Abstract
Proventricular dilatation disease (PDD) is a fatal inflammatory disease that affects mainly, but not exclusively, psittacine birds (Order: Psittaciformes). PDD has long been suspected to be a viral disease, but its causative agent, a novel Bornavirus, was only identified in 2008.
Keywords: Proventricular dilatation disease, Avian borna virus, Management, Diagnosis
Proventricular dilatation disease (PDD; synonyms: proventricular dilatation syndrome, macaw wasting/fading syndrome, neuropathic gastric dilatation of Psittaciformes, psittacine encephalomyelitis, myenteric ganglioneuritis, infiltrative splanchnic neuropathy) is a fatal inflammatory disease that affects mainly, but not exclusively, psittacine birds (Order: Psittaciformes). The disease was first recognized in the 1970s in imported macaws (Ara sp) in Europe and North America,1, 2, 3, 4, 5, 6, 7 but has since been reported from Australia,8, 9 the Middle East,10, 11, 12 and South America.13 PDD is also present in South Africa (Dr Emily Lane, BVSC, MPHIL, MRCVS, DACVP, personal communication, 2009).
PDD has been reported in more than 70 psittacine species.6, 14, 15, 16 These species include members of the most well-known parrot genera in both the Psittacidae and Cacatuidae families, such as macaws (Ara sp), African gray parrots (Psittacus erithacus), cockatoos (Cacatua sp), Amazon parrots (Amazona sp), conures (eg, Aratinga sp), and cockatiels (Nymphicus hollandicus) (Table 1 ). PDD has not been reported in the budgerigar (Melopsittacus undulatus), which may be resistant to the disease.15, 16
Table 1.
Psittacine species that have been diagnosed with PDDa
| Genus | Species | Origin |
|---|---|---|
| Family: Cacatuidae | ||
| Nymphicus | hollandicus | A/P |
| Cacatua | alba, ducrops, galerita, goffini, haematuropygia, moluccensis, sanguine, sulphurea | A/P |
| Eolophus | roseicapillus | A/P |
| Calyptorhynchus | magnificus | A/P |
| Probosciger | atterimus | A/P |
| Family: Psittacidae | ||
| Psittacula | alexandri, derbiana, eupatria, krameri | A/P |
| Eclectus | roratus | A/P |
| Trichoglossus | haematodus | A/P |
| Ara | ararauna, auricollis, chloroptera, glacogularis, macao, maracan, militarisa, nobilis, rubrogenys, severa, (+hybrids) | AM |
| Anodorhyncus | hyacinthinus | AM |
| Cyanopsitta | spixii | AM |
| Aratinga | acuticaudata, aurea, auricapilla, erythrogenys, finschi, guarouba, jandaya, solstitialis, weddellii | AM |
| Nandayus | nenday | AM |
| Cyanoliseus | patagonus | AM |
| Pyrrhura | molinae, rupicola | AM |
| Brotogeris | pyrrhopterus | AM |
| Rhynchopsitta | pachyrhynca | AM |
| Amazona | aestiva, albifrons, amazonica, auropalliata, autumnalis, leucocephala, ochrococephala, tucumana, xantholora | AM |
| Pionopsitta | pileata | AM |
| Pionus | chalcopterus, fuscus, mestruus, senilis | AM |
| Pionetes | leucogaster, melanocephala | AM |
| Deroptyus | accipitrinus | AM |
| Forpus | coelestris | AM |
| Psittacus | erithacus | AF |
| Poicephalus | guliemi, meyeri, rufiventris, senegatus | AF |
| Coracopsis | vasa | AF |
| Agaporis | personata, roseicollia | AF |
In addition to Psittaciformes, pathologic findings identical to those seen in PDD have been reported in several captive and free-ranging birds representing at least 5 additional orders. These birds include canaries (Serinus canaria, order: Passeriformes), greenfinches (Carduelis chloris, order: Passeriformes), long-wattled umbrella birds (Cephalopterus penduliger, order: Passeriformes), Canada geese (Branta canadensis, order: Anseriformes), roseate spoonbills (Ajaja ajaja, order: Pelecaniformes), peregrine falcons (Falco peregrinus, order: Falconiformes), toucans (Ramphastos sp, order: Piciformes), and bearded barbets (Lybius dubius, order: Piciformes).7, 17, 18, 19, 20
Based on the occurrence of case clusters, PDD has been long considered an infectious disease5; however, under most circumstances the disease seems to spread slowly within aviaries. Outbreaks affecting dozens of birds during a short time period (eg, several weeks) have also been described.10, 21, 22 Crowded indoor aviaries as well as nurseries where parrot chicks are being hand-fed seem to be at the highest risk for PDD outbreaks. Although most of the reported PDD cases are of adult birds,6 birds as young as 5 weeks may be affected.22 Female psittacines have previously been reported to be overrepresented in PDD cases at a ratio of 1:0.6 or more,6, 15 whereas in another study males were overrepresented at a ratio of 1:0.9.14 Therefore, it is most likely that males and females are equally susceptible to PDD. Little is known about the occurrence of PDD in wild avian populations. No PDD cases have been reported to date in free-ranging parrots of any continent. PDD is considered the main threat to captive populations of the highly endangered Spix macaw (Cyanopsitta spixii), a species that is now extinct in the wild.12
Etiology
PDD has long been suspected to be a viral disease based on epidemiologic observations, its apparent infectious nature, the typical lesions associated with it, and by ruling out other possible causes.5, 14, 15 Several researchers have attempted to identify the PDD virus using standard virological methods such as culture and electron microscopy (EM). Initially, a virus was recovered from macaws suffering from serositis, and that was later identified as the Eastern equine encephalitis virus, which was suggested to be the candidate causative agent of PDD.23, 24 However, further research did not support this hypothesis.7, 14 Pleomorphic virus-like particles of variable size (30–250 nm) have also been described in tissues of affected birds by EM.25 These particles were suspected to be of the genus avian paramyxovirus (APMV); however, birds affected by PDD have been shown to lack antibodies against APMV of serotypes 1 to 4, 6, and 7, as well as against avian herpes viruses, polyomavirus, and avian encephalitis virus.5, 6 In studies from Germany, APMV-1, closely related to the Hitchner B1 vaccine strain, was isolated from the spinal cords of around 20% of patients with PDD; however, these isolates showed very low pathogenicity and failed to reproduce the disease in African gray parrots.26, 27 Other virus species that have been sporadically documented in tissues or excretions of affected birds include an adeno-like virus, enterovirus, coronavirus, and reovirus.6, 7, 28, 29
More consistently, an unidentified, enveloped virus of about 80 nm in diameter has been demonstrated by EM in feces of affected birds, and a similar virus was isolated from tissues of affected birds using an embryonic cell culture of a macaw.7, 29, 30, 31 This virus was initially suspected to be an alphavirus, but further investigation has ruled out this possibility.32 Tissue homogenates from an affected bird that contained this virus were used to inoculate and successfully reproduce the disease in several psittacine birds,7, 31 but despite this success and nearly 3 decades of PDD research, the identity of the PDD agent remains enigmatic, with some researchers suggesting an autoimmune rather than a viral cause.15, 33
The major breakthrough in identifying what is now widely believed to be the causative agent of PDD only happened recently, when advanced molecular tools, such as panviral DNA microarrays and high-throughput sequencing, were used to test tissues of PDD-positive birds. In 2008, Kistler and colleagues11 and Honkavuori and colleagues34 independently reported on the recovery of a novel Bornavirus from birds with PDD from the United States and Israel. This virus is now designated avian Bornavirus (ABV). Based on 16 ABV isolates, 5 distinct genotypes were identified, each sharing only around 65% nucleotide sequence identity with previously known members of the Bornaviridae family (all originating from mammalian hosts), and around 85% with other ABV genotypes.11
Bornaviruses are negative-encoded, single-stranded, nonsegmented RNA viruses of the order Mononegavirales. The placement of Bornaviruses within a separate family (Bornaviridae) was based on several unique characteristics of their genome and mechanism of replication, most notably that they replicate in the host-cell nucleus rather than in its cytoplasm.35, 36, 37, 38, 39, 40 Before the discovery of ABV, the single known species within this family was the Borna disease virus (BDV). Borna disease is an encephalitic disease found in horses, sheep, and occasionally other domesticated mammals. The disease was first described in the early nineteenth century in Southeast Germany and has since remained endemic in that area. Many additional species, including the chicken (Gallus gallus), are susceptible to BDV infection under experimental conditions, with the outcome ranging from severe encephalomyelitis to persistent asymptomatic infection.37 The lesions seen with BDV are the result of neural invasion by T CD8+ lymphocytes rather than virus-inflicted cellular damage.38
BDV is an enveloped, spherical, medium-sized virus, with most virions being in the range of 70 to 130 nm.39 The approximately 8900 base-pair genome encodes 6 major genes, including a nucleoprotein (N), a nonstructural protein (P10), a regulatory phosphoprotein (P), a matrix protein (M), a membrane-bound glycoprotein (G), and an RNA-dependent RNA polymerase (L).40 BDV strains show remarkable sequence homogeneity and are all derived from mammalian hosts.11 There is only one report on the recovery of partial BDV RNA sequences from wild avian species.41
In the short time since the publication of the 2 pioneering efforts by Kistler and colleagues11 and Honkavuori and colleagues,34 8 additional studies have reported detecting ABV in PDD-positive birds or in birds exposed to PDD cases originating from 4 continents.20, 22, 42, 43, 44, 45, 46, 47 A sixth ABV genotype has been described,44 and ABV has been recovered from at least 28 psittacine species and 1 nonpsittacine species, a canary (S canaria) with typical PDD lesions. Partial sequence analysis has shown the canary ABV strain to be closely related to ABV5.20 Although most of the recoveries of ABV so far have been from clinically affected birds, asymptomatic infection and long-term virus shedding have also been identified and likely play an important role in the epidemiology of PDD.22, 43, 45, 48
PDD has been successfully reproduced in cockatiels (N hollandicus) inoculated with brain homogenate containing ABV4, and the presence of an ABV4, nearly identical to that of the inoculum, was demonstrated in various organs of the inoculees.43 PDD has also been reproduced in cockatiels and Patagonian conures (Cyanoliseus patagonus) using cultured ABV, fulfilling Koch postulates.47 The distribution of ABV in different tissues and organs of PDD-positive birds has been studied by several researchers, using immunohistochemical (IHC) staining, Western blot, and quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR).20, 22, 34, 42, 43, 44, 45, 46 Clear tropism to nervous tissue was demonstrated; however, multiple additional tissue types were involved (see later discussion). The route of transmission of ABV is unknown, but is believed to be feco-oral. Although our understanding of ABV pathogenesis and epidemiology is still in its infancy, the studies published so far provide convincing direct and indirect evidence that the causative agent of PDD has finally been identified.
Pathology
Detailed macroscopic and microscopic lesions in birds with PDD have been described.14, 15, 16 Grossly, many birds suffering from PDD can be dehydrated and mildly to severely emaciated. Atrophied pectoral muscles may especially be seen in birds with a prolonged history of regurgitation or passing of undigested seeds. The proventriculus may or may not be dilated in all birds suffering from PDD, but in nearly 70% of the cases the proventriculus can be distended with seeds and thin walled (Fig. 1 ). In some cases, the proventricular wall may rupture with spillage of food into the celomic cavity, resulting in peritonitis. The duodenum may also be distended and the adrenal glands may be enlarged. In occasional cases, a pale area may be seen on the epicardium. Occasionally, there may be no significant gross lesions in birds that die suddenly without any clinical signs of PDD.
Fig. 1.
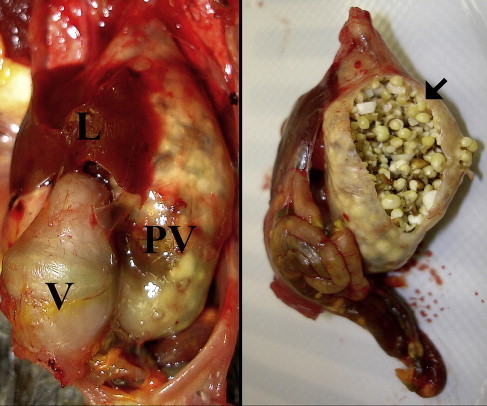
Markedly dilated and thin-walled proventriculus (PV) in a cockatiel (N hollandicus) with experimentally induced PDD. On the right is the PV of the same bird after being removed from the carcass and cut open. Severe impaction with millet seeds is present (arrow). Undigested seeds can also be seen through the wall of the intestine. L, liver; V, ventriculus.
Microscopic lesions can be found in various organs involving the gastrointestinal (GI) tract; central, peripheral, and autonomic nervous systems; heart; adrenal glands; and occasionally in the nerves and ganglia of various visceral organs. It should be pointed out that the lesions in various organs may or may not be present consistently in all birds suffering from PDD. In one study, cases were selected based on lesions in proventriculus and/or gizzard and compared with other organs. The adrenal gland was the second most frequently affected organ, in 89.3% of the psittacines examined, followed by intestine (86.5%), heart (79.3%), brain/spinal cord (78.8%), esophagus/crop (72.1%), peripheral nerves (71.4%), eye (66.7%), and skin (25.0%).14
The microscopic lesions consist of infiltration of the serosal nerves of the proventriculus and/or gizzard, duodenum, and other parts of the intestine by few to large numbers of lymphocytes mixed with some plasma cells (Fig. 2 ). Often in the proventriculus, there is attenuation of glands and fibrosis of the mucosa. In many cases, there is infiltration of lymphocytes mixed with a few plasma cells in and around the nerves of the muscular tunics most prominent in the gizzard. Similar lesions can also be seen in the serosal and subserosal ganglia and nerves of the crop and esophagus, but they tend to be less consistent in these organs. A high percentage of birds can have lesions in the adrenal glands. These lesions can range from the infiltration of a few lymphocytes in the medullary regions to infiltration of a large number of lymphocytes mixed with few plasma cells and heterophils (Fig. 3 ). Often, the adrenocortical cells are vacuolated and hypertrophied. The ganglia, subjacent to the adrenal gland, can also have infiltration of few to large numbers of lymphocytes. In the heart, there is usually infiltration of similar cells either in the epicardial ganglia and nerves or in and around the subendocardial, myocardial, and subepicardial Purkinje fibers. The brain and spinal cord can have similar lesions characterized by mild to severe perivascular cuffing by lymphocytes scattered throughout the cerebral cortex and cerebellum, brain stem, and spinal cord (Fig. 4 ). Vestibulocochlear ganglia along with nerves and spinal ganglia can also have lymphoplasmacytic infiltration. Similarly, perivascular cuffing by lymphocytes in the peripheral nerves, such as sciatic, brachial, vagus, and other nerves can be seen.49 Lesions in the eye, when present, are characterized by moderate to severe perivascular cuffing in the optic nerves and in the choroid, ciliary body, and occasionally in the iris and pecten. Severe retinal lesions and blindness have been reported in a psittacine diagnosed with PDD.50 Lesions in the skin include perivascular infiltration by lymphocytes and plasma cells, and occasional necrosis and infiltration of lymphocytes in the erector pili muscles.
Fig. 2.
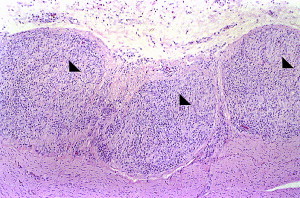
Myenteric ganglioneuritis in an African gray parrot (P erithacus). Three adjacent sections of a large nerve on the serosal surface of the ventriculus are shown (arrowheads). Heavy lymphoplasmacytic infiltration can be seen. This lesion is characteristic for PDD (hematoxylin and eosin staining, original magnification ×100).
Fig. 3.
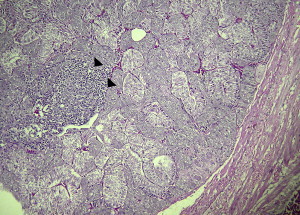
Lymphoplasmacytic infiltration (arrowheads) of medullary areas within the adrenal gland of a cockatiel (N hollandicus) with experimentally induced PDD (hematoxylin and eosin staining, original magnification ×40).
Fig. 4.
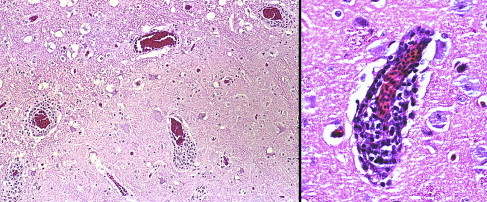
Lymphoplasmacytic perivascular cuffing in the brain of a Blue-and-gold Macaw (Ara ararauna) with PDD (hematoxylin and eosin staining, original magnification for left ×100 and ×400 for right image).
Immunohistochemistry
IHC has been performed by investigators recently to study the tissue distribution and localization of ABV in psittacines42, 43, 44, 46 and canaries20 Antibodies directed against recombinant ABV nucleoprotein, as well as cross-reacting antibodies against the BDV P protein, have been used as reagents for IHC. ABV nucleoprotein was demonstrated primarily in the nuclei, but was also demonstrated in the cytoplasm of neurons including Purkinje cells and glial cells (astrocytes) throughout the brain.43, 44 Studies performed using anti-BDV polyclonal antibodies have demonstrated ABV antigen in the nucleus and the cytoplasm not only of neural tissues (neurons, glial cells, dendrites, axons of brain, myenteric plexus of proventriculus, conduction fibers of the heart, and interstitial nerves in the lung) but also in other cell types including cardiomyocytes, hepatocytes, GI epithelium, and cells in the lamina propria of the intestine.42, 44 Similarly, ABV antigen has also been demonstrated in both neural and extraneural tissues, including tubular epithelia of the kidney in a canary.20 In all studies, ABV antigen was found to be widely distributed among host cells and was not limited only to areas with microscopic lesions (Fig. 5 ).
Fig. 5.
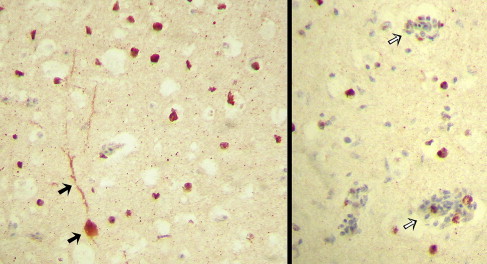
IHC staining directed against avian Bornavirus nucleoprotein in the cerebrum of an African gray parrot (P erithacus) with PDD. The nuclei and cytoplasm of numerous neurons have stained positively (red-brown color), as has the dendritic tree of a large neuron (solid arrows). Viral antigen is widely distributed and can be seen within perivascular cuffs (open arrows) but also in areas without microscopic lesions (original magnification ×400).
Antemortem diagnosis
Clinical Signs
The incubation period of PDD seems to be extremely variable. Under experimental conditions, a minimum of 11 days has been reported in one study,31 whereas in others it was approximately 1 month43 or more.47 The maximum time is certainly in the months range, and possibly even years in some cases.31, 43 Birds clinically affected by PDD may show symptoms related to malfunction of the digestive tract, neurologic signs, or a combination of both.6 Sudden death with no preceding clinical symptoms occurs in some cases.
Birds showing the GI form of PDD often present for marked weight loss, vomiting/regurgitation, and the presence of undigested food (eg, whole seeds, Fig. 6 ) in their feces.6 Any of the symptoms mentioned earlier, and particularly their coexistence, should alert the clinician to a possible diagnosis of PDD; however, none of them should be considered pathognomonic. Furthermore, the severity of these symptoms varies among patients, and the symptoms may not all be noticeable at the time of presentation. Due to the feather coverage, weight loss often goes unnoticed by the bird's owner, and passing of undigested food is difficult to detect in birds that are on a pelleted diet.
Fig. 6.

Large amount of undigested seeds in the feces of an African gray parrot (P erithacus) with PDD.
The range of clinical symptoms possible with the central nervous system (CNS) form of PDD is even greater than that seen in the GI form. The signs may be subtle, ranging from a slightly dim attitude to profound neurologic deficits and/or seizures. Birds may present mildly to severely ataxic, sometimes with only one limb noticeably affected. Paraparesis is also common, and birds may be sternal at presentation with their legs either rigidly flexed or extended. Torticollis and/or abnormal head movements may be present, and central blindness has recently been described in an African gray parrot with PDD.50 The most severe cases are presented in status epilepticus. As with the GI signs, none of these signs are specific for PDD, and other differential diagnoses should always be considered. It should be noted that mixed GI or neurologic PDD cases are common and that most birds have both GI and CNS lesions at necropsy, regardless of the clinical form observed antemortem.14
Hematology and Clinical Chemistry
Birds with PDD often show little or no changes in their blood work.6, 51, 52 Nonregenerative anemia is the most common hematological change seen with PDD. This finding is similar to what is seen in starving birds and is likely related to GI malabsorption. Leukocytosis and heterophilia are present in some patients with PDD, but are not a consistent finding and seem to be related to stress and/or to the existence of secondary infections. Likewise, the biochemistry changes seen in birds with PDD are mainly those associated with their catabolic state. Total protein and albumin levels are often decreased,52 and mild to moderate plasma elevations of enzymes of muscle origin (lactate dehydrogenase, creatine kinase, and aspartate aminotransferase) may be seen. Other changes are possible, but are not consistent; nevertheless, performing a chemistry panel is important for ruling out other disease conditions and for assessing the patient's general health. It is also advisable to test all birds suspected of having PDD for blood lead and zinc levels, because the symptoms of heavy metal toxicosis may mimic those of PDD.51, 53
Fecal and Crop Cytology
There are no fecal or crop cytologic findings that are specific for PDD. However, these simple tests should always be performed as part of the diagnostic workup of birds suspected of having PDD, because they may help rule in or rule out other differential diagnoses or provide important information on changes that are secondary to PDD. It is of particular importance to rule out the presence of avian gastric yeasts (Macrorhabdus ornithogaster) and helminth, because these can cause GI signs similar to those seen with PDD. Changes in normal GI flora (eg, increase in gram-negative bacteria, Clostridium sp, and/or Candida yeasts) should be interpreted with caution, because they may represent a primary or a secondary process; both cases require appropriate therapy.
Diagnostic Imaging
Diagnostic imaging techniques, such as survey radiography, contrast radiography, contrast fluoroscopy, and ultrasonography, are useful aides in the diagnosis of PDD, but cannot be used to confirm or rule it out.51, 53 The most consistent finding in birds with PDD is a moderately to markedly distended proventriculus that contains mainly ingesta and variable amounts of gas. Distention of the proventriculus by gas alone is not typical of PDD. Proventricular diameter has been shown to increase over time in Spix macaws with PDD and has been suggested to be a useful indicator for performing crop biopsy.12 Other GI compartments that may be distended include the crop, ventriculus, and small intestine; however, none of these findings are specific for PDD. The degree of distention of the various GI parts varies among birds with PDD, some showing changes only in the intestine or crop. A relatively large proventriculus may be seen in some healthy eclectus parrots, while distention of the proventriculus and crop can be physiologic in neonate birds.51, 54
For most PDD cases, survey radiographs are the most cost-effective diagnostic imaging procedure and provide sufficient information for the assessment of the size of the relevant GI compartments (Fig. 7 ). In cases where the findings are equivocal or when the overall clinical picture does not fit well with PDD, positive contrast studies may be indicated (Fig. 8 ). The technique for performing GI contrast studies in psittacine birds has been previously described.55, 56 After the birds have been fasted for 4 hours, contrast material is introduced into the crop by gavage. Some investigators recommend dosing the patient at 25 to 50 mL/kg; however, the use of 10 to 15 mL/kg is often sufficient and reduces the risk of regurgitation and aspiration. Either barium sulfate or iodine-based contrast media may be used. Barium sulfate generally provides better and longer-lasting positive contrast compared with iodine-based products, but can cause airway irritation if accidentally aspirated and should be avoided if GI perforation is suspected. The use of barium also necessitates any GI surgery (eg, for collecting a crop biopsy) to be delayed until its complete clearance. Contrast studies may be performed with the patient anesthetized or awake. Although the disadvantages of anesthetizing the patient multiple times are obvious (increased anesthetic risk, risk of aspiration, altering GI motility), images obtained from an awake bird (eg, placed in a cardboard box or on a perch) may not always be sufficiently diagnostic. In many cases, a combination of both options may prove most practical, that is, most of the images are obtained with the bird awake, and the bird is anesthetized for a short period of time to achieve correct positioning, only once or twice, at well-chosen time points.
Fig. 7.
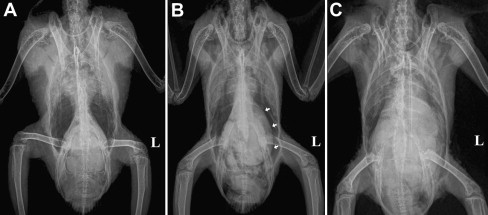
Ventrodorsal survey radiographs. (A) A normal African gray parrot (P erithacus). Note the hourglass appearance of the cardiohepatic waist with abundant and symmetric airsac space on either side of it. (B) Moderate dilation of the proventriculus (arrows) in an African gray parrot with PDD. The proventriculus extends laterally beyond the liver edge at the expense of airsac space on the left. (C) Severe dilation of the proventriculus and ventriculus in a yellow-crested cockatoo (Cacatua sulphurea) with PDD. There is complete loss of the cardiohepatic waist and airsac space is markedly diminished bilaterally. Due to the general loss of peritoneal detail in this bird, a contrast study is indicated.
Fig. 8.
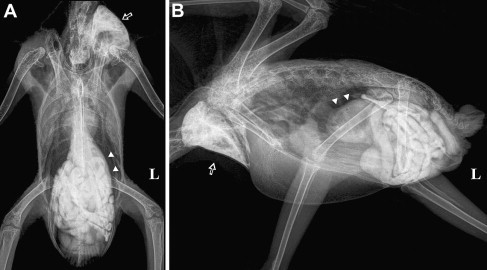
(A) Ventrodorsal and (B) lateral radiographs of the African gray parrot (P erithacus) in Fig. 7B, 50 minutes after administration of 15 mL/kg iodine-based contrast medium (Ultravist 300; Schering AG, Berlin, Germany) by gavage. Although some contrast material is still present in the crop (open arrow), most of it has moved down the GI tract and has already reached the cloaca. It is not unusual for patients with PDD to have normal or even faster than normal GI transition time despite showing advanced clinical signs of PDD (this is the same bird that had passed the feces shown in Fig. 6). The proventriculus of this bird (arrowheads) is moderately dilated and contains mainly ingesta but also some gas. The small intestine of this bird is also mildly to moderately dilated.
Contrast studies provide information not only on the size and relative positioning of the GI compartments but also on the GI transit time. In healthy psittacine birds, barium sulfate should reach the cloaca within 3 hours of administration,55, 56 often taking only 90 minutes to do so. The transit time for iodine-based products has not been well documented but appears to be significantly shorter. In some patients with PDD, transit time may be markedly prolonged,6, 51 whereas in others it is normal or even shortened (see Fig. 8). GI transit time may be altered by many pathologic and physiologic conditions; therefore, GI transit time cannot be considered a sensitive or specific indicator of PDD.
Contrast fluoroscopy can also aid the diagnosis of PDD.52 The procedure is similar to that described earlier. After administration by gavage of 5 to 10 mL/kg barium sulfate mixed 1:1 with commercial hand-feeding formula, the awake bird is placed in a cardboard box or on a perch and observed intermittently with the fluoroscope until the barium reaches its cloaca. The main advantage of fluoroscopy compared with standard contrast studies is that it provides real-time views of the GI motility. Knowing the normal motility patterns is obviously necessary for the detection of changes. In the normal psittacine bird, boluses of ingesta can be clearly seen leaving the crop and traveling along the thoracic esophagus to the proventriculus. These boluses usually occur at an approximate rate of 1 bolus per minute, and should be unidirectional with no significant amount of barium remaining in the esophagus between boluses. The motility of the proventriculus is less pronounced than that of other GI parts, but every few minutes a large contraction followed by partial emptying into the ventriculus should be seen. Little or no proventricular motility may be present in patients with PDD with a grossly distended proventriculus. Most striking of all are the changes in normal ventricular motility seen with PDD. Because of the sequenced contraction of the thick and thin muscle pairs of its wall, a constant washing machine-like turning effect is produced and should be clearly visible in the lateral view of a healthy bird. In patients with PDD this pattern may be completely missing, often being replaced by a shallow and irregular flutter of the ventricular wall. The latter finding is the likely cause of failure of the mechanical food grinding action of the ventriculus, leading to the passing of whole seeds in the feces of patients with PDD. Peristalsis of the small intestine is bidirectional in psittacine species, with waves traveling down to the cecal remnants and back up to the pylorus. Some patients with PDD may show very fast and erratic peristaltic activity and an increase in duodenal diameter, whereas in others motility may be slower than usual. As with other imaging techniques, fluoroscopy findings should be regarded suggestive, but not confirmative, for PDD. Unfortunately, this useful technique requires costly equipment; therefore, it is not readily available to many private practitioners.
Crop Biopsy
The gold standard for diagnosing PDD has been and will likely remain histologic examination. In a live bird, this means that at least one appropriately sized biopsy from a relevant anatomic site must be obtained. Ideally, a biopsy of the serosal surface of the proventriculus and/or ventriculus should be taken because these sites are the most commonly affected by PDD.14, 15, 57 However, these procedures are technically challenging and highly invasive compared with the much simpler and less invasive approach to the crop.57
The sensitivity of crop biopsies for detecting PDD has been a matter of controversy, with the reported prevalence of ganglioneuritis in crops of patients with PDD ranging from 22% to 76%.14, 15, 49, 57, 58 Proper selection of the biopsy site and preparing multiple biopsy sections have been suggested to increase the sensitivity of crop biopsies.54 The surgical approach to the crop has been previously described.59 In brief, under general anesthesia, the bird is placed in dorsal recumbency and the skin above the crop (ie, the ventral area of the lower neck) is aseptically prepared. The skin is then incised along the ventral midline or slightly to the left of it, and the crop wall is exposed by undermining and retracting the skin laterally. The ventral portion of the crop is freed from its fascial attachments and lifted gently. Some investigators suggest that the cranial portion of the left lateral sac of the crop be preferred as the surgical site, because this area is less subject to stress and iatrogenic injury by feeding tubes.57, 59 The biopsy should include a prominent blood vessel (Fig. 9 ), because this increases the chances of obtaining nerve sections.54, 57 Stay sutures may be placed cranially and caudally to the biopsy site, which should measure no less than 12 mm at its long axis (ie, along the blood vessel). It is advisable to obtain an elliptical rather than a round biopsy (eg, 12 × 8 mm), because this enables later identification of the biopsy's original orientation. A second, smaller piece of about 2 × 2 mm should be collected in a sterile container and kept frozen for RT-PCR testing (see later discussion). The crop incision is closed in a continuous inverted (eg, Cushing's) pattern, using synthetic absorbable suture material, and the skin is closed routinely.
Fig. 9.
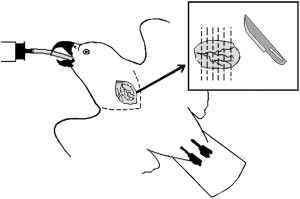
Crop biopsies of about 12 × 8 mm should be collected along a prominent blood vessel. Following fixation in formalin, the biopsy should be carefully sliced perpendicular to its long axis. At least 5 thin slices should be prepared and placed on edge in a histologic cassette. An additional small piece should be frozen (fresh, without fixation) for potential RT-PCR testing.
Following fixation for at least 2 hours in 10% buffered formalin, practitioners are encouraged to either section the biopsy themselves or provide the laboratory with specific modulation instructions. The biopsy should be cut perpendicular to its long axis, using a sharp scalpel or razor blade. Special care should be taken not to drag or compress the adventitial side because it contains most ganglia. At least 5 thin slices should be prepared and placed on edge in a histologic cassette. Under most circumstances this ensures that at least 10 medium to large nerve sections are represented, while a good biopsy includes more than 20 medium to large nerve sections (Fig. 10 ). IHC and RT-PCR for ABV are already offered by some commercial laboratories and can complement the standard histologic examination of crop tissue.
Fig. 10.
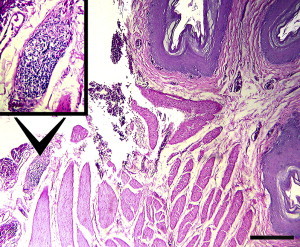
Crop biopsy from a yellow-crested cockatoo (Cacatua sulphurea) with PDD. A ganglion with severe lymphoplasmacytic infiltration (inset) is present on the adventitial surface of the crop (hematoxylin and eosin staining). The biopsy of this bird included 36 sections of nerves and ganglia, of which 22 were diagnostic for PDD. All 6 slices prepared from this biopsy had at least one diagnostic lesion. However, this is not always the case with crop biopsies (original magnification for inset image ×100 and ×40 for actual image).
Molecular Diagnosis and Serology
The recent discovery of ABV and the development of specific molecular and serologic assays for its detection offer new diagnostic tools to avian veterinarians and aviculturists wishing to clear their flocks of this pathogen. However, these tools should be used cautiously, keeping in mind our limited knowledge of this novel virus and the inherent limitations of the techniques.
RT-PCR primers for conserved areas of the L, M, and N genes have been designed, and they successfully detect at least 40 ABV isolates of 5 distinct genotypes.11, 22, 42, 43 Quantitative real-time PCR, based on primers and probes within the P gene, has also been successfully applied to detect and quantify the presence of ABV in various tissues.34, 45 The RT-PCR assays for the highly expressed M and N genes seem to have a similar sensitivity that is somewhat higher than that of the L gene RT-PCR. Based on the limited information available to date, brain, crop, proventriculus, ventriculus, and adrenal glands appear to be the most consistent sites for postmortem detection of ABV RNA.34, 42, 43, 44, 45 Some birds may have ABV RNA present in most major organs, as well as in the plasma,43, 45 whereas others show a more restricted distribution pattern. Therefore, it is important to test several tissue types (brain and stomachs at the least). Specimens that may be tested for ABV RNA antemortem include crop tissue, blood, choanal and cloacal swabs, and feces. Unfortunately, preliminary data show that ABV-infected birds are not consistently viremic43, 45 and shed the virus only intermittently in their saliva/feces, and that crop tissue may test ABV-negative in some patients with PDD.43, 45 Furthermore, some naturally infected birds have been reported to shed the virus without obvious clinical signs.43, 45 One such cockatiel has had ABV RNA present in 90% of its choanal and cloacal swabs during a period of 110 days43 and has remained asymptomatic for at least 1 year thereafter (A.Y. Gancz, unpublished data, 2005–2010). These findings suggest that false-negative and false-positive results may occur when attempting to determine a bird's PDD status based on RT-PCR.
Serum from patients with PDD has been shown by Western blot analysis to contain antibodies against an unidentified ABV protein in the bird's brain. This protein was later identified to be nucleoprotein, 1 of the 2 major immunogenic proteins of Bornaviruses (the second one being P). The protein from the bird's brain was extracted and used to test other sera from birds with PDD and from control birds, with promising results.60 Similarly, Lierz and colleagues45 have used Western blot to test sera from symptomatic and asymptomatic ABV-positive birds. Recombinant ABV N and P proteins, as well as BDV N and P proteins, were used rather than brain extracts, and similar antibody responses were detected, regardless of the birds' clinical status. The strongest reaction was to the recombinant ABV N protein, showing minimal cross-reactivity with BDV N. Responses to both P proteins were relatively weak and variable. It was concluded that serology could not differentiate between patients with PDD and asymptomatic ABV carriers. This conclusion is also supported by the findings of another study that used Western blot and enzyme-linked immunosorbent assay to detect anti-ABV antibodies in asymptomatic ABV-positive macaws.48
Even with the limited data available to date on molecular and serologic assays for ABV detection, the advantages and shortcomings of these tests are already apparent. When used for the diagnosis of PDD, false-positive as well as false-negative results are possible. The tests may detect the ABV status of a bird correctly, but cannot be directly correlated to the patient's clinical status. Therefore, the definitive diagnosis of PDD in the single patient continues to be based on histology, with PCR, serology, and IHC results as supporting evidence (Fig. 11 ). The advantage of these tests is that they offer for the first time practical tools for screening birds for the causative agent of PDD. The optimal screening protocol (eg, serology vs PCR of several swabs collected serially) is yet to be determined. However, it is hoped that these tests greatly improve our ability to clear flocks from ABV and by that significantly reducing the incidence of PDD.
Fig. 11.
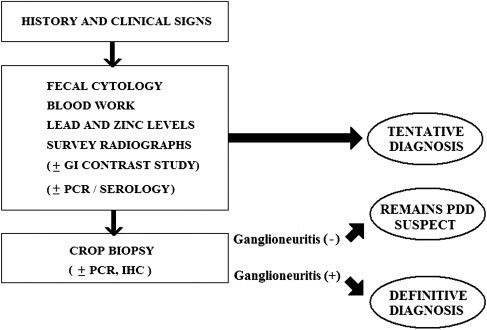
A proposed diagnostic approach to PDD.
Clinical management of PDD
PDD is a devastating disease for affected birds, but it is equally devastating for their owners or caretakers. Furthermore, the disease often becomes a flock management problem, because many owners of psittacine birds have multiple birds. Transmission between birds in the home environment can be problematic and may lead to sequential illnesses and potential deaths, which may occur over a period of years. The social implications of a PDD diagnosis can also be devastating. Owners may be shunned from bird club functions or social interaction with other bird owners. Pet sitters often refuse to provide care while the owner is away.
Likewise, the diagnosis of PDD in an avicultural collection can have severe financial and emotional effects on aviculturists. Counseling the owner and establishing a long-term management plan are important aspects of veterinary care. The first step in management, therefore, is client counseling, and planning not only for the affected bird but also other birds in the flock or home.
Initially the owner may be faced with difficult decisions, choosing between euthanasia and long-term management of affected birds that may remain infectious. Euthanasia may be the best decision if the bird is critically ill; however, many owners may be reluctant to choose this option. A potential compromise that may allow the client to make a more calculated decision is to treat for 3 to 4 weeks and reevaluate for treatment response.
Living with a bird that has a chronic infectious disease, which is a risk to other birds, requires a commitment of time as well as limiting birds coming and going from the home. Long-term care can entail a significant investment of time and money. The bird could be placed in a rescue center that handles birds with PDD, but rescue centers typically request monetary support for long-term treatment of the bird. Placement of the bird in a home without other birds for long-term management is another option if such a home can be found.
Although many birds with clinical PDD can be returned to being clinically normal, effective treatment typically requires months and even years. More research is needed to determine the risk to other birds from birds that have been treated but may still be latently infected.
Counseling for owners needs to include the fact that even using current therapeutic methods, the long-term consequences of treatment and risks associated with the affected bird after treatment are still unknown. It is also important to understand that the disease can take many forms and can have a long incubation period. Clinically healthy birds can be infected with ABV and pose a risk of transmission to others.43, 45, 48 A clinician who only diagnoses PDD in classic emaciated vomiting birds that are passing whole seeds is only seeing the tip of the iceberg.
The second step in clinical management is assessing the disease status of their other birds that are in contact with the affected bird(s). To be in denial and avoid checking other birds in the home or aviary is placing them at risk. If the PDD is diagnosed before the bird is critically ill, most birds can be helped. Conversely, many birds that are diagnosed by either crop biopsy, ABV-PCR, or antibody to ABV may never develop naturally occurring disease.43, 45, 48 ABV diagnostics are currently in their infancy. Extensive, long-term research is needed to provide the client with a reasonable prognosis, especially when multiple birds or flocks are subject to exposure.
In developing a treatment and control plan, each bird should be considered individually. To determine the extent of the problem, it is important to evaluate all birds in contact with an affected bird. Ideally all contact birds should be screened, preferably by a combination of Ag and AB tests, and possibly crop biopsy as well. In this way, asymptomatically infected birds can be identified, isolated, and treated.
Clients should be encouraged to make the commitment not to bring more birds into their homes, placing them at risk. Likewise, transferring birds to other owners without disclosure places other birds at risk.
Treatment Considerations
As an infectious disease that causes inflammation of the central and peripheral nervous system as well as the digestive system, when managing PDD thought must be given to prevention of transmission of the disease to uninfected individuals, reducing inflammation, aiding digestion, and controlling secondary infections. In many cases this must be done for a long time. With prolonged therapy and control of secondary infections, birds that are diagnosed early can return to good physical condition. However, their life expectancy cannot be predicted.
Nonsteroidal Anti-Inflammatory Drugs
Initial reports of treatment using the anti-inflammatory drug celicoxib presented the first real hope for birds with PDD.61 Nonsteroidal anti-inflammatory drugs (NSAIDs) are a group of structurally diverse compounds used clinically for the treatment of pain and/or inflammation. NSAIDs are believed to exert their analgesic and anti-inflammatory effects through inhibition of the cyclooxygenase (COX) enzymes, which catalyze the conversion of arachidonic acid to the various prostaglandins.62
Two isoforms of the COX enzyme have been identified in eukaryotic cells, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). The COX-1 protein is constitutively expressed (ie, it is present under normal conditions and does not need to be induced) and is involved in the maintenance of homeostatic conditions. For example, COX-1 plays a role in blood clotting and elicits a protective role in organs such as the GI tract. The COX-2 protein, on the other hand, is inducible and is involved in the immediate early gene response to various stimuli such as cytokines, growth factors, and ultraviolet light. Older NSAIDs such as aspirin, ibuprofen, and flurbiprofen, inhibit both forms of COX and are referred to as nonselective NSAIDs. Newer NSAIDs, such as celecoxib and rofexocib, are selective for COX-2 and are referred to as selective COX-2 inhibitors.61, 62, 63, 64
In addition to their anti-inflammatory properties, NSAID therapy may have other unexpected effects. Chen and colleagues65 found that NSAID treatment can suppress the propagation of vesicular stomatitis virus (VSV) in mice. The inhibition of COX antagonized VSV propagation using in vitro and in vivo experiments. In addition, aspirin and celecoxib prevented the disruption of the blood-brain barrier in VSV-infected mice. In vitro experiments showed that the effect of COX inhibition was at least partially mediated by increased production of nitric oxide, a molecule that is known to inhibit VSV replication. In another study, Zhu and colleagues66 demonstrated that COX-2 inhibitors could inhibit the production of human cytomegalovirus in human fibroblast cultures. These studies indicate that NSAIDs may have direct antiviral effects.
Celecoxib has been used successfully in treating PDD at the rate of 20 mg/kg body weight (BW) once daily if given directly orally.67 However, unless a bird is extremely tame, stress associated with therapeutic protocols must be considered, especially because of the long-term nature of therapy. Long-term treatment success has been achieved when adding celecoxib to the bird's food at 40 mg/kg BW once daily.67 A 200-mg capsule of celecoxib may be dissolved in 10 mL of water and used at 0.2 mL/100 g BW. The drug should be provided on a small amount of food so that the chance of consuming adequate amounts improves. The stability of this suspension has not been studied. Empirically, it is recommended that a new stock be prepared fresh at least once a week, and that it is stored under refrigeration. Many practitioners prefer to have the drug compounded. In most birds clinical response is slow and gradual, and many birds do not show much benefit for at least 2 weeks.67
Other NSAIDs have also been used to treat PDD. Tepoxalin (Zubrin; Schering Plough, Union, NJ, USA), a combined COX-1, COX-2, and 5-lipoxygenase (LOX) inhibitor, was used successfully in the treatment of a group of crop biopsy positive birds.67 Through its inhibition of the LOX enzymes, this drug potentially reduces the production of leukotrienes, including leukotriene B4, that may contribute to increased GI tract inflammation. Inhibition of LOX may also reduce the GI effects routinely seen in dogs (and possibly in birds) with COX-1 inhibitors.63
In a pilot study comparing the effectiveness of celecoxib and tepoxalin,67 3 treatment groups were compared: (1) Celecoxib 40 mg/kg BW on seed mix (n = 9); (2) tepoxalin 40 mg/kg BW on seed mix (n = 8); and (3) tepoxalin 40 mg/kg BW on an extruded rice-based hypoallergenic diet (n = 14). All birds were positive on crop biopsy before treatment and underwent a second crop biopsy after at least 9 months of therapy. In group 1, 2 birds still had positive crop biopsies after 9 months' treatment, whereas in group 2, 6 birds still had positive crop repeated biopsies. The best results were found in group 3, in which lesions typical of PDD were not found in any of the 14 birds. These results may be attributable to the extruded diet readily absorbing the medication, attributable to its hypoallergenic nature and an enhanced efficacy of tepoxalin on this diet, or possibly because the species in group 3 were easier to treat effectively compared with the species in groups 1 and 2. Palm cockatoos (Probosciger aterrimus) were found to be particularly difficult to treat effectively, accounting for 7 out of 8 cases of treatment failure. Palm cockatoos and hyacinth macaws (Anodorhyncus hyacinthinus) consume much of their calories through nuts and seeds, making consistent dosing difficult.67 In these species, some sort of soft or fresh food, such as fruits and vegetables, should be used as a vehicle for administration of the medication.
Meloxicam is another NSAID that is widely used by avian practitioners. Meloxicam is considered COX-2 preferential (not specific), and at higher dosages its COX-2 specificity is diminished.63 However, in the authors' empiric opinion, the clinical response seen with meloxicam is inferior to that observed with celecoxib therapy.
The most common side effect of celecoxib and other COX-2 inhibitors is bleeding in the gastrointestinal tract. The risk may be higher in the first few weeks of therapy. An adult female hybrid macaw with PDD died within 7 days of initiation of celecoxib therapy, exhibiting acute proventricular bleeding.67 The feces of birds treated with NSAIDs should be monitored daily. Treatment should be discontinued immediately if melena or fresh blood is detected, and the bird should be evaluated. Fecal cytology, including a Gram stain, should be performed to detect Clostridium sp and/or other potential bacterial pathogens.
Some birds seem to develop hypersensitivity to celecoxib. A mature female hyacinth macaw developed severe pruritus locally on the sides of its face while being treated with celecoxib, which subsided in severity when celecoxib therapy was discontinued.67
Most NSAIDs are eliminated by renal clearance and should be used with caution in birds with renal disease. In addition, NSAID-induced renal disease has been documented in birds.68 Therefore, it is recommended that birds on long-term NSAID therapy be monitored on a regular basis for changes in their chemistry panel.
Although the inflammatory lesions in nerves are often reversed in response to NSAID therapy, these drugs are not considered a cure or a prophylactic agent for PDD.
Amantadine Hydrochloride
The prognosis of PDD is especially guarded in patients showing severe CNS disorders. Such cases have been poorly responsive to NSAID therapy alone. In the experience of one of the authors (S.C.), the addition of amantadine hydrochloride (10 mg/kg by mouth once a day or 20 mg/kg once a day on food) to the therapeutic protocol resulted in a vast improvement in outcome.
Amantadine was initially used as an antiviral against influenza viruses.63 Its antiviral mechanism of action involves interference with a viral ion channel. Later, it was also found to have an effect in reducing the severity of symptoms of Parkinson disease, by antagonizing the N-methyl-d-aspartate receptor and other mechanisms that are not yet fully understood.69 Amantadine has many effects on the brain, including release of dopamine and norepinephrine. Because of increased viral resistance, amantadine is no longer recommended for influenza treatment or prophylaxis,70, 71 but is still being used to treat various psychological disorders in humans. Common side effects in humans include appetite loss, diarrhea, nausea, lethargy, and allergic reactions. Amantadine has been used in combination with celecoxib to treat a large number of PDD patients with only rare adverse reactions, which resolved after cessation of therapy (S. Clubb, unpublished data, 2005–2010).
Other Drugs Used to Treat PDD Patients
Because of their impaired GI motility, birds with PDD often develop secondary bacterial and fungal GI infections. These should be diagnosed and treated appropriately. Clostridium infections are more common in birds with PDD than in birds with normal intestinal motility, and can result in bulky, black, foul-smelling feces. Vaccination for Clostridium should be considered. A bovine multivalent Clostridium chauvoei/septicum/haemolyticum/novyisordellii/perfringens types C and D bacterin-toxoid vaccine (Vision 8; Intervet Inc, Millisboro, DE, USA), administered at 0.25 to 1 mL intramuscularly or subcutaneously, has been used with empiric success. Initially, 2 doses are given 2 weeks apart with an annual booster.67
Gas formation and retention in the GI tract is a common finding in birds affected with PDD and can cause discomfort. Gas may be evident radiographically and/or gas bubbles may present in the feces or vomitus. Surfactants (eg, Infant's Mylicon; Johnson & Johnson, Merck Consumer Pharmaceuticals, Ft. Washington, PA, USA) provide some symptomatic relief. Many birds exhibiting GI gas or vomiting respond clinically to combination drug therapy (eg, clarithromycin, metronidazole, and sucralfate) as if they are infected with Helicobacter species; however, the presence of Helicobacter has not been confirmed in these patients.
Metoclopramide (0.5 mg/kg every 12 h by mouth or intramuscularly) is an important adjunct therapy to management of severe PDD cases.67 It is beneficial in cases of reduced intestinal motility or intestinal stasis. Treatment is initiated by injection and later continued orally. An adverse reaction to metoclopramide has been reported in a macaw being treated for PDD.70, 71
Birds with PDD often become anemic and hypoproteinemic. Supplement of vitamins, especially B complex vitamins, is helpful.
Husbandry Considerations
If possible, birds should be kept outside where sunlight and fresh air help in diluting and inactivating the virus; it also enhances the bird’s well-being. The birds should be spread out as much as possible to reduce the concentration of virus in the environment. Stress should be kept to a minimum. The diet should be easily digestible, because ventricular and proventricular function is adversely affected by PDD. Liquid diets and pelleted diets have been developed specifically for birds with PDD, and juvenile hand-feeding formulas can also be used for initial nutritional therapy. Formulated diets are ideal because they are easier to digest than seeds; however, extreme caution should be used in converting an ill bird from a seed-based diet to a formulated diet. Extruded diets also absorb medication well, enabling long-term, stress-free therapy.
Supplementing the diet with vegetables that are high in fiber might be beneficial with early cases of PDD by stimulating intestinal motility. Birds affected by PDD often ingest foreign bodies, especially pieces of wood. These materials may then be passed through vomitus or feces. The bird may be ingesting these materials in an attempt to provide relief from intestinal discomfort. These birds may need toys and cage accessories that cannot be chewed or ingested, and may benefit from high-fiber vegetables to fill this need.
Cruciferous vegetables are beneficial sources of raffinose sugars (rich in oligofructosaccharides), which enhance viability of autochthonous flora (species of Lactobacillus and Bifidobacterium), thereby inhibiting gram-negative bacteria and Clostridium. However, in advanced cases these foods may linger in the intestines and ferment. Periodic supplementation with probiotics may be beneficial.
Because of the inflammatory nature of PDD, supplements that enhance nutrition and provide anti-inflammatory effects may augment conventional therapy. Antioxidants including oils, specific amino acids, and minerals, and some natural herbal anti-inflammatory agents may be beneficial. A balance of omega-3 and omega-6 fatty acids has proved to be beneficial in many inflammatory diseases. Salmon oil, flax seed oil, and safflower oil are used as sources of omega-3 and omega-6 fatty acids. Fatty acid supplementation is provided at 50 to 250 mg/kg BW of omega-3 fatty acids with an omega-3:omega-6 ratio of 1:2 to 1:6. If the bird is primarily on a seed diet, which is naturally high in omega-6 fatty acids, supplementation with salmon oil and flax seed oil helps to correct the omega-3:omega-6 ratio. Nutritional adjuncts to therapy that may be beneficial in cases with CNS signs include Ginkgo biloba, vitamin E, alpha-lipoic acid, acetyl-l-carnitine, and B-complex vitamins. There are no studies in the literature on the effect of various diets and/or nutraceuticals on birds with PDD; therefore, all recommendations made earlier are empiric.
Monitoring Progress of Therapy
Response to therapy can be monitored by periodic physical examination, monitoring body condition and weight, repeated radiographs and hematology, and plasma biochemistry analysis. Increases in body weight can be misleading, because weight gain may be associated with dilation of the proventriculus and intestinal stasis. Monitoring by serial crop biopsies is useful. On repeated biopsy, the site of previous biopsy should be avoided because the presence of old suture material can result in nonspecific inflammatory lesions.
If monitored by radiography, the composition of the diet must be considered in evaluation, especially if the bird is primarily on a seed diet at the time of diagnosis and is converted to a more bulky extruded diet. Birds eating a primarily formulated or extruded diet tend to have a dilated GI tract as evident radiographically, which can complicate radiographic evaluation (S. Clubb, unpublished data, 2005–2010).
Prevention
Early epidemiologic data on PDD as well as recent studies on ABV45 suggest that the disease and its causative agents are not equally distributed among flocks. Although in some aviaries PDD cases occur on a regular basis, the disease appears to be completely absent from other facilities (A.Y. Gancz, unpublished data). With this observation in mind, the obvious goals of PDD prevention are: (a) to avoid introducing the pathogen into new flocks and (b) to clear it from flocks where it is already present. However, until recently these goals were nearly impossible to achieve due to the disease's long incubation period and because its cause was unknown. Even facilities that quarantined all new arrivals for extended periods of time and facilities that performed crop biopsies on all of their birds were not completely safe from PDD. Now, with the discovery of ABV and the development of molecular and serologic assays for its detection, it is hoped that this situation will change.
Regular monitoring of the bird’s body condition and feces (ie, looking for undigested seeds) are simple ways for detecting clinical PDD cases in flocks where the disease already exists. While still valid, these simple measures do not detect birds in early stages of PDD or birds that are asymptomatic ABV carriers.
ABV RT-PCR and serology are already offered by some commercial laboratories, and are expected to become widely available in the near future. At this point, precise recommendations as to the preferred screening protocol cannot be made, but when possible both tests should be used. Because of intermittent shedding of ABV, it is advisable to submit several serially collected oral or cloacal swabs for RT-PCR. If crop biopsies are collected, they too can be submitted for RT-PCR.
As with other infectious diseases, practicing good hygiene and following strict biosafety rules are essential for fighting PDD. Diagnostic necropsies and histopathology should be performed on all birds that die of unknown causes. Overcrowding of aviaries facilitates the spreading of PDD and should be avoided. All new additions should be quarantined and tested (see earlier) as should be any bird suspected to have clinical signs of the disease. Birds that test ABV-positive must not be allowed into existing flocks and should be removed from flocks where they already exist. These birds should be placed in a situation where they cannot infect other birds. Similar principles may be applied to smaller collections such as multiple-bird households.
Summary
PDD is a fatal inflammatory disease that affects mainly psittacine birds (order: Psittaciformes). The disease was first recognized in the 1970s, but it was not until 2008 that the causative agent of PDD, a novel Bornavirus, ABV, was discovered. Since its discovery the number of publications on ABV has been increasing rapidly, with new information becoming available on an almost monthly basis. RT-PCR and serologic and immunohistochemical assays for ABV detection are already commercially available, but the knowledge regarding their optimal application is still lagging behind.
For years, PDD has posed one of the greatest diagnostic and therapeutic challenges to the avian veterinarian. It is hoped that the exciting recent progress in PDD research greatly improves our ability to diagnose, manage, and prevent this disease.
Acknowledgments
The authors thank Juliet Mandelzweig for her assistance with language editing of this article.
References
- 1.Woerpel R.W. Clinical and pathologic features of Macaw wasting disease (proventricular dilatation syndrome) Proc West Poultry Dis Confer. 1984:89–90. [Google Scholar]
- 2.Clark D. Proventricular dilation syndrome in large psittacine birds. Avian Dis. 1984;28(3):813–815. [PubMed] [Google Scholar]
- 3.Graham D.L. An update on selected pet bird virus infections. Proc Annu Conf Assoc Avian Vet. 1984:267–280. [Google Scholar]
- 4.Turner R. Macaw fading or wasting syndrome. Proc West Poultry Dis Confer. 1984:87–88. [Google Scholar]
- 5.Gerlach S. Macaw wasting disease—a 4-year study on clinical case history, epizootology, analysis of species, diagnosis and differential diagnosis, microbiological and virological results. Proc Conf Euro Assoc Avian Vet. 1991:273–281. [Google Scholar]
- 6.Gregory C.R., Latimer K.S., Niagro F. A review of proventricular dilatation syndrome. J Assoc Avian Vet. 1994;8(2):69–75. [Google Scholar]
- 7.Gregory C.R., Ritchie B.W., Latimer K.S. Progress in understanding proventricular dilatation disease. Proc Annu Conf Assoc Avian Vet. 2000:269–275. [Google Scholar]
- 8.Sullivan N.D., Mackie J.T., Miller R.I. First case of psittacine proventricular dilatation syndrome (macaw wasting disease) in Australia. Aust Vet J. 1997;75(9):674. doi: 10.1111/j.1751-0813.1997.tb15371.x. [DOI] [PubMed] [Google Scholar]
- 9.Donelely R.J., Miller R.I., Fanning T.E. Proventricular dilatation disease: an emerging exotic disease of parrots in Australia. Aust Vet J. 2007;85(3):119–123. doi: 10.1111/j.1751-0813.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lublin A., Mechani S., Farnoushi I. An outbreak of proventricular dilation disease in psittacine breeding farm in Israel. Isr J Vet Med. 2006;61(1):16–19. [Google Scholar]
- 11.Kistler A.L., Gancz A., Clubb S. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J. 2008;5:88. doi: 10.1186/1743-422X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyss F., Deb A., Watson R. Radiographic measurements for PDD diagnosis in Spix's macaws (Cyanospitta spixii) at Al Wabra Wildlife Preservation (AWWP), Qatar. Proc Intern Conf Dis Zoo Wild Anim. 2009:349–354. [Google Scholar]
- 13.Marietto-Goncalves G.A., Troncarelli M.Z., Sequeira J.L. Proventricular dilatation disease (PDD) and megaesophagus in a blue-fronted Amazon parrot (Amazona aestiva)—case report. Vet e Zootec. 2009;16(1):69–73. [Google Scholar]
- 14.Shivaprasad H.L., Barr B.C., Woods L.W. Spectrum of lesions (pathology) of proventricular dilatation syndrome. Proc Annu Conf Assoc Avian Vet. 1995:505–506. [Google Scholar]
- 15.Graham D.L. “Wasting/proventricular dilation disease” a pathologists view. Proc Annu Conf Assoc Avian Vet. 1991:43–44. [Google Scholar]
- 16.Reavill D., Schmidt R. Lesions of the proventriculus/ventriculus of pet birds: 1640 cases. Proc Annu Conf Assoc Avian Vet. 2007:89–93. [Google Scholar]
- 17.Daoust P.Y., Julian R.J., Yason C.V. Proventricular impaction associated with nonsuppurative encephalomyelitis and ganglioneuritis in two Canada geese. J Wildl Dis. 1991;27(3):513–517. doi: 10.7589/0090-3558-27.3.513. [DOI] [PubMed] [Google Scholar]
- 18.Shivaprasad H. Proventricular dilation disease in peregrine falcon (Falco peregrinus) Proc Annu Conf Assoc Avian Vet. 2005:107–108. [Google Scholar]
- 19.Perpinan D., Fernandez-Bellon H., Lopez C. Lymphoplasmacytic myenteric, subepicardial, and pulmonary ganglioneuritis in four nonpsittacine birds. J Avian Med Surg. 2007;21(3):210–214. doi: 10.1647/1082-6742(2007)21[210:LMSAPG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Weissenböck H., Sekulin K., Bakonyi T. Novel avian bornavirus in a nonpsittacine species (Canary; Serinus canaria) with enteric ganglioneuritis and encephalitis. J Virol. 2009;83(21):11367–11371. doi: 10.1128/JVI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phalen D. An outbreak of psittacine proventricular dilation syndrome (PPDS) in a private collection of birds and an atypical form of PDS in a nanday conure. Proc Annu Conf Assoc Avian Vet. 1986:27–34. [Google Scholar]
- 22.Kistler A.L., Smith J.M., Greninger A.L. Analysis of naturally occurring avian bornavirus infection and transmission during an outbreak of proventricular dilatation disease among captive psittacine birds. J Virol. 2009;84(4):2176–2179. doi: 10.1128/JVI.02191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaskin J.M., Homer B.L., Eskeland K.H. Preliminary findings in avian viral serositis: a newly recognized syndrome in psittacine birds. J Avian Med Surg. 1991;5(1):27–34. [Google Scholar]
- 24.Gaskin J.M., Homer B.L., Eskeland K.H. Some unofficial thoughts on avian viral serositis. Proc Annu Conf Assoc Avian Vet. 1991:38–42. [Google Scholar]
- 25.Mannl A., Gerlach H., Leipold R. Neuropathic gastric dilatation in psittaciformes. Avian Dis. 1987;31(1):214–221. [PubMed] [Google Scholar]
- 26.Grund C.H., Werner O., Gelderblom H.R. Avian paramyxovirus serotype I isolates from the spinal cord of parrots display a very low virulence. J Vet Med B Infect Dis Vet Public Health. 2002;49(9):445–451. doi: 10.1046/j.1439-0450.2002.00596.x. [DOI] [PubMed] [Google Scholar]
- 27.Grund C.H., Mohn U., Korbel R. Relevance of low virulent avian paramyxovirus serotype 1 for psittacine birds. Proc Annu Conf Assoc Avian Vet. 2005:283–286. [Google Scholar]
- 28.Gough R.E., Drury S.E., Culver F. Isolation of a coronavirus from a green-cheeked Amazon parrot (Amazona viridigenalis Cassin) Avian Pathol. 2006;35(2):122–126. doi: 10.1080/03079450600597733. [DOI] [PubMed] [Google Scholar]
- 29.Gough R.E., Drury S.E., Harcourt-Brown N.H. Virus-like particles associated with macaw wasting disease. Vet Rec. 1996;139(1):24. [PubMed] [Google Scholar]
- 30.Hartcourt-Brown N.H., Gough R.E. Isolation of virus-like particles associated with proventricular dilatation syndrome in macaws. Proc Euro Conf Avian Med Surg. 1997:111–115. [Google Scholar]
- 31.Gregory C.R., Ritchie B.W., Latimer K.S. Proventricular dilatation disease: a viral epornitic. Proc Annu Conf Assoc Avian Vet. 1997:43–52. [Google Scholar]
- 32.Gregory C.R., Latimer K.S., Niagro F.D. Investigation of eastern equine encephalomyelitis virus as the causative agent of psittacine proventricular dilatation syndrome. J Avian Med Surg. 1997;11(3):187–193. [Google Scholar]
- 33.Rossi G., Crosta L., Pesaro S. Parrot proventricular dilation disease. Vet Rec. 2008;163(10):310. doi: 10.1136/vr.163.10.310-b. [DOI] [PubMed] [Google Scholar]
- 34.Honkavuori K.S., Shivaprasad H.L., Williams B.L. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis. 2008;14(12):1883–1886. doi: 10.3201/eid1412.080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cubitt B., Ly C., de la Torre J.C. Identification and characterization of a new intron in Borna disease virus. J Gen Virol. 2001;82(3):641–646. doi: 10.1099/0022-1317-82-3-641. [DOI] [PubMed] [Google Scholar]
- 36.Jordan I., Lipkin W.I. Borna disease virus. Rev Med Virol. 2001;11(1):37–57. doi: 10.1002/rmv.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rott R., Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 38.Stitz L., Bilzer T., Planz O. The immunopathogenesis of Borna disease virus infection. Front Biosci. 2002;7:d541–d555. doi: 10.2741/A793. [DOI] [PubMed] [Google Scholar]
- 39.Kohno T., Goto T., Takasaki T. Fine structure and morphogenesis of Borna disease virus. J Virol. 1999;73(1):760–766. doi: 10.1128/jvi.73.1.760-766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De la Torre J.C. Reverse-genetic approaches to the study of Borna disease virus. Nat Rev Microbiol. 2006;4(10):777–783. doi: 10.1038/nrmicro1489. [DOI] [PubMed] [Google Scholar]
- 41.Berg M., Johansson M., Montell H. Wild birds as a possible natural reservoir of Borna disease virus. Epidemiol Infect. 2001;127(1):173–178. doi: 10.1017/s0950268801005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinder M., Ackermann A., Kempf H. Broad tissue and cell tropism of avian bornavirus in parrots with proventricular dilatation disease. J Virol. 2009;83(11):5401–5407. doi: 10.1128/JVI.00133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gancz A.Y., Kistler A.L., Greninger A.L. Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4. Virol J. 2009;6:100. doi: 10.1186/1743-422X-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissenböck H., Bakonyi T., Sekulin K. Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerg Infect Dis. 2009;15(9):1453–1459. doi: 10.3201/eid1509.090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lierz M., Hafez H.M., Honkavuori K.S. Anatomical distribution of avian bornavirus in parrots, its occurrence in clinically healthy birds and ABV-antibody detection. Avian Pathol. 2009;38(6):491–496. doi: 10.1080/03079450903349238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouyang N., Storts R., Tian Y. Histopathology and the detection of avian bornavirus in the nervous system of birds diagnosed with proventricular dilatation disease. Avian Pathol. 2009;38(5):393–401. doi: 10.1080/03079450903191036. [DOI] [PubMed] [Google Scholar]
- 47.Gray P., Hoppes S., Suchodolski P. Use of avian bornavirus isolates to induce proventricular dilatation disease in conures. Emerg Infect Dis. 2010;16(3):473–479. doi: 10.3201/eid1603.091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Kloet S.R., Dorrestein G.M. Presence of avian bornavirus RNA and anti-avian bornavirus antibodies in apparently healthy macaws. Avian Dis. 2009;53(5):568–573. doi: 10.1637/8828-040209-Reg.1. [DOI] [PubMed] [Google Scholar]
- 49.Berhane Y., Smith D.A., Newman S. Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathol. 2001;30(5):563–570. doi: 10.1080/03079450120078770. [DOI] [PubMed] [Google Scholar]
- 50.Steinmetz A., Pees M., Schmidt V. Blindness as a sign of proventricular dilatation disease in a grey parrot (Psittacus erithacus erithacus) J Small Anim Pract. 2008;49(12):660–662. doi: 10.1111/j.1748-5827.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 51.Hoefer H.L. Diseases of the gastrointestinal tract. In: Altman R.B., Clubb S.L., Dorrestein G.M., Quesenberry K., editors. Avian medicine and surgery. WB Saunders; Philadelphia: 1997. pp. 419–453. [Google Scholar]
- 52.Boutette J.B., Taylor M. Proventricular dilatation disease: a review of research, literature, species differences, diagnostics, prognosis and treatment. Proc Annu Conf Assoc Avian Vet. 2004:175–181. [Google Scholar]
- 53.Lumeij J.T. Gastroenterology. In: Ritchie B.W., Harrison G.J., Harrison L.R., editors. Avian medicine: principles and application. HBD International Inc; Delray Beach (FL): 1999. pp. 482–521. [Google Scholar]
- 54.Ritchie B.W., Gregory C.R., Latimer K.S. Epizootiology of proventricular dilatation disease in breeding cockatiels. Proc Annu Conf Assoc Avian Vet. 2004:41–45. [Google Scholar]
- 55.Smith B.J., Smith S.A. Radiology. In: Altman R.B., Clubb S.L., Dorrestein G.M., editors. Avian medicine and surgery. WB Saunders; Philadelphia: 1997. pp. 170–199. [Google Scholar]
- 56.McMillan M.C. Imaging techniques. In: Ritchie B.W., Harrison G.J., Harrison L.R., editors. Avian medicine: principles and application. HBD International Inc; Delray Beach (FL): 1999. pp. 246–261. [Google Scholar]
- 57.Gregory C.R., Latimer K.S., Campagnoli R.P. Histologic evaluation of the crop for diagnosis of proventricular dilatation syndrome in psittacine birds. J Vet Diagn Invest. 1996;8(1):76–80. doi: 10.1177/104063879600800112. [DOI] [PubMed] [Google Scholar]
- 58.Doolen M. Crop biopsy—a low risk diagnosis for neuropathic gastric dilation. Proc Annu Conf Assoc Avian Vet. 1994:193–196. [Google Scholar]
- 59.Bennett R.A., Harrison G.J. Soft tissue surgery. In: Ritchie B.W., Harrison G.J., Harrison L.R., editors. Avian medicine: principles and application. HBD International Inc; Delray Beach (FL): 1999. pp. 1096–1136. [Google Scholar]
- 60.Hoppes S., Gray P. An update on proventricular dilatation disease. Proc Conf Euro Assoc Avian Vet. 2009:141–146. [Google Scholar]
- 61.Dalhausen B., Aldred S., Colaizzi E. Resolution of clinical proventricular dilation disease by cyclooxygenase 2 inhibition. Proc Annu Conf Assoc Avian Vet. 2002:9–12. [Google Scholar]
- 62.Rao P., Knaus E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11(2):81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 63.Plumb DC. Plumb's veterinary drug handbook. 5th edition. Available at: http://www.vin.com/Members/Drug/VDH.plx. Accessed January, 2010.
- 64.Clement D., Goa K.L. Celecoxib: a review of its use in osteoarthritis, rheumatoid arthritis, and acute pain. Drugs. 2000;59:957–980. doi: 10.2165/00003495-200059040-00017. [DOI] [PubMed] [Google Scholar]
- 65.Chen N., Warner J.L., Reiss C.S. NSAID treatment suppresses VSV propagation in mouse CNS. Virology. 2000;276(1):44–51. doi: 10.1006/viro.2000.0562. [DOI] [PubMed] [Google Scholar]
- 66.Zhu H., Cong J.P., Yu D. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc Natl Acad Sci U S A. 2002;99(6):3932–3937. doi: 10.1073/pnas.052713799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clubb S.L. Clinical management of psittacine birds affected with proventricular dilation disease. Proc Annu Conf Assoc Avian Vet. 2006:85–90. [Google Scholar]
- 68.Echols S. Treatment and management of avian renal disease. Proc Annu Conf Assoc Avian Vet. 2004:77–85. [Google Scholar]
- 69.Danielczyk W. Twenty-five years of amantadine therapy in Parkinson's disease. J Neural Transm Suppl. 1995;46:399–405. [PubMed] [Google Scholar]
- 70.CDC High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents—United States, 2005-06 influenza season. MMWR Morb Mortal Wkly Rep. 2006 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5502a7.htm Available at: Accessed January, 2010. [PubMed] [Google Scholar]
- 71.Massey J.G. Adverse drug reaction to metoclopramide hydrochloride in a macaw with proventricular dilation syndrome. J Am Vet Med Assoc. 1993;203(4):542–544. [PubMed] [Google Scholar]


