Abstract
The prevalence of antibodies to bovine coronavirus (BCV) and bovine respiratory syncytial virus (BRSV) infections was studied in 20 conventional and 20 organic dairy herds. The organic farms had produced ‘certified’ milk for at least 2 years. On two occasions, with a 1-year interval, 699 serum samples from 624 peri-parturient cows were tested by ELISA for antibodies to BCV and BRSV. Accompanying data relating to the sampled animals were collected in order to identify potential factors associated with increased antibody prevalence. The antibody prevalence was high at both sampling times with approximately 85% and 80% of animals positive for antibodies to BCV and to BRSV, respectively. Conventional herds had a significantly higher mean antibody prevalence to BCV and BRSV than the organically managed herds (P < 0.01). Animal age was significantly associated with increased antibody prevalence (P < 0.001). The findings of this study suggest that organic farm management may be effective in reducing the seroprevalence of these viruses relative to conventional farming methods.
Keywords: Bovine coronavirus (BCV), Bovine respiratory syncytial virus (BRSV), Organic, Dairy herds, Seroprevalence
Introduction
Bovine coronavirus (BCV) and bovine respiratory syncytial virus (BRSV) infections are widespread, resulting in a high seroprevalence in cattle herds (Van der Poel et al., 1995, Saif, 2004). Bovine coronavirus is an enveloped, positive-stranded RNA virus classified as an antigenic group II member of the family Coronaviridae (Spaan et al., 1988) and is implicated in both respiratory and enteric disease, including calf diarrhoea, winter dysentery in adult dairy cows, and respiratory infections in cattle of all ages (Alenius et al., 1991, Saif, 2004). BRSV, like its human counterpart, human respiratory syncytial virus (HRSV), is an enveloped, negative-stranded RNA virus classified in the Pneumovirus genus of the Paramyxoviridae family (Stott and Taylor, 1985). Although it is a major cause of respiratory disease in young calves and has a considerable economic impact (Van der Poel et al., 1994, Valarcher and Taylor, 2007), cattle of all ages can be infected with BRSV, and severe morbidity and mortality has been described in adult animals (Elvander, 1996).
Outbreaks of these viral infections usually occur in the autumn and winter months and a humoral immune response persists for at least a year following natural or experimental infection. Epidemiological and experimental studies suggest that antibody levels correlate with immunity (Alenius et al., 1991, Schrijver et al., 1996, Tråvén et al., 2001).
Both BRSV and BCV facilitate secondary pulmonary bacterial infection, which typically necessitates considerable antibiotic use (Hägglund et al., 2007). One of the aims of organic farming is to reduce the use of antibiotics through the provision of optimal husbandry, nutrition and biosecurity but it is currently unclear whether organic management is more successful than conventional management in reducing the risk or prevalence of herd infection with these viruses. This study set out to compare the serological prevalence of BCV and BRSV infection in dairy cows managed under organic and conventional management systems and to investigate potential risk factors associated with these infections.
Materials and methods
Selection of herds and animals
Selected herds had more than 40 cows, were enrolled in the Swedish Official Milk Recording Scheme (SOMRS) and were located in the Uppland, Södermanland, Östergötland, and Småland regions of South-East Sweden. Selected organically managed herds had produced milk in accordance with organic standards for at least 2 years previous to the study. Of the 52 eligible organic farms, 24 were willing to participate and, of these, 20 were randomly selected. Of the 156 eligible conventionally-managed farms, 32 agreed to participate and 20 were randomly selected. All 40 study herds were free of bovine viral diarrhoea virus (BVDV) as defined by The National Control Program (Lindberg and Alenius, 1999).
All 40 herds were visited on two occasions – in the Spring of 2005 and 2006. On each occasion sampled cows were between 7 days before their predicted calving date and 42 days after calving (as sampling was also used to study metabolic parameters in peri-parturient cows; Fall et al., in press). If the number of such animals in a herd was <12, all were sampled. If the herd contained >12 eligible animals, 12 were randomly selected for sampling using a random number table.
A total of 699 serum samples were taken from 624 cows in the 40 herds. At the 2005 sampling, 169 cows were sampled on conventional farms and 169 on the organic farms. At the 2006 sampling, 189 animals were sampled in the conventional herds and 172 in the organic herds. By chance 75 cows were sampled at both sampling times.
Herd and animal data were collected via the SOMRS system and from farmer questionnaires at each sampling time. Data relating to diseases that required veterinary intervention on these premises were retrieved from the national animal disease recording database.
Sample collection and antibody testing
Blood sampling was via the tail vein into 10 mL Vacutainers without anticoagulant. Samples were transported at room temperature within 4 h to a laboratory where they were centrifuged at 2000g for 10 min to produce serum and were then stored at −20 °C.
All sera were tested using commercially available indirect ELISAs (Svanova Biotech) for BCV- and BRSV-specific IgG. Sera were diluted 1:25 and analysis was performed according to the manufacturer’s instructions. Serum samples that generated a corrected optical density (COD) value of ⩾0.2 at 450 nm were considered positive for both viruses. Those with a COD value below this cut-off point were deemed negative (Alenius et al., 1991, Elvander et al., 1995). As determined by the manufacturer, the sensitivity and specificity of the BCV antibody ELISA was 85% and 100%, respectively, and for the BRSV antibody ELISA 94% and 100%, respectively. Both positive and negative control sera were included in each assay. Seroconversion was defined as a negative COD value that converted to a positive COD value in paired sera.
Data and statistical analysis
The Fisher’s exact and Wilcoxon Rank Sum tests were used to investigate possible associations between serological status and animal and farm management details. Animal ages were categorised as: 1-3 years (12-36 months); >3–5 years (37–60 months); >5–7 years (61–84 months); and >7 years (>84 months). The number of serum samples for each of these age categories was 120, 147, 58, and 16, respectively, from the organic herds and 143, 149, 54, and 12, from the conventional herds.
The association between potential risk factors and seroprevalence to BCV or BRSV was analysed using multivariable logistic regression models, with each herd sampling considered the unit of analysis. The regression parameters and their variances were estimated using the generalised estimation equation, which adjusts for clustering of the data at group level (Liang and Zeger, 1986), assuming a compound symmetry correlation structure. Separate analyses were carried out for seroprevalence to either virus, where the dependent variable was number of animals positive to BCV, BRSV, or both, divided by number of animals sampled, and for seroprevalence to both viruses, where the dependent variable was the number of animals positive to both BCV and BRSV divided by number of animals sampled.
Explanatory variables included in the initial models were management type (organic or conventional); artificial insemination (AI) system (AI performed by farm personnel [AIF], or by AI technicians); stall type (‘tied’ or ‘free’ stalls); length of the pasturing period during 2005 (above or below the median pasturing period of 19 weeks for the study); herd average milk yield (above or below the median study yield of 8903 kg); number of cows in the herd (above or below the median herd size of 62.3 cows); number of visits by veterinarians during 2005 (above or below the median number of visits of 17.5), and year of sampling. Data relating to herd size, AI method, stall type and milk yield for September 2005 to August 2006 were used in the initial multivariate models. No co-linearity was found between these variables using the Spearman rank correlation.
These initial models were reduced using a backward stepwise procedure, with P < 0.05 based on the score statistics as the exclusion and re-entering criterion. Confounding between explanatory variables was assessed at each step by examining the change in parameter estimates. No changes >25%, considered indicative of confounding, were identified. Interaction terms were not included in the models because of the relatively few herds studied. The model was validated using the Pearson χ 2 statistic. All statistical analyses were carried out using SAS (Release 9.1, SAS Institute Inc.).
Results
Study herds
Management variables relating to both organic and conventional herds are detailed in Table 1 . The median milk yield and length of pasture period were the only variables that were significantly different between the conventional and organic herds. Milk yield was higher and the pasture period was shorter used in the conventional group (P < 0.01 and P < 0.05, respectively).
Table 1.
Details of management variables relating to 20 conventional and 20 organic dairy herds in which the seroprevalence to BCV and BRSV was studied
| Herd system |
||
|---|---|---|
| Conventional | Organic | |
| Median age in months of cows at sampling (range) | 40 (22–136) | 41 (23–138) |
| Number of herds with/without AIFa | 10/10 | 14/6 |
| Stall type (tied/free) | 13/7 | 6/14 |
| Median number of weeks at pasture (range)b | 17 (0–22) | 20 (8–27) |
| Median herd average milk yieldc (kg/cow/year, range) | 9564 (7804–10,831) | 8383 (5607–11,568) |
| Median herd size (range) | 60 (39–115) | 64 (40–118) |
| Median number of veterinary visits/year (range) | 15 (0–39) | 20 (3–44) |
| Median number of samples/herd (range) | 19 (8–24) | 18 (5–24) |
| Total number of samples (year 2005, 2006) | 358 (169, 189) | 341 (169, 172) |
AIF; AI performed by farm personnel rather than by professional AI technicians.
Significantly different, Wilcoxon Rank Sum test, P = 0.019.
Significantly different, Wilcoxon Rank Sum test, P = 0.003.
Seroprevalence to BCV and BRSV in the sampled population
The seroprevalence to BCV and BRSV at an individual level for all sampled cows was 86% (292/338) and 79% (267/338) in 2005, and 84% (304/361) and 82% (297/361) in 2006. The seroprevalence to these viruses did not differ significantly between the two samplings (Table 2 ).
Table 2.
Mean and median (range) within-herd seroprevalence (%) to BCV and BRSV at a herd level in 20 conventional and 20 organic dairy herds sampled in two consecutive years
| Sampling year | BCV-positive |
BRSV-positive |
BCV and BRSV-positive |
|||
|---|---|---|---|---|---|---|
| Conventional | Organic | Conventional | Organic | Conventional | Organic | |
| 2005 | 96a, 100 | 79a, 100 | 89, 100 | 70, 92 | 85a, 100 | 54a, 46 |
| (42–100) | (30–100) | (25–100) | (0–100) | (25–100) | (0-100) | |
| 2006 | 95b, 100 | 77b, 100 | 93b, 100 | 74b, 85 | 89b, 100 | 59b, 59 |
| (30–100) | (9–100) | (50–100) | (0–100) | (34–100) | (0-100) | |
Numbers within rows and within columns with the same letter are significantly different (Wilcoxon Rank Sum test, P < 0.05).
Seroprevalence in conventional and organic herds
The conventional herds had a significantly higher mean seroprevalence to BCV and BRSV than did the organic herds in both 2005 and 2006. The mean prevalence of cows positive to both BCV and BRSV was 85% in 2005 and 89% in 2006 for the cows in conventional herds, and 54% in 2005 and 59% in 2006 for cows in the organic herds (P < 0.05). No herds were seronegative to BCV and no conventional herds were completely seronegative to BRSV. However, in two organic herds all sampled animals were seronegative to BRSV (Table 2). In organic herds 32% of the youngest cows (1–3 years old) were seropositive to both BCV and BRSV, whereas this figure was 70% in conventional herds (P < 0.001) (Fig. 1 ). In 10 of the conventional herds, but only 1 of the organic herds, all sampled cows were seropositive to both viruses.
Fig. 1.
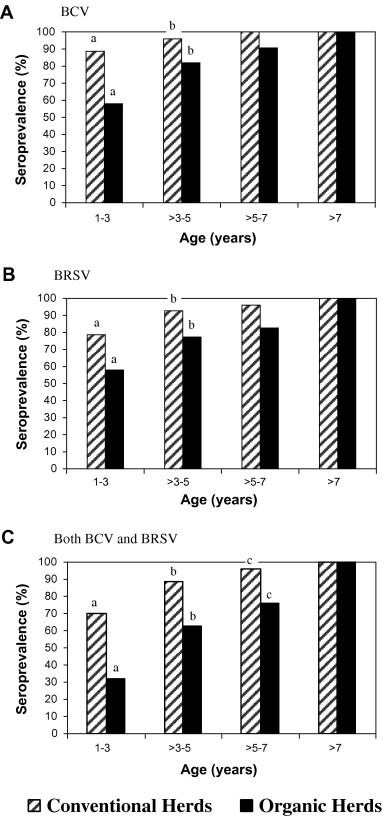
Age-specific prevalence of antibodies to bovine coronavirus (BCV) (A), bovine respiratory syncytial virus (BRSV) (B) and both BCV and BRSV (C) in relation to herd management system. The a–c seroprevalences with the same letter differ significantly between the conventional and organic herds (P < 0.001, Fischer’s exact test).
Age related seroprevalence to BCV and BRSV
The seroprevalence to both BCV and BRSV increased with increasing animal age (Fig. 1). The youngest age group (1–3 years old) had a significantly lower seroprevalence to both viruses than did the older cows (>3–5 years old) (P < 0.001). The mean in each of the four age clusters was 28, 46, 69, and 98 months, respectively.
Seroconversion in paired samples
Seventy-five cows in 15 of the conventional and 17 of the organic herds were sampled in both 2005 and 2006. Of the samples taken in 2005, 92% (69/75) were seropositive to BCV and 79% (59/75) to BRSV. The mean COD was 1.7 (0.53–2.62) for BCV and 1.1 (0.34–1.77) for BRSV in these samples. All the seropositive cows were seropositive at the second sampling with a mean COD of 1.6 (0.85–2.37) for BCV and 1.2 (0.22–2.25) for BRSV. All six cows seronegative to BCV in 2005 remained seronegative in 2006. Of the 16 animals seronegative to BRSV in 2005, 10 had seroconverted by 2006. These seroconverting animals were from seven different herds, five of which were organic and two of which were conventional and in which all 60 samples taken at the second sampling point were seropositive to BRSV.
Modelling of data
Results of logistic regression modelling of seroprevalence and potential risk factors are presented in Table 3, Table 4 . The odds of animals being seropositive to BCV or BRSV or both was significantly lower in organic compared to conventional herds. The odds of animals being seropositive to both BCV and BRSV was significantly higher on farms using tie-stalls and AIF.
Table 3.
Details of associations between management variables and seroprevalence to BCV, BRSV or both as estimated using a logistic regression modela
| Variablec | Level | Serological statusb, number of samples |
Results from logistic regression model |
|||
|---|---|---|---|---|---|---|
| + | − | Odds ratio (OR) | 95% CId of OR | P-value | ||
| Organic | Yes | 317 | 24 | 0.11 | 0.02;0.53 | 0.006 |
| No | 355 | 3 | Reference | |||
| AIFe | Yes | 435 | 10 | nsf | ||
| No | 237 | 17 | ||||
| Stall type | Tie | 300 | 8 | ns | ||
| Free | 372 | 19 | ||||
| Weeks at pasture | Above median | 304 | 19 | ns | ||
| Below median | 368 | 8 | ||||
| Milk yieldg | Above median | 320 | 6 | ns | ||
| Below median | 344 | 21 | ||||
| Herd sizeg | Above median | 377 | 17 | ns | ||
| Below median | 287 | 10 | ||||
| Number of veterinary visits | Above median | 343 | 19 | ns | ||
| Below median | 329 | 8 | ||||
| Year of sampling | 2005 | 326 | 12 | ns | ||
| 2006 | 346 | 15 | ||||
Clustering of observations within herds is taken into account. Pearson χ2 statistic = 149.5, df = 78.
Animals seropositive to either BCV or BRSV or to both are denoted ‘+’, those seronegative to both are denoted ‘−’.
All variables offered to the multivariable logistic regression model.
Confidence interval.
AI was performed by farm personnel rather than by professional AI technicians.
ns: not significantly (P > 0.05) associated with serological status in the logistic regression model.
One herd had missing information.
Table 4.
Details of associations between management variables and seroprevalence to both BCV and BRSV as estimated using a logistic regression modela
| Variablec | Level | Serological statusb, number of samples |
Results from logistic regression model |
|||
|---|---|---|---|---|---|---|
| + | − | Odds ratio (OR) | 95% CId of OR | P-value | ||
| Organic | Yes | 191 | 150 | 0.27 | 0.12;0.60 | 0.001 |
| No | 297 | 61 | Reference | |||
| AIFe | Yes | 315 | 130 | 2.31 | 1.08;4.95 | 0.031 |
| No | 173 | 81 | Reference | |||
| Stall type | Tie | 243 | 65 | 2.21 | 1.02;4.78 | 0.045 |
| Free | 245 | 146 | Reference | |||
| Weeks at pasture | Above median | 211 | 112 | nsf | ||
| Below median | 277 | 99 | ||||
| Milk yieldg | Above median | 233 | 93 | ns | ||
| Below median | 247 | 118 | ||||
| Herd sizeg | Above median | 269 | 125 | ns | ||
| Below median | 211 | 86 | ||||
| Number of veterinary visits | Above median | 249 | 113 | ns | ||
| Below median | 239 | 98 | ||||
| Year of sampling | 2005 | 233 | 105 | ns | ||
| 2006 | 255 | 106 | ||||
Clustering of observations within herds is taken into account. Pearson χ2 statistic 302.4, df = 76.
Animals seropositive to both BCV and BRSV are denoted ‘+’, all other animals are denoted ‘−’.
All variables offered to the multivariable logistic regression model.
Confidence interval.
AI was performed by farm personnel instead of by professional AI technicians.
ns: not significantly (P > 0.05) associated with serological status in the logistic regression model.
One herd had missing information.
Discussion
The results of this study confirm that BCV and BRSV are endemic in dairy cattle in southern Sweden (Elvander, 1996, Tråvén et al., 1999, Hägglund et al., 2006), as they are in most countries with intensive milk production systems (Paton et al., 1998). Although the conventional and organic herds included in this study were similar in many aspects, organic herds had a significantly lower seroprevalence to BCV and BRSV. One explanation for this finding is that conventional herds are at increased risk of such infections as many may have lower biosecurity levels than their organically managed counterparts.
Farm, animal and environmental factors such as stocking density, climate, plane and quality of nutrition and pregnancy status have all been proposed as risk factors for BCV and BRSV infection (Baker et al., 1986, Clark, 1993, Smith et al., 1998). The trading of animals between organic farms is closely regulated so that such premises can only purchase cows from other organic farms and the quarantine of such animals prior to their introduction is also recommended (KRAV, 2007). Such practices could reduce the risk of direct transmission (Wellemans, 1990, Saif, 2004). However, since indirect contact is considered more important in the transmission of both BCV and BRSV (Norström et al., 2000, Tråvén, 2000), further studies will be required to substantiate this hypothesis.
Both veterinarians and AI technicians may play a role in the transmission of BCV and BRSV, especially in large herds which are typically visited more frequently by both (Wellemans, 1990, Tråvén et al., 1993, Norström et al., 2000). However, the results of our study did not indicate such a link and, in contrast, herds using AIF had a higher seroprevalence than herds using professional AI technicians. A similar lack of association between seroprevalence and the total number of visitors on-farm was reported by Hägglund et al. (2006). It is likely that biosecurity measures undertaken by visiting AI technicians, including disinfection and changes of clothing and footwear between herds, and farmer education on the importance of biosecurity all contributed to the lower seroprevalence. Interestingly, one herd had a high number of visits by members of the public, including groups of school children. This organic herd had a low seroprevalence to BRSV, only one positive 5-year-old cow out of 15 animals sampled, suggesting that contacts unrelated to the cattle industry were not a transmission risk.
The increased risk of BCV and BRSV infection on farms practicing the tying of cows in stalls has previously been reported for BCV infection (Smith et al., 1998, Hägglund et al., 2006). In such a system animals are more confined and the stocking density is higher, which may lead to increased handling of cattle by visitors associated with the dairy industry, facilitating indirect inter-herd transmission.
Seropositive animals were also positive at the second sampling time-point and no conversion to seronegativity was observed. This is in line with previous data and indicates that antibodies are detectable for several years following infection with these viruses (Elvander, 1996, Tråvén, 2000). Seroconversion to BRSV was found in some herds, where all animals sampled on the second occasion were seropositive. This finding supports previous observations that following introduction of BCV and BRSV to a herd, most animals seroconvert within a short time period (Alenius et al., 1991, Tråvén, 2000, Hägglund et al., 2006). The fact that in five of the organic herds, only cows in their fourth or later lactation were seropositive to BRSV, suggests such herds can remain free from this infection for several years.
The finding of a significantly lower seroprevalence to BCV and BRSV in 1-3 year old animals relative to animals in the >3–5 year old category, indicates an age-dependent seroprevalence to these viruses. The likelihood of animal seropositivity to both these viruses in either conventional or organic herds gradually increases with age until all cows in the oldest cluster (>7 years old) were seropositive. The finding that mean antibody levels against BCV and BRSV in the oldest cluster were also the highest relative to the other age clusters suggests the oldest cows may have the highest colostral levels of antibodies against BCV and BRSV, increasing the protective value of colostrum from such animals.
The seroprevalences measured in this study corresponded to the incidence of the viral infections over a period of several years, whereas the risk factor information may only be applicable over the more restricted time periods when they were collected. However, a less precise definition of risk factor would reduce the possibility of finding significant associations as such misclassifications are not likely to depend on the type of herd. An alternative approach would be to perform a risk factor analysis based only on data from young cows, where risk factors and seroprevalence are more synchronised. The small sample size, especially of young animals, did not allow for such an approach. However the main conclusion regarding seroprevalence and herd type was supported by the findings in the youngest group of cattle.
Although misclassification of animals due to diagnostic test sensitivity and specificity may affect seroprevalence data, this would not differ between the two herd types under study. The use of young sentinel animals to determine the serological status of herds to BVDV has been found effective (Houe, 1992) and is used in the Swedish National Control Programme for this virus (Lindberg and Alenius, 1999). The repeated sampling of as few as three calves per herd has been used to monitor the herd incidence of BCV and BRSV infection (Hägglund et al., 2006). Of the cows born on the farms in our study, younger animals were typically seronegative and older animals seropositive, confirming the existence of long-lasting antibody responses to these infections. Thus serological status of the two or three youngest cows corresponded to the herd seroprevalence to these viral infections over the 2-year period preceding the sampling. Such a sampling strategy may form the basis of a rapid, economic, and non-labour intensive method of determining if herds are potentially susceptible to these infections.
Conclusions
To our knowledge this is the first survey to compare BCV and BRSV seroprevalence of cattle on organic and conventional dairy farms. Conventional herds had a significantly higher mean antibody prevalence to BCV and BRSV than organically managed herds, and increased animal age was significantly associated with increased antibody prevalence. The findings suggest that organic farm management may be effective in reducing the seroprevalence of these viruses relative to conventional farming methods.
Conflict of interest statement
None of the authors of this paper has a financial relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Mrs. M. Hjort, participating farmers for interest and support, and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) and the Swedish Farmers’ Foundation for Agricultural Research (SLF) for financial support.
References
- Alenius S., Niskanen R., Juntti N., Larsson B. Bovine coronavirus as the causative agent of winter dysentery: serological evidence. Acta Veterinaria Scandinavica. 1991;32:163–170. doi: 10.1186/BF03546976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.C., Ames T.R., Markham R.J.F. Seroepizootiologic study of bovine respiratory syncytial virus in a dairy herd. American Journal of Veterinary Research. 1986;47:240–245. [PubMed] [Google Scholar]
- Clark M.A. Bovine coronavirus. British Veterinary Journal. 1993;149:51–70. doi: 10.1016/S0007-1935(05)80210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvander M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. Veterinary Record. 1996;138:101–105. doi: 10.1136/vr.138.5.101. [DOI] [PubMed] [Google Scholar]
- Elvander M., Edwards S., Näslund K., Linde N. Evaluation and application of an indirect ELISA for the detection of antibodies to bovine respiratory syncytial virus in milk, bulk milk, and serum. Journal of Veterinary Diagnostic Investigation. 1995;7:177–182. doi: 10.1177/104063879500700202. [DOI] [PubMed] [Google Scholar]
- Fall, N., Gröhn, Y., Forslund, K., Essén-Gustafsson, B., Niskanen, R., Emanuelson, U., in press. An observational study on metabolic profiles in early lactation in Swedish organically and conventionally managed dairy cows. Journal of Dairy Science, in press. [DOI] [PubMed]
- Hägglund S., Svensson C., Emanuelson U., Valarcher J.F., Alenius S. Dynamics of virus infections involved in the bovine respiratory disease complex in Swedish dairy herds. The Veterinary Journal. 2006;172:320–328. doi: 10.1016/j.tvjl.2005.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägglund S., Hjort M., Graham D.A., Ohagen P., Törnquist M., Alenius S. A six-year study on respiratory viral infections in a bull testing facility. The Veterinary Journal. 2007;173:585–593. doi: 10.1016/j.tvjl.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houe H. Serological analysis of a small herd sample to predict presence or absence of animals persistently infected with bovine viral diarrhoea virus (BVDV) in dairy herds. Research in Veterinary Science. 1992;53:320–323. [PubMed] [Google Scholar]
- KRAV, 2007. KRAV regler (Standards for organic production) 2007, Uppsala. <http://standards.krav.se/> (accessed 1.03.07.).
- Liang K.Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrica. 1986;73:13–22. [Google Scholar]
- Lindberg A.L.E., Alenius S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Veterinary Microbiology. 1999;64:197–222. doi: 10.1016/s0378-1135(98)00270-3. [DOI] [PubMed] [Google Scholar]
- Norström M., Skjerve E., Jarp J. Risk factors for epidemic respiratory disease in Norwegian cattle herds. Preventive Veterinary Medicine. 2000;44:87–96. doi: 10.1016/s0167-5877(99)00113-0. [DOI] [PubMed] [Google Scholar]
- Paton D.J., Christiansen K.H., Alenius S., Cranwell M.P., Pritchard G.C., Drew T.W. Prevalence of antibodies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. Veterinary Record. 1998;142:385–391. doi: 10.1136/vr.142.15.385. [DOI] [PubMed] [Google Scholar]
- Saif L.J. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Revue Scientifique et Technique. Office International des Epizooties. 2004;23:643–660. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- Schrijver R.S., Langedijk J.P.M., VanderPoel W.H.M., Middel W.G.J., Kramps J.A., VanOirschot J.T. Antibody responses against the G and F proteins of bovine respiratory syncytial virus after experimental and natural infections. Clinical and Diagnostic Laboratory Immunology. 1996;3:500–506. doi: 10.1128/cdli.3.5.500-506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.R., Fedorka-Cray P.J., Mohan R., Brock K.V., Wittum T.E., Morley P.S., Hoblet K.H., Saif L.J. Epidemiologic herd-level assessment of causative agents and risk factors for winter dysentery in dairy cattle. American Journal of Veterinary Research. 1998;59:994–1001. [PubMed] [Google Scholar]
- Spaan W., Cavanagh D., Horzinek M.C. Coronaviruses: structure and genome expression. Journal of General Virology. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Stott E.J., Taylor G. Respiratory syncytial virus: brief review. Archives of Virology. 1985;84:1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- Tråvén, M., 2000. Winter dysentry caused by bovine coronavirus: no rule without an exception. Thesis. University of Agricultural Sciences, Uppsala, Sweden.
- Tråvén M., Sundberg J., Larsson B., Niskanen R. Winter dysentery diagnosed by farmers in dairy herds in central Sweden: incidence, clinical signs and protective immunity. Veterinary Record. 1993;133:315–318. doi: 10.1136/vr.133.13.315. [DOI] [PubMed] [Google Scholar]
- Tråvén M., Björnerot L., Larsson B. Nationwide survey of antibodies to bovine coronavirus in bulk milk from Swedish dairy herds. Veterinary Record. 1999;144:527–529. doi: 10.1136/vr.144.19.527. [DOI] [PubMed] [Google Scholar]
- Tråvén M., Näslund K., Linde N., Linde B., Silván A., Fossum C., Hedlund K.O., Larsson B. Experimental reproduction of winter dysentery in lactating cows using BCV: comparison with BCV infection in milk-fed calves. Veterinary Microbiology. 2001;81:127–151. doi: 10.1016/S0378-1135(01)00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valarcher J.F., Taylor G. Bovine respiratory syncytial virus infection. Veterinary Research. 2007;38:153–180. doi: 10.1051/vetres:2006053. [DOI] [PubMed] [Google Scholar]
- Van der Poel W.H.M., Brand A., Kramps J.A., Van Oirschot J.T. Respiratory syncytial virus-infections in human-beings and in cattle. Journal of Infection. 1994;29:215–228. doi: 10.1016/s0163-4453(94)90866-4. [DOI] [PubMed] [Google Scholar]
- Van der Poel W.H.M., Mourits M.C.M., Nielen M., Frankena K., Van Oirschot J.T., Schukken Y.H. Bovine respiratory syncytial virus re-infections and decreased milk-yield in dairy cattle. Veterinary Quarterly. 1995;17:77–81. doi: 10.1080/01652176.1995.9694537. [DOI] [PubMed] [Google Scholar]
- Wellemans G. Bovine respiratory syncytial virus. In: Dinter Z., Morein B., editors. Virus Infections of Ruminants. Elsevier; Amsterdam: 1990. pp. 363–375. [Google Scholar]


