Abstract
To develop CpG oligodeoxynucleotides (CpG ODNs) based therapy for prevention and treatment of severe acute respiratory syndrome (SARS), we selected a novel CpG ODN (BW001), which displays B-type CpG ODN structure feature at the 5′ and A-type CpG ODN structure feature at the 3′, and tested for its anti-SARS-CoV activity. We found that the supernatants of human PBMCs stimulated by BW001 significantly protected Vero cells from SARS-CoV infection. BW001 could stimulate human PBMCs and pDCs to secrete high level of IFN-α and promote human PBMCs and B cells to proliferate. Furthermore, we demonstrated that BW001 could activate CD19+ B cells and CD56+ NK cells in human PBMCs. In addition, BW001 could enhance NK cytotoxicity and IFN-γ secretion in human PBMCs. Together, BW001 represents a novel type of CpG ODN and may have potential for the development of treatment and prevention for SARS as well as other viral associated diseases.
Abbreviations: SARS, severe acute respiratory syndrome; CpG ODN, oligodeoxynucleotides containing unmethylated CpG motifs; IFN-γ, gamma interferon; PBMCs, peripheral blood mononuclear cells; pDCs, plasmacytoid dendritic cells; SARS-CoV, SARS coronavirus
Keywords: CpG ODN, SARS-CoV, Anit-virus, PBMCs, pDCs, B cells, NK cells, IFN-α, IFN-γ
Introduction
Immunostimulatory activities of CpG oligodeoxynucleotides (CpG ODNs) have been extensively studied in recent years [1], [2], [3], [4], [5], [6], [7], [8], [9]. Based on the functional characteristics, CpG ODNs are divided into three types: A-type CpG ODN is capable of activating human plasmacytoid dendritic cells (pDCs) to produce large amount of type I interferons (IFN-α/β) and strongly activating natural killer cells (NK cells). B-type CpG ODN primarily activates B cells, resulting in their proliferation and antibody secretion. C-type CpG ODN shares the activities of both A- and B-type CpG ODN [3], [8], [9], [10], [11].
CpG ODNs have been widely tested to prevent and treat viral infections [4], [12]. HIV replication in human fetal thymocytes was strongly inhibited by CpG ODN [13], [14]. Subcutaneous injection of CpG ODN in sheep induced a dose-dependent increase in 2′5′-A synthetase, an anti-viral effector molecule, in serum of sheep [15]. Administration of CpG ODN through genital tract enhanced innate immunity and protected mice from herpes virus infection [16], [17], [18]. Mice pretreated with CpG ODN before respiratory syncytial virus (RSV) infection produced a large amount of IFN-γ in the lung that was associated with significantly reduced mucus secretion of the virus [19]. Treatment of Friend virus-infected mice with CpG ODN significantly reduced the virus burden in blood and enhanced virus-specific cellular immune responses [20]. Administration of CpG ODN to SAM-P1 mice (senescence-accelerated model mice) induced a quick clearance of influenza virus and considerably improved the survival of the mice challenged with lethal dose of influenza virus [21]. Recently, treatment of hepatitis C patients with CpG ODN was reported to reduce hepatitis C virus (HCV) RNA in a phase I study, and the reduction was consistent with the elevation of IFN-α and other markers associated with an anti-viral immune response.
In late 2002, severe acute respiratory syndrome (SARS) mysteriously developed in Guangdong Province, the People's Republic of China, and spread to other 24 countries. The infection is characterized by fever, a dry nonproductive cough and shortness of breath. Death from progressive respiratory failure occurs in about 3% to 10% of cases [22], [23]. A novel coronavirus (SARS-CoV) was isolated from SARS patients and identified to be etiologically linked to the outbreak of SARS [24]. The spread and severity of SARS have churned out a surge of developing vaccines and drugs for the control and treatment of SARS.
Our laboratory has developed and tested a large numbers of CpG ODNs in order to develop CpG ODN based therapy for the prevention and treatment of SARS. First, we have systemically screened the function of these CpG ODNs to stimulate human peripheral blood mononuclear cells (PBMCs) to produce anti-viral activity analyzed by an anti-viral bioassay. We have selected a novel type of CpG ODN (BW001), which shows the most potent ability in anti-VSV activity in a bioassay. BW001 displays B-type CpG ODN structure feature at the 5′ and A-type CpG ODN structure feature at the 3′. BW001 showed a potent activity in inhibiting the cytolytic effect of SARS-CoV to Vero E6 cells. We found that BW001 displays the functional activity of C-type CpG ODN because it induces human PBMCs and plasmacytoid dendritic cells (pDCs) to produce high level of IFN-α, stimulates human PBMCs and B cells to proliferate and activates CD19+ B cells and CD56+ NK cells. We suggest that BW001, a novel type of CpG ODN, may have the potential to be developed for anti-SARS prevention and therapy.
Materials and methods
ODNs
Nuclease-resistant phosphorothioate-modified ODNs were synthesized in Sangon Biotech Company (Shanghai, China). The CpG ODNs used in this study are BW001 (5′-tcg tcg ggt gcg acg tcg cag ggg gg-3′), 2216 (5′-ggG GGA CGA TCG TCg ggg gG-3′) and 2006 (5′-tcg tcg ttt tgt cgt tttg tcg tt-3′). BW002 (5′-tgc tgg ggt ggc agc tgc cag ggg gg-3′) is the same as BW001 except the CG sequence is reversed. Lowercase and capital letters represent phosphorothioate and phosphodiester linkage, respectively. All ODNs were diluted in TE buffer (10 mM Tris, 1 mM EDTA, pH 7.0) and tested for endotoxin by using the Limulus amebocyte lysate assay (Associates of Cape Cod, Inc). All reagents used were pyrogen-free reagents.
Cells and cell lines
Human PBMCs were isolated from buffy coats (The Blood Center of Jilin Province, China) by Ficoll–Hypaque density gradient centrifugation (Pharmacia). The viability of the PBMCs was 95–99% as determined by trypan blue exclusion. Plasmacytoid dendritic cells (pDCs) were purified from human PBMCs by depleting lymphocytes, monocytes and neutrophilic granulocytes with 1:1000 dilution of anti-CD3, CD14, CD16, CD19 and CD56 antibodies and 1:4 dilution of anti-mouse IgG magnetic beads (Dynal). The antibody negative cells were sorted for CD4+CD11c−lin− pDCs. CD19+ cells were positively selected using magnetic beads (Dynal). Briefly, a quantity of 1 × 107 beads was added to 1 × 108 PBMCs and gently mixed on a bidirectional specimen mixer for 30 min at 4°C. The CD19+ B cells were isolated by placing the tube in a magnetic particle concentrator for 1 min. The supernatant was removed by pipetting and the remaining CD19-selected cells were washed three times with Iscove's modified Dulbecco's medium (IMDM, GIBCO). Vero E6 cells (African green monkey kidney cell line, American Type Culture Collection) were cultured at 37°C in a 5% CO2 humidified incubator and maintained in IMDM supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; GIBCO) and antibiotics (100 IU of penicillin/ml and 100 IU of streptomycin/ml).
Viruses
Vesicular stomatitis virus (VSV) was grown in Vero E6 cells. After titration, the virus was stored in aliquots at −70°C until use. The SARS-CoV (Sino-5 strain) used was isolated from a SARS patient in Youanmen Hospital of Beijing and identified with RT-PCR, virus neutralization test and DNA sequencing. The virus was propagated in Vero E6 cells and titrated by a cytopathic effect (CPE) method. All experiments involving SARS-CoV were carried out in a BL3 level laboratory in the Chinese Disease Control Center, Beijing, in compliance with the guideline of the government regulation.
Virus protection assays
The anti-viral activities in the culture supernatants of CpG ODN-treated human PBMCs were measured as described [25]. Briefly, the supernatants of PBMCs (3 × 106/ml) stimulated with or without CpG ODN for 36 h were collected and stored in aliquots at −70°C until use. The Vero E6 cells (3 × 104/well) were seeded into 96-well flat-bottomed plates and cultured for 24 h to confluence. The cells were incubated with 100 μl of the diluted supernatants for 24 h and then challenged with 10× TCID50 (50% tissue culture infectious doses) of VSV or 10× TCID50 of SARS-CoV for another 48 h. After staining with 0.5% crystal violet, the cytopathic effect of virus was examined using Multi-well Microtiter Plate Reader at A570 nm and expressed as OD values.
Analysis of cytokines induced by CpG ODN
Human PBMCs (3 × 106/ml) or human pDCs (5 × 104/well in 200 μl medium) were treated with different CpG ODNs (6 μg/ml) for 36 h, and the supernatants were harvested for determination of cytokines. ELISA kits for IFN-α (PIERCE), and IFN-γ (R&D System) were used for detecting IFN-α and IFN-γ in the supernatants according to the manufacture's protocols. The cytokine level in the supernatant was quantitatively calculated based on the standard curve.
Proliferation assays
Proliferation of human PBMCs or human B cells was determined by [3H] thymidine incorporation assay as described previously [26]. Briefly, PBMCs (6 × 105/well) or B cells (5 × 104/well) were plated in 96-well U-bottomed plates (Costar) and cultured with or without CpG ODN (6 μg/ml) for 36 h, followed by pulsing with [3H] thymidine (New England Nuclear, Boston, MA) for 16 h. The cells were harvested on glass fiber filters and detected in a scintillation counter. The cell proliferation was expressed as mean cpm (counts per minute) (from triplet wells) ± SD.
Flow cytometry
PBMCs were incubated with or without CpG ODN (6 μg/ml) for 12 h, washed twice with FACS staining buffer (PBS supplemented with 0.5% BSA, 0.01% NaN3 and 100 mM EDTA) and then analyzed for the expression of various cell surface molecules by dual-color staining with FITC-labeled anti-CD69 mAb (BD Biosciences) and PE-labeled anti-CD19, CD56, CD14 and CD3 mAbs (BD Biosciences) followed by analysis on a FACSCalibur.
NK killing assay
The NK cytotoxicity of human PBMCs stimulated by CpG ODN was assayed using K562 cells as target cells [1]. Briefly, human PBMCs were incubated with CpG ODN (6 μg/ml) for 36 h and then harvested as effector cells. K562 cells were labeled with 51Cr for 2 h and used as target cells. The stimulated PBMCs in various numbers were seeded in a U-bottomed 96-well plate and then incubated with 51Cr-labeled K562 cells (5 × 103/well) in a final volume of 200 μl for 4 h at 37°C. At the end of the incubation, 50 μl of the supernatants was harvested from each well and counted in a γ-counter for 51Cr release. Spontaneous and maximal release was determined in the presence of either medium or 1% Triton X-100 respectively. Results were expressed as mean percentage of specific lysis (triplet wells) ± SD: specific lysis (%) = (experimental counts − mean spontaneous release counts) / (mean maximal release counts − mean spontaneous release counts) × 100.
Statistical analysis
Data are shown as means ± SD. Statistical significance of differences was determined by the paired two-tailed Student's t test. Differences were considered statistically significant for P is less than 0.05.
Results
BW001 induces human PBMCs to produce strong anti-viral activity
To identify CpG ODNs with immune-stimulating activities, we synthesized a series of CpG ODNs and tested their ability to stimulate human PBMCs to produce factors that could protect Vero E6 cells from VSV infection. One of the CpG ODNs, designated as BW001, stood out as possessing anti-viral activity and was studied in detail. Two of well-known prototype CpG ODNs, 2216 as an A-type CpG ODN and 2006 as a B-type CpG ODN, were used as controls. In VSV protection assays, human PBMCs were stimulated with BW001 or control CpG ODNs, and the culture supernatants were tested for the ability to protect Vero E6 cells from VSV infection. As shown in Fig. 1A, BW001-stimulated supernatants protected Vero E6 cells from challenge by 10× TCID50 of VSV. This level of protection was similar to that induced by 2216 (P > 0.05) and significantly higher than that induced by 2006 (P < 0.05). The anti-viral activity was due to the presence of the CG dinucleotide in the BW001 because BW002, which shared the same sequence as the BW001 except the order of the CG was reversed, did not induce the anti-VSV activity in the supernatant of cultured human PBMCs.
Fig. 1.
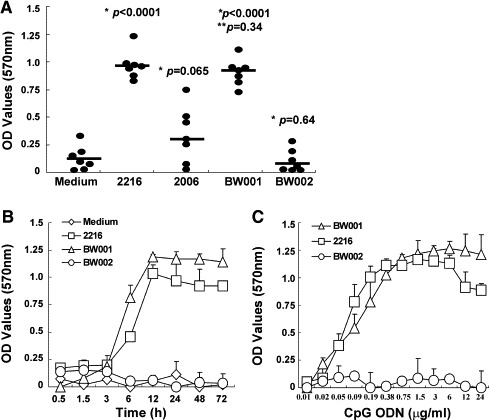
BW001 induces human PBMCs to produce strong anti-viral activity. PBMCs were treated with different CpG ODN (6 μg/ml) or simply cultured in medium for 36 h. The anti-viral activities of the supernatants were tested by a VSV protection assay. (A) Anti-VSV activities of the supernatants from 7 healthy human PBMCs. Each symbol represents anti-VSV activity of one supernatant. Each horizontal line represents median value of anti-VSV activity. 2216 (an A-type CpG ODN), 2006 (a B-type CpG ODN) and BW002 were as control CpG ODNs. The result showed that BW001 could stimulate human PBMCs to produce strong anti-VSV activity. *ODNs vs. Medium; **BW001 vs. 2216. (B) Kinetics of BW001-induced anti-viral activity. The dose of CpG ODN used in the experiment was 6 μg/ml. The result showed that BW001 could induce human PBMCs to produce strong anti-viral activity at 12–72 h. (C) Dose of BW001 in induced anti-viral activity. The human PBMCs were treated for 36 h. The result showed that the optimal dose of BW001 was 0.38–12μg/ml. Data from one representative experiment of three are shown.
To determine the kinetics of anti-viral activity induced by BW001, we stimulated human PBMCs with 6 μg/ml of BW001, 2216 or BW002. At different time after stimulation (45 min to 72 h), the supernatants were assayed for their anti-viral activity. Although 3 h following BW001 stimulation, anti-viral activity was not yet apparent in supernatants, it was quite high 6 h following stimulation (Fig. 1B). The activity reached the peak level at 12 h and was maintained at the peak level until 72 h. 2216-induced anti-viral activity exhibited a similar kinetics, while BW002 induced no observed anti-viral activity. These results show that the anti-viral activities induced by BW001 appear within few hours and maintain for at least 3 days.
Based on the feature of BW001 kinetics, we tested its dose in stimulating anti-viral activity. Human PBMCs were treated with different amounts of BW001, 2216 or BW002 for 36 h, and then the supernatants were used for measuring their anti-viral activities by the VSV protection assay. Significant anti-VSV activity was detected when 0.05 μg/ml of BW001 was used (Fig. 1C). The activity increased sharply when 0.09–0.38 μg/ml of BW001 were used and reached a plateau level when 0.38–24 μg/ml of BW001 were used. The anti-VSV activities induced by 2216 showed a similar profile, except that the activity decreased at high CpG ODN concentration (6–24 μg/ml). The result indicates that BW001 is very potent and sufficient to induce significant anti-viral activity at a very low concentration.
BW001 stimulates human PBMCs to produce strong anti-SARS-CoV activity
To seek an anti-SARS-CoV CpG ODN, we tested the ability of BW001 in inducing anti-SARS-CoV activities. Human PBMCs were stimulated with 6 μg/ml of BW001, and the culture supernatants were tested the ability to protect Vero E6 cells from infection of 10× TICD50 SARS-CoV (Sino-5 strain). As shown in Fig. 2A, BW001-stimulated supernatants significantly protected Vero E6 cells from SARS-CoV infection even when the supernatants were diluted by 80-fold. To estimate the relative level of anti-SARS-CoV activity induced by BW001, we measured the IFN-α in the supernatants by ELISA and found that BW001 could stimulate human PBMCs to produce high level of IFN-α but BW002 could not (Fig. 2B). Meanwhile, we tested the inhibition of SARS-CoV infection of Vero E6 cells in the presence of different amounts of IFN-α2b. The result showed that IFN-α2b could protect Vero E6 cells from SARS-CoV infection in a dose-dependent manner: higher dose of IFN-α2b (1000–2000 U/ml) displayed stronger anti-SARS-CoV activities (Fig. 2C) and lower dose of IFN-α2b (<250 U/ml) lost its anti-SARS-CoV activity. Our results demonstrate that IFN-α in the supernatant of human PBMCs stimulated by BW001 is a major anti-SARS-CoV factor.
Fig. 2.
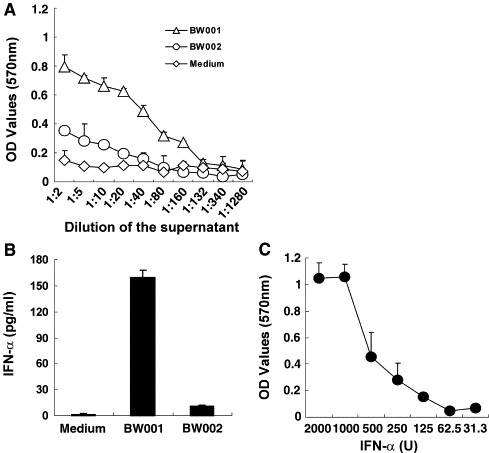
BW001 stimulates human PBMCs to produce strong anti-SARS-CoV activity. (A) The supernatants of human PBMCs treated with BW001 or BW002 were used to protect Vero E6 cells from challenge of 10× TICD50 SARS-CoV (see Materials and methods). The dose of the stimulator was 6 μg/ml. Each symbol represents mean OD values ± SD. Each curve represents anti-SARS-CoV activity induced with or without CpG ODN. The result showed that BW001 could strongly stimulate human PBMCs to produce anti-SARS-CoV activity. (B) IFN-α contents in the supernatants used for anti-SARS-CoV were determined by ELISA method. Each column represents level of IFN-α induced with or without CpG ODN. BW001 could stimulate human PBMCs (3 × 106/ml) to produce 160 pg/ml IFN-α that was much high than that induced by BW002 and medium. (C) Anti-SARS-CoV activity of recombinant IFN-α2b. “U” represents the international unit of IFN-α2b. Data from one representative experiment of three are shown.
BW001 displays the functional feature of C-type CpG ODN
Because CpG ODN has been shown to induce human pDCs to produce type I interferons, including IFN-α/β, important anti-viral factors, we measured IFN-α secretion by human PBMCs and purified pDCs following BW001 stimulation. The result showed that BW001 could stimulate human PBMCs to secrete IFN-α. On average, the IFN-α level in BW001-stimulated supernatants was about 200 pg/ml which was about 30% lower than that induced by 2216 (Fig. 3A, left). Since pDCs are major cells for IFN-α production, we purified pDCs from human PBMCs and stimulated them with different CpG ODN (6 μg/ml) for 36 h. The supernatants were detected for IFN-α production. The result showed that BW001 could stimulate human pDCs to produce high level of IFN-α (3280 pg/ml) that was approximately half of that (7726 pg/ml) induced by 2216 (Fig. 3A, right). This result demonstrates that BW001 can display the functional feature of A-type CpG ODN.
Fig. 3.
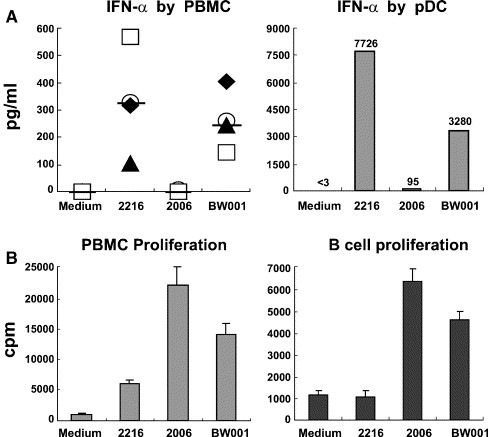
BW001 displays the functional feature of C-type CpG ODN. (A) Detection of IFN-α in the supernatants of human PBMCs or pDCs stimulated by different CpG ODN (6 μg/ml) for 36 h (see Materials and methods) using an ELISA kit. Each symbol represents one experiment with sample from one donor. IFN-α contents in the supernatants of human PBMCs from four donors were tested. Horizontal lines represent median value of IFN-α level. Each column represents IFN-α level in the supernatant of pDCs activated with or without CpG ODN. The results showed that BW001 could stimulate human PBMCs and pDCs to produce high level of IFN-α. (B) Cell proliferation assay. Human PBMCs or purified B cells were treated with different CpG ODN (6 μg/ml) for 36 h and then incorporated with [3H] thymidine for 16 h. Each column represents proliferated cells induced with or without CpG ODN. The proliferation of cells was expressed as mean cpm ± SD. The results showed that BW001 could stimulate human PBMCs and B cells to proliferate obviously. Data from one representative experiment of three are shown.
Next, we measured the ability of BW001 in stimulating B cell proliferation. Human PBMCs or purified B cells were stimulated with various CpG ODN (6 μg/ml) for 36 h and then incorporated with [3H] thymidine for 16 h. The result showed that the proliferation of human PBMCs stimulated by BW001 was obviously higher than that of stimulated by 2216 but lower than that of stimulated by 2006 (Fig. 3B, left). Furthermore, purified B cells were used in proliferation assay. The result showed that BW001 could also stimulate B cell proliferation. The proliferation level of B cells activated by BW001 was lower than 2006-induced but significant higher than 2216-induced (Fig. 3B, right). This result reveals that BW001 can also display functional feature of B-type CpG ODN.
BW001 displays B-type CpG ODN structure feature at the 5′ and A-type CpG ODN structure feature at the 3′ (see Materials and methods). This structure feature might be the reason for BW001 to have functions of inducing anti-viral activity and stimulating the proliferation of B cells. Together, our results show that BW001 is a novel C-type CpG ODN that shares the functional features of both A-type CpG ODN and B-type CpG ODN.
BW001 activates B cells and NK cells in human PBMCs
To identify types of the cells activated by BW001, human PBMCs were cultured with the CpG ODN (6 μg/ml) for 12 h and then stained with PE-labeled anti-CD19 or CD56 or CD14 or CD3 mAbs and FITC-labeled anti-CD69 mAb and analyzed on flowcytometry. BW001 could up-regulate CD69 expression on CD19+ cells (B cells) and CD56+ cells (NK cells). The potency of BW001 on the activation of B cells was stronger than that of 2006 and on activation of NK cells was similar than that of 2216. In contrast, CD3+ T cells and CD14+ monocytes could not be activated by BW001 and other CpG ODN (Fig. 4A). BW002 could not activate immune cells in PBMCs. These results demonstrate that BW001 can activate B cells and NK cells in human PBMCs.
Fig. 4.
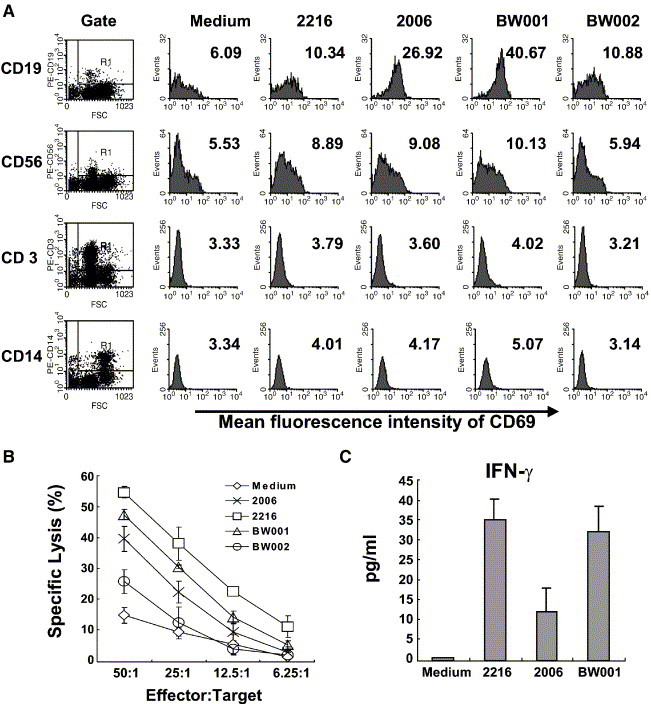
BW001 activates B cells and NK cells. Human PBMCs were treated with different CpG ODN (6 μg/ml) for 12 h. After stimulation, the PBMCs and its supernatants were harvested separately for further study. (A) BW001 stimulates CD69 expression of B cells and NK cells. CpG ODN-treated PBMCs were stained with FITC-labeled anti-CD69 mAb and PE-labeled anti-CD19, CD56, CD14 and CD3 mAb and then analyzed on a FACSCalibur by gating CD19, CD56, CD3 or CD14 positive cells as showed in Fig. 4A. The results represent as mean fluorescent intensity of FITC-CD69. Compare to medium control group, BW001 could up-regulate CD69 expression on CD19+ cells (B cells) and CD56+ cells (NK cells). (B) BW001 enhances NK cytotoxicity of human PBMCs. CpG ODN-treated PBMCs were used as effector cells and 51Cr-labeled K562 cells were used as target cells in NK killing assay. The killing assay was performed for 4 h at 37°C. BW001-induced NK activity was less than that of 2216-induced but stronger than that of 2006-induced. (C) BW001 induces human PBMCs to produce IFN-γ. The supernatants of CpG ODN treated human PBMCs were analyzed for IFN-γ production by ELISA method. Each column represents mean value of IFN-γ level in the supernatant. Data from one representative experiment of three are shown.
To test whether BW001 could promote NK cell killing activity, 51Cr-labeled K562 cells were used as target cells. The result revealed that the NK killing activity from BW001-activated PBMCs was stronger than that from BW002- or 2006-activated PBMCs but weaker than that from 2216-activated PBMCs (Fig. 4B). This result shows that BW001-activated NK cells in human PBMCs display enhanced cytotoxicity.
In the IFN-γ assay, we demonstrated that BW001 could stimulate human PBMCs to produce significant amount of IFN-γ as 2216 did. In contrast, 2006 could not (Fig. 4C). This result suggests that NK cells activated by BW001 may secrete IFN-γ.
Discussion
In this study, we report a novel type of CpG ODN, BW001, which has strong activity against cytolytic activity of SARS-CoV. The anti-SARC-CoV activity of BW001 is correlated with its ability to induce human PBMCs to produce IFN-α in a dose-dependent fashion.
In late 2002, the spread and severity of SARS prompted the search and development of anti-SARS-CoV compounds. Recombinant IFN-β was shown to exhibit the highest anti-viral activity against SARS-CoV in vitro [27]. Pegylated IFN-α was shown to reduce viral replication and excretion in SARS-CoV infected macaques [28]. These findings suggest the possible applications of type I IFN to treat SARS patients. However, direct application of type I IFN has severe drawbacks. First, repeated application of recombinant IFN-α in humans induces antibody responses and results in loss of therapeutic efficacy. Second, there are at least 13 subtypes of IFN-α in humans. It is difficult to produce all of them for human use. In fact, natural IFN-α was found to have higher anti-viral activity against SARS-CoV in vitro than recombinant IFN-α [27], probably due to the presence of multiple subtypes of IFN-α following natural induction. To overcome this difficult, one possibility is to use CpG ODNs to induce the production of IFN by endogenous cells. We show that BW001 induces human PBMCs and pDCs to secrete large amount of IFN-α. Indeed, at least eight subtypes of IFN-α are produced following BW001 stimulation of human PBMCs (data not shown). Consistently, BW001-stimulated PBMCs supernatants exhibit potent anti-SARS-CoV activity.
In innate immune system, NK cells are the first line of defense against virally infected cells and contribute to resistance during the early phases of viral infections. Additionally, activated NK cells and monocytes can also produce cytokines, which may exhibit different anti-virus activities. We found that NK cells (CD56+) in human PBMCs could be activated by BW001. Activation of these cells is probably indirect because studies have shown that CpG ODN-induced type I IFN, cytokines and chemokines trigger a wide range of secondary effects, including NK cell activation [29]. In our study, we also found that BW001 could enhance NK cell cytotoxicity after stimulating human PBMCs, implying that BW001 was capable of triggering all innate anti-virus immunity in vivo including type I IFN production and NK activation.
Previous studies revealed that the innate immune activation by CpG ODN might lead to the generation of adaptive immune responses. We found BW001 could activate B cells of human PBMCs, which might possibly be an indication of priming humoral branch in specific immune system.
In this paper, 2216, the prototype of A-type CpG ODN, was used as a control to study the anti-viral activity of BW001. We found that 2216 induced similar anti-VSV activity in human PBMCs as BW001 did and stimulated the higher level of IFN-α production from both human PBMCs and pDCs. The data suggest that 2216 is also an optimal CpG ODN for inducing anti-SARS-CoV immunity. Comparatively, in light of application, BW001 with full phosphorothioated backbone may be more resistant to the nuclease digestion than 2216 whose backbone is partial phosphorothioated. Furthermore, the C-type CpG ODN properties of BW001 may make it a potential medicament that stimulates more branches of anti-SARS-CoV immunities.
Based on our data, we propose that applications of BW001 may have several advantages in treating SARS and other viral infections: (1) inducing endogenous mixed IFN-α, (2) inducing a rapid anti-virus response, (3) activating NK cells, (4) activating B cell. Collectively, our data suggest that BW001 may be an attractive candidate for further development as an anti-viral drug for treating SARS-CoV and other virus infections.
Acknowledgments
We gratefully acknowledge the Chinese Center for Disease Control (CDC) in Beijing for conducting anti-SARS-CoV experiments. This study was supported by the National Outstanding Young Scientist's Fund from National Nature Scientific Foundation of China (30328010) and by 973 Projects of a National Key Basic Research Program Grant 001CB510007 of China.
Contributor Information
Yongli Yu, Email: ylyu@mail.jlu.edu.cn.
Liying Wang, Email: wlying@mail.jlu.edu.cn.
References
- 1.Ballas Z.K., Rasmussen W.L., Krieg A.M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 2.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 3.Klinman D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev., Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 4.Klinman D.M., Verthelyi D., Takeshita F., Ishii K.J. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity. 1999;11:123–129. doi: 10.1016/s1074-7613(00)80087-4. [DOI] [PubMed] [Google Scholar]
- 5.Krieg A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 6.Krieg A.M., Yi A.K., Matson S., Waldschmidt T.J., Bishop G.A., Teasdale R., Koretzky G.A., Klinman D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 7.Krug A., Rothenfusser S., Hornung V., Jahrsdorfer B., Blackwell S., Ballas Z.K., Endres S., Krieg A.M., Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Latz E., Schoenemeyer A., Visintin A., Fitzgerald K.A., Monks B.G., Knetter C.F., Lien E., Nilsen N.J., Espevik T., Golenbock D.T. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 9.Verthelyi D., Klinman D.M. Immunoregulatory activity of CpG oligonucleotides in humans and nonhuman primates. Clin. Immunol. 2003;109:64–71. doi: 10.1016/s1521-6616(03)00202-x. [DOI] [PubMed] [Google Scholar]
- 10.Marshall J.D., Fearon K., Abbate C., Subramanian S., Yee P., Gregorio J., Coffman R.L., Van Nest G. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leukocyte Biol. 2003;73:781–792. doi: 10.1189/jlb.1202630. [DOI] [PubMed] [Google Scholar]
- 11.Vollmer J., Weeratna R., Payette P., Jurk M., Schetter C., Laucht M., Wader T., Tluk S., Liu M., Davis H.L., Krieg A.M. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer U., Olbrich A.R. Treatment of infectious diseases with immunostimulatory oligodeoxynucleotides containing CpG motifs. Curr. Opin. Microbiol. 2003;6:472–477. doi: 10.1016/j.mib.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Becker Y. A point of view: HIV-1/AIDS is an allergy but CpG ODN treatments may inhibit virus replication and reactivate the adaptive immunity-hypothesis and implications. Virus Genes. 2005;30:127–131. doi: 10.1007/s11262-004-4590-0. [DOI] [PubMed] [Google Scholar]
- 14.Gurney K.B., Colantonio A.D., Blom B., Spits H., Uittenbogaart C.H. Endogenous IFN-alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J. Immunol. 2004;173:7269–7276. doi: 10.4049/jimmunol.173.12.7269. [DOI] [PubMed] [Google Scholar]
- 15.Nichani A.K., Kaushik R.S., Mena A., Popowych Y., Dent D., Townsend H.G., Mutwiri G., Hecker R., Babiuk L.A., Griebel P.J. CpG oligodeoxynucleotide induction of antiviral effector molecules in sheep. Cell. Immunol. 2004;227:24–37. doi: 10.1016/j.cellimm.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Harandi A.M. The potential of immunostimulatory CpG DNA for inducing immunity against genital herpes: opportunities and challenges. J. Clin. Virol. 2004;30:207–210. doi: 10.1016/j.jcv.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Harandi A.M., Eriksson K., Holmgren J. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 2003;77:953–962. doi: 10.1128/JVI.77.2.953-962.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyles R.B., Higgins D., Chalk C., Zalar A., Eiden J., Brown C., Van Nest G., Stanberry L.R. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 2002;76:11387–11396. doi: 10.1128/JVI.76.22.11387-11396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho J.Y., Miller M., Baek K.J., Castaneda D., Nayar J., Roman M., Raz E., Broide D.H. Immunostimulatory DNA sequences inhibit respiratory syncytial viral load, airway inflammation, and mucus secretion. J. Allergy Clin. Immunol. 2001;108:697–702. doi: 10.1067/mai.2001.119918. [DOI] [PubMed] [Google Scholar]
- 20.Olbrich A.R., Schimmer S., Heeg K., Schepers K., Schumacher T.N., Dittmer U. Effective postexposure treatment of retrovirus-induced disease with immunostimulatory DNA containing CpG motifs. J. Virol. 2002;76:11397–11404. doi: 10.1128/JVI.76.22.11397-11404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong L., Mori I., Hossain M.J., Liu B., Kimura Y. An immunostimulatory oligodeoxynucleotide containing a cytidine-guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J. Gen. Virol. 2003;84:1623–1628. doi: 10.1099/vir.0.19029-0. [DOI] [PubMed] [Google Scholar]
- 22.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. National microbiology laboratory, Canada, Canadian severe acute respiratory syndrome study team, identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 23.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 24.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 25.Tokunaga T., Yano O., Kuramoto E., Kimura Y., Yamamoto T., Kataoka T., Yamamoto S. Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovis BCG induce interferons and activate natural killer cells. Microbiol. Immunol. 1992;36:55–66. doi: 10.1111/j.1348-0421.1992.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 26.Mannon R.B., Nataraj C., Pisetsky D.S. Stimulation of thymocyte proliferation by phosphorothioate DNA oligonucleotides. Cell. Immunol. 2000;201:14–21. doi: 10.1006/cimm.2000.1635. [DOI] [PubMed] [Google Scholar]
- 27.Dahl H., Linde A., Strannegard O. In vitro inhibition of SARS virus replication by human interferons. Scand. J. Infect Dis. 2004;36:829–831. doi: 10.1080/00365540410021144. [DOI] [PubMed] [Google Scholar]
- 28.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams O., Besken K., Oberdörfer C., MacKenzie C.R., Rüßing D., Däubener W. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 2004;6:806–812. doi: 10.1016/j.micinf.2004.04.007. [DOI] [PubMed] [Google Scholar]


