Abstract
Mucosal epithelia constitute the first barriers to be overcome by pathogens during infection. The induction of protective IgA in this location is important for the prevention of infection and can be achieved through different mucosal immunization strategies. Lactic acid bacteria have been tested in the last few years as live vectors for the delivery of antigens at mucosal sites, with promising results. In this work, Streptococcus pneumoniae PsaA antigen was expressed in different species of lactic acid bacteria, such as Lactococcus lactis, Lactobacillus casei, Lactobacillus plantarum, and Lactobacillus helveticus. After nasal inoculation of C57Bl/6 mice, their ability to induce both systemic (IgG in serum) and mucosal (IgA in saliva, nasal and bronchial washes) anti-PsaA antibodies was determined. Immunization with L. lactis MG1363 induced very low levels of IgA and IgG, possibly by the low amount of PsaA expressed in this strain and its short persistence in the nasal mucosa. All three lactobacilli persisted in the nasal mucosa for 3 days and produced a similar amount of PsaA protein (150–250 ng per 109 CFU). However, L. plantarum NCDO1193 and L. helveticus ATCC15009 elicited the highest antibody response (IgA and IgG). Vaccination with recombinant lactobacilli but not with recombinant L. lactis led to a decrease in S. pneumoniae recovery from nasal mucosa upon a colonization challenge. Our results confirm that certain Lactobacillus strains have intrinsic properties that make them suitable candidates for mucosal vaccination experiments.
Keywords: Lactic acid bacteria, Streptococcus pneumoniae, PsaA
1. Introduction
Streptococcus pneumoniae is the major agent of pneumonia around the world, causing up to one million deaths per year, mainly in developing countries [1]. The high costs of medical care and the appearance of new clinical isolates with multidrug resistance led to the search for efficient new vaccines to prevent pneumococcal infection. Since the pathogen enters the host through the respiratory mucosa, a vaccine inducing the production of protective secretory IgA at this site, as well as systemic IgG antibodies, would be desirable.
Pneumococcal surface antigen A (PsaA) is a membrane-anchored virulence factor, possibly involved in Mn2+ and Zn2+ transport as predicted by its crystal structure [2]. PsaA deletion mutants display low ability to adhere to mucosal cells and therefore are less pathogenic [3]. This characteristic may be due to differences in the modulation of pneumococcal adhesins caused by the absence of Mn2+ or Zn2+ in the cell [4]. PsaA is conserved among the 90 described S. pneumoniae serotypes and is also immunogenic, which makes it a good candidate for vaccine formulations. In fact, antibodies produced against PsaA by nasal immunization, using cholera toxin B subunit as adjuvant, were shown to protect mice against nasopharyngeal colonization by S. pneumoniae. This protection can be further increased by the co-administration of PsaA with the pneumococcal surface protein A (PspA), another pneumococcal virulence factor [5], [6]. In another approach, oral immunization of mice with PsaA encapsulated in microspheres induced the production of IgG and IgA and resulted in protection against lung colonization and septicemia with five different S. pneumoniae strains [7].
Live bacterial vaccine vectors are being extensively studied for mucosal immunization in the prevention of different infectious diseases [8], [9]. Among them, lactic acid bacteria (LAB) are especially attractive since they are microorganisms present in the gastrointestinal mucosa of healthy individuals, are widely used in dietary products and possess a GRAS (generally recognized as safe) status. This characteristic is not shared by attenuated pathogen derived live vectors, due to the possibility of reversion of the attenuated phenotype, which could be dangerous mainly for immunocompromised individuals.
Interaction of LAB with the immune system and their potential as antigen carriers are the subjects of a number of recently published studies [10], [11], [12], [13], [14], [15]. Different strains and routes of inoculation were evaluated using the fragment C of the tetanus toxin (TTFC), which is so far the best characterized antigen expressed in LAB [9], [16], [17], [18]. Most of these approaches resulted in protection against tetanus toxin lethal challenge [16], [19]. Other antigens like the protective antigen from Bacillus anthracis [20], the E7 protein from human papilloma virus 16 [21], the L7/L12 antigen from Brucella abortus [22], the Env protein from HIV [23], the M protein from Streptococcus pyogenes [24] and the spike glycoprotein from gastroenteritis coronavirus [25] were expressed in LAB and their potential as vaccines against the associated diseases are currently being evaluated. In some cases, such as the immunization with L. lactis expressing the HIV Env protein or the M protein from Streptococcus pyogenes, protection was observed using adequate animal models [23], [24]. In other cases, in vitro neutralizing effects of the antibodies were observed [25].
Using an inducible expression system based on the lactose operon from Lactobacillus casei [26] we expressed the PsaA and the PspA antigens from S. pneumoniae [27] either intracellularly or secreted to the culture media. These recombinant L. casei are being tested as potential anti-pneumococcal vaccines through nasal immunization of mice, but so far we could not detect significant levels of anti-PsaA IgA or IgG (unpublished data). The failure in stimulating the production of antibodies may be a result of the lack of PsaA or PspA expression in the recombinant L. casei after nasal immunization, due to the absence of the inducer in the host mucosa. For this reason, we decided to use a system that allows the constitutive expression of PsaA in different LAB strains. In this work, recombinant Lactococcus lactis, Lactobacillus casei, Lactobacillus plantarum and Lactobacillus helveticus expressing PsaA were evaluated for their ability to induce systemic and mucosal immune responses in nasally immunized C57Bl/6 mice. Nasal colonization of Streptococcus pneumoniae in these mice was also analyzed.
2. Materials and methods
2.1. Bacterial strains and growth conditions
L. casei CECT5275 (formerly ATCC 393 [pLZ15]−), L. plantarum NCDO1193 (kindly provided by Dr K. Thompsom from the Food and Agricultural Microbiology Research Division, Department of Agriculture, Northern Ireland, UK) and L. helveticus ATCC 15009 were routinely grown in MRS medium (Difco), at 37 °C, without shaking. L. lactis MG1363 was grown in M17 medium (Difco) containing 0.5% glucose (GM17) at 30 °C without shaking. Plating of bacteria was performed on the respective media with 1.8% agar. For the selection of transformants, 5 μg/ml of erythromycin was used in the media.
2.2. Plasmids and recombinant DNA procedures
The pT1NX vector, kindly provided by Dr Lothar Steidler (Department of Molecular Biology, Flanders Interuniversity Institute for Biotechnology, Ghent University, Belgium) contains the lactococcal P1 constitutive promoter and the usp-45 signal sequence [28]. The 867 bp gene encoding PsaA from S. pneumoniae serotype 6B (strain St 472/96 from the Instituto Adolpho Lutz, São Paulo, SP, Brazil) was amplified from pCI-psaA plasmid [29] by PCR using the following oligonucleotides:
PsaA-I-forw: 5′ATG CAT CGA TAT CAG CTA GCG GAA AAA AAG AT3′ and
PsaA-I-rev: 5′CCA AGC TTT TAT TTT GCC AAT CCT TCA GC3′
PCR was performed using Taq DNA polymerase (Invitrogen), 200 mM of each deoxynucleoside triphosphate and 20 pmol of each primer. PCR amplification conditions were as follows: 94 °C, 4 min; 30 cycles of 94 °C, 45 s; 45 °C, 45 s; 72 °C 1.5 min; 72 °C, 7 min for final extension. PCR products were cloned into the pGEM-T vector (Promega) for sequence confirmation. One clone was chosen for preparation of the PsaA fragment as follows. The pGEM-T-psaA plasmid was digested with HindIII and treated with Klenow polymerase (Invitrogen) for generation of a blunt end and was further digested with NsiI. The resulting fragment was cloned into pT1NX previously digested with BamHI and treated with Klenow DNA polymerase (Invitrogen) for generation of a blunt end and then digested with PstI, providing a protrude end compatible with the NsiI end from the insert. Nucleic acid manipulation and general cloning procedures were performed according to laboratory manuals [30].
For the preparation of competent L. lactis, an overnight culture was diluted 1:50 in GM17 containing 0.5% glycine and incubated at 30 °C until OD600 reached 0.6. Bacteria were collected by centrifugation at 10,000 × g, for 10 min at 4 °C and the pellet was washed two times with 0.5 M saccharose, 10% glycerol (v/v). Bacteria were resuspended in 1:100 of the same solution and electroporated immediately or kept at −80 °C for further use. L. lactis was electroporated with ligation mixtures at 2.5 kV, 200 Ω, 25 mF in 0.2 cm cuvettes using a BioRad GenePulser (BioRad, Life Science Research Products, CA, USA). Plasmids isolated from L. lactis were used for electroporation of all Lactobacillus strains. Electroporation of L. casei was carried out as previously described [31]. The same protocol was used for the other lactobacillus strains except that the electroporation buffer was three-fold concentrated. Antibiotic resistant L. lactis and Lactobacillus clones were screened for the presence of the insert of interest by PCR, using specific primers as described above. Positive clones were frozen in GM17 or MRS containing 15% glycerol, at −80 °C.
2.3. Protein expression and Western blot analysis
Isolated L. lactis and Lactobacillus clones were grown overnight in GM17 or MRS, respectively. Cultures (10 ml) were collected by centrifugation at 4000 × g for 10 min and bacterial pellet was suspended in 1 ml of 100 mM Tris–HCl, pH 8.0. Cell suspensions were transferred to 2 ml tubes containing the same volume of glass beads and lysates were prepared by vigorous shaking in a Bead-Beater (Biospec, Bartlesville, OK, USA) (four cycles of 30 s at maximal speed). Lysates were centrifuged at 15,000 × g for 5 min and supernatants were maintained at −20 °C for further analysis. For quantification of PsaA, cultures were grown until OD600 reached 2. The CFU values of each culture were determined and extracts corresponding to 109 CFU were analyzed by Western blot. A concentration curve was loaded on the same gel using recombinant PsaA expressed and purified from E. coli [32]. For the analysis of PsaA in the cell wall, bacteria were incubated with 20 mg/ml of lysozyme in 50 mM glucose, 25 mM Tris–HCl pH 8.0 and 10 mM EDTA, at 37 °C for 30 min. The suspension was centrifuged at 15,000 × g for 5 min and the supernatants were collected. Protein extracts or supernatants were fractionated by SDS-PAGE and electrotransferred to nitrocellulose membranes using the Mini Protean II equipment (BioRad, Life Science Research Products, CA, USA). Mouse polyclonal anti-PsaA antiserum was developed against recombinant S. pneumoniae PsaA (from strain St 472/96) expressed in E. coli. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma Chemical, St. Louis, MO, USA) was used according to the manufacturer's instructions. Detection was performed using the chemiluminescent ECL kit (GE Healthcare).
2.4. Immunization and analysis of immune responses
Six- to eight-week-old female C57Bl/6 mice (five per group) received either the recombinant bacteria expressing PsaA or the respective control bacteria harboring the empty vector. An additional control group received saline. LAB strains were grown until cultures reached an OD600 of 2.0; bacteria were collected by centrifugation (4000 × g, 20 min at room temperature), washed with saline and then suspended at 109 cells in 10 μl. For nasal immunization, mice were anesthetized with a mixture of 0.5% xilazine and 0.2% ketamine and 10 μl of a saline suspension containing 109 cells were inoculated into the nostrils with the help of a micropipette on days 0, 1, 14, 15, 28 and 29. Ten days after the last booster mice were bled by the retrorbital plexus. For the collection of saliva, a 0.01% pilocarpine solution was injected intraperitoneally. Nasal and bronchial washes were performed as described elsewhere, using 200 μl and 300 μl of saline, respectively [33]. Anti-PsaA antibodies were detected from sera, saliva, nasal and bronchial washes by enzyme-linked immunoabsorbent assay (ELISA) as previously described [29], using rPsaA purified from E. coli as coating and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG or anti-mouse IgA (Sigma Chemical, St. Louis, MO, USA). Titers were defined as the last dilution in which absorbances at 492 nm reached 0.1.
The maintenance of the LAB strains harboring the pT1NX or the pT1NX-PsaA plasmids in respiratory mucosa was analyzed up to 7 days after a nasal inoculation of 109 bacteria. For this, we performed nasal and bronchial washes in groups of four animals on days 1, 3, 5 and 7 after inoculation (day 0). Dilutions of these washes were plated in MRS or GM17 containing 5 μg/ml of erythromycin. Colonies were counted after 48 h incubation at 37 °C.
2.5. Streptococcus pneumoniae nasal colonization
Six- to eight-week-old C57Bl/6 mice (12 per group) were immunized as described above. Individual serum and pooled saliva were collected for analysis of anti-PsaA IgG and IgA, respectively. Fifteen days after the last inoculation, animals were anesthetized and 10 μl of a suspension containing 5 × 106 CFU of Streptococcus pneumoniae strain 0603 serotype 6B [34] were inoculated nasally. After 5 days, nasal washes were performed using 200 μl of saline. Serial dilutions of the samples were plated in blood agar containing 8 μg/ml gentamicin. Alpha-hemolytic colonies were counted after incubation of the plates for 24 h at 37 °C, considering the volume recovered. For representation in the graphic and statistical analysis log10 was applied to the values and recovery of 0 CFU was considered 1 CFU.
2.6. Statistical analysis
Differences in antibody titers were analyzed by the Mann–Whitney U test. (P ≤ 0.05 was considered significantly different). The same test was used for the analysis of S. pneumoniae colonization. (P ≤ 0.02 was considered significantly different).
3. Results
3.1. Expression of PsaA in different LAB
The psaA gene was cloned into the pT1NX vector PstI site, which produced a fusion of a truncated usp45 signal peptide carried by the vector to the PsaA sequence ( Fig. 1), under the control of a constitutive promoter. Ligation products were used to transform L. lactis and expression of PsaA was analyzed by Western blot of cell lysates. L. lactis carrying the constructed vector showed the expression of a protein around 37 kDa reacting against anti-PsaA antibodies, which was not present in extracts from cells carrying the empty vector ( Fig. 2A). Recombinant plasmid isolated from L. lactis was used to transform different Lactobacillus strains. Western blot analysis of protein extracts from erythromycin resistant colonies showed the expression of PsaA in L. casei, L. plantarum and L. helveticus. The PsaA band was not observed in the respective strains carrying the empty vector (Fig. 2A). Using recombinant PsaA as reference, it could be calculated that 109 L. lactis CFU expressed approximately 20 ng of PsaA, while the amounts synthesized by the different Lactobacillus strains were about 150 ng (L. plantarum), 200 ng (L. casei) and 250 ng (L. helveticus) of PsaA (Fig. 2B). PsaA was not detected in 10-fold concentrated culture media from these clones, indicating that secretion of the protein was not occurring (data not shown). However, analysis of the supernatant obtained after incubation of the cells with lysozyme, showed that at least part of the protein is being directed to the cell wall (Fig. 2C). Moreover, when we incubated whole cells with mouse polyclonal anti-PsaA followed by incubation with anti-mouse IgG-peroxidase conjugate and further revealed with OPD and H2O2, positive reactions were observed in all LAB expressing PsaA, indicating that at least part of the protein is exposed outside of the cell, in contrast to negative reactions observed for the LAB strains carrying the empty vector (data not shown).
Fig. 1.
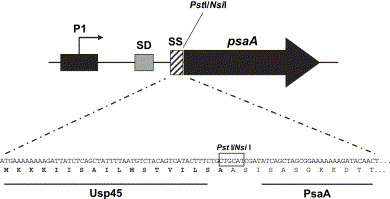
Schematic representation of the PsaA-expressing plasmid and amino acid sequence resulting from the genetic fusion. P1 represents the constitutive promoter; SD, Shine-Dalgarno sequence; SS, first codons of the Usp45 signal peptide fused to the psaA gene.
Fig. 2.
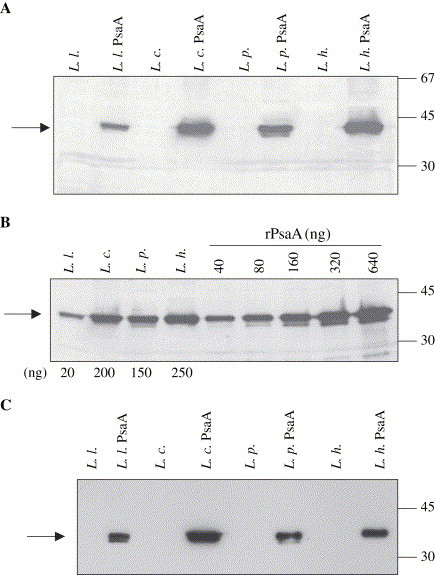
Expression of PsaA in different LAB strains. (A) Western blot analysis from protein extracts show the constitutive expression of PsaA in L. lactis (L. l. PsaA), L. casei, (L. c. PsaA), L. plantarum (L. p. PsaA) and L. helveticus (L. h. PsaA). PsaA bands are pointed by an arrow. Lysates from respective control strains carrying the pT1NX empty vector are also shown in the figure. (B) Lysates from 109 cells of each recombinant LAB were loaded onto SDS-PAGE and transferred to a nitrocellulose membrane. Concentrations from 40 to 640 ng of rPsaA were used as reference. The estimated amounts of PsaA are shown below the panel (20 ng for L. lactis; 200 ng for L. casei, 150 ng for L. plantarum and 250 ng for L. helveticus). (C) Supernatants recovered after treatment of the different strains with lyzosyme. Arrows point to the PsaA band.
3.2. Induction of anti-PsaA antibodies by nasal immunization with recombinant LAB
Recombinant LAB were used for nasal immunization of C57Bl/6 mice. As can be observed in Fig. 3A, nasal inoculation with L. casei, L. plantarum or L. helveticus expressing PsaA induced specific anti-PsaA IgA in pooled saliva. No detectable levels of anti-PsaA IgA antibodies were observed in saliva collected from animals that received L. lactis expressing PsaA, the LAB strains carrying the pT1NX vector or saline. The animals were then subjected to nasal and bronchial washes for the analysis of the presence of IgA in the respiratory tract. In these samples, the highest IgA titers could be observed in animal groups that received either L. plantarum or L. helveticus expressing PsaA (Fig. 3A and B) with means significantly different from groups that received saline (P < 0.001 and P < 0.05, respectively) and the respective controls carrying the empty vector (P < 0.001 and P < 0.05, respectively). Most of the animals that received L. casei expressing PsaA did not display detectable levels of IgA in nasal or bronchial mucosa. In this group, only a single animal showed a very high IgA titer, but the mean of the group was not significantly different from the controls (saline or L. casei carrying the empty vector, P > 0.05). The group that received L. lactis expressing PsaA displayed low levels of anti-PsaA IgA in nasal washes or even no detectable levels in bronchial washes (Fig. 3A and B), with the mean values being not different from the saline group or the L. lactis pT1NX group (P > 0.05). Analysis of the sera collected from the same animals revealed that the animals that displayed the highest levels of anti-PsaA IgA also displayed the highest levels of anti-PsaA IgG, showing correlation in the production of these two classes of antibodies (data not shown).
Fig. 3.
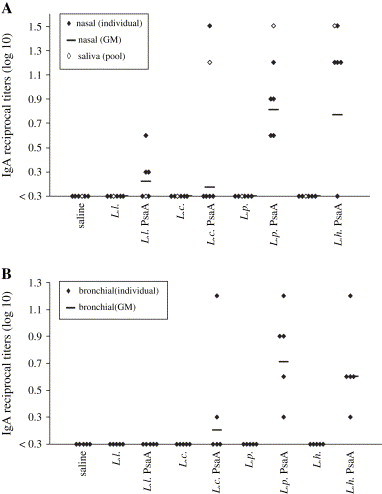
Analysis of the induction of IgA in saliva, nasal and bronchial washes. Saliva and nasal washes (A) and bronchial washes (B) from individual mice were analyzed by ELISA for anti-PsaA antibodies. Log10 of reciprocal antibody titers are shown. Animals that received the respective LAB strains carrying the pT1NX empty vector (L. l.; L. c.; L. p. and L. h.) and saline were used as controls. Results are representative of two independent experiments. The individual sera that displayed non-detectable anti-PsaA IgA titers were represented as <0.3.
For the analysis S. pneumoniae colonization, the same experiment was then performed using 12 animals per group. Before challenge, the induction of anti-PsaA IgG was evaluated in the serum collected from each animal and produced similar results as the previous experiment ( Fig. 4). Nasal administration of recombinant L. plantarum or L. helveticus expressing PsaA led to the induction of the highest titers of anti-PsaA IgG in the serum, whereas low levels of anti-PsaA IgG could be observed in the sera of the animals that received recombinant L. lactis or L. casei (Fig. 4). Statistical analysis showed that the results obtained for the groups that received L. plantarum PsaA or L. helveticus PsaA were significantly higher than the results obtained for the group that received saline (P < 0.01 for L. plantarum and P < 0.001 for L. helveticus) or the respective control strains carrying the pT1NX vector (P < 0.001 for both).The levels of anti-PsaA IgG induced by L. lactis PsaA or L. casei PsaA were not significantly different from the levels found in the saline group (P > 0.05). Comparisons with their control strains carrying the empty vector show that no differences were found for L. lactis (P > 0.05), whereas L. casei PsaA induced higher levels of anti-PsaA IgG when compared with L. casei pT1NX (P = 0.01).
Fig. 4.
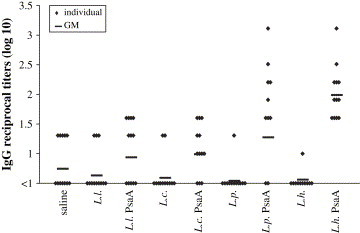
Induction of IgG by nasal immunization of mice with different LAB strains. Sera from individual mice were analyzed by ELISA for anti-PsaA antibodies. Log10 of reciprocal antibody titers are shown. Animals that received the respective LAB strains carrying the pT1NX empty vector (L. l.; L. c.; L. p. and L. h.) or saline were used as controls. Results are representative of two independent experiments. The individual sera that displayed non-detectable anti-PsaA IgG titers were represented as <1.
3.3. Inhibition of Streptococcus pneumoniae nasal colonization
Fifteen days after the last dose, the animals were challenged with S. pneumoniae strain 0603. Results from colonies counting ( Fig. 5) show significant reduction of S. pneumoniae colonization in the L. plantarum PsaA and L. helveticus PsaA groups, when compared to the animals that received saline (P = 0.02 for L. plantarum and P < 0.01 for L. helveticus). When compared with their respective controls, no significant differences were observed for L. plantarum (P > 0.02), but administration of L. helveticus PsaA led to a significant reduction in S. pneumoniae colonization in relation to L. helveticus-pT1NX (P = 0.02). Administration of L. lactis PsaA did not induce significant reduction of S. pneumoniae colonization either when compared with the saline group or the L. lactis pT1NX group (P > 0.02). Interestingly, administration of L. casei pT1NX led to an inhibition in S. pneumoniae colonization when compared with the saline group (P < 0.01) and expression of PsaA in this strain produced a much stronger effect (P < 0.001). However, the results obtained for the L. casei PsaA group were not significantly different from the results obtained for the L. casei pT1NX group (P > 0.02). No other LAB strain carrying the empty vector induced significant reduction in S. pneumoniae colonization when compared with the saline group.
Fig. 5.
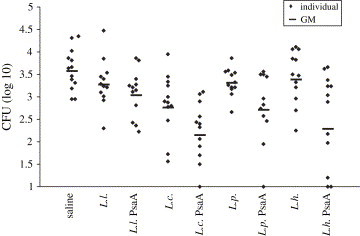
Nasal colonization by S. pneumoniae. Dilutions of individual nasal washes were plated on blood agar and α-hemolytic colonies were counted after 24 h incubation. Log10 of total CFU is shown. Animals that received the respective LAB strains carrying the pT1NX empty vector (L. l.; L. c.; L. p. and L. h.) or saline were used as controls. Absence of colonies in individual nasal washes is represented as 1.
3.4. Recovery of recombinant LAB strains from nasal mucosa
The differences observed in the ability to induce anti-PsaA antibodies as well as the differences in S. pneumoniae colonization led us to analyze possible correlations with the permanency of each recombinant strain in mice respiratory mucosa. All LAB strains could be recovered from nasal washes on day 1 in almost the totality of animals analyzed (3 of 4 animals for L. lactis PsaA, L. casei PsaA and L. helveticus PsaA, 4 of 4 animals for L. plantarum PsaA). On the other hand, erythromycin resistant L. lactis were already absent on day 3, although all Lactobacillus strains were recovered in practically all of the animals analyzed on that day (3 of 4 animals for L. casei and L. plantarum and 4 of 4 animals for L. helveticus). None of the LAB was recovered from nasal washes on days 5 or 7 or from bronchial washes on any of the tested days. Nasal inoculation of the respective strains carrying the empty vector produced similar results, showing that PsaA expression does not exert any effect in bacteria permanency on mice respiratory mucosa (data not shown).
Protein extracts from L. lactis recovered on day 1 and Lactobacillus strains recovered on day 3 were tested for the expression of PsaA. For this, three colonies of each strain were grown overnight and protein extracts were analyzed by Western blot. All recovered colonies tested were still able to express PsaA in vitro (data not shown).
4. Discussion
Several strategies to induce mucosal immune responses against S. pneumoniae antigens are currently being tested [6], [32]. One strategy can be the use of LAB as live vectors for the delivery of specific antigens to mucosal surfaces due to their adhesion to the epithelium and to their claimed adjuvant properties [17], [35], [36].
In order to circumvent a possible bacterial host dependent effect that could compromise the success when designing live vectors for mucosal vaccination, we have expressed the PsaA protein from S. pneumoniae in L. casei CECT 5275, L. plantarum NCDO1193 and L. helveticus ATCC 15009 as well as in the model LAB L. lactis MG1363 strain. After the fusion to the Usp45 signal sequence, the recombinant PsaA protein was directed to the cell wall in the four LAB assayed, with no protein detected in culture supernatants. In a previous report, expression of PsaA in fusion with the leader sequence from the L. casei cell wall proteinase PrtP also resulted in the localization of the protein in the cell wall and no secretion to the culture media [27]. Since the sequence which specifies PsaA covalent anchoring to the cell surface in its natural streptococcal host is absent from our construct, it seems that this protein has the particularity to be retained in the cell wall in different bacteria.
The recombinant LAB inoculated by the nasal route were able to induce different levels of specific anti-PsaA IgA and IgG in C57Bl/6 mice (Fig. 3, Fig. 4). L. plantarum and L. helveticus turned out to be better in the development of mucosal and systemic anti-PsaA immune response than L. casei and L. lactis. Pooled saliva from the L. casei PsaA group displayed an IgA induction similar to those found in the L. plantarum PsaA or the L. helveticus PsaA groups (Fig. 3A). However, when nasal or bronchial washes of individual animals in the L. casei PsaA group were analyzed, most of them displayed low or even no detectable levels of IgA, with only one animal producing a high titer of anti-PsaA IgA (Fig. 3A and B). The high titer in this individual may be the cause of the anti-PsaA IgA observed in pooled saliva from the L. casei PsaA group (Fig. 3A). Since L. casei expressed about the same amount of PsaA as L. plantarum and L. helveticus and was also recovered from mice nasal mucosa in the same period, the differences observed among L. casei and the other lactobacilli may reflect differences in their intrinsic adjuvant potential. In another study, Shaw and collaborators have shown that L. plantarum NCIMB 8826 expressing TTFC produced better results in oral immunization when compared to L. casei 393 [37]. In this case, L. plantarum persisted for up to 12 days in mice gastrointestinal tract whereas L. casei remained for about 72 h [37]. The inability of L. lactis expressing PsaA to significantly induce serum IgG or secreted IgA after nasal inoculation could be explained by the low level of expression in this strain compared to lactobacilli. In addition, it was observed that lactococci carrying the expression vector, remained in mice nasal mucosa only 1 day after the inoculation, while lactobacilli could be detected up to 3 days later.
Nasal inoculation of mice with L. lactis PsaA did not exert any effect on S. pneumoniae colonization, in comparison with inoculation of saline or L. lactis carrying the empty vector (Fig. 5). On the other hand, colonization of S. pneumoniae was significantly reduced in mice immunized with L. helveticus PsaA, when compared with the animals that received saline or L. helveticus pT1NX. A significant decrease in S. pneumoniae colonization is also observed in the group that received L. plantarum PsaA in relation to the group that received saline, but not in relation to the group that received L. plantarum pT1NX. In this case, a possible inhibition effect related to the strain may be taking place. However, it is important to notice that simply inoculation of the L. plantarum strain carrying the empty vector is not sufficient to cause a decrease in S. pneumoniae colonization. Interestingly the group that received L. casei carrying the empty vector displayed a significant reduction in S. pneumoniae colonization and this reduction was more accentuated in the group that received L. casei PsaA. Since the administration of this strain induced low levels of anti-PsaA antibodies, other mechanisms may be causing this reduction. In addition, although a correlation between antibody induction and inhibition of S. pneumoniae colonization was observed when we compared the groups that received L. lactis PsaA, L. plantarum PsaA and L. helveticus PsaA, this correlation is not always maintained when we analyzed the animals individually. Thus, the induction of anti-PsaA antibodies may be only one of the factors contributing to the reduction of S. pneumoniae colonization. Nasal immunization with recombinant PsaA in combination with other pneumococcal proteins and CTB as adjuvant [6] as well as oral immunization with microencapsulated PsaA and CTB [38] were able to induce mucosal and systemic antibodies and protect mice against S. pneumoniae colonization. These studies suggest a correlation between antibody production and inhibition of colonization. In another study using a colonization model in mice, nasal inoculation of S. pneumoniae resulted in mucosal antibody induction that was associated with clearance of the bacteria. However, no correlation was observed between the amount of antibodies detected in the sera or mucosa and the density of colonization of individual animals. In addition, animals with impaired humoral immunity displayed similar densities and durations of S. pneumoniae colonization than their normal counterparts. The authors discuss that other components of the adaptative immune response as well as innate immune response may be the main contributors for pneumococcal clearance in their model [39].
The probiotic effects of certain Lactobacillus strains have been extensively studied [40]. Among these studies, the L. casei strain Shirota has been shown to induce cellular immunity and to reduce influenza virus titers in mice respiratory tract [41]. The administration of Lactobacillus fermentum prior to a S. pneumoniae challenge led to an increase in anti-S. pneumoniae antibodies as well as in activated macrophages in mice lung and a decrease in S. pneumoniae colonization [42]. Although the regimen of administration in these type of studies differ from that used in this work, a possible probiotic effect of the Lactobacillus strains used here cannot be ruled out and may explain the reduction in S. pneumoniae colonization in animals immunized with L. casei.
Our results support the use of lactobacilli for vaccination purposes and experimentally proved the importance of two key issues in the design of live vectors for oral vaccination which include the amount of antigen delivered in relation to the expression system and the strain chosen. The relevance of the immunization protocols in these experiments should be stressed and they could still be improved, perhaps to achieve stimulation of the immune system with fewer doses and more efficiently. As an attempt, co-administration with lactobacilli strains expressing other pneumococcal antigens, such as the PspA, is currently being evaluated.
Acknowledgements
This work was supported by FAPESP, CNPq and Fundação Butantan. We thank Vera Regina F. Ferreira for coordination of the animal facilities from the Centro de Biotecnologia, Instituto Butantan and Erika Regazoli for help with the animals and technical support.
References
- 1.World health Organization WHO position paper. Wkly. Epidemiol. Rec. 1999;74:177–183. [PubMed] [Google Scholar]
- 2.Lawrence M.C., Pilling P.A., Epa V.C., Berry A.M., Ogunniyi D., Paton J.C. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure. 1998;6:1553–1560. doi: 10.1016/s0969-2126(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 3.Berry A.M., Paton J.C. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 1996;68:133–140. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jedrzejas M.J. Pneumococcal virulence factors. Microbiol. Mol. Biol. Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briles D.E., Hollingshead S., Brooks-Walter A., Nabors G.S., Ferguson L., Schilling M. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine. 2000;18:1707–1711. doi: 10.1016/s0264-410x(99)00511-3. [DOI] [PubMed] [Google Scholar]
- 6.Briles D.E., Ade E., Paton J.C., Sampson J.S., Carlone G.M., Huebner R.C. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 2000;68:796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo J.-Y., Seong S.Y., Ahn B.-Y., Kwon I.C., Chung H., Jeong S.Y. Cross-protective immunity of mice induced by oral immunization with pneumococcal surface adhesin A encapsulated in microspheres. Infect. Immun. 2002;70:1143–1149. doi: 10.1128/IAI.70.3.1143-1149.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina E., Guzmán C.A. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine. 2001;19:1573–1580. doi: 10.1016/s0264-410x(00)00354-6. [DOI] [PubMed] [Google Scholar]
- 9.Seegers J.F.M.L. Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol. 2002;20:508–515. doi: 10.1016/s0167-7799(02)02075-9. [DOI] [PubMed] [Google Scholar]
- 10.Cross M.L. Immunoregulation by probiotic lactobacilli: pro-Th1 signals and their relevance to human health. Clin. Appl. Immunol. Rev. 2002;3:115–125. [Google Scholar]
- 11.Cross M.L. Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol. Med. Microbiol. 2002;34:245–253. doi: 10.1111/j.1574-695X.2002.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 12.Drakes M., Blanchard T., Czinn S. Bacterial probiotic modulation of dendritic cells. Infect. Immun. 2004;72:3299–3309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitazawa H., Watanabe H., Shimosato T., Kawai Y., Itoh T., Saito T. Immunostimulatory oligonucleotide CpG-like motif exists in Lactobacillus delbrueckii ssp. bulgaricus NIAI B6. Int. J. Food Microbiol. 2003;85:11–21. doi: 10.1016/s0168-1605(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 14.Maassen C.B.M., van Holten-Neelen C., Balk F., den Bak-Glashouwer M.-J.H., Leer R.J., Laman J.D. Strain-dependent cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine. 2000;18:2613–2623. doi: 10.1016/s0264-410x(99)00378-3. [DOI] [PubMed] [Google Scholar]
- 15.Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 16.Grangette C., Müller-Alouf H., Goudercourt D., Geoffroy M.C., Turneer M., Mercenier A. Mucosal immune response and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect. Immun. 2001;69:1547–1553. doi: 10.1128/IAI.69.3.1547-1553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plant L.J., Conway P.L. Adjuvant properties and colonization potential of adhering and non-adhering lactobacillus spp. following oral administration to mice. FEMS Immunol. Med. Microbiol. 2002;34:105–111. doi: 10.1111/j.1574-695X.2002.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 18.Robinson K.L., Chamberalin M., Lopez M.C., Rush C.M., Marcotte H., Le Page R.W.F. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect. Immun. 2004;72:2753–2761. doi: 10.1128/IAI.72.5.2753-2761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grangette C., Müller-Alouf H., Goudercourt D., Geoffroy M.C., Goudercourt D., Turneer M. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: impact of strain viability and in vivo persistence. Vaccine. 2002;20:3304–3309. doi: 10.1016/s0264-410x(02)00301-8. [DOI] [PubMed] [Google Scholar]
- 20.Zegers N.D., Kluter E., van der Stap H., van Dura E., van Dalen P., Shaw M. Expression of the protective antigen of Bacillus anthracis by Lactobacillus casei: towards the development of an oral vaccine against anthrax. J. Appl. Microbiol. 1999;87:309–314. doi: 10.1046/j.1365-2672.1999.00900.x. [DOI] [PubMed] [Google Scholar]
- 21.Cortes-Perez N.G., Bermudez-Humaran L.G., Le Loir Y., Rodriguez-Padilla C., Gruss A., Saucedo-Cardenas O. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiol. Lett. 2003;229:37–42. doi: 10.1016/S0378-1097(03)00778-X. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro L.A., Azevedo V., Le Loir Y., Oliveira S.C., Dieye Y., Piard J.-Ch. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 2002;68:910–916. doi: 10.1128/AEM.68.2.910-916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin K.Q., Hoshino Y., Toda Y., Igimi S., Kojima Y., Jounai N. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood. 2003;102:223–228. doi: 10.1182/blood-2003-01-0110. [DOI] [PubMed] [Google Scholar]
- 24.Mannam P., Jones K.F., Geller B.L. Mucosal vaccine made from live, recombinant Lactococcus lactis protects mice against pharyngeal infection with Streptococcus pyogenes. Infect. Immun. 2004;72:3444–3450. doi: 10.1128/IAI.72.6.3444-3450.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho P.S., Kwang J., Lee Y.K. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine. 2005;23:1335–1342. doi: 10.1016/j.vaccine.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosalbes M.J., Pérez-Arellano I., Esteban C.D., Galán J.L., Pérez-Martínez G. Use of lac elements for gene expression in Lactobacillus casei. Lait. 2001;81:29–35. [Google Scholar]
- 27.Oliveira M.L.S., Monedero V., Miyaji E.N., Leite L.C.C., Ho P.L., Perez-Martinez G. Expression of Streptococcus pneumoniae antigens, PsaA (pneumococcal surface antigen A) and PspA (pneumococcal surface protein A) by Lactobacillus casei. FEMS Microbiol. Lett. 2003;227:25–31. doi: 10.1016/S0378-1097(03)00645-1. [DOI] [PubMed] [Google Scholar]
- 28.Schotte L., Steidler L., Vandekerckhove J., Remaut E. Secretion of biologically active murine interleukin-10 by Lactococcus lactis. Enzyme Microb. Technol. 2000;27:761–765. doi: 10.1016/s0141-0229(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 29.Miyaji E.N., Dias W.O., Gamberini M., Gerbara V.C.B.C., Schenkman R.P.F., Wild J. PsaA (pneumococcal surface adhesin A) and PspA (pneumococcal surface protein A) DNA vaccines induce humoral and cellular immune responses against Streptococcus pneumoniae. Vaccine. 2001;20:805–812. doi: 10.1016/s0264-410x(01)00395-4. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J., Fritsch E.F., Maniatis T. second ed. Cold Spring Harbor laboratory Press; Cold Spring Harbor, NY: 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 31.Posno M., Leer R.J., van Luijk N., van Giezen M.J.F., Heuvelmans P.T.H.M., Lokman B.C. Incompatibility of lactobacillus vectors with replicons derived from small cryptic lactobacillus plasmids and segregational instability of the introduced vectors. Appl. Environ. Microbiol. 1991;57:1822–1828. doi: 10.1128/aem.57.6.1822-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arêas A.P.M., Oliveira M.L.S., Miyaji E.N., Leite L.C.C., Aires K.A., Dias W.O. Expression and characterization of cholera toxin B-pneumococcal surface adhesin A fusin protein in Escherichia coli: ability of CTB-PsaA to induce humoral immune response in mice. Biochem. Biophys. Res. Commun. 2004;321:192–196. doi: 10.1016/j.bbrc.2004.06.118. [DOI] [PubMed] [Google Scholar]
- 33.Wu H.-Y., Nahm M.H., Guo Y., Russel M.W., Briles D.E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 34.Malley R., Morse S.C., Leite L.C., Areas A.P., Ho P.L., Kubrusly F.S. Multiserotype protection of mice against pneumococcal colonization of the nasopharynx and middle ear by killed nonencapsulated cells given intranasally with a nontoxic adjuvant. Infect. Immun. 2004;72:4290–4292. doi: 10.1128/IAI.72.7.4290-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geoffroy M.-C., Guyard C., Quatannens B., Pavan S., Lange M., Mercenier A. Use of green fluorescent protein to tag lactic acid bacterium strains under development as live vaccine vectors. Appl. Environ. Microbiol. 2000;66:383–391. doi: 10.1128/aem.66.1.383-391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grangette C., Müller-Alouf H., Hols P., Delcour J., Turneer M., Mercenier A. Enhanced mucosal delivery of antigen with cell wall mutants of lactic acid bacteria. Infect. Immun. 2004;72:2731–2737. doi: 10.1128/IAI.72.5.2731-2737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw D.M., Gaerthe B., Leer R.J., Van Der Stap J.G., Smittenaar C., Heijne Den Bak-Glashouwer M. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology. 2000;100:510–518. doi: 10.1046/j.1365-2567.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo J.Y., Seong S.Y., Ahn B.Y., Kwon I.C., Chung H., Jeong S.Y. Cross-protective immunity of mice induced by oral immunization with pneumococcal surface adhesin a encapsulated in microspheres. Infect. Immun. 2002;70:1143–1149. doi: 10.1128/IAI.70.3.1143-1149.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCool T.L., Weiser J.N. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect. Immun. 2004;72:5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clin. Exp. Allergy. 2003;33:1634–1640. doi: 10.1111/j.1365-2222.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- 41.Hori T., Kiyoshima J., Shida K., Yasui H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clin. Diagn. Lab. Immunol. 2002;9:105–108. doi: 10.1128/CDLI.9.1.105-108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez R.C., Santos V., Nader-Macias M.E. Protective effect of intranasally inoculated Lactobacillus fermentum against Streptococcus pneumoniae challenge on the mouse respiratory tract. FEMS Immunol. Med. Microbiol. 2001;31:187–195. doi: 10.1111/j.1574-695X.2001.tb00519.x. [DOI] [PubMed] [Google Scholar]


