Summary
Respiratory tract infections (RTI) are the most common infections transmitted between Hajj pilgrims. The aim of this systematic review was to determine the prevalence of virus carriage potentially responsible for RTI among pilgrims before and after participating in the Hajj. A systematic search for relevant literature was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. 31 studies were identified. Severe Acute Respiratory Syndrome coronavirus and Middle East Respiratory Syndrome coronavirus (MERS) were never isolated in Hajj pilgrims. The viruses most commonly isolated from symptomatic patients during the Hajj by PCR were rhinovirus (5.9–48.8% prevalence), followed by influenza virus (4.5–13.9%) and non-MERS coronaviruses (2.7–13.2%) with most infections due to coronavirus 229E; other viruses were less frequently isolated. Several viruses including influenza A, rhinovirus, and non-MERS coronaviruses had low carriage rates among arriving pilgrims and a statistically significant increase in their carriage rate was observed, following participation in the Hajj. Further research is needed to assess the role of viruses in the pathogenesis of respiratory symptoms and their potential role in the severity of the symptoms.
Keywords: Hajj, Umrah, Respiratory tract infection, Virus
1. Introduction
Every year, more than 10 million pilgrims from over 180 countries arrive in the Kingdom of Saudi Arabia for a pilgrimage to the holy places of Islam [1]. Around 2–3 million Muslims will perform the Hajj at fixed dates over a 6-day period, while the remaining pilgrims will participate in the Umrah, a shorter pilgrimage that can be done at anytime, although most pilgrims perform this activity during the month of Ramadan. The number of pilgrims undertaking the Hajj has increased by a factor 5 from 1920 to 2012 [1].
The crowded conditions within a confined area and the close contact with others, particularly during the circumambulation of the Kaaba (Tawaf) inside the Grand Mosque in Makkah, lead to increased risk of pilgrims acquiring and spreading infectious diseases [2]. Respiratory tract infections are the most common infections transmitted between pilgrims, and the majority of pilgrims will develop one form of respiratory tract infection or another during their few weeks in Makkah and Madinah [3], [4]. Cough attack rates over 90% have been recorded among pilgrims from various nationalities [5], [6], [7]. Among ill pilgrims consulting at Mina primary health structures, 60% present with respiratory tract infection symptoms [8]. Respiratory tract infection is the leading cause of hospitalization in Saudi Hospitals during the Hajj, with a level as high as 57% in one study [9]. Pneumonia accounts for 20–40% of hospitalization in tertiary care structures [9], [10] and for 55–67% of admissions to intensive care units [11], [12].
Several transmissible viral respiratory infections have been reported to cause upper respiratory tract infections in Hajj pilgrims [3], [13]. Published studies over recent years and including large panels of viruses tested by multiplex PCR provided new insights in the epidemiology of viral respiratory tract infections at the Hajj.
The objective of this paper is to summarize available data about the prevalence of virus carriage potentially responsible for respiratory tract infections among Hajj pilgrims as well as data about carriage acquisition and circulation of respiratory viruses among pilgrims before and after participating in the Hajj.
2. Methods
2.1. Search strategy and selection criteria
The systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org). The following databases were searched, attempting to identify all relevant studies published from January 1980 to April 2015: Scopus (http://www.scopus.com/), PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Google Scholar (http://scholar.google.fr/). The latest search was conducted on May 1, 2015. The topic search terms used for searching the databases were as follows:
#1: “hadj” OR “hajj” OR “pilgrimage”;
#2: “respiratory”;
#3: “viral” OR “virus” OR “viruses” OR “pathogens” OR “infection” OR “infections”;
#4: #1 AND #2 AND #3.
The Saudi Epidemiological Bulletin (http://seb.drupalgardens.com/) issues were systematically reviewed for relevant papers.
Only articles published in English were included based on common language charred by the authors.
For inclusion the article needed to fulfill the following criteria [1]: It needed to be related to the Hajj pilgrimage [2], report on screening in asymptomatic or symptomatic participants [3], present virological data and [4] report on virus carriage prevalence. We excluded case reports. The reference lists of reviews were screened to identify studies possibly missed by the search.
Two researchers (P.G. and S.B.) independently performed the screening of the abstracts. Any discordant result was discussed in consensus meetings. After screening the abstracts, the full text of the articles was assessed for eligibility by the same two researchers and selected or rejected for inclusion in the systematic review.
2.2. Data collection process
The following data (if available) were extracted from each article: year, study design, study population, type of sample, microbiological methods, pathogens investigated and their prevalence, influenza vaccination rates and its effect on influenza virus carriage prevalence. We made a distinction between surveys conducted among symptomatic patients only, most of whom were included when consulting for respiratory symptoms at medical structures or were included in other settings on the basis of suffering respiratory symptoms at the time of sampling and, broader surveys conducted among cohorts of pilgrims independently of their clinical status (i.e. symptomatic and asymptomatic individuals). In this latter group sampling in pilgrims presenting respiratory symptoms was not necessarily done at the time of symptoms.
2.3. Data synthesis and analysis
As a result of the design of the studies (cross-sectional studies and cohort studies) and the heterogeneity in patient populations and diagnosis methods, a formal meta-analysis was not possible. Therefore, the study results were summarized to describe the main outcomes of interest (i.e. the prevalence of respiratory viruses before and/or after participation to the Hajj). If possible, percentages not presented in the articles were calculated from the available data.
3. Results
3.1. Study selection
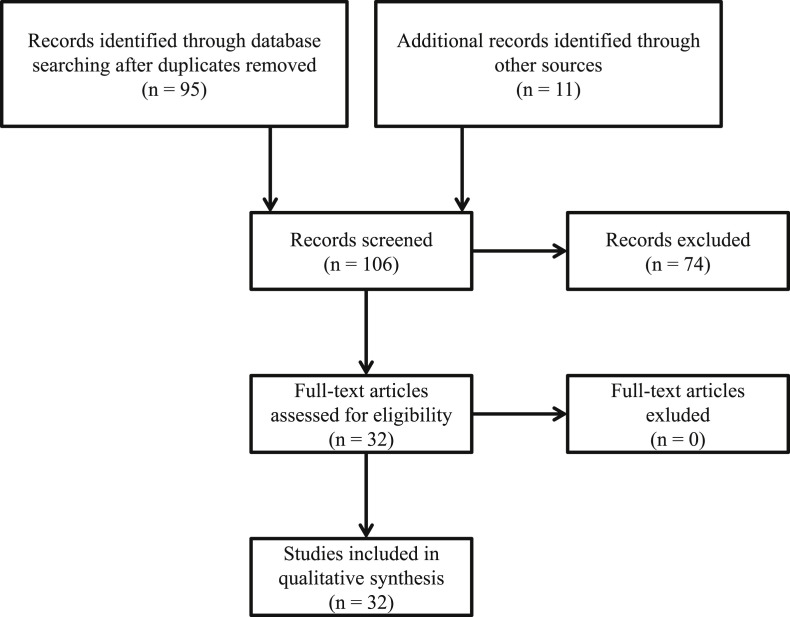
A total of 95 articles were found from the search, eleven additional references were found through manual search. After screening of titles and summaries, 32 articles were selected for full-text assessment [6], [7], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]. All 32 articles were included in the qualitative synthesis of the systematic review (Fig. 1 ).
Figure 1.
Flow diagram of searching strategy.
3.2. Studies conducted among selected population of symptomatic pilgrims suffering respiratory symptoms
See Table 1 .
Table 1.
Prevalence of respiratory viruses in studies conducted among pilgrims suffering from respiratory symptoms.
| Year | Study design | Study population | Type of sample | Microbiological techniques | Pathogens investigated | Prevalence (%) | Influenza vaccination (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| 1991–1992 | Cross-sectional study (Saudi Arabia) | Pilgrims suffering URTIa and LRTIb and seeking care in four hospitals/medical centers (n = 761) | Throat swabs, sputum | Cell culture/cytopathic effect and staining using virus-specific monoclonal antibodies | Influenza A | 4.5 | [14] | |
| Influenza B | 2.0 | |||||||
| Influenza overall | 6.5 | |||||||
| Parainfluenza 1 | 2.0 | |||||||
| Parainfluenza 2 | 1.7 | |||||||
| Parainfluenza 3 | 2.2 | |||||||
| Parainfluenza overall | 5.9 | |||||||
| Adenovirus | 4.7 | |||||||
| Respiratory syncytial virus | 2.4 | |||||||
| Mixed viral infections | ND | |||||||
| No virus | 80.6 | |||||||
| 1994 | Cross-sectional study (Saudi Arabia) | Pilgrims suffering pneumoniac and seeking care in two hospitals (n = 64) | Serum, sputum broncho-alveolar lavage and naso-pharyngeal aspirate | Direct immunofluorescence staining | Influenza A | 3.1 | [15] | |
| Influenza B | 1.6 | |||||||
| Influenza overall | 4.7 | |||||||
| Parainfluenza 1 | 0 | |||||||
| Parainfluenza 2 | 1.6 | |||||||
| Parainfluenza 3 | 0 | |||||||
| Adenovirus | 0 | |||||||
| Respiratory syncytial virus | 0 | |||||||
| Mixed viral infections | 0 | |||||||
| No virus | 89.1% | |||||||
| 2000 | Cross sectional study (Saudi Arabia) | Pilgrims suffering ILId and presenting at outpatient clinics (n = 305) | Serum | ELISA | Influenza A | 5.9 | 4.3 | [16] |
| Influenza B | 11.5 | |||||||
| Influenza overall | 14.8 | |||||||
| Mixed viral infections | ND | |||||||
| No virus | 82.0% | |||||||
| 2003 | Cross-sectional study (Saudi Arabia) | Pilgrims suffering URTIe and seeking care in three hospitals (n = 500) | Throat swabs | Cell culture/cytopathic effect and staining using virus-specific monoclonal antibodies | Influenza A | 0.6 | 4.4% | [17] |
| Influenza B | 5.4 | |||||||
| Influenza overall | 6.0 | |||||||
| Parainfluenza 1,2,3 | 0.8 | |||||||
| Adenovirus | 0 | |||||||
| Respiratory syncytial virus | 1.4 | |||||||
| Enterovirus | 0 | |||||||
| Mixed viral infections | ND | |||||||
| No virus | 89.2% | |||||||
| 2004 | Cross-sectional study (Saudi Arabia) | Pilgrims suffering ILIf and seeking care in hospitals/medical centers (n = 360) | Throat swab | Not described | Influenza overall | 12.8 | 2.2% | [18] |
| (including 70.9% influenza B Sichuan, 1.8% influenza B Hong Kong, 14.6% influenza A untyped, 7.3% influenza A-H1N1, 5.5% influenza A-H3N2 calculated from a mixed sample of 46 positive pilgrims and 9 positives non-pilgrims) | ||||||||
| Mixed viral infections | ND | |||||||
| No virus | 84.7% | |||||||
| 2004–2005 | Cross-sectional study (Saudi Arabia) | Iranian pilgrims suffering respiratory symptomsg and presenting at to medical centers (n = 105) | Gargled pharyngeal secretions, and serum | Cell culture/cytopathic effect and staining using virus-specific monoclonal antibodies - ELISA | Influenza A (pharynx) | 1.9 | [19] | |
| Influenza B (pharynx) | 11.4 | |||||||
| Influenza overall | 13.3 | |||||||
| Parainfluenza (pharynx) | 0 | |||||||
| Adenovirus (pharynx) | 36.2 | |||||||
| Respiratory syncytial virus (pharynx) | 1.9 | |||||||
| Influenza A-H1N1 (serology) | 6.9 | |||||||
| Influenza A-H3N2 (serology) | 6.1 | |||||||
| Influenza B-Hong Kong (serology) | 8.5 | |||||||
| Influenza B-Sichuan (serology) | 12.3 | |||||||
| Influenza overall (serology) | 21.5 | |||||||
| Mixed viral infections | ND | |||||||
| No virus (pharynx) | 48.6% | |||||||
| No virus (serology) | 58.1% | |||||||
| 2005 | Cross-sectional study (Saudi Arabia) | Pilgrimsh suffering respiratory symptomsi attending the British Hajj Delegation clinic in Mecca, or at Mina encampment (n = 202) | Nasal swabs | Rapid diagnostic test (QuickVue) - PCR | Influenza rapid test | 4.5 | 27.7 | [20] |
| Influenza A-H1N1 | 0.5 | |||||||
| Influenza A-H3N2 | 9.9 | |||||||
| Influenza B | 3.5 | |||||||
| Influenza overall | 13.9 | |||||||
| Respiratory syncytial virus | 4.5 | |||||||
| Mixed viral infections | ND | |||||||
| No virus | 86.1 | |||||||
| 2005 | Cross-sectional study (Saudi Arabia) | International pilgrims suffering respiratory symptomsf recruited at two hospitals and at two airports (n = 483) | Throat swab | Not described | Influenza overall | 9.7 | 29.9 | [21] |
| (including 17.0% influenza B Sichuan, 6.4% influenza B Hong Kong, 29.8% influenza A untyped, 34.0% influenza A-H1N1, 12.8% influenza A-H3N2) | ||||||||
| Mixed viral infections | ND | |||||||
| No virus | 90.3 | |||||||
| 2005–2006 | Cross-sectional study (Saudi Arabia and UK) | UK pilgrims suffering respiratory symptomsj during their stay in Saudi Arabia or upon returning to the UK (n = 555) | Nasal swabs | Rapid diagnostic test (QuickVue) - PCR | Influenza (Rapid test) | 3.2 | [22] | |
| Influenza-A (PCR) | 8.2 | |||||||
| Influenza B (PCR) | 2.3 | |||||||
| Influenza overall (PCR) | 10.5 | |||||||
| Mixed viral infections | ND | |||||||
| No virus | 90.1% | |||||||
| 2006 | Cross-sectional study (Saudi Arabia) | British pilgrims suffering respiratory symptomsk attending the British Hajj Delegation clinic in Mecca or at Mina encampment (n = 150) and Saudi pilgrimsk attending the National Guard Health Affair clinic at Mina (n = 110) | Nasal swabs | Rapid diagnostic test (QuickVue) - PCR | Influenza rapid test | 3.5 | 23.1 | [23] |
| Influenza A | 8.1 | |||||||
| Influenza B | 2.3 | |||||||
| Influenza overall | 10.4 | |||||||
| Parainfluenza 1 | 0 | |||||||
| Parainfluenza 2 | 0 | |||||||
| Parainfluenza 3 | 0.4 | |||||||
| Parainfluenza overall | 0.4 | |||||||
| Respiratory syncytial virus | 0.4 | |||||||
| Rhinovirus | 9.2 | |||||||
| Mixed viral infections | 0.8% | |||||||
| No virus | 85.4% | |||||||
| 2006 | Cross-sectional airport study (Iran) | Iranian Returning pilgrims suffering ARIl (n = 255) | Nasal wash | Cell culture/cytopathic effect and staining using virus-specific monoclonal antibodies - PCR | Influenza A | 5.1 | 85.5% | [24] |
| Influenza B | 5.1 | |||||||
| Influenza overall | 10.2 | |||||||
| Parainfluenza 1 | 5.5 | |||||||
| Parainfluenza 2 | 1.6 | |||||||
| Parainfluenza 3 | 0.8 | |||||||
| Parainfluenza overall | 7.9 | |||||||
| Adenovirus | 5.5 | |||||||
| Respiratory syncytial virus | 1.6 | |||||||
| Rhinovirus | 5.9 | |||||||
| Enterovirus | 2.0 | |||||||
| Mixed viral infections | 0.4% | |||||||
| No virus | 67.5% | |||||||
| 2009 | Cross sectional airport survey (Iran) | Iranian returning pilgrims suffering respiratory symptomsm (n = 275) | Throat swabs | Cell culture/cytopathic effect - PCR | Influenza A-H1N1 (culture) | 1.1 | 100% | [25] |
| Influenza A-H3N2 (culture) | 1.8 | |||||||
| Influenza B (culture) | 6.2 | |||||||
| Influenza overall (culture) | 9.1 | |||||||
| Influenza A-H1N1 (PCR) | 1.8 | |||||||
| Influenza A-H3N2 (PCR) | 2.9 | |||||||
| Influenza B (PCR) | 7.3 | |||||||
| Influenza overall (PCR) | 12.0 | |||||||
| Mixed viral infections | ND | |||||||
| No virus (culture) | 90.9 | |||||||
| No virus (PCR) | 88.0 | |||||||
| 2010 | Cross-sectional airport study (Saudi Arabia) | Arriving pilgrimsn suffering from URTIo (n = 713) | Throat swabs | Not described | Influenza A-H1N1 | 3.1 | 26.9 | [26] |
| Influenza A-H3N2 | 1.7 | |||||||
| Influenza B-Hong Kong | 1.1 | |||||||
| Influenza B-Sichuan | 2.1 | |||||||
| Influenza overall | 8.0 | |||||||
| Respiratory syncytial virus | 3.1 | |||||||
| Mixed viral infections | ND | |||||||
| No virus | 88.9% | |||||||
| 2011 | Cross-sectional study (Saudi Arabia) | Australian pilgrims suffering from ILIp, at Mina encampment (n = 80) | Nasal swabs | PCR | Influenza A | 6.3 | [27] | |
| Influenza B | 3.8 | |||||||
| Influenza overall | 10.1 | |||||||
| Parainfluenza 1 | 0 | |||||||
| Parainfluenza 2 | 0 | |||||||
| Parainfluenza 3 | 1.3 | |||||||
| Rhinovirus | 48.8 | |||||||
| Enterovirus | 2.5 | |||||||
| Mixed viral infections | 2.5 | |||||||
| No virus | 41.3 | |||||||
| 2013 | Cross-sectional study (Saudi Arabia) | Pilgrimsq suffering from ILIr, at Mina encampment (n = 112) | Naso-pharyngeal or throat swabs | PCR | Influenza A-H1N1 | 0.9 | 31.3% | [28] |
| Influenza A-H3N2 | 3.6 | |||||||
| Influenza overall | 4.5 | |||||||
| Parainfluenza 1 | 0.9 | |||||||
| Parainfluenza 2 | 0 | |||||||
| Parainfluenza 3 | 1.8 | |||||||
| Parainfluenza overall | 2.7 | |||||||
| Rhinovirus | 26.8 | |||||||
| Adenovirus | 2.7 | |||||||
| Coronavirus OC43 and 229E | 2.7 | |||||||
| Mixed viral infections | 2.7 | |||||||
| No virus | 62.5 | |||||||
| 2013 | Cross sectional study (Saudi Arabie) | Pilgrimss admitted to 15 healthcare facilities with bilateral pneumonia (n = 38) | Sputum | PCR | Influenza A | 15.8 | [29] | |
| Influenza B | 0 | |||||||
| Parainfluenza 1 | 0 | |||||||
| Parainfluenza 2 | 0 | |||||||
| Parainfluenza 3 | 2.6 | |||||||
| Parainfluenza 4 | 0 | |||||||
| Parainfluenza overall | 2.6 | |||||||
| Adenovirus | 0 | |||||||
| Respiratory syncytial virus | 2.6 | |||||||
| MERS-Cov | 0 | |||||||
| CoV-229E | 7.9 | |||||||
| CoV-NL63 | 0 | |||||||
| CoV-HKU1 | 0 | |||||||
| CoV-OC43 | 5.3 | |||||||
| CoV overall | 13.2 | |||||||
| Enterovirus | 0 | |||||||
| Metapneumovirus | 0 | |||||||
| Rhinovirus | 39.5 | |||||||
| Bocavirus | 0 | |||||||
| Mixed viral infections | 15.8 | |||||||
| No virus | 56.7 | |||||||
| 2014 | National survey (Austria) | Returning Austrian pilgrims seeking caret in hospitals/medical centers (n = 7) | Serum, sputum, throat swab, or bronchoalveolar lavage | PCR | MERS-CoV | 0 | 0 | [30] |
| Influenza A-H1N1 | 0 | |||||||
| Influenza A-H3N2 | 28.6 | |||||||
| Influenza B | 42.9 | |||||||
| Influenza overall | 71.4 | |||||||
| Rhinovirus | 26.6 | |||||||
| Mixed viral infections | 0 | |||||||
| No virus | 0 |
Based on history and physical examination.
Evidence of pulmonary consolidation on physical examination and radiograph.
Acute episode associated with respiratory symptoms and radiological evidence of airspace disease of less than 4 weeks' duration.
Fever and at least two of the following: headaches, myalgia, cough, sore throat or coryza.
Sore throat or fever ≥38.3 °C, and cough, headache, runny nose, sneezing, or myalgia.
Fever of at least 38 °C, started within 72 h of presentation; along with history of cough and/or sore throat.
Common cold defined by sore throat, rhinitis, fever, ILI defined by fever >38.5 °C, myalgia, rhinitis and cough, sinubronchitis defined by headaches, purulent postnasal discharge or purulent sputum, cough and fever and pneumonia defined on radiographic findings.
Mainly Indian pilgrims.
Cough, sore throat, rhinorrhea and fever.
Cough, sore throat, rhinorrhea and fever within one week of the onset.
Cough, sore throat, rhinorrhea and fever.
Two of the following conditions: fever, cough, sore throat, congestion, and sinus pain within the past 3 weeks during the Hajj.
Cough, sore throat and fever.
Mainly from Indonesia, Turkey and Pakistan.
Fever and/or headaches and/or myalgia and at least of the following symptoms: runny nose, sneezing, sore throat, cough with or without sputum, difficulty of breathing.
Subjective (or proven) fever plus one respiratory symptom (e.g. dry or productive cough, runny nose, sore throat, shortness of breath) for 3 days or less.
Mainly from Saudi Arabia.
Subjective (or proven) fever and at least one respiratory symptom such as cough, sore throat and rhinorrhea.
Mainly from Asia.
Fever and/or respiratory symptoms.
3.2.1. Study characteristics
A total of 17 studies were conducted from 1991 through 2014 among a total of 5075 pilgrims suffering from upper respiratory tract infections, influenza-like illness, lower tract respiratory infection, or pneumonia. Definition of syndromes differed according to authors. Sample size varied from 7 to 761 individuals. Design of survey included:
-
-
Ten cross-sectional surveys conducted at tertiary care hospitals and primary health care centers in Saudi Arabia [14], [15], [16], [17], [18], [19], [20], [21], [23], [30], two of which included additional pilgrims recruited at Mina encampment [20], [23] and one included additional pilgrims recruited at two airports [21],
-
-
Three cross-sectional surveys conducted at Mina encampment (n = 2) or during the stay in Saudi Arabia and upon returning to the UK (n = 1) [22], [27], [28].
-
-
Three airport cross-sectional surveys conducted at a Saudi airport among arriving pilgrims (n = 1) or at an Iranian airport among returning pilgrims (n = 2) [24], [25], [26].
-
-
One national study conducted in hospitals and medical centers in Austria among returning pilgrims [30].
Most studies were conducted among mixed populations of pilgrims with different nationalities although some countries were predominant in several studies [14], [15], [16], [17], [18], [21], [26], [28], [29]. Some studies were conducted among specific populations including Iranian, British, Australian and Austrian pilgrims [19], [20], [22], [23], [24], [25], [27], [30].
Types of samples included were throat swabs, nasal swabs, naso-pharyngeal swabs, sputum, broncho-alveolar and naso-pharyngeal aspirates, gargled pharyngeal secretions and serum.
Early studies were based on cell culture/cytopathic effect and staining using virus-specific monoclonal antibodies or rapid tests for antigen detection (respiratory samples) and ELISA (serum samples) [14], [15], [16], [17], [19], [20], [22], [23], [24], [25], while most recent studies used PCR (respiratory samples), [20], [22], [23], [24], [25], [27], [28], [29], [30].
3.2.2. Carriage of influenza virus
Prevalence of influenza viruses was investigated in all 16 studies. Two distinct methods of detection were used in some studies, showing a higher sensitivity of ELISA compared to cell culture and of PCR compared to antigen detection and cell culture. When considering the results from the most sensitive methods in each study, the prevalence of influenza viruses ranged 4.5–15.8% (with the exception of one study involving 7 patients only, where it was 71.4% [30]) with prevalence of influenza A ranging 0.6–15.8% and prevalence of influenza B ranging 0–11.5% (with the exception of one study involving 7 patients only, where it was 28.6% for influenza A and 42.9% for influenza B [30]).
Selected analysis of a subset of seven recent studies with similar design using PCR in nasal and or throat swabs from patients suffering ILI or other upper respiratory tract infection symptoms showed prevalence of influenza virus ranging 4.5–13.9%, prevalence of influenza A ranging 4.5–10.4% and prevalence of influenza B ranging 2.3–7.3% [20], [22], [23], [24], [25], [27], [28]. A PCR study of sputum from patients suffering bilateral pneumonia showed 15.8% prevalence of influenza viruses (all influenza A) [29]. In one additional small survey conducted among seven Austrian patients, two were infected with influenza A and three with influenza B [30].
Vaccination rates against influenza were documented in 10 studies ranging from 0 to 100%. Effect of vaccination on influenza detection prevalence was investigated in 6 studies and no statistically significant effect was observed with the following results: 7.7% in vaccinated pilgrims vs. 14.1% in unvaccinated pilgrims (OR = 0.47, 95% CI = 1.01–3.33) [16], 14.3% vs. 13.4% (no statistical test performed) [20], 4.9% vs. 12.0% (p > 0.05) [21], 7.1% vs. 14.3% (p = 0.19) [23], 9.2% vs. 16.5% (p = 0.19) [24], 2.9% vs. 5.2% (p = 1.58) [28].
3.2.3. Carriage of parainfluenza viruses
Parainfluenza prevalence was investigated in nine studies using various methods of detection, four of which used PCR [14], [15], [17], [19], [23], [24], [27], [28], [29]. The prevalence of parainfluenza viruses ranged 0–7.9% with prevalence of parainfluenza 1 ranging 0–5.5%, that parainfluenza 2 ranging 0–1.7% and that of parainfluenza 3 ranging 0–2.6%. Parainfluenza 4 was investigated in one PCR-based study and was not detected.
3.2.4. Carriage of respiratory syncytial virus
Respiratory syncytial virus prevalence was investigated in nine studies [14], [15], [17], [19], [20], [23], [24], [26], [29], four of which included PCR detection [20], [23], [24], [29]. The prevalence of respiratory syncitial virus detection ranged 0–4.5% overall and 0.4–4.5% in PCR-based studies.
3.2.5. Carriage of metapneumovirus
Metapneumovirus was investigated in one PCR-based study and was not detected [29].
3.2.6. Carriage of rhinovirus
Rhinovirus prevalence was investigated in six studies [23], [24], [27], [28], [29], [30] all using PCR detection, in nasal and or throat swabs from patients suffering ILI or other upper respiratory tract infection symptoms in four studies, and in sputum from patients suffering bilateral pneumonia in one study. The prevalence of rhinovirus detection ranged 5.9–48.8%.
3.2.7. Carriage of adenovirus
Adenovirus prevalence was investigated in seven studies [14], [15], [17], [19], [24], [28], [29], three of which included PCR detection [24], [28], [29]. The prevalence of adenovirus detection ranged 0–36.2% overall and 0–5.5% in PCR-based studies.
3.2.8. Carriage of enterovirus
Enterovirus prevalence was investigated in four studies [17], [24], [27], [29], three of which included PCR detection [24], [27], [29]. The prevalence of enterovirus detection ranged 0–2.5%.
3.2.9. Carriage of coronaviruses
Coronaviruses prevalence was investigated in two PCR-based studies [28], [29], with 2.7% and 13.2% prevalence. Only coronaviruses 229E and OC43 were detected.
3.2.10. Mixed viral infections and bacterial surinfections
Co-infection by more than 1 virus were documented in 8 studies [15], [16], [23], [24], [26], [27], [28], [29], [30] and ranged 0–28.9%. Bacterial infections were documented in 5 studies [14], [15], [19], [26], [29], however, surinfection of viral infections by bacteria wad documented in two studies only its prevalence was 0 [15] and 44.7% [29].
3.3. Studies conducted among pilgrims irrespective of the respiratory status
See Table 2 .
Table 2.
Prevalence of respiratory viruses in studies conducted among pilgrims irrespective of the respiratory status.
| Year | Study design | Study population | Type of sample | Microbiological techniques | Pathogens investigated | Prevalence (%) | Influenza vaccination (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| 2003 | Paired cohort survey | UK pilgrims (n = 115) | Serum before and after travel | ELISA | Influenza A-H1N1 | 2.6k | 26.1 | [31] |
| Influenza A-H3N2 | 36.5k | |||||||
| Influenza B | 4.3k | |||||||
| Influenza overall | 38.3k | |||||||
| Mixed viral infections | 5.2k | |||||||
| No virus | 61.7k | |||||||
| 2007–2008 | Paired cohort survey | Iranian pilgrimsa (n = 338) | Serum before and after travel | ELISA | Influenza | 3.6k | 84.0 | [32] |
| Respiratory syncytial virus | 7.4k | |||||||
| Adenovirus | 23.7k | |||||||
| Mixed viral infections | ND | |||||||
| No virus | 65.4k | |||||||
| 2009 | Cross sectional ship port and airport survey (Egypt) | Egyptian returning pilgrims (126 at ship port and 425 at airport) | Nasopharyngeal swabs | PCR | Influenza A-H1N1pdm09 | 0 | 98.1 | [33] |
| Influenza A-H1N1 | 1.0 | |||||||
| Influenza A-H3N2 | 0 | |||||||
| Mixed viral infections | 0 | |||||||
| No virus | 99 | |||||||
| 2009 | Cross sectional airport survey (Iran) | Iranian returning pilgrimsb (n = 305) | Pharyngeal swabs | PCR | Influenza A-H1N1pdm09 | 1.6 | 97.7 | [34] |
| Influenza A-other | 2.6 | |||||||
| Influenza overall | 4.2 | |||||||
| Mixed viral infections | 95.8 | |||||||
| No virus | 0 | |||||||
| 2009 | Cross sectional airport survey (unpaired cohorts) (Saudi Arabia) | Arriving (n = 519) and departing (n = 2699) pilgrimsc | Nasopharyngeal and throat swabs | PCR | Influenza A-H1N1 | 0.2-0.1 | 53.3 (seasonal flu) and 38.8 (pdm09-H1N1) | [35] |
| Influenza A-H3N2 | 0.2-0.3 | |||||||
| Influenza B | 0-0.1 | |||||||
| Influenza overall | 0.4-0.5 | |||||||
| Parainfluenza 1 | 0-0 | |||||||
| Parainfluenza 2 | 0-0 | |||||||
| Parainfluenza 3 | 0-0.1 | |||||||
| Parainfluenza 4 | 0-0 | |||||||
| Parainfluenza overall | 0-0.1 | |||||||
| Adenovirus | 0-0 | |||||||
| Respiratory syncytial virus | 0-0.3 | |||||||
| CoV-229E | 0-0.2 | |||||||
| CoV-NL63 | 0.2-0.1 | |||||||
| CoV-HKU1 | 0-0.1 | |||||||
| CoV-OC43 | 0-0.6 | |||||||
| CoV overall | 0.2-1.0 | |||||||
| Metapneumovirus | 0-0.1 | |||||||
| Rhinovirus | 12.1–13.0 | |||||||
| Bocavirus | 0-0 | |||||||
| Mixed viral infections | 0-0.1 | |||||||
| No virus | 87.5–85.2 | |||||||
| 2009 | Cross sectional survey (Saudi Arabia) | Health care workers serving pilgrims, before and after the Hajj (n = 120) | Combined nasal and throat swabs | PCR | Influenza A-H1N1 | 0-0 | 50.9 (seasonal flu), 21.7 (pdm09-H1N1 | [36] |
| Influenza A-H3N2 | 0-0 | |||||||
| Influenza B | 0-0 | |||||||
| Influenza overall | 0-0 | |||||||
| Parainfluenza 1 | 0-0 | |||||||
| Parainfluenza 2 | 0-0 | |||||||
| Parainfluenza 3 | 0-0 | |||||||
| Parainfluenza 4 | 0-0 | |||||||
| Parainfluenza overall | 0-0 | |||||||
| Adenovirus | 0-0 | |||||||
| Respiratory syncytial virus | 0-0 | |||||||
| CoV-229E | 0-0.8 | |||||||
| CoV-NL63 | 0-0 | |||||||
| CoV-HKU1 | 0-0 | |||||||
| CoV-OC43 | 0-0 | |||||||
| CoV overall | 0-0.8 | |||||||
| Metapneumovirus | 0-0 | |||||||
| Rhinovirus | 7.5–11.7 | |||||||
| Bocavirus | 0-0 | |||||||
| Mixed viral infections | 0-0 | |||||||
| No virus | 92.5–87.5 | |||||||
| 2010 | Cross-sectional airport study (Saudi Arabia) | Arriving pilgrimsd (n = 1600) | Throat swabs | PCR | Influenza A-H1N1 pdm09 | 6.9 | 93.4 | [37] |
| Influenza A-H1N1 non-pdm09 | 0.6 | |||||||
| Influenza overall | 7.5 | |||||||
| Mixed viral infections | – | |||||||
| No virus | 92.5 | |||||||
| 2012 | Paired cohort survey | French pilgrims before arrival, during Hajj and at departuree (n = 165) |
Nasal swabs | PCR | Influenza A-H1N1 | 0-0-0n | 45.6 (2011) | [ [6], [38]] |
| Influenza A-H3N2 | 0-8.6-0 | |||||||
| Influenza B | 0-0-1.3 | |||||||
| Influenza C | 0.6-1.4-0 | |||||||
| Influenza overall | 0.6-10.0-1.3 | |||||||
| Adenovirus | 0.6-1.4-1.9 | |||||||
| Respiratory syncytial virus | 0-1.4-0 | |||||||
| MERS-Cov | 0-0-0 | |||||||
| Enterovirus | 0.6-1.4-0.6 | |||||||
| Metapneumovirus | 0-1.4-0 | |||||||
| Rhinovirus | 3.0-27.1-8.4l | |||||||
| Mixed viral infections | 0-4.3-1.2 | |||||||
| No virus | 95.7-61.4-89.0l | |||||||
| 2013 | Paired cohort survey | French pilgrims before arrival and at departuref (n = 129) | Nasal and throat swabs | PCR | Influenza A-H1N1 | 0-0.8o | 44.2 (2012) | [ [7], [39], [40]] |
| Influenza A-H3N2 | 0-6.2l | |||||||
| Influenza B | 0-0.8 | |||||||
| Influenza C | 1.7–0 | |||||||
| Influenza overall | 1.7–7.8 | |||||||
| Parainfluenza overall | 3.3-0.8 | |||||||
| Adenovirus | 1.7-0.0 | |||||||
| Respiratory syncytial virus | 0-0.8 | |||||||
| MERS-Cov | 0-0 | |||||||
| CoV-229E | 0-12.4l | |||||||
| CoV-NL63 | 0-0.8 | |||||||
| CoV-HKU1 | 0-3.9 | |||||||
| CoV-OC43 | 0-3.9 | |||||||
| CoV overall | 0-20.9 | |||||||
| Cytomegalovirus | 0-0 | |||||||
| Enterovirus | 0.8-2.3 | |||||||
| Metapneumovirus | 1.7-0.8 | |||||||
| Rhinovirus | 14.0–14.7 | |||||||
| Bocavirus | 1.7–0 | |||||||
| Parechovirus | 0-0 | |||||||
| Mixed viral infections | 3.1–5.4 | |||||||
| No virus | 78.5-61.2l | |||||||
| 2013 | Cross sectional airport survey (unpaired cohorts) (Saudi Arabia) | Arriving pilgrimsg (n = 3210) and departing pilgrimsg (n = 2025) | Nasopharyngeal samples | PCR | MERS-CoV | 0-0 | 22.0 | [41] |
| Mixed viral infections | – | |||||||
| No virus | 100-100 | |||||||
| 2013 | Cross-sectional airport study (Ghana) | Returning Ghanaian pilgrimsh (n = 839) | Nasopharyngeal swabs | PCR | Influenza A | 1.3 | [42] | |
| Rhinovirus | 16.8 | |||||||
| Respiratory Syncitial virus | 5.1 | |||||||
| MERS-CoV | 0 | |||||||
| Mixed viral infections | 1.9 | |||||||
| No virus | 78.7 | |||||||
| 2013 | Paired cohort survey/Unpaired cohort survey | Pilgrimsi at arrival and subsequently at Mina (n = 692)/Pilgrims at arrivali (n = 514) Pilgrims at Minaj (n = 470) | Nasal swabs | PCR | Influenza A-H1N1 | 0.1–1.6/0.2-3.0m,l | 21.9 | [43] |
| Influenza A-H3N2 | 0.6-2.0/1.2-1.7 | |||||||
| Influenza B | 0-0/0-0.2 | |||||||
| Influenza C | 0-0/0-0 | |||||||
| Influenza overall | 0.7-3.6/1.4–4.9 | |||||||
| Parainfluenza 1 | 0-0/0.4–0 | |||||||
| Parainfluenza 2 | 0-0.3/0.2-0.4 | |||||||
| Parainfluenza 3 | 0.3-0.1/0-0.2 | |||||||
| Parainfluenza 4 | 0-0.1/0.2–0 | |||||||
| Parainfluenza overall | 0-0.5/0.8-0.6 | |||||||
| Adenovirus | 0-0.6/0-0.4 | |||||||
| Respiratory syncytial virus | 0.6-0.7/0.6-0.2 | |||||||
| MERS-Cov | 0-0/0-0 | |||||||
| CoV-229E | 0.9-14.6l/1.0-10.2l | |||||||
| CoV-NL63 | 0.3-2.0/0-0.2 | |||||||
| CoV-HKU1 | 0.4-1.3/0.2-1.5l | |||||||
| CoV-OC43 | 0.1–1.6/0.4-1.9l | |||||||
| CoV overall | 1.7–19.5/1.6–13.8 | |||||||
| Cytomegalovirus | 0-0/0-0 | |||||||
| Enterovirus | 0.6-0.4/0.8-1.1 | |||||||
| Metapneumovirus | 0-0.1/0.2-0.6 | |||||||
| Rhinovirus | 2.2-34.4l/3.1-30.9l | |||||||
| Bocavirus | 0.1-0/0.2–0 | |||||||
| Parechovirus | 0-0/0-0 | |||||||
| Mixed viral infections | 10.4 overall | |||||||
| No virus | 93.9-50.4l/91.4-54.9l |
Prevalence of cough = 84.3%, hoarseness = 64.6%, sore throat = 62.0%, rhinorrea = 54.2%, wheezing = 53.3%, headache = 44.1%, myalgia = 38.4%, fever = 36.4%, dyspnea = 22.2%.
Prevalence of cough = 48.2%, sore throat = 46.2%, rhinorrhea = 60.7%, fever and cough = 10.5%.
Mainly from Middle-East.
Mainly from Indonesia, India, Algeria and Ivory Coast (prevalence of cough = 92.7% and sore-throat = 3.8%, smokers = 87.7%).
Prevalence of cough = 83.4%, sore throat = 79.7%, rhinorrhea = 68.5%, dyspnea = 19.6%, ILI (fever and cough and sore-throat) = 41.0%.
Prevalence of cough = 86.8%, sore throat = 82.9%, rhinorrhea = 72.1%, dyspnea = 21.7%, ILI (fever and cough and sore-throat) = 47.3%.
Mainly from Asia and Africa.
Prevalence of cough = 70.6%, sore throat = 40.9%, fever = 18.4%, runny-nose or sneezing = 18.1% and breathing difficulty = 14.8%.
From Albania, Bangladesh, Egypt, Ethiopia, India, Indonesia, Malaysia, Pakistan, Somalia, Tanzania, prevalence of ILI = 61.9% at Mina.
From Albania, Bangladesh, Egypt, Ethiopia, France, India, Indonesia, Malaysia, Nigeria, Pakistan, Somalia, Tanzania, prevalence of ILI = 61.9% at Mina.
Seroconversion rate.
Difference statistically significant (p < 0.005, chi-scared test).
Paired cohort at arrival-paired cohort at Mina/unpaired cohort at arrival-unpaired cohort at Mina.
Before arrival-during Hajj-at departure.
Before arrival-at departure (nasal swab)/before arrival-at departure (throat swab).
3.3.1. Study characteristics
A total of 12 studies were conducted from 2003 through 2013 among a total of 12,791 pilgrims [6], [7], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]. Sample size varied from 115 to 3210 individuals. The prevalence of cough was documented in 6 studies and varied from 48.2% to 92.7%. Design of survey included:
-
-
Five paired cohort surveys with longitudinal follow-up of same pilgrims before and after the Hajj [6], [7], [31], [32], [38], [39], [40], [43].
-
-
Three cross-sectional surveys comparing unpaired cohorts of different pilgrims before and after the Hajj, investigated at the airport and/or at Mina encampment [35], [42], [43].
-
-
One cross-sectional survey comparing unpaired cohorts of different health care workers before and after the Hajj, investigated in Saudi hospitals [36].
-
-
One cross-sectional survey among arriving pilgrims conducted in Saudi Arabia [37].
-
-
Three cross-sectional surveys among returning pilgrims conducted in Egypt, Iran and Ghana respectively [33], [34], [42].
Studies were conducted among mixed population of pilgrims from different nationalities [35], [37], [41], [43] or among specific populations including Egyptian, Iranian, British, French and Ghanaian pilgrims [6], [7], [31], [32], [33], [34], [37], [38], [39], [40], [42]. Type of samples included throat swabs, nasal swabs, naso-pharyngeal swabs, and serum. Two studies were based on ELISA seroconversion [31], [32], while other studies used PCR (respiratory samples) [6], [7], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43].
3.3.2. Carriage of influenza virus
Prevalence of influenza viruses was investigated in 11 studies [6], [7], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [42], [43]. A 38.3% seroconversion rate was observed in one UK survey following participation in the Hajj (mostly due to influenza H3N2) while a 3.6% seroconversion rate was observed in one Iranian survey [34]. Differences in influenza vaccination coverage may have accounted for this discrepancy with 26.1% among UK pilgrims compared to 84.0% among Iranian pilgrims. The prevalence by PCR studies among arriving pilgrims ranged 0.6–7.5% and 0.5–7.8% after the Hajj, with most cases being influenza A. The prevalence of influenza after the Hajj was statistically higher than before the Hajj in two studies [39], [43]. Vaccination rates against influenza in these PCR-based studies ranged from 21.9 to 98.1%. Effect of vaccination on influenza detection prevalence was investigated in 5 studies and no statistically significant effect was observed with the following results: 30.0% in vaccinated pilgrims vs. 38.3% in unvaccinated pilgrims (OR = 0.61, p = 0.28) [31], 2.7% vs. 0 (no statistical test performed) [34], 7.4 vs. 8.5% (p > 0.05) [35], no significant differences were observed in two study where details were not provided [38], [43].
Acquisition rate calculated from the largest paired cohort survey was 1.4% for influenza A/H1N1 and 1.9% for influenza A/H3N2 with an overall low 21.9% vaccination rate [43]. The dynamic of influenza carriage was well addressed in one paired cohort survey with 0.6% influenza carriage prevalence in arriving pilgrims (none symptomatic), 10.0% carriage during the Hajj among symptomatic pilgrims and 1.3% carriage after the Hajj (90.4% symptomatic during the Hajj prior sampling, 2.0% still symptomatic at sampling) [38]. In the one study conducted among health care workers, all samples were negative for influenza.
3.3.3. Carriage of parainfluenza viruses
Parainfluenza prevalence was investigated in five studies using PCR [6], [7], [35], [36], [38], [39], [40], [43]. The prevalence of parainfluenza virus detection among pilgrims ranged 0–3.3% before the Hajj and 0.1–0.8% after the Hajj. Acquisition rate calculated from the largest paired cohort survey was 0.1% [43]. In the one study conducted among health care workers, all samples were negative for parainfluenza [36].
3.3.4. Carriage of respiratory syncytial virus
Respiratory syncytial virus prevalence was investigated in seven studies [6], [7], [32], [35], [38], [40], [42], [43] six of which including PCR detection [5], [6], [35], [36], [38], [39], [40], [42], [43]. The prevalence of respiratory virus detection among pilgrims ranged 0–0.6% before the Hajj and 0–5.1% after the Hajj in PCR-based studies. Acquisition rate calculated from the largest paired cohort survey was 0.7% [43]. In the one study conducted among health care worker, all samples were negative for respiratory syncytial virus [36].
3.3.5. Carriage of metapneumovirus
Metapneumovirus was investigated in five PCR-based study, [6], [7], [35], [36], [38], [39], [40], [43]. The prevalence of respiratory virus detection among pilgrims ranged 0–1.7% before the Hajj and 0–0.8% after the Hajj. Acquisition rate calculated from the largest paired cohort survey was 0.1% [43]. In the one study conducted among health care worker, all samples were negative for metapneumovirus [36].
3.3.6. Carriage of rhinovirus
Rhinovirus prevalence was investigated in six PCR studies [6], [7], [35], [36], [38], [39], [40], [42], [43]. The prevalence of rhinovirus detection among pilgrims ranged 2.2–14.0% before the Hajj and 8.4–34.4% after the Hajj. The prevalence of rhinovirus after the Hajj was statistically higher than before the Hajj in two studies [38], [43]. Acquisition rate calculated from the largest paired cohort survey was 34.1% [43]. The dynamic of rhinovirus carriage was well addressed in one paired cohort survey with 3.0% rhinovirus carriage prevalence in arriving pilgrims (none symptomatic), 27.1% carriage during the Hajj among symptomatic pilgrims and 8.4% carriage after the Hajj (90.4% symptomatic during the Hajj prior sampling, 2.0% still symptomatic at sampling) [38]. In the one study conducted among health care workers, 7.5% of individuals tested positive before the Hajj and 11.7% after the Hajj [36].
3.3.7. Carriage of adenovirus
Adenovirus prevalence was investigated in six studies [6], [7], [32], [35], [36], [38], [39], [40], [43], five of which included PCR detection [6], [7], [35], [36], [38], [39], [40], [43]. The prevalence of respiratory virus detection among pilgrims ranged 0–1.7% before the Hajj and 0–0.6% after the Hajj in PCR-based studies. Acquisition rate calculated from the largest paired cohort survey was 0.6% [43]. In the one study conducted among health care worker, all samples resulted negative for adenovirus [36].
3.3.8. Carriage of enterovirus
Enterovirus prevalence was investigated in three PCR studies [6], [7], [38], [39], [40], [43]. The prevalence of enterovirus detection among pilgrims ranged 0.6–0.8% before the Hajj and 0.4–2.3% after the Hajj. Acquisition rate calculated from the largest paired cohort survey was 0.4% [43].
3.3.9. Carriage of coronaviruses
Coronaviruses prevalence was investigated in seven PCR studies [6], [7], [35], [36], [38], [39], [40], [41], [42], [43]. The prevalence of non-coronavirus detection among pilgrims ranged 0.2–1.7% before the Hajj and 1.0–20.9% after the Hajj, with most infections due to coronavirus 229-E. The prevalence of non MERS-coronaviruses after the hajj was statistically higher than before the Hajj in two studies [39], [43]. Acquisition rate calculated from the largest paired cohort survey was 4.9% [43]. In the one study conducted among health care worker, 0% individuals tested positive before the Hajj and 0.8% after the Hajj [36]. MERS coronavirus was never isolated.
3.3.10. Carriage of bocavirus
Bocavirus was investigated in four PCR studies and detected among arriving pilgrims only in a few cases [7], [35], [36], [38], [39], [40], [43].
3.3.11. Carriage of cytomegalovirus and parechovirus
Cytomegalovirus and parechovirus were investigated in two PCR studies and never detected [7], [38], [39], [40], [43].
4. Discussion
The viruses primarily associated with upper respiratory tract infections commonly include rhinoviruses, enteroviruses, adenoviruses, parainfluenza viruses, influenza viruses, respiratory syncytial viruses and coronaviruses 229E and OC43. Non-respiratory viruses including measles virus, herpes simplex virus 1, varicella zoster virus and cytomegalovirus may occasionally be responsible for respiratory involvement. In recent years new human respiratory viruses have been reported including human metapneumovirus, bocavirus, new human coronaviruses including Severe Acute Respiratory Syndrome coronavirus, human coronavirus NL63, and HKU1 and Middle East Respiratory Syndrome coronavirus [44] and parechovirus [45]. New variants of viruses have also emerged including human adenovirus-14, variant swine-like influenza H3N2, avian influenza H7N9 and H10N8 [46].
In this review we show that the viruses most commonly isolated from symptomatic patients during the Hajj are rhinovirus (5.9–48.8% prevalence), followed by influenza virus (4.5–13.9%) and coronaviruses (2.7–13.2%) with most infections due to coronavirus 229E; other viruses are less frequently isolated. We also show that these viruses have low carriage rates among arriving pilgrims while an increase is observed in the carriage rate following participation in the Hajj. This increase was statistically significant for several viruses in certain studies including influenza AH1N1 [43], influenza AH3N2 [39], rhinovirus [38], [43], coronavirus 229E [39], [43], coronavirus HKU1 and OC43 [43]. These cosmopolitan viruses are probably easily transmitted between pilgrims given the crowded conditions at the Holy Mosque and other religious sites around Mecca. The Holy Mosque's total capacity is 2 million and the average crowd density is at least four people per square meter and can reach levels of 6–8 people per square meter as people get closer to the Kaaba [47]. It is also likely that housing conditions at Mina encampment in tents of 50–100 pilgrims play a role in the transmission of respiratory viruses [27]. Overall, these results show an acquisition of viruses following the Hajj with high carriage prevalence among departing pilgrims. This suggests that an international mass gathering such as the Hajj may contribute to the globalization of common respiratory pathogens. Of note, emerging pathogens like SARS coronavirus and MERS coronavirus were not isolated from Hajj pilgrims until now, although rare cases of MERS have been reported among Umrah pilgrims [48]. Mixed viral infections were relatively rare; however it depends mostly on the number of viruses investigated. The proportion of mixed viral infections reached 10.4% in a study where 21 different viruses were tested [43].
Since therapeutic options are limited in the context of respiratory viral infections [49] prevention is of paramount importance. Influenza vaccination is recommended to Hajj pilgrims by the Saudi Arabian Ministry of Health [50]. In this review, no significant effect of influenza vaccination was observed on the carriage of influenza virus in individual studies and the uptake of seasonal influenza vaccine was very variable. The apparent lake of efficacy of vaccination against influenza in the context of the Hajj may result of a mismatch of circulating strains with vaccine strains [51]. The effectiveness of influenza vaccine was assessed in pooled metadata from six studies included in the present review [16], [20], [21], [23], [30], [36] by other authors [51]; influenza was significantly effective against laboratory-confirmed influenza (risk ratio 0.56; 95% CI 0.41–0.75; p < 0.001). However a definitive conclusion should be not drawn from this analysis [51]. Non-pharmaceutical interventions, such as hand hygiene, wearing a face mask, cough etiquette, social distancing, and contact avoidance can be effective in reducing the spread of respiratory viruses from person to person [52] and are therefore recommended to Hajj pilgrims by national public health agencies [50]. However, evidence of their effectiveness at the Hajj are limited and inconclusive and prospective cohort studies are required to confirm whether or not such non-pharmaceutical interventions are relevant for interrupting or reducing the spread of respiratory viruses during the annual Hajj pilgrimage [53]. A cluster-randomized controlled trial is being conducted to provide valuable evidence on the efficacy of facemasks in preventing viral respiratory tract infections during the Hajj [54].
Our review was limited to papers written in English which may have been a source of bias. There was an in important heterogeneity in studies in regards of study populations, clinical criteria for respiratory infections and diagnostic methods applied. The design of surveys conducted so far among pilgrims does not allow us to ascertain the role of viruses in the pathogenesis of respiratory symptoms and their potential role in the severity of the symptoms. The frequency of secondary bacterial infection could not be evaluated from the available studies since most surveys only addressed virus carriage and the number of viruses investigated was limited in many studies. The role of co-infection with viruses and bacteria warrants further investigation using ideally a paired cohort-survey design, allowing assessment of acquisition rate of a large panel of pathogens with sequential sampling and careful recording of clinical data associated with the highly common “Hajj cough” [55].
Conflict of interest
No competing interest.
Funding
No funding source.
References
- 1.Memish Z.A., Zumla A., Alhakeem R.F., Assiri A., Turkestani A., Al Harby K.D. Hajj: infectious disease surveillance and control. Lancet. 2014 Jun 14;383(9934):2073–2082. doi: 10.1016/S0140-6736(14)60381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed Q.A., Arabi Y.M., Memish Z.A. Health risks at the Hajj. Lancet. 2006 Mar 25;367(9515):1008–1015. doi: 10.1016/S0140-6736(06)68429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Tawfiq J.A., Zumla A., Memish Z.A. Respiratory tract infections during the annual Hajj: potential risks and mitigation strategies. Curr Opin Pulm Med. 2013 May;19(3):192–197. doi: 10.1097/MCP.0b013e32835f1ae8. [DOI] [PubMed] [Google Scholar]
- 4.Shafi S., Booy R., Haworth E., Rashid H., Memish Z.A. Hajj: health lessons for mass gatherings. J Infect Public Health. 2008;1(1):27–32. doi: 10.1016/j.jiph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Deris Z.Z., Hasan H., Sulaiman S.A., Wahab M.S., Naing N.N., Othman N.H. The prevalence of acute respiratory symptoms and role of protective measures among Malaysian hajj pilgrims. J Travel Med. 2010 Mar-Apr;17(2):82–88. doi: 10.1111/j.1708-8305.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 6.Gautret P., Charrel R., Belhouchat K., Drali T., Benkouiten S., Nougairede A. Lack of nasal carriage of novel corona virus (HCoV-EMC) in French Hajj pilgrims returning from the Hajj 2012, despite a high rate of respiratory symptoms. Clin Microbiol Infect. 2013 Jul;19(7):E315–E317. doi: 10.1111/1469-0691.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautret P., Charrel R., Benkouiten S., Belhouchat K., Nougairede A., Drali T. Lack of MERS coronavirus but prevalence of influenza virus in French pilgrims after 2013 Hajj. Emerg Infect Dis. 2014 Apr;20(4):728–730. doi: 10.3201/eid2004.131708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzahrani A.G., Choudhry A.J., Al Mazroa M.A., Turkistani A.H., Nouman G.S., Memish Z.A. Pattern of diseases among visitors to Mina health centers during the Hajj season, 1429 H (2008 G) J Infect Public Health. 2012 Mar;5(1):22–34. doi: 10.1016/j.jiph.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Al-Ghamdi S.M., Akbar H.O., Qari Y.A., Fathaldin O.A., Al-Rashed R.S. Pattern of admission to hospitals during muslim pilgrimage (Hajj) Saudi Med J. 2003 Oct;24(10):1073–1076. [PubMed] [Google Scholar]
- 10.Madani T.A., Ghabrah T.M., Albarrak A.M., Alhazmi M.A., Alazraqi T.A., Althaqafi A.O. Causes of admission to intensive care units in the Hajj period of the Islamic year 1424 (2004) Ann Saudi Med. 2007 Mar-Apr;27(2):101–105. doi: 10.5144/0256-4947.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baharoon S., Al-Jahdali H., Al Hashmi J., Memish Z.A., Ahmed Q.A. Severe sepsis and septic shock at the Hajj: etiologies and outcomes. Travel Med Infect Dis. 2009 Jul;7(4):247. doi: 10.1016/j.tmaid.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Mandourah Y., Al-Radi A., Ocheltree A.H., Ocheltree S.R., Fowler R.A. Clinical and temporal patterns of severe pneumonia causing critical illness during Hajj. BMC Infect Dis. 2012 May;16(12):117. doi: 10.1186/1471-2334-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzeer A.H. Respiratory tract infection during Hajj. Ann Thorac Med. 2009 Apr;4(2):50–53. doi: 10.4103/1817-1737.49412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SM1 El-Sheikh, El-Assouli S.M., Mohammed K.A., Albar M. Bacteria and viruses that cause respiratory tract infections during the pilgrimage (Haj) season in Makkah, Saudi Arabia. Trop Med Int Health. 1998 Mar;3(3):205–209. [PubMed] [Google Scholar]
- 15.Alzeer A., Mashlah A., Fakim N., Al-Sugair N., Al-Hedaithy M., Al-Majed S. Tuberculosis is the commonest cause of pneumonia requiring hospitalization during Hajj (pilgrimage to Makkah) J Infect. 1998 May;36(3):303–306. doi: 10.1016/s0163-4453(98)94315-8. [DOI] [PubMed] [Google Scholar]
- 16.Kholeidi A.N., Baksh M.F., A1 Hamdan N.A., A1 Mazam A.A., Mohammed A.G., Ghazi H. Seropositivity in clinical influenza cases among pilgrims during Hajj, 1421 H. Saudi Epidemiol Bull. 2001;8(4):27–28. [Google Scholar]
- 17.Balkhy H.H., Memish Z.A., Bafaqeer S., Almuneef M.A. Influenza a common viral infection among Hajj pilgrims: time for routine surveillance and vaccination. J Travel Med. 2004 Mar-Apr;11(2):82–86. doi: 10.2310/7060.2004.17027. [DOI] [PubMed] [Google Scholar]
- 18.AlSaleh E., A1 Mazroua M., Choudhiy A.J., Turkistani A., A1 Hunzrlan N., Azhur E. Serotypes of influenza during Hajj season, 1424 (2004) Saudi Epidemiol Bull. 2005;12(1):1–7. [Google Scholar]
- 19.Razavi S.M., Ziaee H., Mokhtari-Azad T., Hamkar R., Doroodi T., Mirsalehian A. Surveying respiratory infections among Iranian Hajj pilgrims. Iran J Clin Infect Dis. 2007;2(2):67–70. [Google Scholar]
- 20.Rashid H., Shafi S., Booy R., El Bashir H., Ali K., Zambon M. Influenza and respiratory syncytial virus infections in British Hajj pilgrims. Emerg Health Threats J. 2008;1:e2. doi: 10.3134/ehtj.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Anzi F., AI Mazroa M., Chaudhry J., Humdan N., Azhur E. Distribution of influenza virus during Hajj season 1426 Hijri (2005 G) Saudi Epidemiol Bull. 2006;13:10–13. [Google Scholar]
- 22.Rashid H., Shafi S., Haworth E., El Bashir H., Ali K.A., Memish Z.A. Value of rapid testing for influenza among Hajj pilgrims. Travel Med Infect Dis. 2007 Sep;5(5):310–313. doi: 10.1016/j.tmaid.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Rashid H., Shafi S., Haworth E., El Bashir H., Memish Z.A., Sudhanva M. Viral respiratory infections at the Hajj: comparison between UK and Saudi pilgrims. Clin Microbiol Infect. 2008 Jun;14(6):569–574. doi: 10.1111/j.1469-0691.2008.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alborzi A., Aelami M.H., Ziyaeyan M., Jamalidoust M., Moeini M., Pourabbas B. Viral etiology of acute respiratory infections among Iranian Hajj pilgrims, 2006. J Travel Med. 2009 Jul-Aug;16(4):239–242. doi: 10.1111/j.1708-8305.2009.00301.x. [DOI] [PubMed] [Google Scholar]
- 25.Moattari A., Emami A., Moghadami M., Honarvar B. Influenza viral infections among the Iranian Hajj pilgrims returning to Shiraz, Fars province. Iran Influenza Other Respir Viruses. 2012 Nov;6(6):e77–e79. doi: 10.1111/j.1750-2659.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdulrahman N.K., Chaudhry A.J., Al Mazroa M. Etiology of upper respiratory tract infection among international pilgrims arriving for Hajj 2010 G. Saudi Epidemiol Bull. 2011;19(2):14–15. [Google Scholar]
- 27.Barasheed O., Almasri N., Badahdah A.M., Heron L., Taylor J., McPhee K. Pilot randomised controlled trial to test effectiveness of facemasks in preventing influenza-like illness transmission among Australian Hajj Pilgrims in 2011. Infect Disord Drug Targets. 2014;2:110–116. doi: 10.2174/1871526514666141021112855. [DOI] [PubMed] [Google Scholar]
- 28.Barasheed O., Rashid H., Alfelali M., Tashani M., Azeem M., Bokhary H., Hajj Research Team Viral respiratory infections among Hajj pilgrims in 2013. Virol Sin. 2014 Dec;29(6):364–371. doi: 10.1007/s12250-014-3507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Memish Z.A., Almasri M., Turkestani A., Al-Shangiti A.M., Yezli S. Etiology of severe community-acquired pneumonia during the 2013 Hajj-part of the MERS-CoV surveillance program. Int J Infect Dis. 2014 Aug;25:186–190. doi: 10.1016/j.ijid.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aberle J.H., Popow-Kraupp T., Kreidl P., Laferl H., Heinz F.X., Aberle S.W. But not MERS-CoV in Hajj Pilgrims, Austria, 2014. Emerg Infect Dis. 2015 Apr;21(4):726–727. doi: 10.3201/eid2104.141745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Bashir H., Haworth E., Zambon M., Shafi S., Zuckerman J., Booy R. Influenza among U.K. pilgrims to hajj, 2003. Emerg Infect Dis. 2004 Oct;10(10):1882–1883. doi: 10.3201/eid1010.040151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imani R., Karimi A., Habibian R. Acute respiratory viral infections among Tamattu' Hajj pilgrims in Iran. Life Sci J. 2013;10(3s):449–453. [Google Scholar]
- 33.Kandeel A., Deming M., Elkreem E.A., El-Refay S., Afifi S., Abukela M. Pandemic (H1N1) 2009 and Hajj Pilgrims who received predeparture vaccination, Egypt. Emerg Infect Dis. 2011 Jul;17(7):1266–1268. doi: 10.3201/eid1707.101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziyaeyan M., Alborzi A., Jamalidoust M., Moeini M., Pouladfar G.R., Pourabbas B. Pandemic 2009 influenza A (H1N1) infection among 2009 Hajj Pilgrims from Southern Iran: a real-time RT-PCR-based study. Influenza Other Respir Viruses. 2012 Nov;6(6):e80–e84. doi: 10.1111/j.1750-2659.2012.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Memish Z.A., Assiri A.M., Hussain R., Alomar I., Stephens G. Detection of respiratory viruses among pilgrims in Saudi Arabia during the time of a declared influenza A(H1N1) pandemic. J Travel Med. 2012 Jan-Feb;19(1):15–21. doi: 10.1111/j.1708-8305.2011.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Memish Z.A., Assiri A.M., Alshehri M., Hussain R., Alomar I. The prevalance of respiratory viruses among healthcare workers serving pilgrims in Makkah during the 2009 influenza A (H1N1) pandemic. Travel Med Infect Dis. 2012 Jan;10(1):18–24. doi: 10.1016/j.tmaid.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashshi A., Azhar E., Ayman Johargy A., Asghar A., Momenah A., Turkestani A. Demographic distribution and transmission potential of influenza A and 2009 pandemic influenza A H1N1 in pilgrims. J Infect Dev Ctries. 2014;8(9):1169–1175. doi: 10.3855/jidc.4204. [DOI] [PubMed] [Google Scholar]
- 38.Benkouiten S., Charrel R., Belhouchat K., Drali T., Salez N., Nougairede A. Circulation of respiratory viruses among pilgrims during the 2012 Hajj pilgrimage. Clin Infect Dis. 2013 Oct;57(7):992–1000. doi: 10.1093/cid/cit446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benkouiten S., Charrel R., Belhouchat K., Drali T., Nougairede A., Salez N. Respiratory viruses and bacteria among pilgrims during the 2013 Hajj. Emerg Infect Dis. 2014 Nov;20(11):1821–1827. doi: 10.3201/eid2011.140600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benkouiten S., Gautret P., Belhouchat K., Drali T., Nougairede A., Salez N. Comparison of nasal swabs with throat swabs for the detection of respiratory viruses by real-time reverse transcriptase PCR in adult Hajj pilgrims. J Infect. 2015 Feb;70(2):207–210. doi: 10.1016/j.jinf.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Memish Z.A., Assiri A., Almasri M., Alhakeem R.F., Turkestani A., Al Rabeeah A.A. Prevalence of MERS-CoV nasal carriage and compliance with the Saudi health recommendations among pilgrims attending the 2013 Hajj. J Infect Dis. 2014 Oct 1;210(7):1067–1072. doi: 10.1093/infdis/jiu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Annan A., Owusu M., Marfo K.S., Larbi R., Sarpong F.N., Adu-Sarkodie Y. High prevalence of common respiratory viruses and no evidence of Middle East Respiratory Syndrome Coronavirus in Hajj pilgrims returning to Ghana, 2013. Trop Med Int Health. 2015 Jun;20(6):807–812. doi: 10.1111/tmi.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Memish Z.A., Assiri A., Turkestani A., Yezli S., Al Masri M., Charrel R. Mass gathering and globalization of respiratory pathogens during the 2013 Hajj. Clin Microbiol Infect. 2015 Feb;21(6):571.e1–571.e8. doi: 10.1016/j.cmi.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berry M., Gamieldien J., Fielding B.C. Identification of new respiratory viruses in the new millennium. Viruses. 2015 Mar 6;7(3):996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvala H., Simmonds P. Human parechoviruses: biology, epidemiology and clinical significance. J Clin Virol. 2009 May;45(1):1–9. doi: 10.1016/j.jcv.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Gautret P., Gray G.C., Charrel R.N., Odezulu N.G., Al-Tawfiq J.A., Zumla A. Emerging viral respiratory tract infections–environmental risk factors and transmission. Lancet Infect Dis. 2014 Nov;14(11):1113–1122. doi: 10.1016/S1473-3099(14)70831-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alnabulsi H., Drury J. Social identification moderates the effect of crowd density on safety at the Hajj. Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9091–9096. doi: 10.1073/pnas.1404953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sridhar S., Brouqui P., Parola P., Gautret P. Imported cases of Middle East respiratory syndrome: an update. Travel Med Infect Dis. 2015 Jan-Feb;13(1):106–109. doi: 10.1016/j.tmaid.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunning J., Baillie J.K., Cao B., Hayden F.G. International severe acute respiratory and emerging infection Consortium (ISARIC). Antiviral combinations for severe influenza. Lancet Infect Dis. 2014 Dec;14(12):1259–1270. doi: 10.1016/S1473-3099(14)70821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alqarni H., Memish Z.A., Assiri A.M. Health conditions for travellers to Saudi Arabia for the pilgrimage to Mecca (Hajj) – 2015. J Epidemiol Glob Health. 2015 Jul;13 doi: 10.1016/j.jegh.2015.07.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alqahtani A.S., Rashid H., Heywood A.E. Vaccinations against respiratory tract infections at Hajj. Clin Microbiol Infect. 2015 Feb;21(2):115–127. doi: 10.1016/j.cmi.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 52.Jefferson T., Del Mar C.B., Dooley L., Ferroni E., Al-Ansary L.A., Bawazeer G.A. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011 Jul;6(7) doi: 10.1002/14651858.CD006207.pub4. CD006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benkouiten S., Brouqui P., Gautret P. Non-pharmaceutical interventions for the prevention of respiratory tract infections during Hajj pilgrimage. Travel Med Infect Dis. 2014 Sep-Oct;12(5):429–442. doi: 10.1016/j.tmaid.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M., Barasheed O., Rashid H., Booy R., El Bashir H., Haworth E. A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims. J Epidemiol Glob Health. 2015 Jun;5(2):181–189. doi: 10.1016/j.jegh.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gautret P., Benkouiten S., Griffiths K., Sridhar S. The inevitable Hajj cough: surveillance data in French pilgrims, 2012–2014. Travel Med Infect Dis. 2015 Nov-Dec;13(6):485–489. doi: 10.1016/j.tmaid.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]