = 43) | 10 (23) | 3 (7) | 30 (70) |
| PIV-3 (n = 66) | 7 (11) | 14 (21) | 45 (68) | |
| BCoV (n = 45) | 7 (16) | 1 (2) | 37 (82) | |
| BVDV (n = 15) | 7 (47) | 1 (7) | 7 (47) | |
| Sample 2a | BRSV (n = 56) | 5 (9) | 6 (11) | 45 (80) |
| PIV-3 (n = 76) | 4 (5) | 9 (12) | 63 (83) | |
| BCoV (n = 72) | 0 (0) | 2 (3) | 70 (97) | |
| BVDV (n = 15) | 4 (27) | 2 (13) | 9 (60) | |
Only herds with three sampled animals are included.
Percentage out of total number of herds with at least one animal seropositive for respective virus.
The combinations of infections that were diagnosed in herds with different BVDV status are illustrated in Fig. 2 . Whereas single PIV-3 infections was the most prevalent event in BVDV negative herds in Sample 1, the combination BRSV/PIV-3/BCoV was most prevalent in Sample 2, when the sampled animals were older. Two or three (additional) infections were significantly more common in BVDV positive than in BVDV negative herds in Sample 2 (94% vs. 62%, P = 0.01, Fisher’s exact test), and similarly BCoV infections were significantly more common in BVDV positive than in BVDV negative herds in Sample 2 (94% vs. 61%, P = 0.01, Fisher’s exact test).
Fig. 2.
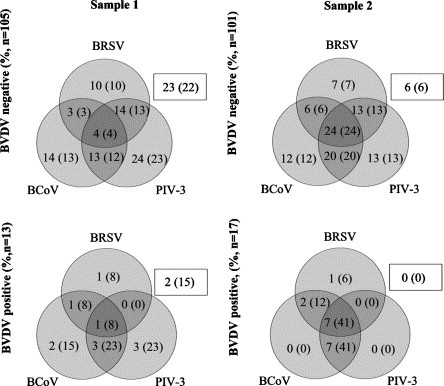
Number of herds (% within parentheses) classified as infected with different combinations of BRSV, PIV-3 and BCoV in 118 Swedish BVDV positive or negative dairy herds. Herds shown in squares are classified as negative for BRSV, PIV-3 and BCoV. For definition of infection, see text.
In Sample 1, 23 herds were classified as negative for all four infections (Fig. 2). Six of these herds were still negative for all infections in Sample 2, whereas three, four and seven herds became single infected with BRSV, PIV-3 or BCoV, respectively, two herds became infected with PIV-3/BCoV and one herd with BRSV/BCoV/BVDV. Of 18 herds in which Sample 2 was obtained between May and August 1999 (outside the period when the infections are most commonly detected), seroconversions to BRSV, PIV-3, BCoV and BVDV were observed in 3 (17%), 4 (22%), 1 (6%) and 0 (0%) herds. Of the 15 herds in which no calves had a recorded history of respiratory disease at first sampling (see selection criteria), nine herds (60%) were classified as negative for all four viruses in Sample 1. This was a significantly higher proportion than among herds with at least one calf with a previous record of respiratory disease (14%, P = 0.0002, Fisher’s exact test).
3.3. Interview data
Estimated air-distances to closest cattle-rearing herd varied between 0.1 and 7 (mean 1) km. Herds with such distance of >1.0 km (n = 24) were at less risk to be classified as positive for BCoV in Sample 1 than herds in more densely populated areas (P = 0.03, χ 2-test), but corresponding associations for BCoV in Sample 2, for any of the other viruses or for number of infections, were not significant.
Fifty of 112 farmers (45%) reported a purchase of animals during the period 1998–2000. No significant association was found between this factor and herd classification to any of the four viruses or with number of infections in each herd (χ 2-test). Fifty-eight of the 62 herds (94%) that stated no purchase during the study period were classified as infected with 1–4 infections in Sample 2, compared to 48/50 herds (96%) that stated purchase.
Results of estimations of number of visitors were distributed as follows: >8 visitors/month in 37 herds, 5–8 visitors/month in 37 herds, 3–4 visitors/month in 27 herds and 1–2 visitors/month in 12 herds. Fourteen herds used co-pasture with cattle from other herds, whereas 99 herds did not. None of these factors was significantly associated with herd classification to any of the four viruses or with number of infections in each herd. However, a positive correlation was found between herd size and number of infections (P = 0.04, χ 2-test).
3.4. Bulk tank milk data
Bulk tank milk collected in 2000 was BRSV and BCoV antibody positive in all 115 sampled herds, with COD values ranging between 0.56–2.12 and 0.39–1.47, respectively (Fig. 3 ). Only 44 of 115 herds (38%) were antibody positive for BVDV in bulk tank milk (Fig. 3, mean COD 0.2), which contrasted with 107/116 herds (92%) in 1993 (mean COD 0.7; data obtained from the Swedish Dairy Association) and 35/100 herds (35%) in 2002 (mean COD 0.1; data obtained from the Swedish Dairy Association).
Fig. 3.
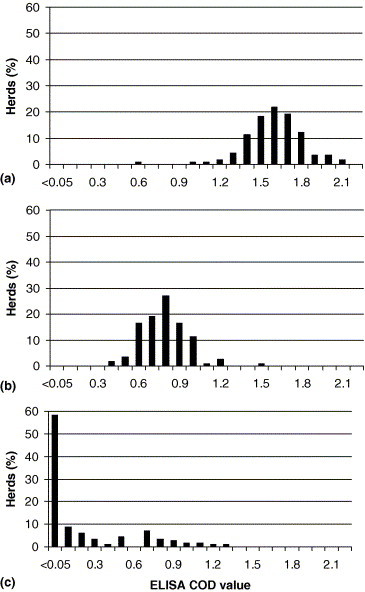
Distribution of ELISA results for antibodies to: (a) BRSV; (b) BCoV; (c) BVDV in bulk tank milk from 115 Swedish dairy herds. Values are given as corrected optical density (COD) values at 450 nm.
3.5. Geographical distribution of infected herds
Herds classified as infected with BRSV, BCoV and BVDV in Sample 1 were mainly located in eastern parts of the area, whereas herds classified as infected with PIV-3 were more evenly distributed throughout the area. Seroconversions between samplings appeared to occur more commonly in herds adjacent to already infected herds (Fig. 4 ).
Fig. 4.
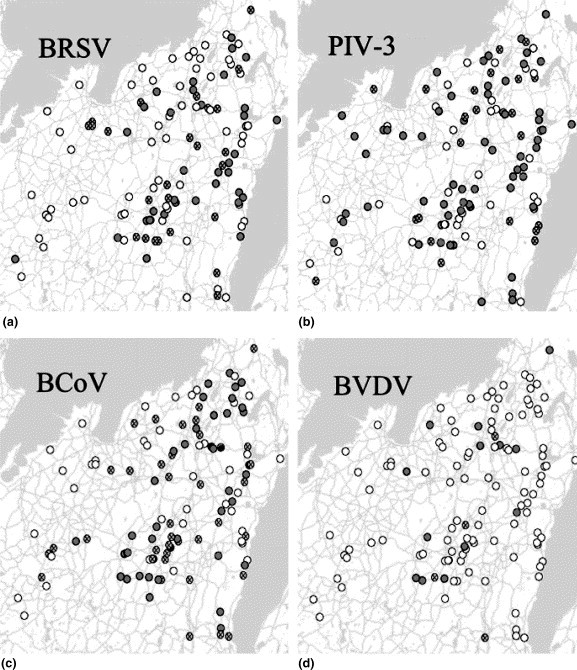
Geographical distribution of 118 Swedish dairy herds with regard to serological status for: (a) BRSV, (b) PIV-3, (c) BCoV and (d) BVDV. Herds classified as infected with respective virus in Sample 1 are shown as filled circles (for classification of infection, see Section 2). Herds not classified as infected in Sample 1, but in which seroconversions were detected between Samples 1 and 2 are shown as circles with cross (for definition of seroconversion, see text). Herds classified as not infected with respective virus in Samples 1 and 2 are shown as empty circles.
4. Discussion
In this study, we investigated the serum antibody status of dairy calves in an area of Sweden with verified high BRSV and BCoV herd-prevalences (71–73% and 90–94%) (Elvander, 1996, Tråvén et al., 1999) and with a cattle density of 11.4 heads/km2 (Swedish official statistics 2003). Higher densities occur in European countries with more intensive cattle rearing. The applied sampling strategy also allowed us to study the propagation of BRSV, PIV-3, BCoV and BVDV infections over approximately 1 year. Considering the recent introduction of a vaccine against BRSV and PIV-3 on the Swedish market in 2004, similar serological studies might be difficult to perform in the future.
BRSV and PIV-3 have been estimated to infect 70–85% of animals in British beef-rearing units by the age of nine months (Stott et al., 1980) and closed Dutch dairy herds seem to encounter these viruses almost annually (Kimman et al., 1988, Van der Poel et al., 1993, Verhoeff and van Nieuwstadt, 1984). In contrast, only some young animals included in the present study were seropositive to BRSV, PIV-3 and BCoV after the winter season 1999. However, at least 74% of calves had experienced one or more of the infections at this time point. A substantial number of the calves that were seronegative in Spring seroconverted before the next sampling, suggesting a constant presence of naïve young animals that render possible a continuous circulation of virus in the area. Accordingly, in some herds, seroconversions to BRSV, PIV-3 and BCoV were observed in March to August, i.e., outside the peak period when clinical signs of disease caused by these infections are most often seen (Stott et al., 1980, Van der Poel et al., 1993). It was not possible within the scope of this study to conclude whether co-infections of the monitored viruses were frequent; nevertheless, in the herds with a short sample interval only seroconversions to a single agent were detected (data not shown).
Antibody positive bulk tank milk implied that both BRSV and BCoV had been present in many or all herds. These endemic infection patterns might be the result of: (i) new introduction of virus, (ii) continuous within herd virus circulation, (iii) re-excretion of virus from carrier animals, or (iv) a combination of these alternatives. Both within herd circulation and re-excretion of BRSV or BCoV remain hypotheses, since this has, to the authors’ knowledge, not been described in animals that were strictly isolated. Although the data in the present study do not allow an extensive interpretation of the source of infections, a continuous within herd-circulation of virus at least did not occur in 49 and 34% of the herds, respectively, as these were classified as not infected with BRSV and BCoV on both sampling occasions.The geographical distribution of recently infected herds, with several clusters of herds in which seroconversions were detected, indicated a horizontal spread of virus. Since infections occurred in many herds that did not introduce new animals, the transmission was possibly indirect (e.g., with human, milk tanker or unknown vectors), or direct during pasture (possible contact between adjacent herds). Many authors underline the lability of BRSV in the environment (Stott and Taylor, 1985), but cell culture stocks of this virus has been reported to remain infective at 5 °C for 300 days (Tjornehoj, 2000) and re-isolation in vitro from contaminated surfaces has been demonstrated for human RSV (HRSV) after 6 h (Hall et al., 1980).
Investigations are lacking on the disinfection procedures or time of quarantine required to avoid transmission to sentinel animals after visiting an infected herd: the infection dose might be much smaller than is required for isolation in vitro. Interestingly, 101 of 113 farmers (89%) estimated the average number of visitors with probable contact with other herds within the previous 24 h (excluding milk tanker drivers) to be more than two per month. These estimations were, however, not significantly correlated with our herd infection status. Collection of milk occurred in all herds and may have been involved in indirect transmission, reinforcing the potential role of visitors in the spread of infectious diseases. Further investigations need to be performed to evaluate the real impact of this mode of transmission. Careful precautions were undertaken to prevent spread of viruses with the researchers (disinfection of material/hands and change of clothing/boots between each herd).
A decreased risk of infection in herds with increased air-distance to the closest cattle-rearing herd was shown only for BCoV. Although this finding might have been due to reduced contacts between animals from different herds on pasture or to increased distances for small vectors such as rodents; the possibility of airborne spread of BCoV between herds should be considered. Indeed, SARS-CoV is believed to have spread by air between buildings 60 m apart (Yu et al., 2004) and BCoV may spread by aerosol within buildings (Niskanen et al., 2002). Investigations on single infants have shown that small particle aerosol spread is unlikely for HRSV (Hall and Douglas, 1981); however, during an outbreak involving many naïve individuals, virus shedding is probably many times greater and airborne spread of RSV should not be ruled out. Airborne transmission within a building has been documented for BRSV using a group of donor calves (Mars et al., 1999). The impact of wind spread is difficult to interpret in the field for viruses that are easily carried between herds by man and therefore also easily spread to more isolated herds.
The sample calves in a herd were in general either all seropositive or all seronegative for the different infections, indicating that infections spread to all calves in the herd when introduced or activated. A complete random selection of calves without regard to previous disease would thus probably not have changed the results. This infection-pattern has previously been described for BRSV, PIV-3, BCoV and to some extent for BVDV in herds with persistently infected animals (PI) (Alenius et al., 1991, Houe, 1992, Verhoeff and van Nieuwstadt, 1984). The exceptions from ‘none seropositive’ or ‘all seropositive’ in this study might have been due to infections in the herd before all calves were born, or to maternal antibodies in certain calves. Houe (1992) estimated the probability of obtaining at least two BVDV antibody positive animals in a test sample of three in a herd containing PI animals to be between 0.725 and 0.992. Activities within the BVDV program, such as removal of PI animals, probably affected the within-herd spread of BVDV in this study.
Seropositive animals remained positive at second sampling in the majority of cases, confirming earlier data that antibodies are detectable for long periods after infections (Elvander, 1996). Declining antibodies from a positive to a seronegative status (2–10 animals per virus, Table 1) were possibly of maternal origin, but could have originated from an early infection occurring under the influence of maternal antibodies that are known to suppress the humoral response to BRSV (Kimman et al., 1987, Uttenthal et al., 2000). This phenomenon can also have contributed to a possible underestimation of infected animals. In dairy calves, maternal antibodies to BRSV have been documented to remain until, on average, 3.3 months of age, up to a maximum of 7 months of age (Baker et al., 1986) and data from suckling beef cattle show a mean time to seronegative status of 6.2 (maximum 7.3), 6.3 (8.5) and 6.4 (7.9) months for BRSV, PIV-3 and BVDV, respectively (Fulton et al., 2004).
The low number of diagnosed BVDV infections clearly shows that the Swedish eradication scheme against BVDV is in progress in areas with prevalent livestock trade. Only 38% of sampled herds had BVDV antibody positive bulk tank milk, in contrast to a figure of 91% when the program was initiated in 1993. BVDV infected herds were at greater risk of being exposed to BCoV, possibly because these herds were managed in ways that lowered their level of biosecurity. The limited geographical spread of BVDV, however, demonstrates that the transmission of this virus is controllable. Influenza virus may also be involved in respiratory disease of cattle (Graham et al., 2002) and this will be the scope of further studies.
5. Conclusion
By repeated sampling of only three calves per herd in a large number of herds, a relatively instant picture was obtained on BRSV, PIV-3, BCoV and BVDV infections in an area of Sweden. The applied sampling strategy also allowed a surveillance of the spread of infections geographically on a herd level. Our findings inferred that dairy calves are already exposed to BRSV, PIV-3 and BCoV at a young age and that a constant presence of seronegative animals supports the circulation of virus between herds throughout the year. The results suggest indirect spread, possibly in combination with latency or circulation of virus within herds. Further knowledge of viral epidemiology in the cattle population and modes of virus transmission is needed for successful applications of preventive measures. An increased effort should also be made to evaluate bovine vaccines in blinded, randomized field trials (Perino and Apley, 1998, Perino and Hunsaker, 1997).
Acknowledgements
The authors gratefully acknowledge the technical assistance of Mrs. M. Hjort, participating farmers for interest and support and Dr. A. Lindberg, Swedish Dairy Association, for supplying BVDV data from 1993 and 2002. This work was supported by grants from AGRIA research fund, the Swedish Farmers’ Foundation for Agricultural Research and the Swedish Council for Forestry and Agricultural Research.
References
- Alenius S., Niskanen R., Juntti N., Larsson B. Bovine coronavirus as the causative agent of winter dysentery: serological evidence. Acta Veterinaria Scandinavica. 1991;32:163–170. doi: 10.1186/BF03546976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.C., Ames T.R., Markham R.J. Seroepizootiologic study of bovine respiratory syncytial virus in a dairy herd. American Journal of Veterinary Research. 1986;47:240–245. [PubMed] [Google Scholar]
- Collins J.K., Riegel C.A., Olson J.D., Fountain A. Shedding of enteric coronavirus in adult cattle. American Journal of Veterinary Research. 1987;48:361–365. [PubMed] [Google Scholar]
- Elvander M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. The Veterinary Record. 1996;138:101–105. doi: 10.1136/vr.138.5.101. [DOI] [PubMed] [Google Scholar]
- Fulton R.W., Briggs R.E., Payton M.E., Confer A.W., Saliki J.T., Ridpath J.F., Burge L.J., Duff G.C. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine. 2004;22:643–649. doi: 10.1016/j.vaccine.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Graham D.A., Calvert V., McLaren E. Retrospective analysis of serum and nasal mucus from cattle in Northern Ireland for evidence of infection with influenza A virus. The Veterinary Record. 2002;150:201–204. doi: 10.1136/vr.150.7.201. [DOI] [PubMed] [Google Scholar]
- Hall C.B., Douglas R.G., Jr. Modes of transmission of respiratory syncytial virus. The Journal of Pediatrics. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- Hall C.B., Douglas R.G., Jr., Geiman J.M. Possible transmission by fomites of respiratory syncytial virus. Journal of Infectious Diseases. 1980;141:98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- Heckert R.A., Saif L.J., Myers G.W., Agnes A.G. Epidemiologic factors and isotype-specific antibody responses in serum and mucosal secretions of dairy calves with bovine coronavirus respiratory tract and enteric tract infections. American Journal of Veterinary Research. 1991;52:845–851. [PubMed] [Google Scholar]
- Houe H. Serological analysis of a small herd sample to predict presence or absence of animals persistently infected with bovine viral diarrhoea virus (BVDV) in dairy herds. Research in Veterinary Science. 1992;53:320–323. [PubMed] [Google Scholar]
- Kimman T.G., Westenbrink F., Schreuder B.E., Straver P.J. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. Journal of Clinical Microbiology. 1987;25:1097–1106. doi: 10.1128/jcm.25.6.1097-1106.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimman T.G., Zimmer G.M., Westenbrink F., Mars J., van Leeuwen E. Epidemiological study of bovine respiratory syncytial virus infections in calves: influence of maternal antibodies on the outcome of disease. The Veterinary Record. 1988;123:104–109. doi: 10.1136/vr.123.4.104. [DOI] [PubMed] [Google Scholar]
- Larsen L.E., Tjornehoj K., Viuff B. Extensive sequence divergence among bovine respiratory syncytial viruses isolated during recurrent outbreaks in closed herds. Journal of Clinical Microbiology. 2000;38:4222–4227. doi: 10.1128/jcm.38.11.4222-4227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A.L., Alenius S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Veterinary Microbiology. 1999;64:197–222. doi: 10.1016/s0378-1135(98)00270-3. [DOI] [PubMed] [Google Scholar]
- Mars M.H., Bruschke C.J., van Oirschot J.T. Airborne transmission of BHV1, BRSV, and BVDV among cattle is possible under experimental conditions. Veterinary Microbiology. 1999;66:197–207. doi: 10.1016/s0378-1135(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Niskanen R. Relationship between the levels of antibodies to bovine viral diarrhoea virus in bulk tank milk and the prevalence of cows exposed to the virus. The Veterinary Record. 1993;133:341–344. doi: 10.1136/vr.133.14.341. [DOI] [PubMed] [Google Scholar]
- Niskanen R., Alenius S., Larsson B., Juntti N. Evaluation of an enzyme-linked immunosorbent assay for detection of antibodies to bovine virus diarrhoea virus in milk. Zentralblatt für Veterinärmedizin [B] 1989;36:113–118. doi: 10.1111/j.1439-0450.1989.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Niskanen R., Lindberg A., Traven M. Failure to spread bovine virus diarrhoea virus infection from primarily infected calves despite concurrent infection with bovine coronavirus. The Veterinary Journal. 2002;163:251–259. doi: 10.1053/tvjl.2001.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton D.J., Christiansen K.H., Alenius S., Cranwell M.P., Pritchard G.C., Drew T.W. Prevalence of antibodies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. The Veterinary Record. 1998;142:385–391. doi: 10.1136/vr.142.15.385. [DOI] [PubMed] [Google Scholar]
- Perino L.J., Apley M.D. Clinical trial design in feedlots. Veterinary Clinics of North American Food Animal Practice. 1998;14:343–365. doi: 10.1016/s0749-0720(15)30258-9. [DOI] [PubMed] [Google Scholar]
- Perino, L.J., Hunsaker, B.D., 1997. A review of bovine respiratory disease vaccine field efficacy. In: Proceedings of the 30th Annual Convention, American Association of Bovine Practitioners, Montreal, Quebeck, Canada, pp. 59–66.
- Sandvik T. Progress of control and prevention programs for bovine viral diarrhea virus in Europe. Veterinary Clinics of North American Food Animal Practice. 2004;20:151–169. doi: 10.1016/j.cvfa.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Storz J., Lin X., Purdy C.W., Chouljenko V.N., Kousoulas K.G., Enright F.M., Gilmore W.C., Briggs R.E., Loan R.W. Coronavirus and Pasteurella infections in bovine shipping fever pneumonia and Evans’ criteria for causation. Journal of Clinical Microbiology. 2000;38:3291–3298. doi: 10.1128/jcm.38.9.3291-3298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E.J., Taylor G. Respiratory syncytial virus. Brief review. Archives of Virology. 1985;84:1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- Stott E.J., Thomas L.H., Collins A.P., Crouch S., Jebbett J., Smith G.S., Luther P.D., Caswell R. A survey of virus infections of the respiratory tract of cattle and their association with disease. Journal of Hygiene. 1980;85:257–270. doi: 10.1017/s0022172400063294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Lundborg K., Emanuelson U., Olsson S.O. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Preventive Veterinary Medicine. 2003;58:179–197. doi: 10.1016/s0167-5877(03)00046-1. [DOI] [PubMed] [Google Scholar]
- Tjornehoj, K., 2000. Development of a model for experimental infection of calves with bovine respiratory syncytial virus, and use of the model in vaccine efficacy studies. Doctoral thesis, The Royal Veterinary and Agricultural University, Copenhagen.
- Tråvén M., Bjornerot L., Larsson B. Nationwide survey of antibodies to bovine coronavirus in bulk milk from Swedish dairy herds. The Veterinary Record. 1999;144:527–529. doi: 10.1136/vr.144.19.527. [DOI] [PubMed] [Google Scholar]
- Uttenthal A., Larsen L.E., Philipsen J.S., Tjornehoj K., Viuff B., Nielsen K.H., Nielsen T.K. Antibody dynamics in BRSV-infected Danish dairy herds as determined by isotype-specific immunoglobulins. Veterinary Microbiology. 2000;76:329–341. doi: 10.1016/S0378-1135(00)00261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valarcher J.F., Bourhy H., Lavenu A., Bourges-Abella N., Roth M., Andreoletti O., Ave P., Schelcher F. Persistent infection of B lymphocytes by bovine respiratory syncytial virus. Virology. 2001;291:55–67. doi: 10.1006/viro.2001.1083. [DOI] [PubMed] [Google Scholar]
- Van der Poel W.H., Kramps J.A., Middel W.G., Van Oirschot J.T., Brand A. Dynamics of bovine respiratory syncytial virus infections: a longitudinal epidemiological study in dairy herds. Archives of Virology. 1993;133:309–321. doi: 10.1007/BF01313771. [DOI] [PubMed] [Google Scholar]
- Verhoeff J., van Nieuwstadt A.P. BRS virus, PI3 virus and BHV1 infections of young stock on self-contained dairy farms: epidemiological and clinical findings. The Veterinary Record. 1984;114:288–293. doi: 10.1136/vr.114.12.288. [DOI] [PubMed] [Google Scholar]
- Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H., Leung D.Y., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. New England Journal of Medicine. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]


