Abstract
Aim:
To estimate management cost of NSCLC ALK+ patients with and without brain metastasis (BM), and to compare annual costs in patients treated with alectinib or crizotinib.
Methods:
Management cost/year (€ 2018) in patients with and without BM was estimated with disaggregated resource consumption provided by local oncologists, including medical visits, hospitalizations, diagnostic/laboratory tests, imaging techniques and surgical procedures. The comparison of costs/year with alectinib and crizotinib, considered the cumulative 12-month incidence of BM in ALEX trial (9.4 and 41.4%, respectively).
Results:
Management cost was €6173.42/patient-year without BM and €21,637.50/patient-year with BM. With alectinib, average cost/patient was lower than crizotinib (€4948.51/patient-year).
Conclusion:
Prevention of BM with alectinib may result in reductions of cost/year in the management of advanced ALK+ NSCLC.
Keywords: : alectinib, ALK NSCLC, costs, crizotinib, economic evaluation, metastasis CNS
Practice points.
The annual cost derived from the management of advanced ALK+ NSCLC patients with CNS metastases compared with patients without CNS metastases was estimated.
An expert panel formed by medical oncologists estimated the healthcare resources consumption of each type of patient (with vs without CNS metastases).
Data of cumulative incidence of brain metastases from pivotal Phase III clinical trial ALEX of alectinib (9.4%) versus crizotinib (41.4%) were used to estimate annual cost of managing patients with ALK+ NSCLC treated with alectinib or crizotinib.
Total cost of managing patients without CNS metastases was €6173.42 patient/year, compared with €21,637.50 patient/year in case of having CNS metastases.
The cost per patient treated with alectinib was lower than patients treated with crizotinib, resulting in annual cost savings of €4949 per patient.
An alternative analysis was carried out, including the cost of grade 3–5 adverse event related to the treatments, whose result showed even more cost savings compared with base case, in patients treated with alectinib versus crizotinib (€5044).
This analysis could help health decision makers to make decisions regarding the first-line treatment of advanced ALK+ NSCLC, due to alectinib, through its protective effect on the development of CNS metastases, could result in savings of costs/year related to management of advanced ALK+ NSCLC patients.
Lung cancer, in addition to being the most commonly diagnosed cancer (with 29,503 new cases estimated for 2019) [1], is the leading cause of cancer-related death both worldwide (18.4% of total cancer-related deaths) [1,2] and in Spain (22,121 in 2017), according to the latest available mortality data for the group of patients with malignant tumors [1]. NSCLC accounts for up to 85% of all lung cancers [3] and, of these, approximately 3–7% of patients have a rearrangement in a gene called ALK [4,5]. This gene produces a protein called ALK, which causes cells to grow and spread. Currently, the guidelines by the European Society of Medical Oncology [6] and the Spanish Society of Medical Oncology [3] recommend first-line treatment of ALK+ patients with alectinib, brigatinib, crizotinib or ceritinib. The National Comprehensive Cancer Network guidelines recommend alectinib as the preferred treatment, category 1 [7]. The asymptomatic nature of NSCLC in the early stages of the disease [8] contributes to the diagnosis occurring in the advanced stages of the disease (stages IIIB and IV), making therapeutic options difficult. The prognosis for patients with advanced disease is very poor, with a 5-year survival rate for stage-IV NSCLC of only 4% [7]. Metastases in the CNS represent a frequent complication in ALK+ NSCLC, developing in up to 40% of patients during the course of their disease [9,10], and they are associated with a high rate of morbidity and mortality [11], which entails a high clinical and economic burden in this type of patient [12,13]. In the course of first-line therapy, either with crizotinib or chemotherapy, the frequency of brain metastases may increase up to 60% [10]. Brain metastases are associated with a multitude of complications, particularly neurocognitive, psychological and physical as well as with severe comorbidities and a decrease in life expectancy [10], with average survival ranging from 3 to 14 months [9]. The treatment of brain metastases generally involves holocranial or stereotactic radiotherapy and may include surgical resection and/or chemotherapy (in isolated cases). Additionally, patients sometimes need rehabilitation therapy after treatment, as well as support from caregivers in everyday tasks in the case of convalescent patients [9,10]. The objective of this study was to carry out a cost analysis of the management of brain metastases in patients with advanced ALK+ NSCLC, treated with alectinib or crizotinib. In a first phase, the cost associated with the management of patients with advanced ALK+ NSCLC, with and without metastasis in the CNS was estimated, and in a second phase an analysis of the annual cost of managing patients with advanced ALK+ NSCLC treated with alectinib or with crizotinib was performed, the former being the standard treatment for these patients. To carry out this second phase, we took into account the data on cumulative annual incidences of metastasis in the CNS obtained in the ALEX study, a pivotal Phase III clinical trial that directly compared alectinib and crizotinib [14] and the only study performed in relation to the standard treatment at the time of the analysis. As compared with crizotinib, ALEX study showed superior efficacy and lower toxicity with alectinib treatment in first line of ALK+ NSCLC patients.
The available therapies that were approved for the treatment of patients with ALK+ NSCLC at the time of this analysis were alectinib (Alecensa®, Roche), crizotinib (Xalkori®, Pfizer) and ceritinib (Zykadia®, Novartis).
Material & methods
In a first phase of the analysis, information was collected on the consumption of health resources by patients with ALK+ NSCLC with and without metastasis in the CNS, over a period of 1 year. A cost analysis was performed to compare the annual cost of managing patients with ALK+ NSCLC treated with alectinib or crizotinib and the appearance of brain metastases was considered with each of the treatments. A panel of three medical oncologists from across Spain, who were specialists in lung cancer, provided the disaggregated consumption of resources following a standardized methodology widely accepted in economic evaluations [15], both for patients with metastasis in the CNS and those without metastasis in the CNS, due to the lack of possibility at the time of the study of carrying out a retrospective or prospective analysis.
The healthcare resources collected included specific tests for the diagnosis of ALK+ NSCLC (Table 1), medical visits, hospitalizations, laboratory tests, imaging techniques and surgical procedures (Table 2), depending on the presence or absence of metastases in the CNS. Drug costs were excluded from the analysis.
Table 1. . Consumption of resources associated with the diagnosis of ALK+ NSCLC and unit costs.
| Specific tests to diagnose NSCLC | Patients with suspected diagnosis of ALK+ NSCLC | Unit cost (updated €, 2018) [16] | |
|---|---|---|---|
| Patients (%) | Tests (n) | ||
| Bronchoscopy | 90 10 |
1 2 |
€264.75 |
| Tumor biopsy | 80 | 1 | €342.08 |
| Spirometry | 100 | 1 | €40.38 |
| Bone scan | 10 | 1 | €129.80 |
| Fine needle aspiration | 20 | 1 | €190.17 |
| Chest x-ray | 100 | 1 | €22.23 |
| Cerebral MRI | 80 | 1 | €280.31 |
| Chest computed tomography | 100 | 1 | €131.15 |
| Abdomen/pelvis computed tomography | 100 | 1 | €356.00 |
| Brain computed tomography | 20 | 1 | €135.75 |
| Positron emission tomography | 80 | 1 | €720.26 |
| Transthoracic core needle biopsy | 25 | 1 | €282.47 |
| Analytics, biochemistry, coagulation | 100 | 1 | €22.48 |
| Arterial gasometry | 100 | 1 | €4.27 |
| Electrocardiogram | 100 | 1 | €17.65 |
| Echocardiogram | 20 | 1 | €76.51 |
| Stress test | 20 | 1 | €159.99 |
| Specific ALK+ screening tests | |||
| Immunohistochemistry | 100 | 1 | €48.16 |
| FISH | 20 | 1 | €481.75 |
| Next-generation sequencing | 5 | 1 | €1510.60 |
Table 2. . Consumption of resources associated with the management of patients with versus without metastasis in the CNS and unit costs.
| Rescources | Patients ‘without’ CNS metastasis | Patients ‘with’ CNS metastasis | Unit cost (€, 2018) [16] | ||
|---|---|---|---|---|---|
| Patients (%) | Tests/year (n) | Patients (%) | Tests/year (n) | ||
| Specific procedures for the treatment of metastasis | |||||
| Holocranial brain radiotherapy | NA | NA | 15 | 5 | €4714.59 |
| Radiosurgery or stereotactic radiotherapy | NA | NA | 35 | 3 | €9954.17 |
| Surgical resection | NA | NA | 2 | 3 | €769.87 |
| None | NA | NA | 48 | 0 | €0 |
| Hospitalizations | |||||
| Medical oncology | 10 | 1 (5 days) | 20 | 1 (5 days) | €448.38 |
| Radiation oncology | 2 | 3 (5 days) | €1452.90 | ||
| Visitors | |||||
| Medical oncology | 100 | 12 | 100 | 15 | €141.28 (first visit) €84.76 (successive visits) |
| Emergencies | 100 | 3 | 100 | 5 | €88.58 |
| Radiation oncology | 15 15 |
5 3 |
€173.46 (first visit) €104.08 (successive visits) |
||
| Laboratory tests | |||||
| Blood count | 100 | 12 | 100 | 12 | €4.68 |
| Biochemistry | 100 | 12 | 100 | 12 | €3.72 |
| Thoracentesis | 10 | 1 | 10 | 1 | €104.43 |
| Imaging techniques | |||||
| Bone scan | 5 | 2 | 5 | 2 | €129.80 |
| Cerebral MRI | 0 | 0 | 50 | 4 | €280.31 |
| Thorax/abdomen computed tomography | 100 | 4 | 100 | 4 | €356.00 |
| Brain computed tomography | 70 | 4 | 100 | 4 | €135.75 |
NA: Not applicable.
The estimation of the annual cost of managing patients with ALK+ NSCLC with or without the development of cerebral metastases was made by multiplying resource consumption by the corresponding unit cost. The unit costs (updated to €, 2018) of the health resources were extracted from a Spanish healthcare cost database (Tables 1 & 2) [16]. In a second phase of the analysis, the annual cost of managing patients with ALK+ NSCLC treated with alectinib or crizotinib was estimated considering the cumulative incidences for the development of metastasis in the CNS after 12 months, for each treatment, as observed in the ALEX clinical trial [14]. Of the total patients treated with alectinib, after 12 months, 9.4% had metastasis in the CNS, compared with 41.4% of patients treated with crizotinib. The estimated annual total cost for each of the alternatives was calculated by weighting the annual cost of managing patients with ALK+ NSCLC with a previously estimated development of metastasis multiplied by the incidence of metastasis, and the annual cost of managing patients with ALK+ NSCLC who do not develop metastasis multiplied by the remaining proportion of patients. Finally, a comparison was made of the weighted annual costs for each of the alternatives in order to estimate the difference in annual costs of managing patients with ALK+ NSCLC treated with alectinib versus patients treated with crizotinib.
Alternative analysis
An alternative analysis was carried out to supplement the main analysis considering the cost associated with the management of grade 3–5 adverse events (AE) related to the treatments (reported in at least 10% of patients in either treatment arm). The frequency of AE for each treatment alternative was extracted from the ALEX clinical trial [14] comparing alectinib and crizotinib. The costs associated with managing each of the AEs included in the analysis were identified by a search through the scientific literature and review of studies that included the management of AE in cancer patients with solid tumors (lung, kidney and pancreatic cancer). All the costs specified in the literature were harmonized to 2018, applying the CPI since the year of origin corresponding to each study, published by the National Institute of Statistics (INE) [17]. The costs for those AEs for which a cost could not be identified in the literature were obtained from a Spanish healthcare cost database (Table 3) [16].
Table 3. . Incidence of adverse events and aggregate average cost related to their management.
| Adverse events grades 3–5 (reported in at least 10% of patients) | Incidence alectinib ALEX (%) [14] | Incidence crizotinib ALEX (%) [14] | Average aggregate cost (updated €, 2018) | Ref. |
|---|---|---|---|---|
| Anemia | 5 | 1 | €1720.99 | [18] |
| Arthralgia | 0 | 1 | €1345.88 | [19] |
| Diarrhea | 0 | 2 | €298.32 | [18] |
| Peripheral edema | 0 | 1 | €489.60 | [16] |
| Fatigue | 1 | 0 | €118.95 | [20] |
| Increased ALT | 5 | 15 | €1313.96 | [20] |
| Increased AST | 5 | 11 | €623.74 | [20] |
| Increased bilirubin in blood | 2 | 0 | €1085.64 | [20] |
| Nausea | 1 | 3 | €266.68 | [18] |
| Rash | 1 | 0 | €1898.28 | [20] |
| Vomiting | 0 | 3 | €266.68 | [18] |
Results
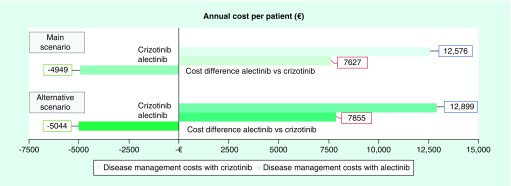
From the consumption of resources estimated by the panel of experts and the corresponding unit costs, an annual management cost of €6173.42 per patient was estimated for patients with ALK+ NSCLC but without metastasis in the CNS, and €21,637.50 per patient for patients with ALK+ NSCLC with metastasis in the CNS. The presence of brain metastases was associated with an annual cost increase of €15,464.08 per patient with ALK+ NSCLC (€1288.67 per month; Figure 1). In the case of patients with brain metastases, the cost item with the greatest bearing on the overall cost (€21,637.50) corresponded to surgical procedures (€14,034), followed by imaging techniques (€2541), diagnostic tests (€2437), medical visits (€1631), hospitalizations (€884) and, lastly, laboratory tests (€111). In patients without cerebral metastases (€6173.43), diagnostic testing had the greatest economic impact (€2437) followed by imaging techniques (€1817) and medical visits (€1584), hospitalizations (€224) and laboratory tests (€111). Considering the cumulative incidence of brain metastasis development in patients treated with alectinib and with crizotinib observed in the ALEX trial [14], the average annual cost of management would be €7627.04 per patient with ALK+ NSCLC treated with alectinib and €12,575.55 per patient with ALK+ NSCLC treated with crizotinib. Therapy with alectinib was associated with a reduction in the cost of managing patients with ALK+ NSCLC, which would produce savings of €4948.51 per patient (Figure 2). For a cohort of 100 patients, treatment with alectinib would produce annual savings of €494,850.76. In the alternative analysis considering the management of possible AEs observed in the ALEX trial [14], an annual management cost of €7854.53 was estimated with alectinib and €12,898.79 with crizotinib, with the annual cost relative to the management of AE being €227.49 with alectinib and €323.24 with crizotinib. The average annual total cost per patient with ALK+ NSCLC treated with alectinib was lower than the cost per patient treated with crizotinib (€5044.26 lower), increasing the saving compared with the main scenario (€4948.51; Figure 3). For a cohort of 100 patients, treatment with alectinib in this alternative scenario would produce annual savings of €504,425.85.
Figure 1. . Annual cost associated with the management of patients with ALK+ NSCLC without metastasis compared with patients with metastasis in the CNS.
The cost for the management of the patients without metastases in the CNS was €6173 per patient/year, in contrast with the management of the patient with metastases in the CNS, which was €21,638 per patient/year (producing an increase of €15,464 per patient/year).
Figure 2. . Results of the main scenario: average cost per patient treated with alectinib versus crizotinib.
Annual cost per patient treated with crizotinib was higher than patients treated with alectinib (€12,575 vs €7627, respectively), which resulted in a cost difference of €4949.
Figure 3. . Results of the alternative scenario (including adverse event): average cost per patient treated with alectinib versus crizotinib.
Results of alternative scenario showed that total cost per patient/year in patients treated with alectinib was lower than patients treated with crizotinib (€7855 vs €12.899, respectively), generating additional savings in comparison with the main scenario.
Discussion
Lung cancer is the leading cause of death related to cancer worldwide and is often not diagnosed until the tumor reaches an advanced stage, in which case the patient’s survival is low. For these reasons, the management of patients with lung cancer currently has a significant economic impact on national health systems around the world, reaching €18.8 billion per year in the EU [21]. Lung cancer treatment in Spain accounts for 15% of the total cost of cancer management in the EU, as it is the type of cancer associated with the highest cost, with total health costs estimated at €1258 million (2009) [21]. According to a study conducted in Spain at the regional level, the average cost of a patient with advanced NSCLC is €12,482, with lower costs related to surgical and hospital care compared with those patients with nonadvanced disease, and higher costs related to chemotherapy treatment [22].
The appearance of brain metastases in the CNS is frequent in patients with advanced NSCLC and is associated with a high clinical and economic burden [9,10,12]. According to several international studies, the incremental cost derived from the consumption of resources related to the presence of brain metastases ranges from $6029 to $20,301 per patient/month [10,13,23,24]. This disparity in the range of results could mainly be due to differences in the consumption of resources assessed, to population heterogeneity in the study and to different data capture periods and methods [12]. Additionally, it is also important to note that healthcare costs and reimbursement policies may vary significantly across different health systems in the world [12]. In the pivotal ALEX trial [14], alectinib was associated with a preventive effect on the appearance of brain metastases with respect to the standard treatment at the time (crizotinib). The results of this analysis establishing a direct relationship between the lower incidence of metastasis in the CNS due to said protective effect and a lower consumption of healthcare resources suggest that alectinib therapy in patients with advanced ALK+ NSCLC could reduce the cost associated with managing the patient as a consequence of its effect on preventing the appearance of metastasis in the CNS. Taking into account this analysis, compared with crizotinib there would be estimated savings of up to €4948.51 per patient/year for the health system (€5044.26, including AE). Crizotinib, despite being the first drug approved in the treatment of ALK+ NSCLC, has the limitation that half of the patients progress during the first year of treatment (average PFS of 10.9 months) [25], so that new therapeutic options for treating these patients are required. This study has a series of strengths. This is the first study conducted in Spain, which presents data on the resource consumption associated with the management of patients with ALK+ NSCLC depending on the presence or absence of metastasis in the CNS. It is also the first study to inter-relate the costs arising from this resource consumption with cumulative data on the incidence of CNS metastases arising from treatment with alectinib and crizotinib to estimate the difference in patient management costs between both the treatments. Other studies carried out in other countries have shown the benefit of alectinib versus crizotinib, in terms of quality-adjusted life-years and cost savings related to brain metastases [26–30]. The choice of crizotinib as a comparator in this analysis made it possible to use ALEX clinical trial data, in which a head-to-head comparison between both the treatments was carried out.
The use of data from direct comparison studies, with high methodological robustness, can be understood as a strength that limits the possible bias associated with the use of information from various studies, where differences may be found in the profile of the patients included. Pharmacoeconomic analyses and the modeling intrinsic there to imply the adoption of premises, which may be the source of limitations. Ideally, to carry out this analysis, we would have used published data on the consumption of resources in the two types of patients analysed (with metastasis in the CNS vs no metastases in the CNS) in Spain, and corroborated these data with the panel of experts. However, in the absence of Spanish publications, the information was collected directly from the panel of experts. The participation of a panel of experts who, based on their experience, determined the consumption of resources associated with patients with versus without metastases in the CNS, instead of extrapolating the data on the consumption of resources from international studies [13], decreased possible bias due to differences in the management of patients between different healthcare settings. Moreover, drug costs were not considered in the analysis because the main objective of this study was to estimate and quantify the direct costs associated with healthcare consumption associated with the development of CNS metastases.
Despite the limitations, the results of this analysis provide information that could facilitate decision-making in clinical practice in Spain, due to the choice of therapies that reduces the incidence of brain metastases, could lead to a decrease in the management costs of these patients for national health system. This information can be used alongside international recommendations on the treatment of NSCLC recently published by scientific societies, such as the European Society of Medical Oncology guide [6], National Comprehensive Cancer Network NSCLC guide [9], national recommendations from the Spanish Society of Medical Oncology [3], Spanish Society of Medical Oncology evaluation reports and therapeutic positioning reports by the Spanish Agency for Medicines and Health Products. Taking into account the clinical (for patients) and economic (for healthcare systems) losses arising from the appearance of metastasis in the CNS in patients with advanced NSCLC, and in line with the results obtained in this analysis, there is an unquestionable need to adopt effective strategies that prevent the appearance of brain metastases and to implement screening strategies to detect ALK rearrangements that would help the physician to prescribe the most effective treatment at an early stage. This would, thus, make it possible to control and minimize the cost arising from their management, thereby contributing to the sustainability of the CNS and improving the prognosis for the disease and the patients’ quality of life. The scarcity of disaggregated data on the actual cost or consumption of resources in these patients demonstrates the value of carrying out future studies in which actual unbundled data on the consumption of health resources in these patients are collected, whether with a retrospective or prospective approach.
Conclusion
In conclusion, according to the results obtained with the methodology described, treatment with alectinib would reduce the annual cost of managing patients with advanced ALK+ NSCLC due to its preventive effect on the appearance of metastasis in the CNS compared with crizotinib.
Footnotes
Author contributions
D Isla, B Massuti, M Lázaro, L Ruiz de Alda, R Gordo, N Ortega-Joaquín & I Oyagüez, L Ruiz de Alda, R Gordo, N Ortega-Joaquín and I Oyagüez conceived the study. N Ortega-Joaquín and I Oyagüez developed the analysis and drafted the manuscript. D Isla, B Massuti and M Lázaro, as clinical experts, contributed to the validation of the necessary parameters for the analysis. All the experts were involved in collecting the necessary data and parameters for conducting the analysis through a panel of experts. All the authors participated in the interpretation of results. All the authors had full access to the data, revisions of the manuscript and approved the final version thereof for submission to this journal.
Financial & competing interests disclosure
This work was financed in an unconditional way by Roche Farma. N Ortega-Joaquín and I Oyagüez are the employees of Pharmacoeconomics & Outcomes Research Iberia (PORIB), a consultancy specializing in the economic assessment of health technologies, which has received unconditional funding from Roche Farma to carry out this analysis. L Ruiz de Alda and R Gordo are the employees of Roche Farma. D Isla, B Massuti and M Lázaro collaborated as experts in validating the parameters used in the analysis, having received unconditional funding for such purpose from Roche Farma, which at no time influenced their opinions or the conclusions obtained. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Sociedad Española de Oncología Médica (SEOM). Cancer figures in Spain (2019). https://seom.org/dmcancer/wp-content/uploads/2019/Informe-SEOM-cifras-cancer-2019.pdf
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Majem M, Juan O, Insa A. et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin. Transl. Oncol. 21(1), 3–17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Spanish Society of Medical Oncology recommendations for first-line treatment of ALK+ patients.
- 4.Sun YW, Xu J, Zhou J, Liu WJ. Targeted drugs for systemic therapy of lung cancer with brain metastases. Oncotarget 9(4), 5459–5472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reckamp KL. Targeted therapy for patients with metastatic non-small cell lung cancer. J. Natl Compr. Cancer Netw. 16(5S), 601–604 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Planchard D, Popat S, Kerr K. et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29(4), iv192–iv237 (2018). [DOI] [PubMed] [Google Scholar]; • European Society of Medical Oncology recommendations for first-line treatment of ALK+ patients.
- 7.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (2019). www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 8.American Cancer Society. Learn about lung cancer (2019). www.cancer.org/cancer/non-small-cell-lung-cancer.html
- 9.Economopoulou P, Mountzios G. Non-small cell lung cancer (NSCLC) and central nervous system (CNS) metastases: role of tyrosine kinase inhibitors (TKIs) and evidence in favor or against their use with concurrent cranial radiotherapy. Transl. Lung Cancer Res. 5(6), 588–598 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guérin A, Sasane M, Zhang J. et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J. Med. Econ. 18(4), 312–322 (2015). [DOI] [PubMed] [Google Scholar]; • An important article describing treatment patterns, symptoms, healthcare resource utilization, and costs, before and after brain metastases diagnosis.
- 11.Nicoś M, Jarosz B, Krawczyk P. et al. Screening for ALK abnormalities in central nervous system metastases of non-small-cell lung cancer. Brain Pathol. 28(1), 77–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan PC, Rodrigues MM, Lim FM. et al. Economic burden of brain metastases in patients with non-small cell lung cancer: costs and implications. Ann. Palliat. Med. 8(2), 210–214 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Burudpakdee C, Wong W, Seetasith A, Corvino FA, Yeh W, Gubens M. Economic impact of preventing brain metastases with alectinib in ALK-positive non-small cell lung cancer. Lung Cancer 119, 103–111 (2018). [DOI] [PubMed] [Google Scholar]; • Baseline article where alectinib reduced costs related to brain metastases by preventing or delaying the occurrence of brain metastases compared with crizotinib.
- 14.Peters S, Camidge DR, Shaw AT. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377(9), 829–838 (2017). [DOI] [PubMed] [Google Scholar]; •• Primary results from the pivotal Phase III ALEX trial where alectinib showed superior efficacy and lower toxicity in primary treatment of ALK-positive NSCLC, compared with crizotinib
- 15.Spanish Society of Hospital Pharmacy. Economic evaluation guide and budget impact on drug assessment reports (2016). http://gruposdetrabajo.sefh.es/genesis
- 16.eSalud. Oblikue Consulting. eSalud database of health costs in Spain (2018). www.oblikue.com/bddcostes/
- 17.National Statistics Institute. Consumer Price Index (2018). www.ine.es/calcula/
- 18.Banz K, Bischoff H, Brunner M. et al. Comparison of treatment costs of grade ¾ adverse events associated with erlotinib or pemetrexed maintenance therapy for patients with advanced non-small-cell lung cancer (NSCLC) in Germany, France, Italy, and Spain. Lung Cancer 74(3), 529–534 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Frías C, Cortés J, Seguí MÁ, Oyagüez I, Casado MÁ. Cost–effectiveness analyses of docetaxel versus paclitaxel once weekly in patients with metastatic breast cancer in progression following anthracycline chemotherapy, in Spain. Clin. Transl. Oncol. 12(10), 692–700 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Martín-Escudero V, García-Muro X, Trigo JM. et al. Use of resources and costs related to the management of adverse events associated with the use of targeted therapies in the treatment of metastatic renal cell carcinoma in Spain. Presented at: Health Economics Association (AES) – 30th Health Economics Conference. P082 (2010). www.aes.es/jornadas [Google Scholar]
- 21.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 14(12), 1165–1174 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Corral J, Espinàs JA, Cots F. et al. Estimation of lung cancer diagnosis and treatment costs based on a patient-level analysis in Catalonia (Spain). BMC Health Serv. Res. 15, 70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes AW, Wu B, Turner RM. Brain metastases in non-small cell lung cancer patients on epidermal growth factor receptor tyrosine kinase inhibitors: symptom and economic burden. J. Med. Econ. 20, 1136–1147 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Guérin A, Sasane M, Dea K. et al. The economic burden of brain metastasis among lung cancer patients in the United States. J. Med. Econ. 19, 526–536 (2016). [DOI] [PubMed] [Google Scholar]; • Describes economic burden of brain metastasis in lung cancer (direct healthcare costs to payers and indirect costs to patients, payers and employers) in the USA.
- 25.Solomon BJ, Mok T, Kim DW. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 373(16), 1582 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Sivignon M, Monnier R, Tehard B, Roze S. Cost–effectiveness of alectinib compared to crizotinib for the treatment of first-line ALK+ advanced non-small-cell lung cancer in France. PLoS ONE 15(1), e0226196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan H, Sheng Y, Guo W, Han S, Shi L. Cost–effectiveness of alectinib for patients with untreated ALK-positive non-small cell lung cancer in China. Adv. Ther. 36(5), 1114–1125 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Carlson JJ, Suh K, Orfanos P, Wong W. Cost–effectiveness of alectinib vs crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Pharmacoeconomics 36(4), 495–504 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Li H, Lai L, Wu B. Cost–effectiveness of ceritinib and alectinib versus crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Clin. Drug Investig. 40(2), 183–189 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Zhang L, Huang Q, Li N, Zheng B, Cai H. Cost–effectiveness analysis of ceritinib and alectinib versus crizotinib in the treatment of anaplastic lymphoma kinase-positive advanced non-small cell lung cancer. Cancer Manag. Res. 11, 9195–9202 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]