Summary
Background
The number of travelers in Oman has increased significantly in the last 2 decades with an increase in the expatriate population workforce leading to the emergence of infections related to travel. This paper aims to highlight the burden of travel-related infections in Oman.
Method
Our study is a descriptive record-based review and analysis of travel-associated diseases over a 14 year time period from 1999 to 2013. The data was sourced from the communicable disease surveillance system, and central public health laboratory results.
Results
From 1999 to 2013 there were a combined total of 7022 cases of cholera, chikungunya, dengue, filariasis, leptospirosis, meningococcal infection, poliomyelitis, measles, schistosomiasis, viral hepatitis (A), typhoid and para-typhoid reported to and subsequently investigated by the Department of Communicable Diseases. Among these cases, 558 (7.9%) were attributed to travel. Fifty percent of these patients were admitted to hospitals.
Conclusion
Travel-associated infections account for about 8% of notifiable infections in Oman and have low mortality rate. However, some travel-associated infections are considered as a threat to polio eradication and measles elimination programs. Furthermore, some can cause outbreaks that can overwhelm the healthcare system.
Keywords: Imported infections, Oman, Dengue, Typhoid, Measles
1. Introduction
Since the advent of modern commercial aviation in the 1950s, and over the past 6 decades, international civilian travel globally has experienced continued expansion and virtually uninterrupted growth from 25 million in 1950 to 278 million in 1980, 528 million in 1995, and 1087 million in 2013. It is expected that international tourist arrivals globally will increase by 3.3% a year from 2010 to 2030 to reach 1.8 billion by 2030 [1].
International travel exposes individuals to new cultural, psychological, physiological and microbiological exposures and challenges. The tourists' and migrants abilities to adopt, cope and survive are influenced by many variables such as the visited region, the length of the trip and the diversity of planned activities [2]. These variables are modified by personality, experiences and behavior; and they differ according to age, gender, culture, race, social status and education [3]. Infectious disease risks for international travelers are moving targets, as new diseases have emerged and old ones have re-emerged. The diseases that travelers come in contact with have changed. Some countries have become safer overall, but other countries are experiencing new diseases or the re-emergence of past diseases. For example, travelling to the developing world necessarily puts travellers and migrants at risk for infectious diseases, with 20%–70% of returned travellers suffering some sort of illness [4], [5], [6].
International travelers can experience travel-related morbidity during and after travel. Of the approximately 50 million people who travel from industrialized countries to developing countries each year, 8% report becoming ill enough to seek health care either during or after travel [7]. Many other travelers also experience health problems that often go unreported [8].
Travel-related illnesses may have important calamitous public health consequences if conditions for re-introduction of diseases are met [9]. Traveling contributes to the global spread of infectious diseases, including novel and emerging pathogens; the global epidemic of Severe Acute Respiratory Syndrome (SARS) in 2003 was a prime example [10]. More recently, international travelers infected with novel H1N1 influenza played a major role in the rapid global spread of the virus [11]. Travelers have also carried pathogens to areas of the world where these pathogens were rare or had been eliminated. Recent outbreaks of vaccine-preventable diseases such as measles [12] and mumps [13] in the United States have been traced to contact with persons who had traveled to locations where vaccination was less prevalent. In addition, travel and migration have contributed to recent introduction or reintroduction of vector-borne diseases in places that had been free from these diseases, such as locally acquired dengue in Florida [14], malaria in Greece [15] and also in Great Exuma Island in the Caribbean [16].
The Sultanate of Oman is located in the south-eastern part of the Arabian Peninsula with land area of 309,500 square kilometers. The country is divided into 11 administrative governorates; with a with a total population of 4,156,967 and about 43.5% of them are expat [17]. Most of the population is located in the north and south of the country. Oman has achieved remarkable developments in health care within a relatively short span of 4 decades and has developed a good infrastructure for health services. Health care in Oman is largely the responsibility of the state and the cost is borne by the government. As of 2013, the Ministry of Health (MOH) had 195 primary care centers and 49 hospitals. Out of these 49 hospitals, there are 4 in the capital area of Muscat, which offer tertiary care services. The primary health centers offer primary care services to the population residing in the assigned catchment area of the center. The secondary and tertiary care services are provided through a referral process.
The objective of this retrospective descriptive study of the travel-associated infections in Oman between 1999 and 2013 is to identify the burden of travel-associated infections and to develop risk profiles for the most common travel-related infections in Oman.
2. Methods
A retrospective descriptive record-based review and analysis of travel-associated diseases was conducted over the period from 1999 to 2013.
All travel-associated infectious diseases that were reviewed and included in this study were detected by the routine communicable disease surveillance and the central public health laboratory results during the specified study period.
The studied travel-related infections include: dengue, chikungunya, cholera (classical form), lymphatic filariasis, Neisseria meningitides, measles, Schistosoma haematobium, poliomyelitis, leptospirosis and typhoid. The patient should be a resident in Oman who met the case definition for the specific disease. In addition, subjects must have reported traveled to a country endemic with that disease during the investigation, and should have been out of Oman during the full incubation period of the disease. Case definitions were based on the National Communicable Diseases Surveillance and Control SOP manual [18]. As malaria surveillance in Oman is a separate program, data about malaria was not included in this study. However, all malaria cases reported during the study period were imported [19].
Included subjects are residents of Oman (Omani and non-Omani), based on the clinical presentation and incubation period of each disease, included subject has to be free from the infection under consideration (manifesting or incubating) before travelling, and has to meet the case definition for the specific disease.
The primary dependent variables for the surveillance data were the diagnosed travel-associated diseases. Independent variables include: basic demographics (age, sex and nationality), country of exposure and disease progression (admission and outcome).
Data analysis was conducted using SPSS (statistical program for the social science, version 18). Frequency distributions were used to determine the percent of travel-associated infections.
This study is free from any ethical constraints as it is a secondary analysis of the data collected routinely for the purpose of public health surveillance and reporting. The data analysis was conducted at the Department of Communicable Diseases. No personal identifying information linking the patients with the results of the study were disclosed with the findings of the study.
3. Results
From 1999 to 2013 there were a total of 7022 cases of cholera, chikungunya, dengue, filariasis, leptospirosis, meningococcal infection, poliomyelitis, measles, schistosomiasis, viral hepatitis (A), typhoid and para-typhoid reported and subsequently investigated by the Department of Communicable Diseases. Among these cases, 558 (7.9%) met the case definition for travel-related illnesses.
Typhoid was the most frequent travel-associated infection, 300 (53.8%), followed by measles 80 (14.3%), and the least reported travel-associated infection was poliomyelitis with 2 cases (0.4%) (Table 1 ).
Table 1.
Number and percent of diagnosed travel-associated infections in Oman (1999–2013).
| Diagnosis | Travel-associated infections no. (%) | Total infections | % Of travel-associated infection |
|---|---|---|---|
| Cholera | 14 (2.5%) | 34 | 41.2% |
| Chikungunya | 9 (1.6%) | 9 | 100% |
| Dengue | 78 (14.0%) | 78 | 100% |
| Filariasis | 4 (0.7%) | 5 | 80% |
| Leptospirosis | 2 (0.4%) | 6 | 33.3% |
| Meningococcal | 25 (4.5%) | 80 | 31.3% |
| Poliomyelitis | 2 (0.4%) | 2 | 100% |
| Measles | 80 (14.3%) | 80 | 100% |
| Schistosomiasis (H) | 4 (0.7%) | 24 | 16.7% |
| Viral Hepatitis A | 18 (3.2%) | 5625 | 0.3% |
| Typhoid | 300 (53.8%) | 991 | 30.3% |
| Para-typhoid | 22 (3.9%) | 88 | 25% |
| Total | 558 (100%) | 7022 | 7.9% |
The majority of travel-associated infections were reported among non-Omani and affected predominantly male population (Table 2). The greater proportion was among those aged between 19 and 35, and most of the cases reported South Asia as the region of exposure.
Table 2.
Distribution of travel-associated infections by nationality, sex, age groups and country of exposure in Oman (1999–2013).
| Character | No. (%) |
|---|---|
| Nationality | |
|
77 (13.7%) |
|
481 (86.3%) |
| Sex | |
|
392 (70.3%) |
|
166 (29.7%) |
| Age | |
|
149 (26.7%) |
|
24 (4.3%) |
|
220 (39.4%) |
|
119 (21.3%) |
|
38 (6.8%) |
|
6 (1%) |
| Geographical region of exposure | |
|
130 (23.3%) |
|
401 (71.9%) |
|
4 (0.7%) |
|
23 (4.1%) |
| Total | 558 (100%) |
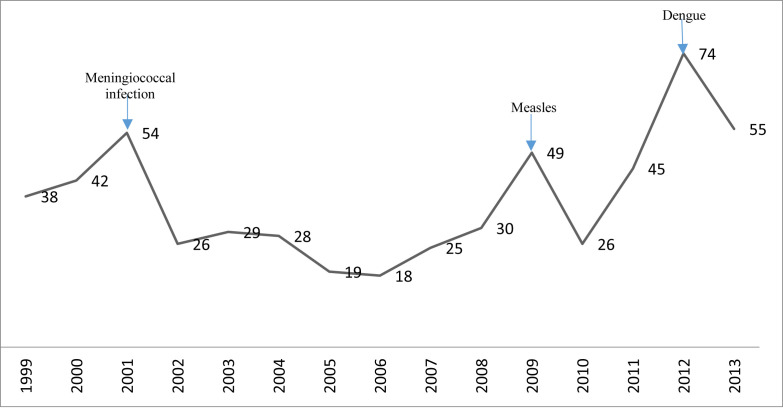
The trend of travel-associated infections in Oman between 1999 and 2013 (Fig. 1 ) shows three peaks, and each one is related to the rise in the number of certain infections.
Figure 1.
Trend of travel-associated infections in Oman (1999–2013).
The first peak was in 2001 (54 cases) and was related to a cluster of 7 cases of meningococcal infections among pilgrims during Hajj with additional 15 cases of measles. In 2009 the rise (49 cases) was due to imported cases of measles from Yemen and the last peak in 2012 (74 cases) was mainly related to cases of dengue imported from India. Excluding peak years, the annual average of travel-associated infections was 31.7 ± 11.1 cases.
Deaths attributed to travel-associated infections were reported in only 9 cases (less than 2%) with case fatality rate ranging from 5.1% for dengue fever to 20% for meningococcal disease. Disease admission rates were different among different infections. All reported travel-associated poliomyelitis cases were admitted to hospitals as they presented with acute flaccid paralysis and the majority of cholera, dengue and meningococcal infections as well as Paratyphoid cases were admitted; however, no admissions were reported for measles or viral hepatitis A cases.
3.1. Dengue
A total of 78 cases of dengue were reported. Fifty-two (66.7%) of theses case were non-Omani and 59 (75.6%) were males. Thirty cases (38.5%) were in the age group between 19 and 50 years. South Asian Countries, predominantly India accounted for 57 (73.1%) of the cases. Fever presented in 69 (88.5%) of the cases and 66 (84.6%) patients were hospitalized with 4 (5.1%) reported deaths. The first reported case was in 2001, and there was an upsurge in the number of cases in 2012 with 15 cases reported (Table 3 ).
Table 3.
Number and percentage of admissions and deaths (CFR) among travel-associated infections in Oman (1999–2013).
| Travel-associated infection | Total | Inpatient | % Admission rate | Death | % (CFR) |
|---|---|---|---|---|---|
| Cholera | 14 | 12 | 85.7% | – | – |
| Chikungunya | 9 | – | – | – | |
| Dengue | 78 | 66 | 84.6% | 4 | 5.1% |
| Filariasis | 4 | 2 | 50% | – | – |
| Leptospirosis | 2 | 1 | 50% | – | – |
| Meningococcal | 25 | 21 | 84% | 5 | 20% |
| Poliomyelitis | 2 | 2 | 100% | – | – |
| Measles | 80 | – | – | – | – |
| Schistosomiasis (H) | 4 | 1 | 25% | – | – |
| Viral Hepatitis A | 18 | – | – | – | – |
| Typhoid | 300 | 176 | 58.6% | – | – |
| Para-typhoid | 22 | 19 | 86.4% | – | – |
| Total | 558 | 300 | 53.8% | 9 | 1.6% |
3.2. Chikungunya
There were a total of 9 cases of chikungunya. All of the cases were attributed to foreign travel and were non-Omani females coming from India. Four cases (44.4%) were between 35 and 50. None of the cases were hospitalized and there were no reported deaths.
Chikungunya was first reported in 2007 with 7 cases (77.7%), 2 more cases were reported in 2008 and no more cases reported since then.
3.3. Cholera
There were 14 cases of cholera; all of these cases were in non-Omani males, and 9 (64.3%) were between 19 and 35 years of age; 13 (92.9%) were from South Asia. The cholera admission rate was 85.7% with no reported mortality. Cholera represents 2.5% of travel-associated infections. The first imported cluster of 7 cases was reported in 1999 among immigrants entering illegally into Oman. Vibrio cholera O1, Ogawa, El Tor was isolated. The cases presented with classical clinical picture. Few sporadic cases were reported among Asian workers acquiring infection abroad and travelling to Oman during the incubation period.
3.4. Meningococcal infection
There were 25 cases of meningococcal infections, 13 (52%) of them were Omani and 15 (60%) were males, 9 (36%) occurred in patients under 12 years of age. Fourteen (56%) of the cases are linked to the Hajj season in April, 2000. Meningococcal infection showed the highest mortality rate with 5 deaths (20%) among all travel-associated infections and a 21 inpatient (84%) admission rate.
3.5. Typhoid
There were 300 typhoid cases and this is the most common travel-related illness following malaria. Typhoid represents more than half the cases of travel-associated infections (53.8%). Among these cases, 294 (98.0%) were non-Omani and the majority of the cases were in males, 226 cases (75.3%). The most frequent age distribution was between 19 and 35 years, and most of the 262 (87.3%) cases had travelled to South Asian countries. One hundred and seventy-six (58.6%) cases were admitted to hospitals and no deaths were reported.
3.6. Measles
Eighty cases of measles were reported representing 14.3% of the travel-associated infections. Forty-four (55%) cases were Omanis, 52 (65%) were females and 54 (67.5%) presented in patients under 12 years of age. Fifty-four (67.5%) of the cases have travelled to Middle Eastern countries. None of these cases were admitted to a hospital and there were no deaths.
An upsurges of measles occurred in 2001 with 15 cases; 12 of them had travel history to United Arab Emirates. Another surge occurred in 2009 with 19 cases in travelers to Yemen.
4. Discussion
The distribution of nationality and country of exposure in this study can be explained as most of the travel-associated infections occurred among expatriates from South Asia who represent the majority of the foreign work force in Oman. Travelling for the purpose of visiting friends and relatives is a documented risk factor for the acquisition of travel-related illnesses, as people travelling for this reason tend to stay in local homes, travel for longer durations and may fail to recognize the health risks inherent to travelling to their country of origin [4], [20]. This was comparable to other studies where India was one of the most common countries of exposure among travel-associated infections [21], [22].
Age and sex distribution among cases in this study with male and working-aged group predominance is affected by the characteristics of the work force in Oman. Other explanations may be related to the scope and nature of activities conducted by males between 19 and 35, exposing them to infections more than females and people in childhood and older age. However, sex distribution in this study was in contrast with other studies where travel-associated infections were divided almost equally between males and females [21], [22].
Although a very low mortality rate was detected, more than half of the cases of travel-associated infections were admitted to hospital which causes a burden on the health care system raising the bed occupancy rates. This high rate of admission is in contrast with other studies where cases treated as inpatients were only 5.1% and 11.1% [21], [22], respectively. This contrast can be related to the difference in the spectrum of the infectious diseases and the severity of cases.
Dengue is an increasingly prevalent tropical arbovirus infection with significant morbidity and mortality [23]. It is documented that dengue infection has been known to be endemic in India for over two centuries. India witnessed a widespread dengue fever outbreak in the year 2012 [24]. The increased number of reported cases in Oman in 2012 are likely to be related to this outbreak in India.
Chikungunya, which presents as a dengue-like illness, is an emerging arboviral infection of travelers to South Asia and the Indian Ocean islands. Cases reported in 2007 were related to the upsurge of cases in India where a major outbreak of chikungunya fever during 2006–2007 occurred and Kerala was the worst affected state contributed to 55.8% cases in the country [25]. This seroprevalence rate is higher than similar chikungunya outbreaks in many other parts of the world [26], [27], [28], reflecting the severity and far reach of this outbreak, thereby explaining its impact in Oman.
The most travel-associated cases of cholera and typhoid came from South Asia and this is similar to reports from different parts of the world [29], [30], [31].
Measles and meningococcal meningitis are important notifiable diseases in Oman due to their associated complications, mortality and potential risk for outbreaks. The Middle East was reported as the country of origin of most of cases of these diseases. During the 2000 Hajj season, an outbreak of meningococcal disease principally involved serogroup W-135 [32], [33], [34]. Cases were reported in returning pilgrims in countries throughout the world including Europe, the United States, Asia, Africa and the Middle East [32], [34]. More than half of the travel-related cases occurred in Oman was part of this outbreak in year 2000 with 20 local infections in Oman as secondary infections and serotype W-135 was implicated for the first time. Another 8 local cases occurred just after Hajj season in 2001. Since May 2001, quadrivalent vaccine is a requirement for all pilgrims [35].
Our study had several limitations: it is retrospective and uncontrolled in design, it is based on notifications received at the Department of Communicable Disease and the test results for samples referred to the Central Public Health Laboratory. Missing and incomplete data about some cases and some variables were limiting factors, and some private health institutions may not notify all the cases, this may lead to an underestimation of the burden of travel-associated infections in Oman.
However, this study collected data about travel-associated infections for 14 years reflecting the large database and good record keeping including travel history. This is a national database and it is mandatory to report certain types of travel related illnesses with standardized data collection for the whole country.
5. Conclusions
Travel-associated infections account for about 8% of infections in Oman with a low mortality rate. These infections are considered as a threat to polio eradication and measles elimination programs and some can cause outbreaks. Travel-associated infections have the potential to overwhelm the healthcare system—more than 50% of these studied cases were admitted to hospitals.
This study shows the need to establish a travel health service that is integrated within the primary health care system, to establish a surveillance system for travel-associated infections and to implement laboratory-based surveillance as well. In addition, there is a need to increase awareness of physicians regarding travel-associated infections with an emphasis on addressing travel history. There is a need for more research on knowledge, attitude and practice of both healthcare workers and the public about travel health. Further research on travel-associated infection focusing on the expat workforce in Oman with more detailed analysis of demographics will be of great benefit.
Conflict of interest
None.
Contributor Information
Seif S. Al-Abri, Email: salabri@gmail.com.
Doaa M. Abdel-Hady, Email: doaahady2000@gmail.com.
Salem S. Al Mahrooqi, Email: salem.mahrooqi@gmail.com.
Hanan S. Al-Kindi, Email: drhananalkindi@gmail.com.
Amina K. Al-Jardani, Email: draljardani@hotmail.com.
Idris S. Al-Abaidani, Email: dr.idris.oman@gmail.com.
References
- 1.United Nations World Tourism Organization . vol. 10. United Nations World Tourism Organization; Madrid: 2012. http://dtxtq4w60xqpw.cloudfront.net/sites/all/files/pdf/unwto_highlights14_en.pdf (UNWTO world tourism barometer). (September) [accessed 09.02.15] [Google Scholar]
- 2.Lee A.W., Kozarsky P.E. CDC Health Information For International Travel; 2012. Planning for healthy travel: responsibilities & resources.http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-1-introduction/introduction-to-travel-health-and-the-yellow-book [accessed 12.04.15] [Google Scholar]
- 3.Cossar J. Travellers' health: an epidemiological overview. In: Wilks J., Page S.J., editors. Managing tourist health and safety in the new millennium. Elsevier Science Ltd; Oxford: 2003. pp. 19–33. [Google Scholar]
- 4.Ryan E.T., Wilson M.E., Kain K.C. Illness after international travel. N Engl J Med. 2002;347(7):505–516. doi: 10.1056/NEJMra020118. [DOI] [PubMed] [Google Scholar]
- 5.Ryan E.T., Kain K.C. Health advice and immunizations for travelers. N Engl J Med. 2000;342(23):1716–1725. doi: 10.1056/NEJM200006083422306. [DOI] [PubMed] [Google Scholar]
- 6.Steffen R., Rickenbach M., Wilhelm U., Helminger A., Schär M. Health problems after travel to developing countries. J Infect Dis. 1987;156(1):84–91. doi: 10.1093/infdis/156.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Hill D.R. Health problems in a large cohort of Americans traveling to developing countries. J Travel Med. 2000;7:259–266. doi: 10.2310/7060.2000.00075. [DOI] [PubMed] [Google Scholar]
- 8.Steffen R., deBernardis C., Baños A. Travel epidemiology—a global perspective. Int J Antimicrob Agents. 2003;21:89–95. doi: 10.1016/S0924-8579(02)00293-5. [DOI] [PubMed] [Google Scholar]
- 9.Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A.C., Panning M. Infection with Chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 10.Center for Disease Control and Prevention (CDC) Update: outbreak of severe acute respiratory syndrome-worldwide, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(12):241–248. [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention (CDC) Update: novel influenza A (H1N1) virus infections-worldwide. MMWR Morb Mortal Wkly Rep 2009. 2009;58(17):453–458. [PubMed] [Google Scholar]
- 12.Center for Disease Control and Prevention (CDC) Measles—United states, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(15):253–257. [PubMed] [Google Scholar]
- 13.Center for Disease Control and Prevention (CDC) Update: mumps outbreak—New York and New Jersey, June 2009–January 2010. MMWR Morb Mortal Wkly Rep. 2010;59(05):125–129. [PubMed] [Google Scholar]
- 14.Center for Disease Control and Prevention (CDC) Locally acquired dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59(19):577–581. [PubMed] [Google Scholar]
- 15.Odolini S., Gautret P., Parola P. Epidemiology of imported malaria in the Mediterranean region. Mediterr J Hematol Infect Dis. 2012;4:e2012031. doi: 10.4084/MJHID.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Center for Disease Control and Prevention (CDC) Malaria—Great exuma, Bahamas, May–June 2006. MMWR Morb Mortal Wkly Rep. 2006;55(37):1013–1016. [PubMed] [Google Scholar]
- 17.National center for statistics and information, Sultunate of Oman. http://www.ncsi.gov.om/Pages/NCSI.aspx [accessed 15.07.15]
- 18.Directorate General for Health Affairs, MoH . 2nd ed. Ministry of Health, Sultanate of Oman; 2005. Standard operating procedures manual for communicable disease surveillance and control.http://www.cdscoman.org/uploads/cdscoman/CDS%20Manual.pdf [accessed 29.03.15] [Google Scholar]
- 19.Minsitry of Health . Annual health report 2013. Ministry of Health, Sultanate of Oman; 2013. Health domains; pp. 8–72. [Google Scholar]
- 20.Leder K., Torresi J., Libman M.D., Cramer J.P., Castelli F., Schlagenhauf P. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med. 2013;158(6):456–468. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boggild A.K., Geduld J., Libman M., Ward B.J., McCarthy A.E., Doyle P.W. Travel-acquired infections and illnesses in Canadians: surveillance report from CanTravNet surveillance data, 2009–2011. Open Med. 2014;8(1):e20–e32. PMCID: PMC4085092. [PMC free article] [PubMed] [Google Scholar]
- 22.Odolini S., Parola P., Gkrania-Klotsa E., Caumes E., Schlagenhauf P., López-Vélez R. Travel-related imported infections in Europe, EuroTravNet 2009. Clin Microbiol Infect. 2012;18(5):468–474. doi: 10.1111/j.1469-0691.2011.03596.x. [DOI] [PubMed] [Google Scholar]
- 23.Gubler D.J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandyopadhyay B., Bhattacharyya I., Adhikary S., Konar J., Dawar N., Sarkar J. ISRN Virology; 2013. A comprehensive study on the 2012 dengue fever outbreak in Kolkata, India. Article ID 207580. Available at: [accessed 02.04.15] [DOI] [Google Scholar]
- 25.Kumar N.P., Joseph R., Kamaraj T., Jambulingam P. A226V mutation in virus during the 2007 Chikungunya outbreak in Kerala, India. J Gen Virol. 2008;89:1945–1948. doi: 10.1099/vir.0.83628-0. [DOI] [PubMed] [Google Scholar]
- 26.Gerardin P., Perrau J., Fianu A., Favier F. Determinants of Chikungunya virus infection in the Reunion Island: results of the SEROCHIK seroprevalence survey in the population, August–October 2006. Bull Epidemiol Hebd. 2008;38:39–40. [Google Scholar]
- 27.Moro M.L., Gagliotti C., Silvi G., Angelini R., Sambri V., Rezza G. Chikungunya virus in north-eastern Italy: a seroprevalence survey. Am J Trop Med Hyg. 2010;82(3):508–511. doi: 10.4269/ajtmh.2010.09-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sissoko D., Moendandze A., Malvy D., Giry C., Ezzedine K., Solet J.L. Seroprevalence and risk factors of Chikungunya virus infection in Mayotte, Indian Ocean, 2005–2006: a population-based survey. PloS One. 2008;3(8):e3066. doi: 10.1371/journal.pone.0003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Public Health England. Cholera. Available at: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/TravelHealth/EpidemiologicalData/GastrointestinalInfections/travCholera/ [accessed 5 .02.15].
- 30.Loharikar A., Newton A.E., Stroika S., Freeman M., Greene K.D., Parsons M.B. Cholera in the United States, 2001–2011: a reflection of patterns of global epidemiology and travel. Epidemiol Infect. 2015;143(4):695–703. doi: 10.1017/S0950268814001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor B.A., Schwartz E. Typhoid and paratyphoid fever in travellers. Lancet. 2005;5:623–628. doi: 10.1016/S1473-3099(05)70239-5. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Serogroup W-135 meningococcal disease among travelers returning from Saudi Arabia–United States, 2000. MMWR Morb Mortal Wkly Rep. 2000;49(16):345–346. http://www.ncbi.nlm.nih.gov/pubmed/10817480 [accessed 04.04.15] [PubMed] [Google Scholar]
- 33.Samuelsson S., Handysides S., Ramsay M., Lyytikainen O., Nygard K., Perrocheau A. Meningococcal infection in pilgrims returning from the Hajj: update from Europe and beyond. Eurosurveillance Wkly. 2000;17:1–5. [Google Scholar]
- 34.Taha M.K., Achtman M., Alonso J.M., Greenwood B., Ramsay M., Fox A. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet. 2000;356:2159. doi: 10.1016/S0140-6736(00)03502-9. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO) Meningococcal disease, serogroup W135. Wkly Epidemiol Rec. 2001;19:141–142. http://www.ncbi.nlm.nih.gov/pubmed/11383502 [accessed 04.04.15] [PubMed] [Google Scholar]