Highlights
-
•
An etiological agent was found in 60% of the acute respiratory infections.
-
•
Human metapneumovirus showed an association with severe infection.
-
•
The importance of atypical bacteria in severe cases, in particular L. pneumophila.
-
•
The low effectiveness of the influenza vaccine.
Keywords: Elderly, Respiratory infections, Respiratory viruses, Legionella pneumophila, Elderly care centers, Real time PCR
Abstract
Objective
The aim of this study was to analyze the etiology and clinical consequences of viral respiratory infections in 18 elderly care centers (ECC) in Lisbon, which housed a total of 1022 residents.
Methods
Nasopharyngeal swabs were collected whenever an elderly had symptoms of acute respiratory infections (ARI). PCR and RT-PCR were performed for influenza A/B, human parainfluenza virus 1–4, adenovirus, human metapneumovirus (HMPV), respiratory syncytial virus (RSV), rhinovirus, enterovirus, human coronavirus and human Bocavirus (HBoV). Array cards for atypical bacteria were also used in severe cases.
Results
In total, 188 episodes of ARI were reported, being rhinovirus the most frequently detected (n = 53), followed by influenza A(H3) (n = 19) and HBoV (n = 14). Severe infections were reported in 19 patients, 11 of which were fatal, Legionela pneumophila, rhinovirus, HMPV and RSV associated with these fatalities. Nine influenza strains were analyzed, all antigenically dissimilar from vaccine strain 2013/14. “Age”, “HMPV” and “Respiratory disease” showed an association with severe infection.
Conclusions
In this study an etiologic agent could be found in 60% of the acute respiratory episodes. These data provides information about the circulating viruses in ECC and highlights the importance of searching both viruses and atypical bacteria in severe ARI.
Introduction
Ageing is a natural process that impairs several physiological systems, including the immune system and results in increased susceptibility to infectious, autoimmune and neoplastic diseases. The number of elderly is expected to increase from 901 million to an estimated 1.4 billion between 2015 and 2030 (UN, 2015). Therefore, emphasis on the overall quality of life of the older person represents a major public health challenge and is now a global issue.
Pneumonia is a common illness that continues to be a major cause of death in young children in developing countries and elderly people in developed countries. About 200 million cases of viral community-acquired pneumonia occur every year, equally distributed between children and adults, with the elderly being the most affected group among the adult population (Ruuskanen et al., 2011). However, despite the potential severity of viral respiratory infections (VRI), the spectrum of clinical presentations in elderly patients is extremely broad, and many of them have only mild upper respiratory infections (Nicholson et al., 1997).
In Portugal, 65.3% of hospital admissions for respiratory diseases in 2013 were due to bacterial or viral pneumonias (DGS, 2014).
The list of viruses responsible for ARI in elderly people includes influenza A and B virus, adenovirus, RSV, rhinovirus, enterovirus, human coronavirus (HCoVs), HMPV, human parainfluenza virus (HPIVs), HBoV and varicella, among other less common viruses (Ruuskanen et al., 2011). However, the attribution of the pneumonia to viruses can sometimes be difficult, because viruses may be found in respiratory secretions due to asymptomatic viral shedding, and co-infection with two organisms may occur (Cesario, 2012).
The current article reports the microbiology results from the GERIA Project “Geriatric Study on Health Effects of Air Quality in ECC” conducted in Portugal, the country with the 5th oldest population in the world (UN, 2015) and the 4th oldest in Europe, with 20% of the population older than 65 years (Eurostat, 2016). Results of chronic respiratory diseases and quality of life from this project have already been published (Carreiro-Martins et al., 2016). The team involved was the same of a previous one that studied the health impact of indoor air environment in Children Day Care Centers (ENVIRH) (Araújo-Martins et al., 2014, Paixão et al., 2014). Here, we aimed to study the viral role in ARI in residents of ECC of Lisbon, during the 2013/14 winter. In addition, in order to better clarify the role of the respiratory viruses in severe infections, atypical bacteria were also searched in a subset of patients.
Methods
Study design
Within the scope of this study, 33 ECC were selected through proportional stratified random sampling (by parish) from the 95 included in the Portuguese Social Charter of Lisbon. Of these 33, 18 agreed to participate.
Considering the particular characteristics of the surrounding environment, these 18 ECC were classified as urban and housed a total of 1022 residents, with a range of 5 to 334 occupants. The majority of these elderly spend all of their time indoors.
Before starting the study, open sessions were performed in all ECC, conducted by a virologist of the GERIA team. In these open sessions, a brief review of the importance of the ARI in elderly was provided to the local staff, along with a description of the GERIA project.
Participants’ epidemiological data (sociodemographic and clinical information including data on underlying conditions and co-morbidities) were collected by an interviewer with a dedicated questionnaire, which included the burden of obstructive lung disease questionnaire (BOLD). Clinical files were reviewed using International Classification of Primary Care, 2nd edition (ICPC-2). These interviews were performed by health technicians who were trained and certified by the GERIA team.
The viral study included phone contact by the ECC staff to the research team each time an elderly person had symptoms of ARI (ECDC criteria): sudden onset of symptoms, at least one respiratory symptom (cough, sore throat, shortness of breath and coryza), and a clinician’s judgement that the illness was due to an infection (OJ, 2012).
Sample collections were performed between November 2013 and April 2014 at the ECC, by members of the research team within the first 48 h after the phone call. Two swabs were collected from each patient (nasopharyngeal and oropharyngeal) and immediately pooled into viral transport medium (Vircell's Transport Medium for virus, Chlamydia and Mycoplasma).
The clinical outcome of each patient was assessed by completing a structured questionnaire, two weeks after the specimen collection, conducted for a phone call to the respective ECC staff. In this follow-up questionnaire, infections were classified as severe if there was a worsening in the initial symptoms, resulting in hospitalization and/or death.
In order to encourage participation, every week the research team called the ECC that did not report any cases.
The study was approved by the Ethics Committee of Nova Medical School (nr.08/2013/CEFCM) and National Data Protection Commission (nr. 1279/2013). The participants, or their legal representatives, were informed about the study objectives and their signed consents were obtained previously to the sample collection.
Laboratory methods
Viral nucleic acid was extracted using the QIAamp MinElute Virus Spin Kit (Qiagen), according to the manufacturer’s instructions. A starting volume of 200 μL was used to elute 60 μL of viral RNA/DNA, and 5 μL of this eluate was used for each PCR reaction, with a 25 μL final reaction volume.
PCR for respiratory viruses was performed using “in house” real-time Taqman PCR and RT-PCR. Briefly, four panels were used, a pentaplex and three additional multiplex real-time PCR assays: panel 1 (influenza A, A(H1), A(H3), B, internal control), panel 2 (RSV A/B, HPIVs types 1/3, adenovirus), panel 3 (HPIVs types 2/4, enterovirus, rhinovirus, HMPV), panel 4 (HCoVs group 1 (229E, NL63), HCoVs group 2 (OC43, HKU1)) (Ellis and Curran, 2011, Clark et al., 2014, Clark et al., 2015). Superscript™III Platinum one-step enzyme (Invitrogen) was used for the reverse transcription. All assays shared identical amplification conditions (50 °C for 30 min, 95 °C for 2 min, 45 cycles at 95 °C for 15 s, and 60 °C for 1 min), allowing all four panels to run simultaneously on the same instrument. An additional monoplex assay specific for HBoV was also included in this study (Table 1 ). Amplification and detection of PCR products were performed using the Rotorgene™ PCR system (Qiagen) acquiring the fluorescence on FAM, JOE, CY5, and ROX channels at each cycle. Samples and negative control (molecular grade water) were individually spiked with MS2 bacteriophage internal control (4,600 pfu per extraction) prior to nucleic acid extraction to identify any inhibitors. Positive controls for all panels were made from a combination of recombinant plasmids and from known positive specimens, diluted to give a cycle threshold value of 20–25.
Table 1.
Primers and probe used for Bocavirus real-time PCR.
| Target | Sequence (5′ → 3′) | Function | Product size | Primer source |
|---|---|---|---|---|
| HBoV | CACKCCCAGGAARTGACGTAT | Forward primer | 118 bp | This study |
| 3′ non-coding region | CCAGAGATGTTCACTCGCCGGA | Reverse primer | ||
| TCAGACTGCATCCGGTCTa | Probe |
Platinum ® Quantitative PCR SuperMix-UDG Invitrogen enzyme (Cat.No. 11730-005) was used for PCR amplification of the above targets in a monoplex format in a final 25ul volume reaction (containing 20 ul of master mix and 5ul of nucleic acid extract) with 3 mM MgCl2, 400 nM primers and 120 nM probe concentration.
Cycling conditions were 50 °C for 2 min, 95 °C for 2 min, 40 cycles at 95 °C for 15 s and 60 °C for 1 min and acquiring on FAM for both assays.
= MGB probe. Fluorophore = FAM, Quencher = NFQ (Life technologies).
In a second phase of the study, “in house” TaqMan array cards (Steensels et al., 2015) were used for several respiratory viruses and atypical bacteria in severe cases. The cards included adenovirus, HBoV, influenza A(H1, H3, H7, H9) and B, HPIVs 1/4, RSV, enterovirus, rhinovirus, HMPV, HCoVs, Legionella pneumophila (L. pneumophila), Mycoplasma pneumoniae, Chlamydophila pneumoniae and Coxiella burnetii. To confirm the L. pneumophila cards results, an “in house” real-time PCR was also used (Welti et al., 2003).
Influenza positive samples were sent to the National Influenza Reference Laboratory for virus isolation and characterization. Virus isolation was performed in MDCK-Siat1 cell line (WHO, 2011). Antigenic characterization was accomplished by hemagglutination inhibition assay (HIA) using reference viruses and monoclonal antiserums gently provided by WHO (2014) collaborating for European region. HIA was performed with guinea pig red blood cells. Isolated viruses were compared with 2013/14 and 2014/15 A(H3) vaccine strains, genetic groups reference strains and circulating viruses (ECDC, 2014, ECDC, 2015), genetic analysis was based on the HA1 subunit of the viral hemagglutinin gene. Nucleotide sequence assembly was carried out using the Seqman program (Lasergene99 package, DNASTAR) and for multiple alignments of nucleotide and deduced amino acid sequences the MEGA Software 6.0. Module (Tamura et al., 2013).
Statistical analysis
Categorical data were presented as frequencies (percentages), and continuous variables as mean and standard deviation (SD). Proportions were compared using Fisher’s exact test. To study the association between severe infection and socio-demographic characteristics, clinical information and co-morbidities, logistic regression models were used. The variables attaining a p-value < 0.25 in the univariable analysis were selected as candidates for the multivariable model. A level of significance α = 0.05 was considered. The statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL).
Results
Between November 2013 and April 2014, 188 episodes of ARI from 163 patients were reported to the research team (49 men and 114 women), with a mean age of 83 years (SD = 9.2; range: 59–101) for men and 85 years (SD = 7.2; range: 62–101) for women (Table 2 ). These patients were from 14 institutions, as four institutions did not report any episode (Table 3 ).
Table 2.
Distribution of global attack by age groups and gender.
| Age groups | Gender |
Totala | |||
|---|---|---|---|---|---|
| Male |
Female |
||||
| Positive cases | Negative cases | Positive cases | Negative cases | ||
| <65 | 1 | 1 | 2 | 0 | 3/4 |
| ≥65 < 75 | 2 | 1 | 5 | 1 | 7/9 |
| ≥75 < 85 | 15 | 7 | 27 | 16 | 42/65 |
| ≥85 < 95 | 12 | 6 | 27 | 29 | 39/74 |
| ≥95 | 0 | 4 | 6 | 1 | 6/11 |
| TOTAL | 30 | 19 | 67 | 47 | 97/163 |
Number of positive cases/Total of patients.
Table 3.
Number of residents, episodes and distribution of the respiratory viruses by ECC.
| ECC | Number of residents | Number of episodes | Rhinovirus | Influenza A(H3) | HBoV | HCoVs group 1 | HMPV | RSV A/B | HCoVs group 2 | HPIVs 1/3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | 34 | 4 | 11 | 5 | 5 | 1 | 1 | ||
| 2 | 148 | 27 | 8 | 7 | 3 | 3 | 1 | |||
| 3 | 21 | 1 | 1 | |||||||
| 4 | 51 | 0 | ||||||||
| 5 | 44 | 1 | ||||||||
| 6 | 44 | 13 | 7 | 3 | 3 | 1 | ||||
| 7 | 14 | 2 | 1 | |||||||
| 8 | 39 | 1 | 1 | 1 | ||||||
| 9 | 33 | 0 | ||||||||
| 10 | 334 | 76 | 30 | 1 | 5 | 5 | 2 | 1 | 1 | 1 |
| 11 | 16 | 3 | 2 | 1 | ||||||
| 12 | 47 | 0 | ||||||||
| 13 | 48 | 20 | 6 | 1 | 1 | 2 | ||||
| 14 | 5 | 1 | ||||||||
| 15 | 38 | 5 | 1 | 1 | ||||||
| 16 | 72 | 3 | 1 | 3 | ||||||
| 17 | 9 | 1 | ||||||||
| 18 | 14 | 0 | ||||||||
| Total | 1022 | 188 | 53 | 26 | 19 | 14 | 11 | 5 | 3 | 1 |
Overall, there was a significant difference between ECC (p < 0.001) concerning the ratio of the number of episodes to the number of residents.
One hundred and thirteen episodes were positive by PCR and/or array for at least one respiratory virus, co-infections with two and three viruses were detected in multiple episodes (Table 4 ). Rhinovirus was detected in 53 samples, followed by influenza A(H3), HBoV, HCoVs group 1, HMPV, RSV, HCoVs group 2, and HPIVs types 1/3, respectively with 26, 19, 14, 11, 5, 3 and 1 positive samples (Table 3).
Table 4.
Single and mixed viral infections.
| Viruses | Single infection | Double co-infection | Triple co-infection |
|---|---|---|---|
| Rhinovirus | 46 | 7 | 0 |
| Influenza A(H3) | 20 | 5 | 1 |
| HBoV | 8 | 11 | 0 |
| HCoVs group 1 | 7 | 6 | 1 |
| HMPV | 10 | 1 | 0 |
| RSV A/B | 3 | 2 | 0 |
| HCoVs group 2 | 1 | 1 | 1 |
| HPIVs 1/3 | 0 | 1 | 0 |
Regarding the severity of the infections, most were clinically mild, although some degree of dyspnea was reported in 50 episodes at the time of collection. The clinical situation deteriorated in 19 (11.7%) patients (severe infection: hospitalization and/or death). Of these, 14 hospitalizations were reported, and 6 of them died. Five additional deaths in non-hospitalized patients were reported, giving a total of 11 (6.7%) patients dying with ARI during this study.
All of the samples from these patients with severe infections were later tested by the array card technique and by a monoplex PCR technique for L. pneumophila. Results of these two approaches in severe patients are presented in Table 5 .
Table 5.
Results of patients with severe infections (PCR and array cards).
| Patients (ECC/Ep) | Hospitalization | Death | Pathogens |
|---|---|---|---|
| 2/3 | Yes | Yes | Negative |
| 2/32 | Yes | No | Rhinovirus |
| 2/44 | No | Yes | HMPV, L. pneumophila |
| 2/45 | No | Yes | HMPV, L. pneumophila |
| 2/67 | No | Yes | L. pneumophila |
| 2/76 | Yes | Yes | Negative |
| 2/133 | Yes | Yes | Rhinovirus |
| 6/134 | Yes | No | HMPV, HBoVb |
| 10/40 | No | Yes | Rhinovirusa, L. pneumophila |
| 10/60 | Yes | No | HMPV |
| 10/125 | Yes | No | Negative |
| 10/126 | Yes | Yes | Rhinovirus |
| 10/137 | No | Yes | L. pneumophila |
| 10/142 | Yes | No | Rhinovirus |
| 10/163 | Yes | Yes | L. pneumophila |
| 10/164 | Yes | No | Negative |
| 10/169 | Yes | No | Negative |
| 10/171 | Yes | No | Negative |
| 15/159 | Yes | Yes | RSVa |
Ep: Episode number.
Detected only by array card.
Detected only by PCR.
To study the association between severe infection and socio-demographic characteristics, clinical information and co-morbidities, a univariable analysis was performed (see Supplementary Table S1).
In the final multivariable model, variables “age”, “HMPV” and “Respiratory disease” showed an association with severe infection. For each 5 years increase in age there was a 49% increase in the odds of severe infection (OR = 1.49; 95% CI: 1.02–2.17; p = 0.042). The residents infected with HMPV had a seven-fold increase in the odds of severe infection (OR = 6.99; 95% CI: 1.96–24.95; p = 0.003). Also, the odds of severe infection was four times higher in residents with “Respiratory disease” (OR = 4.09; 95% CI: 1.51–11.06; p = 0.006).
Information about vaccination status for influenza viruses was obtained from 159 patients (159 from 163 with reported infections). The rates of influenza A(H3) infections among vaccinated and unvaccinated patients were quite similar (p = 0.682) (Table 6 ). Moreover, influenza was not associated with serious infections (p = 0.215 and p = 0.357 for hospitalization and death, respectively).
Table 6.
Distribution of influenza A(H3) infections among vaccinated and unvaccinated residents.
| Influenza A(H3) positive | Influenza A negative | |
|---|---|---|
| Vaccinated | 20 | 107 |
| Non-vaccinated | 6 | 26 |
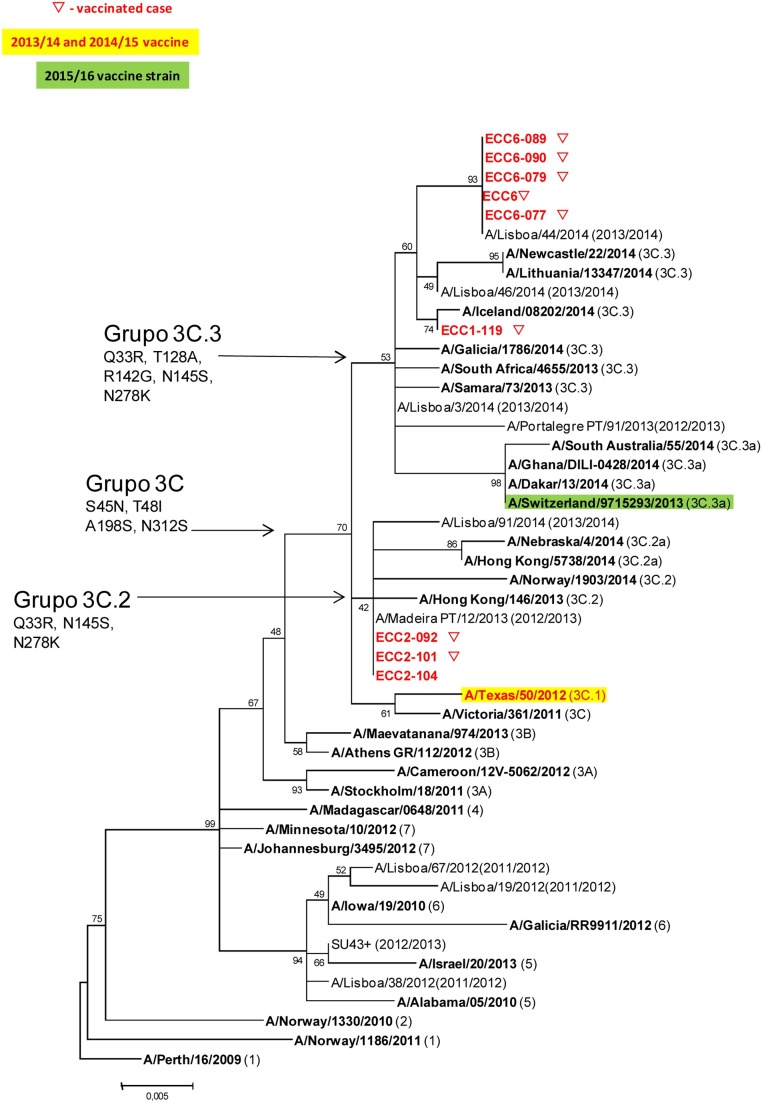
Concerning the genetic characterization of influenza strains, typing was obtained only for 9 strains A(H3), from patients living in 3 different ECC (ECC1, ECC2 and ECC6). Eight viruses were from vaccinated patients and 1 from an unvaccinated patient. All the strains belong to the genetic group 3C, which includes also the strain A(H3) of the vaccine 2013/14 season. However, they were divided into different subgroups: samples of ECC1 (n = 1) and ECC06 (n = 5) belong to the genetic subgroup 3C.3 and samples of the ECC02 (n = 3) belong to the 3C.2 (the vaccine strain belongs to subgroup 3C.1). The virus detected in the unvaccinated patient shows the same sequence of strains detected in vaccinated patients of the same ECC (ECC02) (Figure 1 ).
Figure 1.
Phylogenetic comparison based on the similarity of HA1 subunit of influenza strains from this study and circulating/vaccine viral strains in 2013/14 and 2014/15. Nucleotide sequences were assembled with the Seqman program (Lasergene99 package, DNASTAR). The phylogenetic tree was created with MEGA software version 6, using the maximum likelihood method, Hasegawa–Kishino–Yano nucleotide distances and bootstrapped with 500 replicates.
Relating to the amino acid substitutions, 9 amino acid position changes were identified in the HA1 subunit of the influenza strains from this study, when compared with the strain included in the 2013/14 influenza vaccine, A/Texas/50/2012. Samples of the ECC02 share the same substitutions of amino acids present in the reference strains of the 3C.2 subgroup (N128T, N145S, V186G, P198S and F219S), which distinguishes them from the vaccine strain. The viruses detected in ECC01 and ECC06 share 4 of the substitutions in 3C.2 subgroup (N145S, V186G, P198S and F219S) and acquire three new replacements (N128A, R142G and L157S). Regarding viruses from ECC06, all accumulate the R261Q substitution. All these mentioned amino acid substitutions occurred in the hemagglutinin antigenic sites, and have already been observed in other strains that circulated in the season 2013/14.
Discussion
ECC represent an increasing form of caring for older persons in developed countries. However, these institutions can also provide the requisite ingredients for outbreaks of infectious diseases. Prolonged lengths of stay of residents with multiple chronic diseases and functional impairments, limited capacities for diagnosis and inadequate infection-control programs, often allow outbreaks of respiratory viruses to propagate in these facilities (Strausbaugh et al., 2003).
The present study aimed to identify the viruses responsible for ARI in a resident sample of ECC in the city of Lisbon during the winter of 2013/14. One of the strengths of this study was the relatively large sample size of elderly people. Eighteen of the 95 ECC registered in the Lisbon area (19% of the total ECC) agreed to participate, resulting in a total sample size of 1022 residents. Moreover, every resident who was able to answer the questionnaire was invited to participate in the survey. On the other hand, we could not interview many residents, as they were too disabled to participate in the study. For this reason, our sample is not entirely representative of the elderly who attended the ECC.
Another weakness of our study was the low staff compliance with the project for some of the ECC, despite an intensive campaign of information and contacts with them. In fact, four of them did not report a single event, which cannot be explained by the lower number of residents (ECC01 and ECC04 are good examples, with rates of 34/45 and 0/51, respectively). Therefore, any study or campaign including ECC should take into consideration the variability of staff compliance between centers, which can affect significantly the results obtained.
One hundred and thirteen of 188 episodes of reported ARI were positive for at least one respiratory virus. Most of the respiratory viruses searched for in this project were detected, confirming the diversity on the aetiology of ARI in elderly patients. In fact, only adenovirus, enterovirus, and HPIVs types 2/4 were never detected in this study, which is in accordance with reports that suggest that these viruses are not frequently detected in this age group (Falsey and Walsh, 2006), although adenovirus can occasionally be responsible for severe infections in long-term care facilities for the elderly (Kandel et al., 2010).
Rhinoviruses were the most frequently detected viruses, but were also detected in 5 severe infections, 3 of them with fatal outcomes (although one of these was associated with L. pneumophila). Therefore our results corroborate the notion that these viruses can be responsible for severe ARI in elderly patients (Pop-Vicas and Gravenstein, 2011).
Influenza remains a leading cause of infectious death among the elderly (Pop-Vicas and Gravenstein, 2011). Influenza vaccination rates of 80% of residents at a given institution have been described to be associated with a decreased risk of an outbreak, although some outbreaks can occur even when resident vaccination rates are 90% (Strausbaugh et al., 2003). In our study, the vaccination rate within the group of patients with episodes was 78% (all vaccinations were performed at least one month before the first reported episode). The vaccination rate of the entire population could not be determined, because the initial questionnaire that was made for the 1022 residents (BOLD) did not include the question about the vaccination status, and therefore only in the follow-up questionnaire for the group of infected patients this question was addressed.
Influenza A(H3) was the second most prevalent virus detected. Interestingly, A(H1) was not detected in this study, although this virus circulated in the Portuguese population during this winter and in a proportion equivalent to that of A(H3) (Cristovão et al., 2014). The rates of influenza A(H3) infections between vaccinated and unvaccinated residents were quite similar, suggesting a low effectiveness of the vaccine. As a possible explanation, we observed that all analyzed strains were antigenically dissimilar from vaccine strain 2013/14. Interestingly, influenza cases in vaccinated elderly were not associated with serious infections in this study, which is in accordance with the information from Public Health authorities that vaccination for influenza is aimed mainly to prevent complications (CDC, 2016). Nevertheless, it remains unclear if this was the result of vaccine protection, because the six unvaccinated residents with influenza infection did not experience serious illness as well.
As expected, the samples of the same ECC share identical sequences of influenza virus, indicating a probable common source of infection and sustained transmission between residents.
In the present study, HBoV was detected in 19 episodes and ranked third in prevalence, in contrast with another study that presented very low virus prevalence in the respiratory tract of adults (Schildgen et al., 2008). Other report suggests that HBoV might be associated with upper and lower respiratory disease in elderly patients (Liu et al., 2011), although in our study most of these detections were co-infections (11/19) with other viruses, and only in 8 episodes HBoV was detected alone. Therefore, results suggest that HBoV detection by PCR can be a frequent event in elderly patients, but the pathogenic role of these viruses in many of these infections is still unclear.
Among the other respiratory viruses detected in this study, HCoVs group 1/2 were commonly detected, particularly the first, but not associated with severe infections. HMPV was detected in 11 patients, with 4 of these having severe infections. However, the 2 fatalities associated with this virus had mixed infection and therefore the contribution of the HMPV is not clear. Nevertheless, these results suggest that HMPV can be responsible for severe infections in older populations, as described by others (Panda et al., 2014).
RSV was positive only in 5 patients, but associated with 1 fatality, being the only pathogen detected in this case, but with a late CT value. No conclusions can be drawn due to the small sample size, although the association with severe infections in elderly patients has been well known for several years (Walsh and Falsey, 2012).
In the current study, 40% of the samples were negative for respiratory viruses. False negative results with the PCR and RT-PCR, or infections by other respiratory viruses not searched, are possible explanations for some of the negative results. Another possibility is the presence of bacterial infections, considering their important role in elderly with pneumonia.
To confirm this last possibility, patients with severe infections were also tested for atypical bacteria, as previously described. The Taqman arrays cards allowed us to detect 2 more viruses in these severe infections, suggesting that if they were used in all the patients, the rate of viral detection could have been higher than using PCR alone. But the major contribution of this technique was for the atypical bacteria: L. pneumophila was detected in 6 patients (in 2 different ECC), and all of them subsequently died. When comparing Taqman array cards and multiplex PCR results for viral detection, some differences were detected (Table 5), but only in samples with low viral load. Therefore, sensitivity of these techniques can be compromised when studying samples with low viral or bacterial load.
L. pneumophila is a well-known cause of severe ARI and the population in this study belongs to the group with high susceptibility in acquiring infections with this bacterium. Moreover, the mortality rate, usually between 8–12%, is higher in elderly people (Phin et al., 2014). Therefore, despite the fact that 3 of the fatal cases had mixed infections with viruses (2 with HMPV and 1 with rhinovirus) there is a high probability that this bacteria could have contributed to these fatalities. Nevertheless, we cannot exclude that viruses could have had an important contribution as well; autopsy was not ordered in any of the fatalities, and post-mortem lung specimens were not collected, which could have helped to clarify this situation. Infections caused by L. pneumophila have been described in ECC but often go undetected (Seenivasan et al., 2005), most facilities do not have the level of microbiology laboratory support that is available to acute care hospitals, and only if the resident is transferred to the hospital will he/she have access to different methods to detect this bacterium. Another important point is the fact that the L. pneumophila infection rate was probably underestimated, for the following reasons: L. pneumophila was searched only in severe infections, and more cases could have been found if this bacterium were searched in all patients; L. pneumophila infections show a seasonal pattern, with peak activity in late summer to autumn, and this study was held during the winter (Phin et al., 2014); and finally, upper respiratory samples are not the best samples for the diagnosis of these infections (Cho et al., 2012). All of the detected cases were notified to the National surveillance scheme so that environmental research could be triggered. At the end of the study a recommendation for the use of a urinary antigen test for Legionella was made to the ECC medical teams involved, but also a public discussion of these findings was conducted by our team.
Interestingly, the variables “Age”, “HMPV” and “Respiratory disease” showed an association with severe infection in the final multivariable model. These associations are not unexpected, since increased age and previous respiratory disease are known risk factors for respiratory infections (Ruuskanen et al., 2011), and HMPV has been associated with severe disease, as previously mentioned (Panda et al., 2014). Despite the suggestive results obtained with L. pneumophila, no conclusions can be drawn from this study because this bacterium was searched only in severe infections.
Conclusion
This is the first study performed in Portugal, with elderly populations in ECC, aimed to study the viral aetiology of the ARI. An aetiological agent could be found in 60% of the acute respiratory episodes, a rate higher than usually reported (Ruuskanen et al., 2011). Most of the infections were initially considered mild, but worsening was observed in a significant proportion of patients. “Age”, “HMPV” and “Respiratory disease” were associates with severe infection. The data highlight the importance of searching both viruses and atypical bacteria in severe ARI in elderly patients, in particular L. pneumophila, which was associated with 6 fatalities in the studied population. The study also provided information about the circulating influenza viruses in this season.
Financial support
This work was supported by Foundation for Science and Technology (Fundação para a Ciência e Tecnologia – FCT, grant number PTDC/SAU-SAP/116563/2010) through Operational Competitiveness Programme (COMPETE) as part of the National Strategic Reference Framework.
Conflict of interest
None.
Ethical approval
The study was approved by the Ethics Committee of Nova Medical School, Lisbon and the National Data Protection Commission.
Acknowledgements
The authors express their deepest gratitude to all the elderly residents for their important contribution. We also thank all the other ECC staff and authorities involved in the study.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2018.01.012.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Araújo-Martins J., Carreiro Martins P., Viegas J., Aelenei D., Cano M.M., Teixeira J.P. Environment and health in children day care centres (ENVIRH) — study rationale and protocol. Rev Port Pneumol. 2014;20:311–323. doi: 10.1016/j.rppneu.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiro-Martins P., Gomes-Belo J., Papoila A.L., Caires I., Palmeiro T., Gaspar-Marques J. Chronic respiratory diseases and quality of life in elderly nursing home residents. Chron Respir Dis. 2016;(March) doi: 10.1177/1479972316636990. pii: 1479972316636990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2016. Seasonal influenza (flu) http://www.cdc.gov/flu/protect/keyfacts.htm. [Accessed December 2016] [Google Scholar]
- Cesario T.C. Viruses associated with pneumonia in adults. Clin Infect Dis. 2012;55:107–113. doi: 10.1093/cid/cis297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M.C., Kim H., An D., Lee M., Noh S.A., Kim M.N. Comparison of sputum and nasopharyngeal swab specimens for molecular diagnosis of Mycoplasma pneumoniae, Chlamydophila pneumoniae and Legionella pneumophila. Ann Lab Med. 2012;32:133–138. doi: 10.3343/alm.2012.32.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T.W., Medina M.J., Batham S., Curran M.D., Parmar S., Nicholson K.G. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69:507–515. doi: 10.1016/j.jinf.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T.W., Medina M.J., Batham S., Curran M.D., Parmar S., Nicholson K.G. C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation of COPD. Eur Respir J. 2015;45:76–86. doi: 10.1183/09031936.00092214. [DOI] [PubMed] [Google Scholar]
- Cristovão P., Pechirra P., Conde P., Maia A., Roque C., Carpinteiro D. Vigilância da gripe em Portugal no inverno 2013/2014. Instituto Nacional de Saúde Doutor Ricardo Jorge Boletim Epidemiológico. 2014;8:20–24. [Google Scholar]
- Direcção Geral de Saúde (DGS) Portugal doenças respiratórias em números 2014. Programa nacional para as doenças respiratórias; Lisboa, Dezembro de; 2014. https://www.dgs.pt/estatisticas-de-saude/estatisticas-de-saude/publicacoes/portugal-doencas-respiratorias-em-numeros-2014.aspx. [Accessed 21 January 2016] [Google Scholar]
- Ellis J.S., Curran M.D. Simultaneous molecular detection and confirmation of influenza AH5, with internal control. Methods Mol Biol. 2011;665:161–181. doi: 10.1007/978-1-60761-817-1_10. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2014. Influenza in Europe — season 2013–2014. http://ecdc.europa.eu/en/publications/Publications/Influenza-2013-14-season-report.pdf. [Accessed 15 December 2015] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2015. Seasonal influenza in the EU/EEA countries, 2014–2015. http://ecdc.europa.eu/en/publications/Publications/seasonal-influenza-risk-assessment.pdf. [Accessed 15 December 2015] [Google Scholar]
- Eurostat . 2016. Population structure and ageing. http://ec.europa.eu/eurostat/statistics-explained/index.php/Population_structure_and_ageing. [Accessed 21 January 2017] [Google Scholar]
- Falsey A., Walsh E. Viral pneumonia in older adults. Clin. Inf. Dis. 2006;42:518–524. doi: 10.1086/499955. PMID:16421796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel R., Srinivasan A., D’Agata E.M., Lu X., Erdman D., Jhung M. Outbreak of adenovirus type 4 infection in a long-term care facility for the elderly. Infect Control Hosp Epidemiol. 2010;31:755–757. doi: 10.1086/653612. [DOI] [PubMed] [Google Scholar]
- Liu W.K., Chen D.H., Liu Q., Liang H.X., Yang Z.F., Qin S. Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis. 2011;11:345–352. doi: 10.1186/1471-2334-11-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G., Kent J., Hammersley V., Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. Br Med J. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Official Journal of European Union (OJ). 2012, 55; L262. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012D0506&qid=1428573336660&from=EN#page=16. [Accessed 1 September 2013].
- Paixão P., Piedade C., Papoila A., Caires I., Pedro C., Santos M. Improving influenza surveillance in Portuguese preschool children by parents’ report. Eur J Pediatr. 2014;173:1059–1065. doi: 10.1007/s00431-014-2285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Mohakud N.K., Pena L., Kumar S. Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis. 2014;25:45–52. doi: 10.1016/j.ijid.2014.03.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phin N., Parry-Ford F., Harrison T., Stagg H.R., Zhang N., Kumar K. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect Dis. 2014;14:1011–1021. doi: 10.1016/S1473-3099(14)70713-3. [DOI] [PubMed] [Google Scholar]
- Pop-Vicas A., Gravenstein S. Influenza in the elderly — a mini-review. Gerontology. 2011;57:397–404. doi: 10.1159/000319033. [DOI] [PubMed] [Google Scholar]
- Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O., Müller A., Allander T., Mackay I.M., Völz S., Kupfer B. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21:291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seenivasan M.H., Yu V.L., Muder R.R. Legionnaires’ disease in long-term care facilities: overview and proposed solutions. J Am Geriatr Soc. 2005;53:875–880. doi: 10.1111/j.1532-5415.2005.53270.x. [DOI] [PubMed] [Google Scholar]
- Steensels D., Reynders M., Descheemaeker P., Curran M.D., Jacobs F., Denis O. Clinical evaluation of a multi-parameter customized respiratory TaqMan®array card compared to conventional methods in immunocompromised patients. J Clin Virol. 2015;72:36–41. doi: 10.1016/j.jcv.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbaugh L., Sukumar S., Joseph C. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36:870–876. doi: 10.1086/368197. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (UN). Department of Economic and Social Affairs, Population Division (2015). World Population Ageing 2015: 29 (ST/ESA/SER.A/390). http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf. [Accessed 21 January 2016].
- Walsh E.E., Falsey A.R. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets. 2012;12:98–102. doi: 10.2174/187152612800100116. [DOI] [PubMed] [Google Scholar]
- Welti M., Jaton K., Altwegg M., Sahli R., Wenger A., Bille J. Development of a multiplex real-time quantitative PCR assay to detect Chlamydia pneumoniae, Legionella pneumophila and Mycoplasma pneumoniae in respiratory tract secretions. Diagn Microbiol Infect Dis. 2003;45:85–95. doi: 10.1016/s0732-8893(02)00484-4. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO; 2011. Manual for the laboratory diagnosis and virological surveillance of influenza. http://www.who.int/influenza/gisrs_laboratory/manual_diagnosis_surveillance_influenza/en/. [Accessed 15 December 2014] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.