Highlights
-
•
Equine coronavirus has only recently been associated with emerging infections in adult horses.
-
•
Seroprevalence data is needed to better understand the epidemiology of equine coronavirus in adult horses.
-
•
The seroprevalence to equine coronavirus was 9.6% in 5247 healthy, adult horses from 18 states in the USA.
-
•
Seropositivity was significantly associated with horses from the Mid-West, draft horses and ranch/farm and breeding use.
Keywords: Equine coronavirus, ELISA, Horses, Risk factors, Seroprevalence
Abstract
Equine coronavirus (ECoV) is considered an enteric pathogen of foals and has only recently been associated with infections in adult horses. Seroprevalence data is needed to better understand the epidemiology of ECoV in adult horses, evaluate diagnostic modalities and develop preventive measures. The objective of this study was to investigate the seroprevalence and selective risk factors for ECoV in 5247 healthy adult horses in the USA, using a recently established and validated IgG enzyme-linked immunosorbent assay. Prevalence factors analysed in this study included geographic region, age, breed, sex and use.
A total of 504/5247 horses (9.6%) horses tested seropositive. Geographic region (Mid-West; P = 0.008), breed (Draft horses; P = 0.003) and specific uses of horses (ranch/farm, P = 0.034; breeding use, P = 0.016) were all statistically significant risk factors for seropositivity.
Introduction
Coronaviruses are part of the coronaviridae family, which are positive-strand RNA viruses. The coronaviridae subfamily is grouped into four genera, based on genetic differences and serological cross-reactivity (Woo et al, 2009, Woo et al, 2012). Equine coronavirus (ECoV) is a β-coronavirus, as are human coronavirus OC43 and HKU1, murine hepatitis virus, bovine coronavirus (BCoV), porcine haemagglutinating encephalomyelitis virus, canine respiratory coronavirus and rat coronavirus (Zhang et al., 2007). ECoV is considered an enteric pathogen of foals (Guy et al., 2000) and has only recently been associated with infections in adult horses in Japan, the USA and Europe (Oue et al, 2011, Oue et al, 2013, Pusterla et al, 2013, Miszczak et al, 2014). Reported morbidity rates during outbreaks range from 20–83% (Pusterla et al, 2013, Fielding et al, 2015, Giannitti et al, 2015).
Despite the sporadic occurrence of ECoV outbreaks in adult horses, the overall seroprevalence of this virus in horse populations remains largely unknown. Seroprevalence data is needed to better understand the epidemiology of ECoV, evaluate diagnostic modalities and develop preventive measures. Therefore, the objective of this study was to investigate the seroprevalence and selective risk factors to ECoV in 5247 healthy adult horses originating from 18 US states using a recently established and validated ELISA (Kooijman et al., 2016).
Materials and methods
Study population
The study population consisted of 5247 healthy horses from 18 states (CA, CO, FL, KY, MD, MN, MS, MO, NY, NC, OH, PA, TN, TX, VA, WA, WI, WY) representing the four regions of the USA (North-East, South, Mid-West, West). States sampled from the four regions were as follows: North-East (NY, MD, PA); South (FL, MS, KY, TN, NC, VA, TX); Mid-West (MO, OH, WI, MN); West (WA, CA, CO, WY). Banked sera collected by Zoetis USA for the purposes of another study were used. Blood samples were collected by convenience sampling from healthy horses by equine veterinarians from participating veterinary clinics across the USA during the autumn of 2013. Clinics enrolled voluntarily and each clinic sampled approximately 110 horses; no more than 10 horses sampled from each clinic resided on the same farm. Jugular venipuncture was used to collect 10 mL of whole blood from each horse. Additionally, age, breed, primary use, and sex data were collected for each horse by questionnaire. Blood samples were centrifuged, serum was separated, shipped on ice, processed and stored at −20 °C.
Serological analysis
All sera were tested for IgG to ECoV using an ELISA based on a recombinant protein containing two immunodominant areas of the spike protein of ECoV, including the area with the highest predicted antigenic sequence (Kooijman et al., 2016). Briefly, microtiter plates were coated with 0.0156 µg recombinant protein per well and sera were tested at a dilution of 1 µL serum in 100 µL of buffer. Each plate contained known negative and positive controls. Negative controls consisted of sera collected from healthy adult horses from the university herd with no history of systemic disease and qPCR-negative feces for ECoV. Positive controls consisted of sera collected from horses 30 days after they developed clinical signs compatible with ECoV infection and tested qPCR-positive for ECoV in the faeces. A secondary horseradish-peroxidase labelled IgG was used to bind with ECoV-specific antibodies from the serum samples. An enzyme substrate (Bethyl ELISA starter accessory kit II) was used to detect the secondary antibody. The optical density (OD) of each well was measured and corrected by subtracting the average of the OD values of the negative control samples. Sera were considered positive if the corrected value was above the average plus three times the standard deviation of the OD of the negative control samples (Kooijman et al., 2016).
Statistical methods
Risk factors analysed in this study included geographic regions (North-East, South, Mid-West, West), age, breed (Quarter horse, Warmblood, Thoroughbred, Paint horse, Arabian, Draft horse, Pony/miniature, other), sex (female, gelding, male) and use (competition, ranch/farm, breeding, other). Age was analysed in 5-year increments as a categorical variable.
Univariate logistic regression (controlling for the random effect of farm of origin) of each prevalence factor was performed to determine the odds ratios associated with region, age, breed, sex and use. Further, a mixed effects logistic regression model was developed to include significant risk factors and the random effects parameter of horses originating from the same farm. All statistical evaluations analyses were performed in Stata 12 (Stata statistical software, Version 12) and statistical significance was set at P < 0.05.
Results
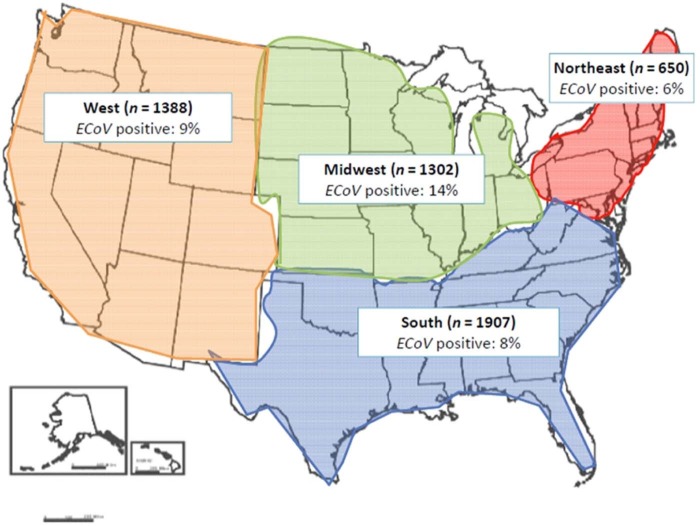
The total number of ECoV positive horses was 504/5247 (9.6%). The seroprevalence per state ranged from 4.0–19.7% (Table 1 ). Fig. 1 shows the number of horses sampled in each region and the rates of seropositive samples (%). Horses aged >20 years (13.0%), Draft horses (17.6%) and ranch/farm horses (12.0%) had the highest seroprevalence (Table 2 ). The median age of the study population was 11 years (range, 2–40 years).
Table 1.
Frequency of equine coronavirus (ECoV) seropositivity per state as defined by ELISA in 5247 equine sera collected from 18 different states.
| State (n) | ECoV positive samples (%) |
|---|---|
| Maryland (100) | 4(4) |
| Colorado(321) | 15(5) |
| Virginia(262) | 13(5) |
| Pennsylvania(220) | 13(6) |
| Kentucky(328) | 22(7) |
| Washington(320) | 21(7) |
| Florida(327) | 25(8) |
| New York(330) | 25(8) |
| Tennessee(220) | 18(8) |
| Texas(330) | 26(8) |
| California(328) | 30(9) |
| Missouri(330) | 34(10) |
| North Carolina(330) | 32(10) |
| Ohio(320) | 34(11) |
| Wyoming(419) | 53(13) |
| Mississippi(110) | 17(15) |
| Wisconsin(322) | 57(18) |
| Minnesota(330) | 65(20) |
Fig. 1.
Frequency of equine coronavirus (ECoV) seropositivity by ELISA per region in 5247 equine sera collected across the USA.
Table 2.
Equine coronavirus (ECoV) serologic status of 5247 healthy horses reported by geographic region, age, breed, sex and use.
| ECoV positive n (%) | ECoV negative n (%) | Total n (%) |
|
|---|---|---|---|
| Regiona (n) | |||
| North-East (650) | 42(6) | 608(94) | 650(12) |
| South(1907) | 153(8) | 1754(92) | 1907(36) |
| Mid-West(1302) | 190(15) | 1112(85) | 1302(25) |
| West(1388) | 119(9) | 1269(91) | 1388(26) |
| Age in years(n) | |||
| <1(0) | 0 | 0 | 0 |
| 1–5(1006) | 83(8) | 923(92) | 1006(19) |
| 6–10(1490) | 140(9) | 1350(91) | 1490(28) |
| 11–15(1284) | 121(9) | 1163(91) | 1284(24) |
| 16–20(761) | 77(10) | 684(90) | 761(15) |
| >20(599) | 78(13) | 521(87) | 599(11) |
| Not reported(107) | 5(5) | 102(95) | 107(2) |
| Breed(n) | |||
| Quarter horse (1567) | 166(11) | 1401(89) | 1567(30) |
| Warmblood(561) | 46(8) | 515(92) | 561(11) |
| Thoroughbred(844) | 41(5) | 803(95) | 844(16) |
| Paint(344) | 37(11) | 307(89) | 344(7) |
| Arabian(266) | 33(12) | 233(88) | 266(5) |
| Draft horse (238) | 42(18) | 196(82) | 238(5) |
| Pony/Miniature(269) | 21(8) | 248(92) | 269(5) |
| Other(1046) | 115(11) | 931(89) | 1046(20) |
| Not reported(112) | 3(3) | 109(97) | 112(5) |
| Sex(n) | |||
| Female(2196) | 200(9) | 1996(91) | 2196(42) |
| Maleb(2885) | 294(10) | 2591(90) | 2885(55) |
| Not reported(166) | 10(6) | 156(94) | 166(3) |
| Use(n) | |||
| Competition(2043) | 156(8) | 1887(92) | 2043(39) |
| Ranch/farm horse(2288) | 274(12) | 2014(88) | 2288(44) |
| Breeding(653) | 63(10) | 590(90) | 653(12) |
| Other(5) | 1(20) | 4(80) | 5(<1) |
| Not reported(258) | 10(4) | 248(96) | 258(5) |
Regions were defined as follows: North-East, NY, MD, PA; South, FL, MS, KY, TN, NC, VA, TX; Mid-West, MO, OH, WI, MN; West, WA, CA, CO, WY.
Male includes geldings and stallions.
Statistically significant risk factors in the mixed effects univariate analysis included region (Mid-West), age (>20 years old), breed (Draft horse, Thoroughbred) and use (ranch/farm). There was no significant difference in risk for ECoV seropositivity between male and female horses.
In the mixed effects multivariate model, horses from the Mid-West displayed the highest odds ratio of seropositivity in the region category when compared to the reference region (Table 3 ). Draft horse showed the highest odds ratio of seroprevalence in the breed category, while Thoroughbreds had the lowest odds ratio of seroprevalence. Ranch/farm and breeding horses displayed the highest odds ratio of seroprevalence in the use category. There was no significant difference between male and female horses and between the reference age group and the other age groups.
Table 3.
Results of the mixed effects logistic regression model for equine coronavirus (ECoV) infection, including significant predictor variables and random effects parameters expressed as odds ratios (OR) and 95% confidence intervals (95% CI) for risk factors studied (region, age, breed, sex and use) in 5247 healthy US horses.
| ECoV positivea | ECoV negativeb | P | |||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Regionc | |||||
| North-East | Ref | Ref | Ref | Ref | |
| South | 1.04 | 0.68–21.62 | 0.95 | 0.62–1.47 | 0.831 |
| Mid-West | 1.84 | 1.17–2.87 | 0.54 | 0.35–0.85 | 0.008 |
| West | 1.25 | 0.80–1.95 | 0.80 | 0.51–1.25 | 0.323 |
| Age in years | |||||
| 1–5 | Ref | Ref | Ref | Ref | |
| 5–10 | 1.12 | 0.82–1.53 | 0.89 | 0.65–1.21 | 0.472 |
| 11–15 | 1.13 | 0.82–1.58 | 0.88 | 0.64-1.22 | 0.437 |
| 16–20 | 1.18 | 0.82–1.70 | 0.85 | 0.59–1.21 | 0.375 |
| >20 | 1.42 | 0.97–2.08 | 0.70 | 0.48–1.03 | 0.070 |
| Breed | |||||
| Quarter horse | Ref | Ref | Ref | Ref | |
| Warmblood | 0.98 | 0.65–1.46 | 1.02 | 0.68–1.52 | 0.924 |
| Thoroughbred | 0.48 | 0.32–0.74 | 2.07 | 1.36–3.15 | 0.001 |
| Paint | 0.99 | 0.65–1.50 | 1.01 | 0.67–1.53 | 0.957 |
| Arabian | 1.20 | 0.76–1.90 | 0.83 | 0.53–1.31 | 0.430 |
| Draft horse | 1.95 | 1.25–3.05 | 0.51 | 0.33-0.80 | 0.003 |
| Pony/miniature | 0.81 | 0.48–1.37 | 1.24 | 0.73–2.09 | 0.426 |
| Other | 1.04 | 0.78–1.40 | 0.96 | 0.71–1.30 | 0.790 |
| Sexd | |||||
| Female | Ref | Ref | Ref | Ref | |
| Male | — | — | — | — | — |
| Use | |||||
| Competition | Ref | Ref | Ref | Ref | |
| Ranch/farm horse | 1.32 | 1.02–1.72 | 0.76 | 0.58–0.98 | 0.034 |
| Breeding | 1.59 | 1.09–2.31 | 0.63 | 0.43–0.92 | 0.016 |
| Other | 0.79 | 0.07–8.71 | 1.26 | 0.11–13.9 | 0.848 |
Ref, parameter used as reference category.
Random effects parameter: OR = 2.39; 95% CI (2.00–2.99); Likelihood ratio test vs. logistic regression: X2(1) = 40.42, P < 0.05.
Random effects parameter: OR = 2.39; 95% CI (2.00–2.99); Likelihood ratio test vs. logistic regression: X2(1) = 40.42, P < 0.05.
North-East was considered reference for region; Quarter horse was considered reference for breed; Competition was considered reference for use; Female was considered reference for sex; 1–5 year old horses were considered reference for age.
Sex was not included in multivariate model due to non-significance in univariate model.
The total number of ECoV negative horses was 4743/5247 horses (90.4%). Table 2 shows the number (%) of seropositive and seronegative horses sampled for each of the risk factor categories.
In the univariate model, horses aged 1–5 years old had 91.7% seronegativity, Thoroughbreds had the highest % seronegativity (95.1%) and competition horses had the highest % seronegativity (92.4%).
In the mixed effects multivariate model, horses from the Mid-West were least likely to be seronegative, Thoroughbreds were most likely to be seronegative and Draft horses were least likely to be seronegative (Table 3). There was no significant difference in odds for seronegative status by age group, sex and use.
Discussion
To the authors' knowledge, this is the first seroprevalence study of ECoV in adult healthy horses. The study focused on the detection of IgG-specific antibodies against ECoV because the IgG isotype has been shown to persist in the blood of cattle experimentally infected with BCoV for up to 22 months, while the IgM isotype response to BCoV only persisted up to 43 days (Tråvén et al., 2001). In our study, the seroprevalence of ECoV was 9.6% in 5247 healthy, adult horses from 18 different US states. Since follow-up data to determine the duration of antibodies against ECoV in seropositive horses were unavailable, it is possible that our cross-sectional study underrepresented the true seroprevalence of ECoV in our sample set. Following exclusion of the effect of farm origin, seropositivity was significantly associated with horses from the Mid-West, Draft horses and specific uses including ranch/farm and breeding use. A Swedish study of BCoV in 2007 also found region was a significant variable, in addition to herd size, biosecurity measures (i.e. boots for visitors) and distance to the nearest herd (animal density; Ohlson et al., 2010). It would be interesting to investigate whether these risk factors also apply to horses, but such information was unavailable for our study.
According to a study conducted in 1998 by the US Department of Agriculture's National Animal Health Monitoring System (USDA NAHMS)1 , the percentage of Draft horses was the highest in the North-Eastern and Central regions. In our study population, 7% of the horses from the Mid-West were Draft horses, compared to 2.6%, 3.7% and 6.3% from the West, South and North-East, respectively. Furthermore, 60% of the study horses were used as ranch or farm horses in the Mid-West and 33% of all Draft horses were used for ranch and farm work. Horses used for breeding in the Mid-West accounted for 43% of the total Draft horses used for breeding across regions. Therefore, factors contributing to the higher ECoV seroprevalence in the Mid-West could be related to the higher numbers of ECoV seropositive Draft horses used for farm/ranch work and breeding in that region.
Age was not a risk factor for seropositivity in the present study, suggesting that infection rate among the various age groups was probably similar. Previous studies have shown a wide range of age susceptibility to clinical ECoV, including young Draft horses in Japanese outbreaks and middle-aged horses in North American outbreaks (Oue et al, 2011, Oue et al, 2013, Pusterla et al, 2013). One of our study limitations was the lack of availability of blood samples from horses <1 year old. Previous studies have shown that ECoV can frequently be detected in the faeces of healthy foals and foals with diarrhoea (Slovis et al., 2014). Further work is required to determine the seroprevalence of ECoV in horses <1 year old.
Apart from the possible correlation between Draft horses and the Mid-West region, breed could also be correlated with specific uses. Previous Japanese studies have reported ECoV outbreaks predominantly in racing Draft horses (Oue et al., 2013). It remains to be determined if breed is directly linked to increased susceptibility to ECoV infection or if the observations are confounded by specific husbandry practices.
Seropositivity as it relates to use could possibly be related to farm size. According to the NAHMS2 , breeding farms are often relatively large (>19 animals). More horses housed together and higher stocking densities could increase the risk of ECoV transmission. This is supported by a NAHMS study which reported that more horses/farm increased the risk of illness. Because there are more foals and young horses at breeding farms, such operations represent relatively large, high-risk populations, which could contribute to increased spread of ECoV and, hence, higher seroprevalence. Additionally, up to 6% of the horses used for breeding in our study population were Draft horses, which was higher than for other breeds.
Climatic differences in the different geographical regions studied could affect the environmental viability of the virus, thereby influencing the risk of spread and seropositivity rates. The virus responsible for severe acute respiratory syndrome (SARS) in humans, also a β-coronavirus, is known to survive > 5 days outside the host at a temperature of 22–25 °C and relative humidity of 40–50%. Higher temperatures and/or humidity levels resulted in a rapid loss of viability (Chan et al., 2011). To the authors' knowledge, there is no information on the environmental viability of ECoV.
As with BCoV, clinical cases of ECoV in adult horses are diagnosed more frequently during the colder months of the year in the USA (Pusterla et al., 2013). This observation probably relates to husbandry-specific factors and the temperature-sensitivity of the virus, which are likely to affect transmission rates of ECoV. The major limitation of this study is the estimation of overall seroprevalence of ECoV using a single data collection point, which could have underestimated the general exposure rate. Robust longitudinal studies are needed to further investigate the observations and risk factors presented herein.
Conclusions
In this sample of 5247 healthy, adult horses from 18 states across the USA, ECoV seroprevalence was 9.6%. Seropositivity was significantly associated with region (Mid-West), breed (Draft horses) and specific uses (ranch/farm and breeding). Longitudinal studies are required to further investigate these findings.
Conflict of interest statement
Zoetis USA supplied the equine sera used in this study. Zoetis USA played no role in the analysis and interpretation of data, or in the decision to submit the manuscript for publication. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
The study was supported by the Center for Equine Health, School of Veterinary Medicine, University of California, Davis, with additional contributions from public and private donors. The authors gratefully acknowledge the support of Zoetis USA for providing the sera required for this study.
Footnotes
See: Part I: Base line Reference of 1998 Equine Health and Management www.aphis.usda.gov/animal_health/nahms/equine/downloads/equine98/Equine98_dr_PartI.pdf (Accessed 3 January 2017).
See: USDA Equine 2005 Part I: Baseline Reference of Equine Health and Management, 2005 www.aphis.usda.gov/animal_health/nahms/equine/downloads/equine05/Equine05_dr_PartI.pdf (accessed 3 January 2017).
References
- Chan K.H., Malik Peiris J.S., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Advances in Virology. 2011;2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C.L., Higgins J.K., Higgins J.C., McIntosh S., Scott E., Giannitti F., Mete A., Pusterla N. Disease associated with equine coronavirus infection and high case fatality rate. Journal of Veterinary Internal Medicine. 2015;29:307–310. doi: 10.1111/jvim.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannitti F., Diab S., Mete A., Stanton J.B., Fielding L., Crossley B., Sverlow K., Fisch S., Mapes S., Scott L. Necrotizing enteritis and hyperammonemic encephalopathy associated with equine coronavirus infection in equids. Veterinary Pathology. 2015;52:1148–1156. doi: 10.1177/0300985814568683. [DOI] [PubMed] [Google Scholar]
- Guy J.S., Breslin J.J., Breuhaus B., Vivrette S., Smith L.G. Characterization of a coronavirus isolated from a diarrheic foal. Journal of Clinical Microbiology. 2000;38:4523–4526. doi: 10.1128/jcm.38.12.4523-4526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman L.J., Mapes S., Pusterla N. Development of an equine coronavirus-specific enzyme-linked immunosorbent assay to determine serological responses in naturally infected horses. Journal of Veterinary Diagnostic Investigation. 2016;28:414–418. doi: 10.1177/1040638716649643. [DOI] [PubMed] [Google Scholar]
- Miszczak F., Tesson V., Kin N., Dina J., Balasuriya U.B.R., Pronost S., Vabret A. First detection of equine coronavirus (ECoV) in Europe. Veterinary Microbiology. 2014;171:206–209. doi: 10.1016/j.vetmic.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson A., Heuer C., Lockhart C., Tråvén M., Emanuelson U., Alenius S. Risk factors for seropositivity to bovine coronavirus and bovine respiratory syncytial virus in dairy herds. Veterinary Record. 2010;167:201–207. doi: 10.1136/vr.c4119. [DOI] [PubMed] [Google Scholar]
- Oue Y., Ishihara R., Edamatsu H., Morita Y., Yoshida M., Yoshima M., Hatama S., Murakami K., Kanno T. Isolation of an equine coronavirus from adult horses with pyrogenic and enteric disease and its antigenic and genomic characterization in comparison with the NC99 strain. Veterinary Microbiology. 2011;150:41–48. doi: 10.1016/j.vetmic.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oue Y., Morita Y., Kondo T., Nemoto M. Epidemic of equine coronavirus at Obihiro Racecourse, Hokkaido, Japan in 2012. Journal of Veterinary Medical Science. 2013;75:1261–1265. doi: 10.1292/jvms.13-0056. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Mapes S., Wademan C., White A., Ball R., Sapp K., Burns P., Ormond C., Butterworth K., Bartol J. Emerging outbreaks associated with equine coronavirus in adult horses. Veterinary Microbiology. 2013;162:228–231. doi: 10.1016/j.vetmic.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovis N.M., Elam J., Estrada M., Leutenegger C.M. Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: A comprehensive molecular study. Equine Veterinary Journal. 2014;46:311–316. doi: 10.1111/evj.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tråvén M., Näslund K., Linde L., Linde B., Silván A., Fossum C., Hedlund K.O., Larsson B. Experimental reproduction of winter dysentery in lactating cows using BCV – Comparison with BCV infection in mild-fed calves. Veterinary Microbiology. 2001;81:127–151. doi: 10.1016/S0378-1135(01)00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Huang Y., Yuen K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Experimental Biology and Medicine. 2009;234:1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Lam C.S.F., Lau C.C.Y., Tsang A.K.L., Lau J.H.N., Bai R., Teng J.L.L., Tsang C.C.C., Wang M. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. Journal of Virology. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.X., Guy J.S., Snijder E.J., Denniston D.A., Timoney P.J., Balasuriya U.B. Genomic characterization of equine coronavirus. Virology. 2007;369:92–104. doi: 10.1016/j.virol.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]