Abstract
Diseases of the urinary tract are reviewed, covering infectious (bacterial, viral, parasitic), degenerative, congenital, metabolic, nutritional, neoplastic, obstructive, and toxic causes. Some clinical presentations and diagnostic procedures are described for ferrets, rabbits, guinea pigs, hamsters, mice, rats, chinchillas, hedgehogs, and sugar gliders, as well as therapies.
Keywords: Small mammals, Pathology, Kidney, Urinary bladder, Tumors, Renal, Uroliths, Viral infections
Key points
-
•
There are some species-specific disease conditions and anatomic structures of the urinary tract, including the composition of uroliths, an open inguinal ring in male rabbits and rodents, a cloaca in sugar gliders, and the presence of a baculum.
-
•
Hematuria is a common clinical sign for various urinary tract diseases.
-
•
Therapy for most urinary tract diseases is based on procedures common to domestic species (dogs and cats).
Introduction
The anatomy of the kidney is conserved among mammals. The kidneys are located within the sublumbar peritoneal space, with the right kidney positioned cranially to the left. In female rodents, there is a separate external orifice for the urinary tract and the reproductive tract. In male and female sugar gliders (Petaurus breviceps), the urinary tract terminates into a cloaca.1 Male chinchillas (Chinchilla lanigera), guinea pigs (Cavia porcellus), rats (Rattus norvegicus), mice (Mus musculus), hamsters, gerbils, and ferrets (Mustela putorius furo) have an os penis or baculum.
There are a few histologic differences of the kidney, including the number of papilla and length of the nephrons. Most differences are related to the natural environment of the animal. These differences are noted between desert-dwelling species requiring optimal ability to concentrate urine, and other species in which water deprivation is not common. Of clinical significance is the specific gravity of urine in desert-dwelling species, which is generally higher.2
Ferret
Common Problems
The most common problems affecting the ferret urinary system described in the literature are Aleutian disease of the kidney, renal tumors, renal cysts, urolithiasis, and bacterial cystitis. In one author’s database (DRR), hydronephrosis, nephritis (all causes), and renal mineralization are the most common lesions of submitted kidneys.
Infectious/inflammatory
Aleutian disease is caused by a parvovirus.3 The lesions are immune mediated and vary in severity. Gross lesions may be minimal and nonspecific. Emaciation and organ enlargement can be seen. In the kidney, membranous glomerulonephritis is common and a lymphocytic-plasmacytic inflammatory infiltrate is seen in the interstitium. This infection is uncommon in ferrets and the mortality is generally low.
Systemic coronavirus can also affect the kidneys, and the primary lesion is granulomatous inflammation.4 In a study showing ultrasonography results in ferrets with systemic coronavirus, nephromegaly was found in 4 out of 11 patients.5
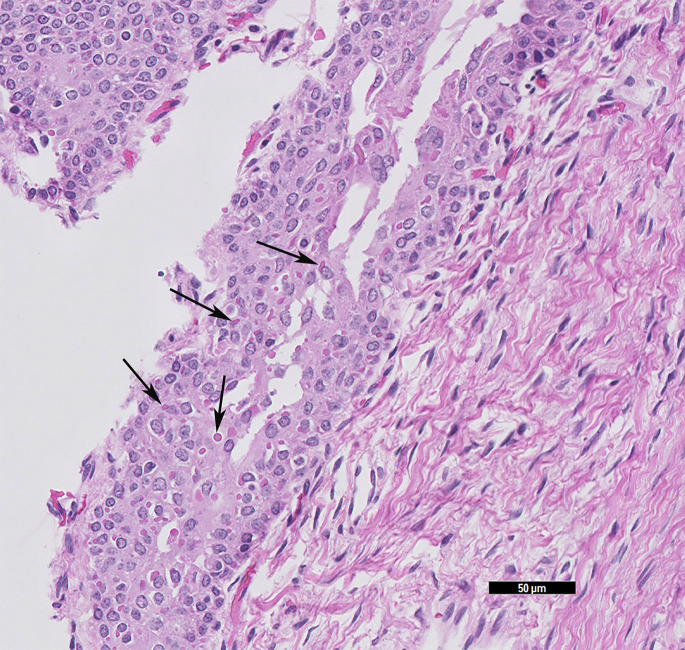
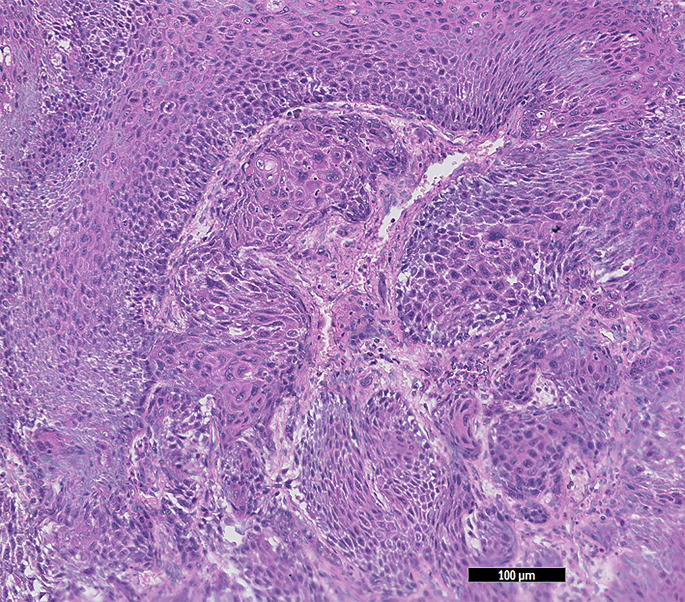
Canine distemper is essentially 100% fatal. Affected ferrets present with neurologic clinical signs, bronchopneumonia, hyperkeratosis of the planum nasale and footpads, and a papular rash starting on the chin. However, the most productive tissues to evaluate for viral inclusions are the urinary bladder, renal pelvis, and biliary epithelium, and in suspected cases these tissues should be submitted (Fig. 1 ). Immunohistochemistry can be applied for confirmation.
Fig. 1.
Ferret (M putorius furo) urinary bladder with intracytoplasmic inclusions of a canine distemper infection. Confirmed with immunohistochemistry. Black arrows indicate a few of the intracytoplasmic eosinophilic viral inclusions (HE stain, 20× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Bacterial nephritis and pyelonephritis occur in ferrets and can be severe. These conditions are thought to be caused by ascending infections of the lower urinary tract. A variety of organisms can be the cause. Grossly, the kidney may be abscessed and histologically there is necrosis and a neutrophilic infiltrate. Organisms may be seen but culture is necessary for a definitive diagnosis. Bacterial infection of the kidney, prostate, or urinary bladder can result in inflammatory cells (often undergoing degeneration) and bacteria in the urine.
Degenerative/congenital
Nephrosis is considered a degenerative disease of geriatric ferrets, and is often an incidental finding.6 Nephrosis is characterized by damage of tubular epithelial cells. It is a nonspecific lesion that can have many causes, including ischemia, toxic exposure, and shock.
Nephrocalcinosis is reported more frequently in European pet ferrets than laboratory ferrets, and lesions include calcium deposition in the renal tubules.7 The cause of renal mineralization is usually not determined. Soft tissue mineralization is usually considered dystrophic (secondary to tissue injury) or metastatic (associated with excessive calcium in the blood), but neither cause is obvious in most cases seen at necropsy. The lesion is usually in the renal papilla. Rare cases of lymphoma-associated hypercalcemia have also resulted in renal dystrophic mineralization. The mechanism is most likely azotemia and the nephrotoxic effects of prolonged hypercalcemia.8
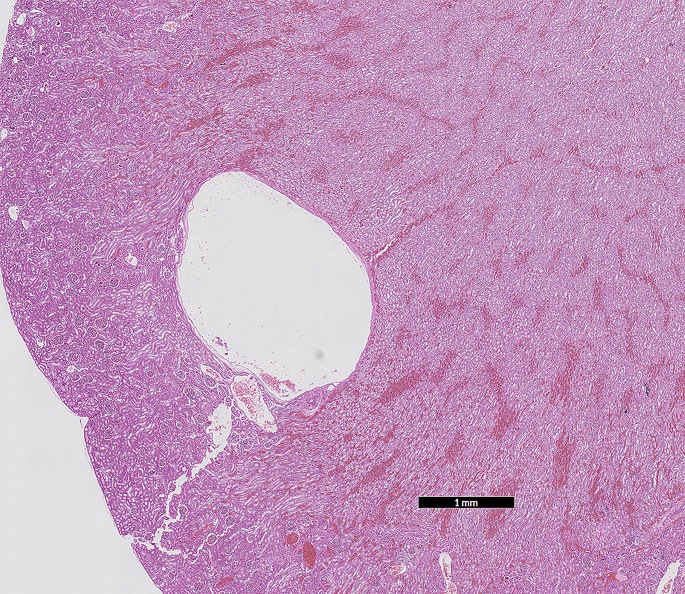
Renal cysts are a common and usually incidental finding in the ferret (Fig. 2 ). A 17-year retrospective analysis of 54 ferrets with cystic kidney disease noted that 69% had renal cysts, and 26% primary polycystic disease.9 Cysts may be single or multiple and may be present in the cortex of 1 or both kidneys. It is speculated that acquired cysts arise from gradual distention of the nephron, caused by obstruction by exudates or fibrosis tissue, although the cause is seldom determined. Rare cases of true polycystic disease have been reported in the ferret. This congenital lesion may result in markedly enlarged cystic kidneys that fill the posterior abdomen. Many small cysts throughout the cortex and medulla characterize polycystic kidneys.
Fig. 2.
Renal cysts within the cortex of the ferret (M putorius furo) kidney (HE stain, 1× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Another rare congenital lesion is an extramural ectopic ureter. This condition is the most common congenital cause of urinary incontinence in domestic canines. Young ferrets present with urinary incontinence and urine scalding around perineal and inguinal areas. The ectopic ureter can be visualized via excretory urography. In 1 case, nephroureterectomy was chosen instead of ureteral transplant. The ureter progressed extramurally to enter the distal urethra, caudal to the urethral sphincter, or vagina (on contrast).10
Neoplastic
The most common renal neoplasm is malignant lymphoma. Lymphoma usually involves multiple organs and can be severe in the kidney. Grossly, there is variable replacement and distortion of the kidney. Histology is typical, with a diffuse monomorphic sheet of lymphoid cells. Impression smears can often provide a diagnosis. Other neoplasms have been reported, although primary tumors of the urinary tract system are uncommon.11 From 1 study of 574 ferret tumors, only 6 urinary tumors were recognized. There were 3 renal carcinomas and 3 unspecified renal tumors.11 Another study evaluating 856 ferret tumors identified only 1 renal tumor listed as a sarcoma, not otherwise specified, and lymphoma involving the kidney.12 One renal adenocarcinoma is described in detail collected from an adult neutered female. This pleomorphic renal adenocarcinoma had multiple metastases to the lung, liver, greater omentum, right renal pelvis, and systemic lymph nodes. The tumor cells were pleomorphic with a large number of giant cells and arranged in tubular and cystic patterns. It was confirmed of renal origin by immunohistochemistry with strong positive CD10 and cytokeratin.13 Two cases of transitional cell carcinoma arising from the renal pelvis, with 1 completely replacing the kidney, have been reported. The presenting complaint was hematuria.14 , 15
Obstructive disorders
Urolithiasis
The clinical signs of urolithiasis include stranguria and dysuria. Uroliths in ferrets commonly consist of sterile struvite or magnesium ammonium phosphate. Based on 1 study evaluating 272 cases, neutered males have a significantly increased risk of developing sterile struvite urolithiasis.16 These uroliths are more likely to be retrieved from the lower urinary tract than from the upper urinary tract.16 Stone formation is suspected to be caused by a vegetable-based protein diet that increases urine pH, leading to struvite stone formation.17
Cystine uroliths are uncommon, with a reported incidence of 16% in 1 study evaluating 70 cases.18 Again, males are predisposed to urolithiasis, possibly because the distal portion of the urethra has a J-shaped bend with a decreased diameter of the urethra. All cystine uroliths have been found in the lower urinary tract.18 The uroliths are ovoid and smooth, light yellow to tan, and range in number from 1 to more than 100. Cystine uroliths are composed of nonessential sulfur-containing amino acid; there may be a familial cause based on studies in other mammals.18
Calcium oxalates are the next most common type of urolith.19
Prostatic disease
Other causes of urinary obstruction in the male ferret are abnormalities of the prostate caused by adrenocortical disease. Males can develop prostatic hyperplasia, cysts, and abscesses. Prostatic enlargement is caused by increased levels of the hormones estradiol, testosterone, and 17-hydroxyprogesterone produced by the neoplastic adrenal cortex from an adrenal gland tumor. Some animals develop concurrent urinary tract infection, including small urethral or cystic calculi. Urethral blockages can also be caused by sloughed proteinaceous debris from squamous metaplasia of prostatic tissue and by small struvite calculi, which develop from the increase in pH caused by bacterial cystitis.20
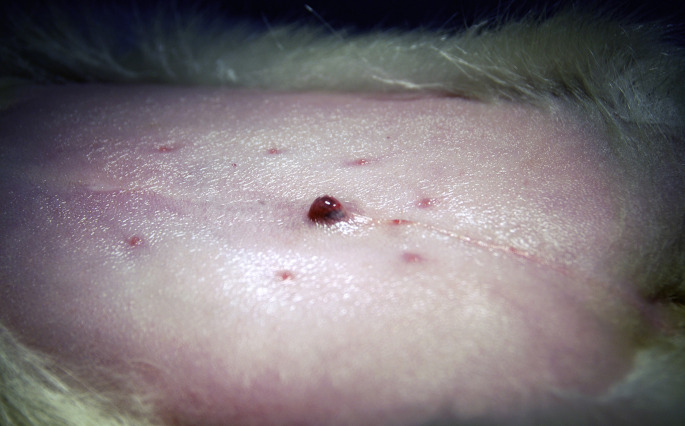
Preputial tumors in male ferrets can also result in urethral obstruction. These aggressive tumors are poorly responsive to both surgery and radiation therapy. Nevertheless, wide surgical resection has been recommended, although the procedure may necessitate partial or total penile resection (Fig. 3 ).21
Fig. 3.
Preputial tumor on a ferret (M putorius furo), prepped for surgical removal.
(Courtesy of S. A. Kelleher, DVM, Deerfield Beach, FL).
Treatment
Any disease of the kidneys can result in renal insufficiency or failure. Symptoms may include polyuria/polydipsia (PU/PD), weight loss, weakness, nausea, and decreased appetite, as can be seen in other traditional pet species. Physical examination findings are also consistent and often include dehydration, although hydration status can be challenging to determine by testing skin turgor in patients experiencing rapid weight loss. Some clinicians have anecdotally reported halitosis and oral ulcers in association with renal failure, but this is poorly documented.
Treatment protocols are based on those for traditional pet species, and include fluid diuresis for renal insufficiency and failure, antibiotic therapy for bacterial nephritis, and basic surgical approaches to the kidney.
Treatment of urinary tract disease follows guidelines established for traditional pet species, such as treatment of bacterial disease based on results of culture and sensitivity. General principles for treatment of renal insufficiency and renal failure are described later in this article. Treatment of adrenocortical disease is well described in any current literature about ferret endocrine diseases. Large associated prostatic cysts have been managed with marsupialization or omentalization of the cyst.20
Successful nephrotomy for removal of unilateral renoliths has been reported in the ferret.22
Rabbit
Common Problems
The most common causes of renal disease reported in the literature are Encephalitozoon cuniculi, chronic renal failure in older rabbits (Oryctolagus cuniculus), and urolithiasis. Other renal conditions seen in rabbits include bacterial nephritis, hydronephrosis, and papillary necrosis with renal mineralization.
Infectious/inflammatory
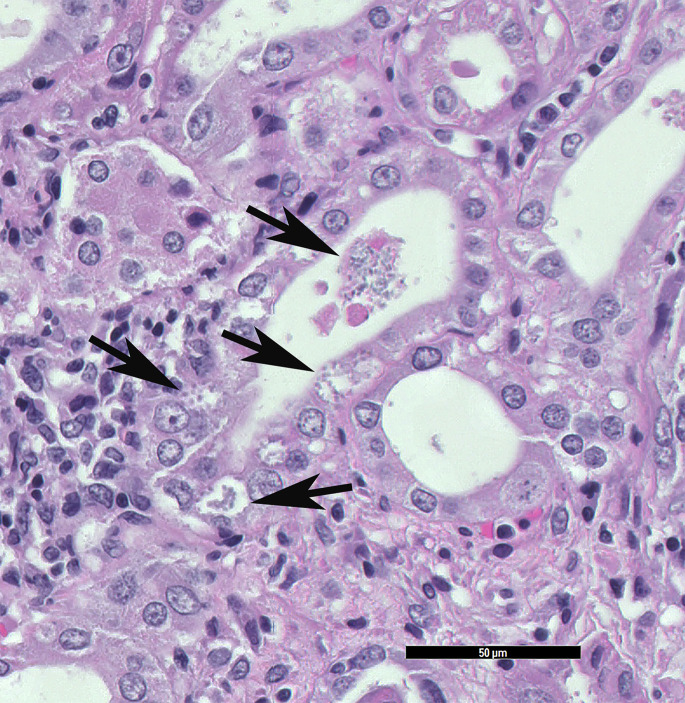
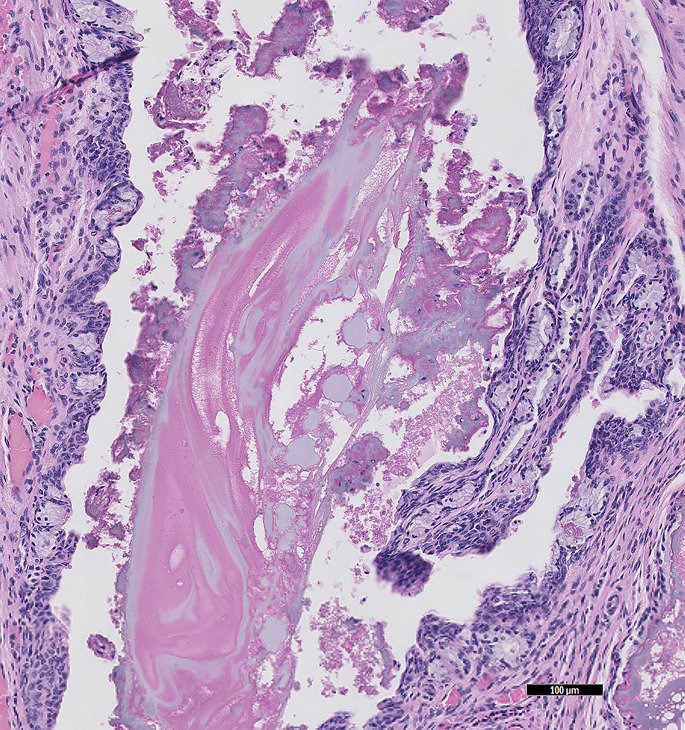
The most common renal disease of rabbits is infection caused by E cuniculi.23, 24, 25 Although disease is often occult, rabbits with severe E cuniculi–associated renal disease may present with PU/PD and a host of other nonspecific signs, such as decreased appetite and weight loss, dehydration, and lethargy. In some rabbits, only renal changes are seen at necropsy. Grossly, there may be no lesions or the kidneys may be pitted. On histology, there is a chronic active lesion, which may be granulomatous or pyogranulomatous. Aggregates of organisms are found rarely in or around tubules as small, basophilic structures (Fig. 4 ).
Fig. 4.
E cuniculi microbes are within the tubular epithelial cells and these are sloughing into the lumen of the tubules. The black arrows indicate intratubular and intraepithelial densely packed microbes, which are small round/oval amphophilic structures. Samples from a dwarf rabbit (O cuniculus) (HE stain, 40× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Rare cases of polypoid cystitis and urethritis have been described in rabbits. The masses are white to transparent, spheroidal masses protruding into the lumen. On histology, the polyps are lined by variably hyperplastic to attenuated transitional epithelium over cores of edematous inflamed fibrovascular stroma. There may be transitional epithelium lining cysts within the stroma. These polyps are suspected to arise from a combination of inflammation and a hyperplastic reaction to chronic irritation of the urinary mucosa. Bacterial isolates have included Proteus and Enterococcus spp. The clinical signs associated with polypoid cystitis include perineal scalding, urinary sludge, and possibly hematuria.26 Hematuria is frequently described as a clinical sign with obstructive urolithiasis, cystitis, bladder polyps, and pyelonephritis.27 Red urine in rabbits may not necessarily be hematuria but may be the more common, and normal, porphyrin pigments. Urine dipstick is an easy method to differentiate porphyrinuria from hematuria.
Degenerative/congenital
Chronic renal disease or end-stage kidney disease seem to be common in older rabbits. Rabbits with renal failure can have lesions similar to those seen in old rats. The kidneys are grossly scarred and histologically there is marked interstitial fibrosis and glomerulosclerosis. This condition may be a sequela of encephalitozoon infection, but the cause is usually not determined.
Renal cysts are uncommon in rabbits. In a few reports, some cases were speculated to be inherited and to resemble human polycystic kidney disease.28 , 29 In experimental studies, methylprednisolone acetate has been injected into newborn rabbits to induce polycystic kidneys.30 The few cases of renal cysts (7 out of 796 rabbit renal submissions) in 1 author’s database (DRR) have been in older rabbits (>5 years) and associated with lesions of chronic renal disease.
Neoplasia
Tumors of the urinary tract are rare in rabbits, with most involving the kidney. These tumors include benign embryonal nephroma, renal carcinoma,31 renal adenocarcinoma, nephroblastoma (Fig. 5 ), and kidney hamartoma. A case of renal and ureteral transitional cell carcinoma produced hydronephrosis.32 A urinary bladder transitional cell carcinoma in a female rabbit presented with acute hematuria, partial vaginal prolapse, and inappropriate urination. The gross appearance was a nodular cystic mass at the cranial central apex of the urinary bladder.33
Fig. 5.
Rabbit (O cuniculus) nephroblastoma is effacing the kidney (HE stain, 10× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Obstructive disorders
Urolithiasis and urinary sludge
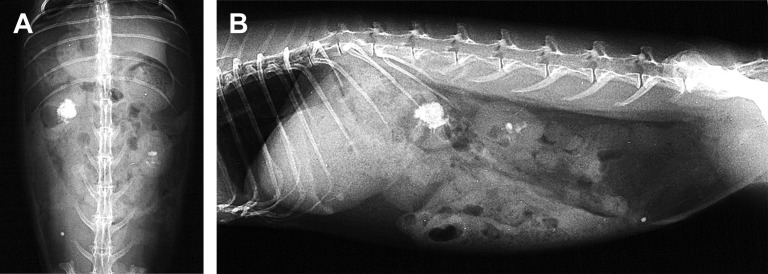
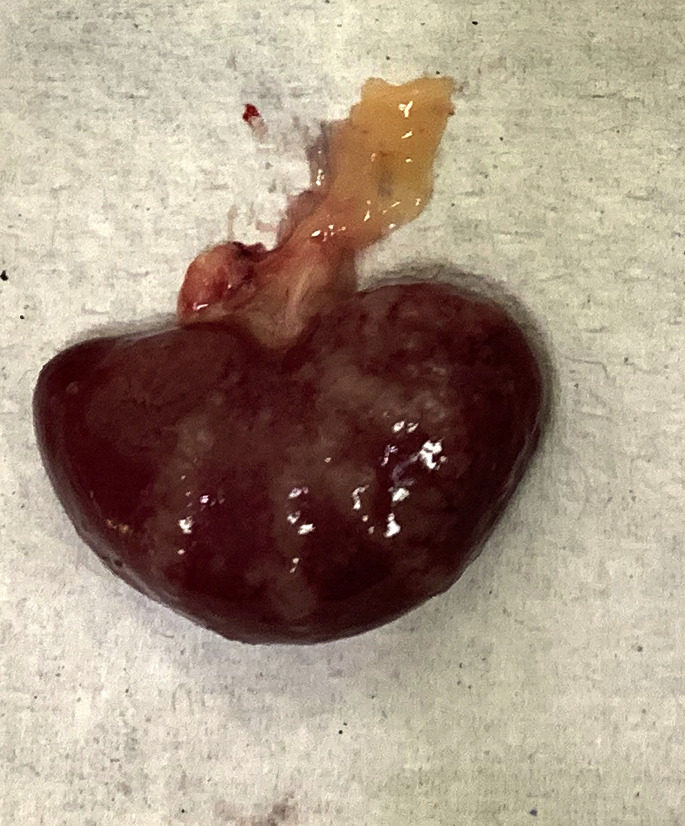
Uroliths are described in the kidney and urinary bladder of rabbits. Renoliths can be mild with few stones, or severe and produce renomegaly and complete destruction the kidney (Fig. 6 ).
Fig. 6.
(A) Bilateral nephrolithiasis in a rabbit (O cuniculus). The radiographs highlight the radiodense materials within the pelvis of the both kidneys. (B) Lateral view.
(Courtesy of A. Lennox, DVM, DABVP (Avian Practice, Exotic Companion Mammal Practice), Diplomate ECZM (Small Mammal), Indianapolis, IN).
Uroliths and urinary sludge in rabbits are usually composed of various calcium salts, predominately calcium carbonate. From a study evaluating more than 100 rabbit uroliths, the most common were calcium carbonate (69.4%), compound (23%), and mixed (3.3%).19 Radiographs are usually confirmatory because calcium carbonate is radiopaque (Fig. 7 ). The predominance of calcium-based uroliths is most likely caused by the unique calcium metabolism of rabbits. Excess dietary calcium is absorbed unregulated, resulting in increased plasma calcium concentrations and excretion of excess calcium in the urine. Therefore, serum and urinary calcium levels are related to dietary intake. Urinary calcium can bind with other constituents when conditions are met for crystallization, such as dehydration, changes in pH, and urine retention, to form both the sludge (which can appear as thick white to yellow material) and uroliths. Abnormal accumulations of calcium (sludge) are more common in older rabbits, possibly because of decreased mobility or water consumption.34 There are rare reports of uroliths composed of silica, struvite, and calcium sulfate dehydrate or gypsum.35 The gypsum urolith was associated with the rabbit eating gypsum-based plaster that was covered by a white paint containing barium sulfate.
Fig. 7.
Uroliths in the bladder can be of variable size and shape; although many are smooth, this urolith contained sharp points. The rabbit (O cuniculus) presented with anorexia and hunched posture, suggesting discomfort.
(Courtesy of A. Lennox, DVM, DABVP (Avian Practice, Exotic Companion Mammal Practice), Diplomate ECZM (Small Mammal), Indianapolis, IN).
Hernia
Hernias can be classified by their anatomic location (body cavities or external, such as inguinal or femoral), cause (true hernia [through an anatomic structure and surrounded by peritoneum] or false hernia [traumatic through induced defects]), and contents.
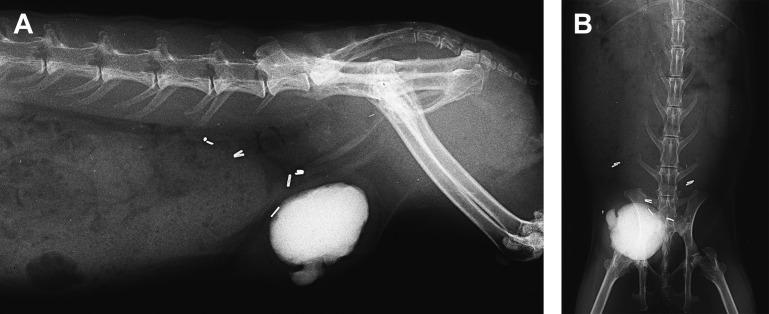
Inguinal or scrotal hernias develop when abdominal contents, such as the urinary bladder, enter the inguinal canal or scrotum through a wide or defective inguinal canal. In male rabbits, the inguinal rings are open throughout life, increasing the risk of herniation. The typical appearance of bladder herniation is a fluid-filled mass in the scrotum and no bladder palpable in the abdomen. Some cases have concurrent lesions of urinary sediment and multiple uroliths; 1 case featured a testicular tumor on the opposite side.36 Inguinal herniation of the bladder seems uncommon in female rabbits (Fig. 8 ). Trauma was suspected to be the cause in 1 case of a female rabbit presenting with hematuria.37
Fig. 8.
Inguinal herniation of the bladder in a female rabbit (O cuniculus). The bladder is filled with microuroliths (bladder sludge), which are acting as natural contrast. Surgery to replace the bladder and correct the herniation was successful. (A) Lateral and (B) frontal view.
(Courtesy of A. Lennox, DVM, DABVP (Avian Practice, Exotic Companion Mammal Practice), Diplomate ECZM (Small Mammal), Indianapolis, IN).
Female rabbits may also evert the urinary bladder through the urethra. Recent kindling or a history of multiple litters is common. Rabbits present with a vaginal mass, which is the mucosal surface of the everted bladder.38 Rarely, males may evert and prolapse the urinary bladder. This finding seems more likely in castrated males because penis length is reduced after castration.39
Another unusual presentation is diaphragmatic herniation of retroperitoneal fat and a kidney.40 A case of kidney herniation occurred in conjunction with a diaphragmatic lipoma.41
Treatment
General management of renal insufficiency and renal failure is described later.
Encephalitozoonosis
Treatment of E cuniculi is problematic because there is a lack of well-controlled scientific studies linking various treatment protocols with documented clearing of organisms. The problem relates to difficulties in establishing a diagnosis, and the observation that many affected rabbits recover spontaneously without treatment.42 Rabbits with severe renal disease secondary to E cuniculi may not harbor renal organisms, and treatment is focused on managing acute or chronic renal failure. Treatment of E cuniculi has relied on benzimidazoles, such as fenbendazole,42 but recently severe disease and deaths have been reported in rabbits treated with this class of drugs.43 Benzimidazoles are radiomimetic and toxicity has been observed in many species; Graham and colleagues43 recently reported deaths in 13 rabbits treated with various dosages of several drugs, including fenbendazole, albendazole, and oxibendazole. Clinical signs in rabbits were nonspecific and histopathology showed depletion of all bone marrow cell lines. For this reason, treatment should not be approached lightly, and serial monitoring of the complete blood count may be beneficial.
Surgical management of renal disease
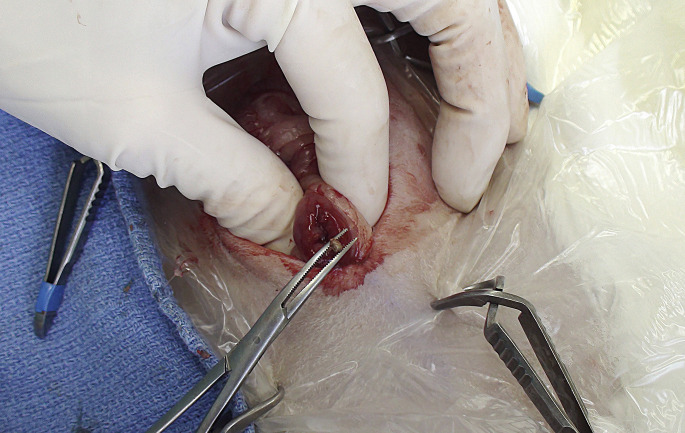
Various surgical approaches to the kidney have been described. Martorell and colleagues44 described a lateral flank approach for access to the kidney for removal of uroliths; 1 author (AML) also found this approach simple and straightforward compared with a traditional midline abdominal approach (Fig. 9 ). Nephrectomy, performed through a ventral abdominal approach, was reported to manage hydronephrosis in a pet rabbit45 and to remove a renal carcinoma.32 Cases of diaphragmatic herniation were successfully treated by replacing the herniated kidney and fat followed by diaphragmatic herniorrhaphy,40 and use of polypropylene mesh.41
Fig. 9.
Unilateral flank approach nephrotomy allowed removal of uroliths from the right kidney.
(Courtesy of A. Lennox, DVM, DABVP (Avian Practice, Exotic Companion Mammal Practice), Diplomate ECZM (Small Mammal), Indianapolis, IN).
Management of bladder sludge
Strategies for managing urinary sludge include encouraging water consumption and exercise paired with dietary modification as needed. Accumulation of sludge can be temporarily managed by bladder catheterization and flushing, or sometimes by gently agitating the bladder to manually resuspend the mineral, followed by repeated expression of urine.
Guinea pig
Common Problems
Urolithiasis is commonly reported in the guinea pig. This condition usually involves the lower urinary tract. Inflammatory, degenerative, and other conditions are also reported.
Infectious/inflammatory
Chronic nephritis, interstitial nephritis, and pyelonephritis have been reported.46 , 47 In these conditions the kidneys may be irregular grossly and the ureters may be dilated in pyelonephritis. On histology, the inflammation depends on the cause and the duration of the disease.
Cystitis in guinea pigs is common. Guinea pigs, like chinchillas and degus, have adapted to a semiarid habitat with varying, species-specific adaptations to water deprivation. However, several diseases have been linked to insufficient water intake, such as cystitis, urolithiasis, or obstipation. A decrease in urination frequency may favor bacterial infection and formation of uroliths.48 The clinical signs of bacterial cystitis include dysuria, polyuria, hematuria, lethargy, or urethral discharge. The urinary bladder may be painful on palpation, small, firm, with a thickened wall. The most common isolate in a review series of adult female guinea pigs was Escherichia coli. Relapses were common. Two guinea pigs were diagnosed with encrusted cystitis (further description in relation to hedgehogs later).49
Degenerative/congenital
Renal cysts are a rarely reported and usually incidental finding.50 Given the few reports of renal cysts in guinea pigs, it seems most cases are acquired cysts associated with chronic renal disease. One author (DRR) has seen 21 cases in adult guinea pigs (3–7 years of age), all with chronic renal lesions (fibrosing interstitial nephritis) out of 430 case submissions with kidneys (4.8%).
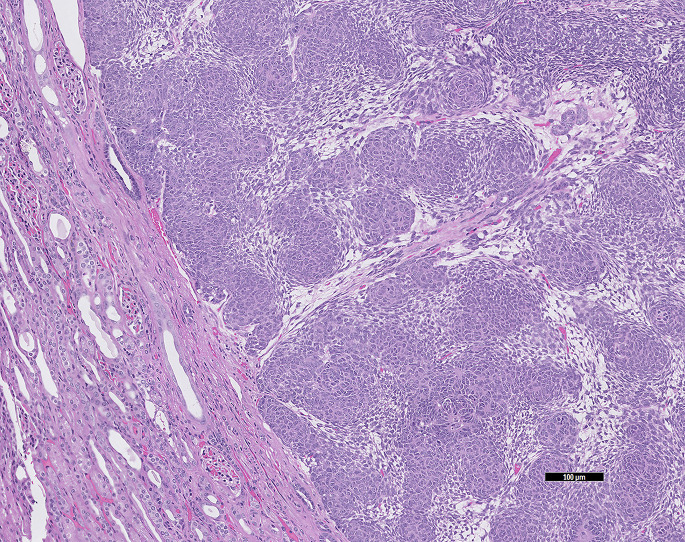
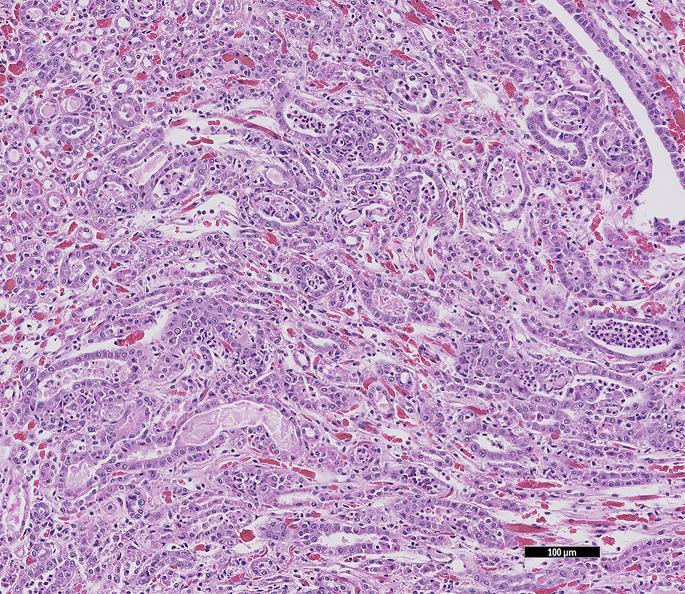
Chronic (segmental) nephrosclerosis is renal scarring of undetermined cause, which may be the result of vascular disease, infection, or immune-mediated disease. Grossly, the renal cortex is irregularly pitted and histologically there is interstitial fibrosis, variable glomerulosclerosis, tubular dilatation, and variable mononuclear inflammation (Fig. 10 ).
Fig. 10.
Gross view of chronic renal disease in a guinea pig (C porcellus). Bands of blotchy white areas radiate out from the pelvis. The histology findings were of severe diffuse fibrosing lymphoplasmacytic interstitial nephritis with periglomerular sclerosis.
(Courtesy of S. Brenner, MS, DVM, San Mateo, CA).
Renal mineralization can be incidental or associated with renal failure, resulting in uremia and soft tissue mineralization. The mechanism is complex but involves tissue death and secondary mineralization. In guinea pigs, exposure to excessive vitamin D is a common mechanism if renal failure is not present. Such exposure occurs when feeding guinea pigs a commercial diet with errors in formulation or commercial rodent diets. Commercial rodent diets are formulated with more vitamin D than is safe for guinea pigs.51 , 52
Neoplasia
Primary tumors are rare. Lymphoma occasionally involves the kidneys. Grossly, there is a diffuse white-gray infiltrate that replaces normal parenchyma. On histology, this is composed of immature lymphoid cells.
Obstructive disorders
Uroliths may be present from the renal pelvis to the urinary bladder. If in the ureter or bladder, there may be ureteral dilatation and renal swelling or shrinkage. The most common urolith is calcium carbonate, which is radiopaque.19 Similar to rabbits and other hindgut-fermenting herbivores, guinea pigs excrete excess calcium53 and they can eliminate up to 75% of their absorbed calcium via urine. Urine specific gravity and pH highly influence crystallization. Herbivore urine is commonly alkaline, which complicates dietary modification efforts to change urine pH and reduce urolith formation.54
Treatment
Strategies for treatment of acute and chronic renal failure are described later. Surgery of the kidney has not been described in pet guinea pigs.
The urethra of the female guinea pig is short and wide. Uroliths in the urethra and in the vaginal vestibulum can be visualized and removed manually with the aid of the Lone Star retractor.55
Hamster
Common Problems
Primary renal disease is uncommonly reported in pet hamsters, and mostly includes infectious and degenerative causes.
Infectious/inflammatory
Sporadic cases of bacterial cystitis and pyelonephritis are seen. Gross changes include thickening and exudation in the bladder and yellow-white foci of necrosis and inflammation in the kidney. Culture is usually necessary to establish a definitive cause.
Degenerative/congenital
The most common problem seen in the kidneys is amyloidosis, which has been reported in more than 50% of hamster necropsies in some colonies.56 , 57 Amyloid is an insoluble pathologic proteinaceous substance, deposited between cells in various tissues and organs of the body. Systemic amyloidosis can be classified as primary (amyloid light chain), secondary (amyloid associated), or familial. Secondary amyloidosis is most commonly described in animals and has been described as a reaction to diverse inflammatory stimuli. Amyloid deposits are more common in female hamsters more than 18 months of age. Deposits can also be seen in almost any other organ. Amyloidosis can be associated with nephritic syndrome.58 Grossly, the kidneys are pale and irregular. Microscopically, amyloid is eosinophilic to amphophilic and can be seen in glomeruli, interstitium, or both.
Glomerulonephropathy occurs in hamsters as well as other rodents.59 Progressive nephropathy can result in renal failure. Grossly, kidneys are pitted and variably shrunken, and histologically there may be variable stages of mesangial and glomerular capillary thickening, periglomerular sclerosis, and variable interstitial nephritis. This condition has been described in both Chinese (Cricetulus griseus) and Siberian hamsters (Phodopus sungorus).60
Neoplasia
Primary tumors are rare in hamsters. Two cases of renal adenoma have been reported in aged Siberian hamsters.59 , 61 One author (DRR) has seen 2 cases of lymphoma involving the kidney as well as 1 case of a renal adenoma out of 60 submissions that included the kidney.
Obstructive disorders
Uroliths are also uncommon in hamsters. From 1 study evaluating only 14 samples, the most common uroliths were calcium phosphate (28.6%), compound (28.6%), and calcium oxalate (21.4%).19
Treatment
Strategies for treatment of acute and chronic renal failure are described later. Surgery of the kidney has not been described in pet hamsters.
Mouse
Common Problems
Renal disease is common in mice. Some conditions are strain related, and determining the genetic heritage of a pet mouse (if possible) may be of value in diagnosis. Amyloidosis is seen primarily in older animals.
Degenerative/congenital
Chronic progressive nephropathy or chronic nephritis in mice is similar to the condition in rats and other rodents.62 , 63 Pathogenesis is unknown. Interstitial nephritis and pyelonephritis are also reported.64 , 65 Klossiella muris infection is seen primarily in wild mice.66
Grossly, kidneys affected by these conditions have an irregular pitted cortex. Microscopically, there is glomerular sclerosis, tubular dilatation and degeneration, interstitial inflammation, and fibrosis. Lesions vary in severity according to their duration. In cases of klossiellosis, organisms can usually be seen on histology and may not be associated with significant inflammation. This organism is a parasitic protozoa of the phylum Apicomplexa. Species in this genus infect the renal tract of mammals and intestinal tract of snakes. This genus of the phylum Apicomplexa is unusual in having only a single host in its life cycle.
Polycystic kidneys occur in BALB/c mice, which are used as a model for human disease. Cysts vary in size and number, but the condition is progressive and results in loss of renal function and early mortality. The renal surface is irregular and fluid-filled cysts are visible. Cysts are also obvious in cut sections. Dilated tubules are seen histologically.
Neoplasia
Renal neoplasia occurs sporadically. Malignant lymphoma can efface the kidney and carcinomas are occasionally seen. Gross lesions are not specific. On histology, lymphoma is typical and carcinomas are usually composed of poorly differentiated cells with a loss of organized structure.
Obstructive disorders
Hydronephrosis can be unilateral or bilateral. Unilateral hydronephrosis is often an incidental necropsy finding and may be strain related or secondary to obstruction or inflammation of the renal pelvis. It is usually progressive and grossly there is variable dilatation of the renal pelvis with loss of renal parenchyma. One cause of obstructive hydronephrosis in male mice, as well as in rats and guinea pigs, is the retrograde movement of seminal plugs in the urethra (Fig. 11 ). These seminal vesicle secretions may coagulate and become impacted in the urethra and urinary bladder.67
Fig. 11.
Seminal plugs within the lumen of the urethra in a male guinea pig (C porcellus). The acellular coagulum is within the lumen. (HE stain, 20× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Rat
Common Problems
Common renal conditions reported include hydronephrosis, polycystic kidneys,68 and pyelonephritis, as well as neoplasms.69, 70, 71
Infectious/inflammatory
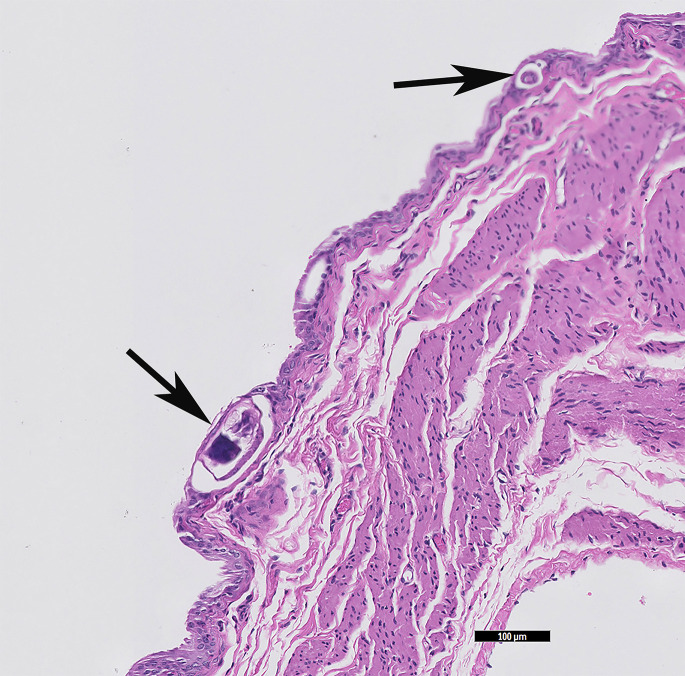
Trichosomoides crassicauda is a nematode that affects the urinary tract of rats. Adult worms can be seen in the renal pelvis and urinary bladder, but the condition is usually asymptomatic. Eggs are passed in the urine. Nematodes are present in the epithelium and there is variable inflammation (Fig. 12 ).72
Fig. 12.
T crassicuada, the rat (R norvegicus) urinary bladder nematode can be found in the lumen as well as in the mucosal epithelium (black arrows). (HE stain, 10× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Degenerative/congenital
The most common renal disease in rats is chronic progressive nephropathy or old rat nephropathy.73, 74, 75 The incidence can be up to 75% in affected strains. This disease affects older animals and is more common and severe in males, with a much higher incidence in S-D and Fischer 344 rats. High-protein diets are a factor in pathogenesis, as are immunologic factors, such as mesangial deposition of immunoglobulin M, and high levels of prolactin. Lesions of progressive nephropathy are characterized by irregular and pitted renal cortices. The cut surface is also irregular. Microscopic changes vary with duration. Glomeruli are thickened, tubules are dilated and may contain proteinaceous fluid, and there is interstitial fibrosis and inflammation.
Nephrocalcinosis is seen, usually as an incidental finding but also with diets low in magnesium and/or high in calcium and phosphorous. Mineral is deposited within the interstitium and tubules. Grossly, there are pale streaks and, histologically, basophilic mineral deposits are seen.
Neoplasia
Spontaneous tumors of the urinary tract are uncommon in rats. Tumors reported in the kidney include renal cell adenoma, lipoma/liposarcoma, renal cell carcinoma (with metastasis to the lung and liver), and a nephroblastoma with metastasis to the lung and local lymph nodes. Transitional cell carcinoma of the urinary bladder also occurs spontaneously and in 1 case there was metastasis to the lungs.70 , 71 , 76
Obstructive disorders
Uroliths in rats are generally struvite (80.4%) with fewer calcium phosphate (5.9%).19
Chinchillas
Common Problems
Primary renal diseases are poorly described in chinchillas. Urolithiasis has been described, and, although rarely reported, suppurative nephritis is common in younger animals (Fig. 13 ). E coli has frequently been isolated based in 1 author’s experience (DRR).
Fig. 13.
Histology of a suppurative tubular nephritis in a chinchilla (C lanigera). A bacterial culture collected at the time of necropsy isolated E coli. (HE stain, 10× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Obstructive disorders
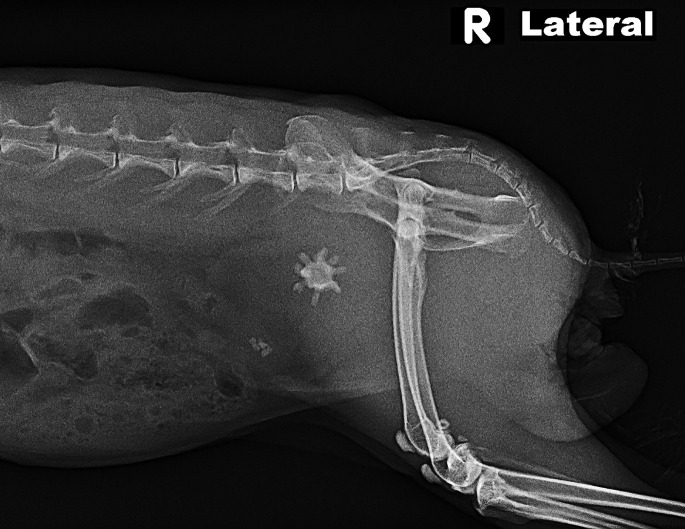
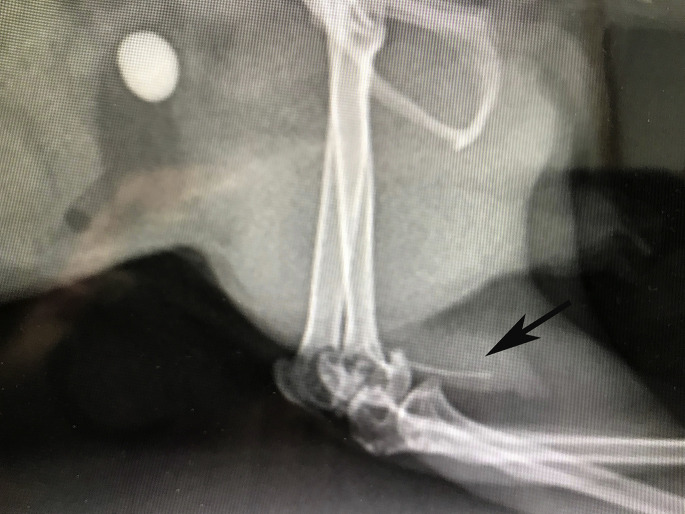
Unlike rabbits and guinea pigs, chinchillas eliminate excessive calcium approximately 80% via feces and only 1% to 3% via urine. Nevertheless, their urine calcium concentration may show great individual variation. Urolithiasis is reported in chinchillas and most are calcium carbonate (87.7%) and miscellaneous (6.8%).19 The clinical signs are as expected, with irritation around the preputial orifice, as well as hematuria, pollakiuria, and stranguria. Most uroliths are found in the urinary bladder, with fewer within the urethra. In males, most are in the proximal urethra near the pelvis and cranial to the os penis (Fig. 14 ). Prognosis is guarded with urethral stones and recurrence is common.77 Within the urinary bladder there may be thickening of the bladder wall with an inflamed mucosa.78
Fig. 14.
A chinchilla (C lanigera) with a urinary stone and the os penis can be seen (black arrow). Lateral view.
(Courtesy of C. Griffin, DVM, ABVP (avian practice), Kannapolis, NC).
Perineal hernias are described in 2 sexually intact males. The hernia contents were urinary bladder in 1 animal and lobules of fat in the other. In both cases, the testicles could be moved freely in and out of the inguinal canal.79
Hedgehogs
Common Problems
Hedgehogs (Atelerix albiventris) have been reported to have cystitis and uroliths, but the most common renal problem is chronic nephritis.80 As in other species, chronic renal disease is likely to have a large number of causes, such as infections, immune-mediated disorders, and nephrotoxins. Renal disease may also be a part of a systemic condition. Grossly and histologically, the lesion is similar to others that have been previously described in other animals.
Inflammatory/infectious
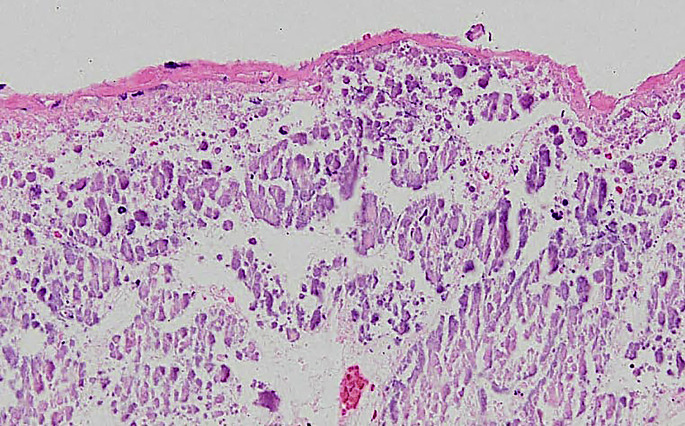
Cystitis is uncommon in hedgehogs, with scarce descriptions in the literature. In females, uterine tumors are a much more common cause of hematuria. However, 1 author (DRR) has diagnosed cases of hemorrhagic acute to subacute cystitis. Lesions of the urinary system collected from the database of 1 author (DRR) are summarized in Table 1 . One case was of encrusted cystitis. There was extensive mineralization of the urinary bladder with an ulcerative and/or necrotic cystitis. This type of lesion is uncommon and has been associated with alkaline urine and several bacteria, including Staphylococcus pseudintermedius and Corynebacterium urealyticum (Fig. 15 ).81
Table 1.
Data from Zoo/Exotic Pathology Service of 85 hedgehog (Atelerix albiventris) urinary lesions from 878 case submissions 1998 to 2019
| Lesion Category | Lesion | Number of Hedgehogs |
|---|---|---|
| Inflammatory | Nephritis, chronic fibrosing interstitial | 46 |
| Cystitis | 7 | |
| Cortical infarct | 6 | |
| Nephritis, interstitial | 5 | |
| Glomerulitis | 4 | |
| Nephritis, suppurative | 2 | |
| Neoplastic | Lymphoma | 3 |
| Sarcoma, renal (not differentiated) | 1 | |
| Miscellaneous | Renal mineralization | 9 |
| Renal tubular necrosis (nephrosis) | 8 | |
| Nephrosis pigmentary | 3 | |
| Renal cysts | 3 |
Fig. 15.
Hedgehog (A albiventris) encrusted cystitis (HE stain, 20× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Sugar gliders
Common Problems
There are very few reports of urinary disease in sugar gliders.
Inflammatory/infectious
Renal klossiellosis has been described in pet sugar gliders. In affected sugar gliders there is tubular dilatation and interstitial nephritis. The various life cycle stages can be identified within the renal tubular epithelium. Based on morphologic evaluation, the name Klossiella dulcis n. sp was proposed. No treatment has been described.82
Obstructive disorders
Bilateral hydronephrosis in a male sugar glider developed secondarily to a functional obstruction suspected to be from hyperplasia and inflammation of the urinary bladder and ureteral epithelium. The animal presented with acute caudal abdominal swelling.83
One author (AML) has noted several cases of disease of the cloacal and paracloacal glands that resulted in apparent urinary obstruction; obstruction resolved with treatment of the primary cause. Both sexes have paracloacal glands (anterior, dorsal, and ventral), which are more developed in the male. Under the influence of testosterone, the dorsal paracloacal glands and testes increase in size.1 Inflammation and neoplastic disease of these glands can result in functional obstruction of the digestive and urinary tract.84 There are reports of a paracloacal cyst,85 sebaceous nodular hyperplasia, and carcinomas in male sugar gliders. Self-mutilation, stranguria, and pericloacal swelling are the typical clinical signs. One case had a dorsal paracloacal gland carcinoma84 and the other a transitional cell carcinoma with squamous differentiation.86
Toxic exposure
Copper toxicosis has been described in a sugar glider voiding red-brown urine and showing possible seizurelike activity. The kidneys were enlarged and dark red with marked, acute, multifocal renal tubular degeneration and necrosis with hemoglobin casts. Icterus was noted and the liver had marked, acute, centrilobular hepatocellular degeneration and necrosis with hepatocellular copper accumulation confirmed with rubeanic stain and a hepatic copper concentration of 709 parts per million (wet-weight basis). The provided mineral and vitamin supplement had 2.26 and 250 μg/mL respectively. It was unknown whether the vitamin supplement could have led to the toxicosis.87
In a review of sugar glider case submissions (DRR) from 1998 to 2019, out of 337 there were 86 urinary tract lesions. Interstitial nephritis was common, as was the nonspecific finding of renal tubular necrosis (nephritis). Other lesions included membranoglomerulopathy, renal cysts, and transitional cell carcinoma of the urinary bladder (Fig. 16 ). Lesions are summarized in Table 2 .
Fig. 16.
Transitional cell carcinoma in the urinary bladder of a sugar glider (P breviceps) (HE stain, 10× magnification).
(Courtesy of D. R. Reavill, DVM, DABVP (Avian and Reptile & Amphibian Practice), DACVP, Carmichael, CA).
Table 2.
Data from Zoo/Exotic Pathology Service of 86 sugar glider (Petaurus breviceps) urinary lesions from 377 case submissions 1998-2019
| Lesion Category | Lesion | Number of Sugar Gliders |
|---|---|---|
| Inflammatory | Nephritis, interstitial | 43 |
| Membranoglomerulopathy | 6 | |
| Bacteremia | 2 | |
| Nephritis, tubular, bacteria | 2 | |
| Neoplastic | Transitional cell carcinoma, urinary bladder | 2 |
| Lymphoma | 1 | |
| Miscellaneous | Renal tubular necrosis (nephrosis) | 18 |
| Nephrosis pigmentary | 4 | |
| Hydronephrosis | 1 | |
| Renal cysts | 1 |
Treatment of renal insufficiency and failure
Without benefit of evidence-based therapies for exotic mammals, treatment of renal insufficiency and failure is based on therapies considered most advantageous in traditional pet species.
Chronic Kidney Disease
The 2016 International Society of Feline Medicine guidelines for management of chronic kidney disease (CKD) may be useful. Even in cats, simple and accurate markers for renal function are currently unavailable; biomarkers such as blood urea nitrogen (BUN), creatinine, and urine specific gravity are useful, but interpretation can be difficult. The principles of treatment are supportive, and the overall goal is maintenance of quality of life. Even the goal of maintaining hydration has not been formally associated with increased longevity and improved quality of life in cats with CKD; however, the benefits of normohydration are considered obvious enough to continue recommendation of fluid therapy.88
Unstable or decompensated patients benefit from hospitalization and intravenous (IV) fluid therapy. Fluid rates are based on calculation of hydration deficit: body weight (kg) times estimated dehydration (%) times 1000. Hydration deficit is added to maintenance fluids (50 mL/kg/24 h in cats) and provided over 24 to 48 hours. Once normohydration is achieved, fluid therapy continues at maintenance rate, monitoring the patient carefully for evidence of fluid overload. Fluid therapy is tapered over several days once condition and appropriate biomarkers have improved.
For long-term maintenance of hydration, many recommendations for cats are appropriate in exotic mammals, and include free access to high-quality water using multiple water sources. In exotic pets, this means providing water in bowls along with traditional water bottles. Although moist canned food is not available for rabbits and rodents, moistening greens and hay may aid water intake, as well as supplementing with liquid foods designed for convalescing exotic pets; for example, Oxbow Critical Care or Carnivore Care depending on the nutritional needs of the species. In cats, placement of feeding tubes for oral hydration may be used long term; this approach is not useful for exotic species. However, subcutaneous fluid administration may have utility, because some owners can be taught to administer fluids to small patients at home. Commercial indwelling subcutaneous catheters have been used in cats, and 1 author (AML) has used one in a large rabbit with some success.
Acute Renal Failure
Acute renal failure (ARF) may be reversible if identified and treated early and aggressively. As in other species, ARF may be prerenal (shock, hypovolemia), renal (renal disease), or postrenal (obstruction). Urine specific gravity plus changes in BUN and creatinine levels can be helpful. ARF is treated by correcting perfusion deficits (discussed later) and dehydration, and diuresis, along with correction of an identified underlying cause. Once the patient is rehydrated and normotensive, continue fluid administration as maintenance (3–4 mL/kg/h) plus estimated losses (diarrhea/vomiting, if present, and estimated urine volume). Based on information in domestic species, patients with ARF may become 3% to 5% dehydrated each day because of ongoing losses. Fluid administration is decreased when the patient maintains hydration, begins to eat and drink, and BUN/creatinine levels normalize.89
IV catheterization is a standard procedure in rabbit medicine; IV access is also possible in large guinea pigs and some chinchillas. IV access may not be possible in any patient that is hypovolemic. For these cases, intraosseous (IO) catheterization is indicated. Placement sites in mammals include the proximal humerus, femur, and tibia.
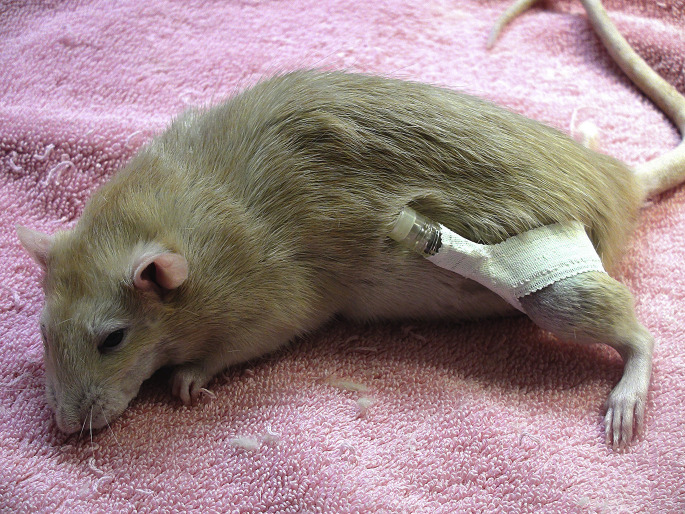
Placement is best performed in a sedated patient with parenteral analgesia, and a local block (2–3 mg/kg lidocaine into the skin, subcutaneous tissue, and periosteum). Although short spinal needles are useful in rabbits and large rodents, most IO catheters are simple hypodermic needles, 25 to 18 gauge depending on the size of the bone cavity. Seat the needle into the proximal bone, and then orient the needle into the bone cavity. Cover with an IV catheter cap and secure to the limb with tape (Fig. 17 ).
Fig. 17.
An intraosseous catheter in place for fluid diuresis for a pet rat (R norvegicus). A 25-gauge needle was used for the catheter, which was taped into place and capped with an IV catheter cap.
(Courtesy of A. Lennox, DVM, DABVP (Avian Practice, Exotic Companion Mammal Practice), Diplomate ECZM (Small Mammal), Indianapolis, IN).
Treatment of shock in rabbits and rodents has been described.89 Management of fluid deficits is maintenance fluids estimated at 3 to 4 mL/kg/h in exotic mammals, or via allometric calculations of resting energy requirements (weight [kg]75 × 70) plus hydration deficit, adjusting fluid rates for ongoing losses, such as polyuria in cases of renal insufficiency.
Other therapies include erythropoietin for treatment of anemia, analgesia as indicated, and support feeding.
Acknowledgments
Histologic images were digitally processed by Animal Reference Pathology, Salt Lake City, UT.
Footnotes
Disclosure: The authors have no commercial or financial conflicts of interest nor any funding sources.
References
- 1.Bradley A.J., Stoddart D.M. The dorsal paracloacal gland and its relationship with seasonal changes in cutaneous scent gland morphology and plasma androgen in the marsupial sugar glider (Petaurus breviceps; Marsupialia: Petauridae) J Zoology. 1993;229(2):331–346. [Google Scholar]
- 2.Mbassa G.K. Mammalian renal modifications in dry environments. Vet Res Commun. 1988;12(1):1–18. doi: 10.1007/BF00396399. [DOI] [PubMed] [Google Scholar]
- 3.Welchman Dde B., Oxenham M., Done S.H. Aleutian disease in domestic ferrets: diagnostic findings and survey results. Vet Rec. 1993;132(19):479–484. doi: 10.1136/vr.132.19.479. [DOI] [PubMed] [Google Scholar]
- 4.Lindemann D.M., Eshar D., Schumacher L.L., et al. Pyogranulomatous panophthalmitis with systemic coronavirus disease in a domestic ferret (Mustela putorius furo) Vet Ophthalmol. 2016;19(2):167–171. doi: 10.1111/vop.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez E., Novellas R., Moya A., et al. Abdominal radiographic and ultrasonographic findings in ferrets (Mustela putorius furo) with systemic coronavirus infection. Vet Rec. 2011;169(9):231. doi: 10.1136/vr.d4705. [DOI] [PubMed] [Google Scholar]
- 6.Johnson-Delaney C. Ferret medicine and surgery. CRC Press; Boca Raton (FL): 2017. Applied clinical anatomy and physiology; p. 29. [Google Scholar]
- 7.Johnson-Delaney C. In: Ferret medicine and surgery. Johnson-Delaney C., editor. CRC Press; 2017. Disorders of the urogenital system; pp. 366–370. [Google Scholar]
- 8.Bean A.D., Fisher P.G., Reavill D.R., et al. Hypercalcemia associated with lymphomas in the ferret (Mustela putorius furo): four cases. J Exot Pet Med. 2019;29:147–153. [Google Scholar]
- 9.Jackson D.N., Rogers A.B., Maurer K.J., et al. Cystic renal disease in the domestic ferret. Comp Med. 2008;58(2):161–167. [PMC free article] [PubMed] [Google Scholar]
- 10.MacNab T.A., Newcomb B.T., Ketz-Riley C., et al. Extramural ectopic ureter in a domestic ferret (Mustela putorius furo) J Exot Pet Med. 2010;19(4):313–316. [Google Scholar]
- 11.Li X., Fox J.G., Padrid P.A. Neoplastic disease in ferrets: 574 cases (1968–1997) J Am Vet Med Assoc. 1998;212(9):1402–1406. [PubMed] [Google Scholar]
- 12.Avallone G., Forlani A., Tecilla M., et al. Neoplastic diseases in the domestic ferret (Mustela putorius furo) in Italy: classification and tissue distribution of 856 cases (2000-2010) BMC Vet Res. 2016;12(1):275–283. doi: 10.1186/s12917-016-0901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawaguchi H., Miyoshi N., Souda M., et al. Renal adenocarcinoma in a ferret. Vet Pathol. 2006;43(3):353–356. doi: 10.1354/vp.43-3-353. [DOI] [PubMed] [Google Scholar]
- 14.Bell R.C., Moeller R.B. Transitional cell carcinoma of the renal pelvis in a ferret. Lab Anim Sci. 1990;40(5):537–538. [PubMed] [Google Scholar]
- 15.Volgenaeu T., Greenacre C.B., Smith J., et al. Challenging cases in internal medicine: what's your diagnosis? Vet Med. 1998;9(93):797–804. [Google Scholar]
- 16.Nwaokorie E.E., Osborne C.A., Lulich J.P., et al. Epidemiology of struvite uroliths in ferrets: 272 cases (1981-2007) J Am Vet Med Assoc. 2011;239(10):1319–1324. doi: 10.2460/javma.239.10.1319. [DOI] [PubMed] [Google Scholar]
- 17.Fox J.G., Pearson R.C., Bell J.A. In: Biology and diseases of the ferret. 2nd edition. Fox J.G., editor. Williams and Wilkens; Baltimore (MD): 1998. Diseases of the genitourinary system; pp. 247–272. [Google Scholar]
- 18.Nwaokorie E.E., Osborne C.A., Lulich J.P., et al. Epidemiological evaluation of cystine urolithiasis in domestic ferrets (Mustela putorius furo): 70 cases (1992-2009) J Am Vet Med Assoc. 2013;242(8):1099–1103. doi: 10.2460/javma.242.8.1099. [DOI] [PubMed] [Google Scholar]
- 19.Osborne C.A., Albasan H., Lulich J.P., et al. Quantitative analysis of 4468 uroliths retrieved from farm animals, exotic species, and wildlife submitted to the Minnesota Urolith Center: 1981 to 2007. Vet Clin North Am Small Anim Pract. 2009;39(1):65–78. doi: 10.1016/j.cvsm.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Powers L.V., Winkler K., Garner M.M., et al. Omentalization of prostatic abscesses and large cysts in ferrets (Mustela putorius furo) J Exot Pet Med. 2007;16(3):186–194. [Google Scholar]
- 21.van Zeeland Y.R.A., Lennox A., Quinton J.F., et al. Prepuce and partial penile amputation for treatment of preputial gland neoplasia in two ferrets. J Small Anim Pract. 2014;55(11):593–596. doi: 10.1111/jsap.12243. [DOI] [PubMed] [Google Scholar]
- 22.Fisher P. Exotic mammal renal disease: diagnosis and treatment. Vet Clin North Am Exot Anim Pract. 2006;9(10):69–96. doi: 10.1016/j.cvex.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Hinton M. Kidney disease in the rabbit: a histological survey. Lab Anim. 1981;15(3):263–265. doi: 10.1258/002367781780893849. [DOI] [PubMed] [Google Scholar]
- 24.Csokai J., Gruber A., Künzel F., et al. Encephalitozoonosis in pet rabbits (Oryctolagus cuniculus): pathohistological findings in animals with latent infection versus clinical manifestation. Parasitol Res. 2009;104(3):629–635. doi: 10.1007/s00436-008-1239-2. [DOI] [PubMed] [Google Scholar]
- 25.Leipig M., Matiasek K., Rinder H., et al. Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J Vet Diagn Invest. 2013;25(1):16–26. doi: 10.1177/1040638712466394. [DOI] [PubMed] [Google Scholar]
- 26.Di Girolamo N., Bongiovanni L., Ferro S., et al. Cystoscopic diagnosis of polypoid cystitis in two pet rabbits. J Am Vet Med Assoc. 2017;251(1):84–89. doi: 10.2460/javma.251.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Garibaldi B.A., Fox J.G., Otto G., et al. Hematuria in rabbits. Lab Anim Sci. 1987;37(6):769–772. [PubMed] [Google Scholar]
- 28.Maurer K.J., Marini R.P., Fox J.G., et al. Polycystic kidney syndrome in New Zealand white rabbits resembling human polycystic kidney disease. Kidney Int. 2004;65(2):482–489. doi: 10.1111/j.1523-1755.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 29.Fox R.R., Krinsky W.L., Crary D.D. Hereditary cortical renal cysts in the rabbit. J Hered. 1971;62(2):105–109. doi: 10.1093/oxfordjournals.jhered.a108132. [DOI] [PubMed] [Google Scholar]
- 30.Ojeda J.L., García-Porrero J.A. Structure and development of parietal podocytes in renal glomerular cysts induced in rabbits with methylprednisolone acetate. Lab Invest. 1982;47(2):167–176. [PubMed] [Google Scholar]
- 31.Durfee W.J., Masters W.G., Montgomery C.A., et al. Spontaneous renal cell carcinoma in a New Zealand white rabbit. Contemp Top Lab Anim Sci. 1999;38(10):89–91. [PubMed] [Google Scholar]
- 32.Rose J.B., Vergneau-Grosset C., Steffey M.A., et al. Adrenalectomy and nephrectomy in a rabbit (Oryctolagus cuniculus) with adrenocortical carcinoma and renal and ureteral transitional cell carcinoma. J Exot Pet Med. 2016;25(4):332–341. [Google Scholar]
- 33.Cikanek S.J., Eshar D., Nau M., et al. Diagnosis and surgical treatment of a transitional cell carcinoma in the bladder apex of a pet rabbit (Oryctolagus cuniculus) (AEMV Forum) J Exot Pet Med. 2018;27(2):113–117. [Google Scholar]
- 34.Clauss M., Burger B., Liesegang A., et al. Influence of diet on calcium metabolism, tissue calcification and urinary sludge in rabbits (Oryctolagus cuniculus) J Anim Physiol Anim Nutr. 2012;96(5):798–807. doi: 10.1111/j.1439-0396.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 35.Kucera J., Koristkova T., Gottwaldova B., et al. Calcium sulfate dihydrate urolithiasis in a pet rabbit. J Am Vet Med Assoc. 2017;250(5):534–537. doi: 10.2460/javma.250.5.534. [DOI] [PubMed] [Google Scholar]
- 36.Thas I., Harcourt-Brown F. Six cases of inguinal urinary bladder herniation in entire male domestic rabbits. J Small Anim Pract. 2013;54(12):662–666. doi: 10.1111/jsap.12120. [DOI] [PubMed] [Google Scholar]
- 37.Grunkemeyer V.L., Sura P.A., Baron M.L., et al. Surgical repair of an inguinal herniation of the urinary bladder in an intact female domestic rabbit (Oryctolagus cuniculus) J Exot Pet Med. 2010;19(3):249–254. [Google Scholar]
- 38.Greenacre C.B., Allen S.W., Ritchie B.W. Urinary bladder eversion in rabbit does. Comp Cont Educ Pract Vet. 1999;21(6):524–528. [Google Scholar]
- 39.Szabó Z. Transurethral urinary bladder eversion and prolapse in a castrated male pet rabbit. Acta Vet Hung. 2017;65(4):556–564. doi: 10.1556/004.2017.054. [DOI] [PubMed] [Google Scholar]
- 40.Wu R.S., Chu C.C., Wang H.C., et al. Clinical diagnosis and surgical management of diaphragmatic retroperitoneal perirenal fat and kidney herniation in a pet rabbit. J Am Vet Med Assoc. 2016;248(12):1399–1403. doi: 10.2460/javma.248.12.1399. [DOI] [PubMed] [Google Scholar]
- 41.Carrasco DC, Skarbek A, Lopez M. Surgical management of diaphragmatic lipoma and kidney herniation in a rabbit (Oryctolagus cuniculus) with a polypropylene mesh. Proceedings of the annual conference of the association of exotic mammal Veterinarians. Atlanta, 2018. p. 757.
- 42.Künzel F., Fisher P. Clinical signs, diagnosis, and treatment of Encephalitozoon cuniculi infection in rabbits. Vet Clin North Am Exot Anim Pract. 2018;21(1):69–82. doi: 10.1016/j.cvex.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Graham J.E., Garner M.M., Reavill D.R. Benzimidazole toxicosis in rabbits: 13 cases (2003 to 2011) J Exot Pet Med. 2014;23(2):188–195. [Google Scholar]
- 44.Martorell J., Bailon D., Majo N., et al. Lateral approach to nephrotomy in the management of unilateral renal calculi in a rabbit (Oryctolagus cuniculus) J Am Vet Med Assoc. 2012;240(7):863–868. doi: 10.2460/javma.240.7.863. [DOI] [PubMed] [Google Scholar]
- 45.Rhody J.L. Unilateral nephrectomy for hydronephrosis in a pet rabbit. Vet Clin North Am Exot Anim Pract. 2006;9(3):633–641. doi: 10.1016/j.cvex.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Steblay R.W., Rudofsky U. Spontaneous renal lesions and glomerular deposits of IgG and complement in guinea pigs. J Immunol. 1997;107(4):1192–1196. [PubMed] [Google Scholar]
- 47.Takeda T., Grollman A. Spontaneously occurring renal disease in the guinea pig. Am J Pathol. 1970;60(1):103–118. [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf P., Schröder A., Wenger A., et al. The nutrition of the chinchilla as a companion animal – basic data, influences and dependences. J Anim Physiol Anim Nutr. 2003;87(3–4):129–133. doi: 10.1046/j.1439-0396.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- 49.Schuhmann B., Kück G., Cope I. Bacterial cystitis in four female guinea pigs (Cavia porcellus) resembling necrotising bacterial cystitis. Vet Rec Case Rep. 2015;3(1):e000136. [Google Scholar]
- 50.Steele H. Subcutaneous fibrosarcoma in an aged guinea pig. Can Vet J. 2001;42(4):300–302. [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen J.A., Brice A.K., Bagel J.H., et al. Hypervitaminosis D in guinea pigs with α-mannosidosis. Comp Med. 2013;63(2):156–162. [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons JL. Feeding pocket pets: Nutritional physiology of rodents and lagomorphs. Proceedings of the annual conference of the American association of Zoo Veterinarians. Atlanta, 2016.
- 53.Clauss M, Hummel J. Getting it out of the (digestive) system: hindgut fermenters and calcium. Proceedings of the Annual Conference of the Comparative Nutrition Society, 2008. p. 30–36.
- 54.Jolánkai R., Guija de Arespacochaga A., Iben C. Urolithiasis in guinea pigs — Nutritional aspects. Cereal Res Commun. 2006;34(1):743–746. [Google Scholar]
- 55.Lennox AM. A simple technique for removal of urethroliths in the female guinea pig. Proceedings of the Annual Conference of the association of exotic mammal Veterinarians. Orlando, 2014.
- 56.Hubbard G.B., Schmidt R.E. In: Laboratory hamsters. Van Hoosier G.L. Jr., McPherson C.W., editors. Academic Press; Orlando (FL): 1987. Noninfectious diseases; pp. 169–178. [Google Scholar]
- 57.Sobh M., Moustafa F., Hamed S., et al. Effect of colchicine on schistosoma-induced renal amyloidosis in Syrian golden hamsters. Nephron. 1995;70(4):478–485. doi: 10.1159/000188648. [DOI] [PubMed] [Google Scholar]
- 58.Murphy J.C., Fox J.G., Niemi S.M. Nephrotic syndrome associated with renal amyloidosis in a colony of Syrian hamsters. J Am Vet Med Assoc. 1984;185:1359–1362. [PubMed] [Google Scholar]
- 59.McKeon G.P., Nagamine C.M., Ruby N.F., et al. Hematologic, serologic, and histologic profile of aged Siberian hamsters (Phodopus sungorus) J Am Assoc Lab Anim Sci. 2011;50(3):308–316. [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamin S.A., Brooks A.L. Spontaneous lesions in Chinese hamsters. Vet Pathol. 1977;14(5):449–462. doi: 10.1177/030098587701400504. [DOI] [PubMed] [Google Scholar]
- 61.Pour P., Mohr U., Althoff J., et al. Spontaneous tumors and common diseases in two colonies of 6. Syrian hamsters. III. Urogenital system and endocrine glands. J Natl Cancer Inst. 1976;56(5):949–961. doi: 10.1093/jnci/56.5.949. [DOI] [PubMed] [Google Scholar]
- 62.Miyazawa S., Saiga K., Nemoto K., et al. A repeat biopsy study in spontaneous crescentic glomerulonephritis mice. Ren Fail. 2002;24(5):557–566. doi: 10.1081/jdi-120013958. [DOI] [PubMed] [Google Scholar]
- 63.Imai H., Nakamoto Y., Asakura K., et al. Spontaneous glomerular IgA deposition in ddY mice: an animal model of IgA nephritis. Kidney Int. 1985;27(5):756–761. doi: 10.1038/ki.1985.76. [DOI] [PubMed] [Google Scholar]
- 64.Montgomery C.A. In: Monographs on pathology of laboratory animals: urinary system. Jones T.C., Hard G.C., Mohr U., editors. Springer-Verlag; New York: 1998. Interstitial nephritis, mouse; pp. 238–243. [Google Scholar]
- 65.Montgomery C.A. In: Monographs on pathology of laboratory animals: urinary system. Jones T.C., Hard G.C., Mohr U., editors. Springer-Verlag; New York: 1998. Suppurative nephritis, pyelonephritis, mouse; pp. 244–248. [Google Scholar]
- 66.Barthold S.W. In: Monographs on pathology of laboratory animals: urinary system. Jones T.C., Hard G.C., Mohr U., editors. Springer-Verlag; New York: 1986. Klossielosis, kidney, mouse, rat; pp. 276–278. [Google Scholar]
- 67.Ninomiya H., Inomata T., Ogihara K. Obstructive uropathy and hydronephrosis in male KK-Ay mice: a report of cases. J Vet Med Sci. 1999;61(1):53–57. doi: 10.1292/jvms.61.53. [DOI] [PubMed] [Google Scholar]
- 68.Shoieb A., Shirai N. Polycystic kidney disease in Sprague-Dawley rats. Exp Toxicol Pathol. 2015;67(5–6):361–364. doi: 10.1016/j.etp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Hard G.C., Betz L.J., Seely J.C. Association of advanced chronic progressive nephropathy (CPN) with renal tubule tumors and precursor hyperplasia in control F344 rats from two-year carcinogenicity studies. Toxicol Pathol. 2012;40(3):473–481. doi: 10.1177/0192623311431948. [DOI] [PubMed] [Google Scholar]
- 70.Dontas I.A., Khaldi L. Urolithiasis and transitional cell carcinoma of the bladder in a Wistar rat. J Am Assoc Lab Anim Sci. 2006;45(4):64–67. [PubMed] [Google Scholar]
- 71.Izumi K., Kitaura K., Chone Y., et al. Spontaneous renal cell tumors in Long-Evans Cinnamon rats. Jpn J Cancer Res. 1994;85(6):563–566. doi: 10.1111/j.1349-7006.1994.tb02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bowman M.R., Pare J.A., Pinckney R.D. Trichosomoides crassicauda infection in a pet hooded rat. Vet Rec. 2004;154(12):374–375. doi: 10.1136/vr.154.12.374. [DOI] [PubMed] [Google Scholar]
- 73.Burek J.D., Duprat P., Owen R., et al. Spontaneous renal disease in laboratory animals. Int Rev Exp Pathol. 1988;30:231–319. doi: 10.1016/b978-0-12-364930-0.50009-9. [DOI] [PubMed] [Google Scholar]
- 74.Solleveld H.A., Boorman G.A. Spontaneous renal lesions in five rat strains. Toxicol Pathol. 1986;14(2):168–174. doi: 10.1177/019262338601400204. [DOI] [PubMed] [Google Scholar]
- 75.Kondo S., Yoshizawa N., Wakabayashi K. Natural history of renal lesions in spontaneously hypercholesterolemic (SHC) male rats. Nihon Jinzo Gakkai Shi. 1995;37:91–99. [PubMed] [Google Scholar]
- 76.Chandra M., Riley M.G., Johnson D.E. Spontaneous renal neoplasms in rats. J Appl Toxicol. 1993;13(2):109–116. doi: 10.1002/jat.2550130207. [DOI] [PubMed] [Google Scholar]
- 77.Martel-Arquette A., Mans C. Urolithiasis in chinchillas: 15 cases (2007 to 2011) J Small Anim Pract. 2016;57(5):260–264. doi: 10.1111/jsap.12479. [DOI] [PubMed] [Google Scholar]
- 78.Spence S., Skae K. Urolithiasis in a chinchilla. Vet Rec. 1995;136(20):524. doi: 10.1136/vr.136.20.524. [DOI] [PubMed] [Google Scholar]
- 79.Thöle M., Schuhmann B., Köstlinger S., et al. Treatment of unilateral perineal hernias in 2 male chinchillas (Chinchilla lanigera) J Exot Pet Med. 2018;27(3):43–49. [Google Scholar]
- 80.Gardhouse S., Eshar D. Retrospective study of disease occurrence in captive African pygmy hedgehogs (Atelerix albiventris) Isr J Vet Med. 2015;70(1):32–36. [Google Scholar]
- 81.Raab O., Béraud R., Tefft K.M., et al. Successful treatment of Corynebacterium urealyticum encrusting cystitis with systemic and intravesical antimicrobial therapy. Can Vet J. 2015;56(5):471–475. [PMC free article] [PubMed] [Google Scholar]
- 82.Ardiaca M., Bennett M.D., Montesinos A., et al. Klossiella dulcis n. sp. (Apicomplexa: Klossiellidae) in the kidneys of Petaurus breviceps (Marsupialia: Petauridae) J Zoo Wildl Med. 2016;47(2):622–627. doi: 10.1638/2015-0258.1. [DOI] [PubMed] [Google Scholar]
- 83.Cusack L., Schnellbacher R., Howerth E.W., et al. Bilateral hydronephrosis in a sugar glider (Petaurus breviceps) J Zoo Wildl Med. 2016;47(3):886–889. doi: 10.1638/2016-0007.1. [DOI] [PubMed] [Google Scholar]
- 84.Ju-Chi Chen, Pin-Huan Yu, Chen-Hsuan Liu, et al. Paracloacal gland carcinoma in a sugar glider (Petaurus breviceps) J Exot Pet Med. 2018;27(1):36–40. [Google Scholar]
- 85.Thomas M., Parkinson L., Shaw G., et al. Paracloacal cyst in a sugar glider (Petaurus breviceps) J Exot Pet Med. 2019;29(0):40–44. [Google Scholar]
- 86.Marrow J.C., Carpenter J.W., Lloyd A., et al. A transitional cell carcinoma with squamous differentiation in a pericloacal mass in a sugar glider (Petaurus breviceps) J Exot Pet Med. 2010;19(1):92–95. [Google Scholar]
- 87.Kuroki K., DeBey B.M., Pollock C.G., et al. Pathology in practice. Copper toxicosis. J Am Vet Med Assoc. 2011;239(12):1549–1551. doi: 10.2460/javma.239.12.1549. [DOI] [PubMed] [Google Scholar]
- 88.Sparkes A.H., Caney S., Chalhoub S., et al. ISFM consensus guidelines on the diagnosis and management of feline chronic kidney disease. J Feline Med Surg. 2016;18(3):219–239. doi: 10.1177/1098612X16631234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lennox AM, Gladden J. Emergency and critical care of small mammals. In: Quesenberry K, Mans C, Orcutt C, editors. Ferrets, rabbits, and rodents, 4th edition Saunders, in press.