Summary
Background
A case control study to better characterize the clinical features, laboratory, and radiological abnormalities associated with MERS-CoV infection in order to help with early identification of this syndrome from other respiratory infections.
Methods
Eighty patients admitted to a hospital in Riyadh, diagnosed with MERS-CoV infection based on RT-PCR were matched on age, sex, and the presence of a co-morbid condition on a basis of 1:2 to other patients admitted with respiratory symptoms and tested negative for MERS-CoV on RT-PCR.
Results
None of the reported MERS-CoV presenting symptoms was significantly associated with being infected with MERS-CoV. On the other hand, WBC count was significantly lower in patients with confirmed MERS-CoV infection (median 5.7 vs 9.3, P: 0.0004). Neutrophil count was as well significantly lower in MERS-CoV patients (median 3.7 vs 6.7, P: 0.0001). Both AST, and ALT values were significantly higher in MERS-CoV infected group (AST median 42 vs 36, P: 0.03, and ALT median 33 vs 28, P: 0.003). Overall our MERS-CoV mortality rate was (10%) below the national figure of (40%).
Conclusions
None of the presenting symptoms are specific for MERS-CoV infection. And out of all the investigations WBC, neutrophil counts, AST and ALT values have some predictive utility.
Keywords: Middle East Respirator Syndrome, MERS-CoV, Coronavirus, Predictors, Mortality
1. Introduction
MERS-CoV is a novel betacoronavirus that was discovered in 2012 after it was isolated from the respiratory secretions of a patient in Saudi Arabia who died with acute respiratory syndrome [1]. So far, all reported cases were diagnosed in the Arabian Peninsula (mainly Saudi Arabia) or epidemiologically linked to it [2], [3], [4], [5], [6], [7]. Up to April 2016 more than 1360 cases have been reported in Saudi Arabia alone with a mortality rate that exceeded 40% [8]. Though the disease is believed to be zoonotic with strong evidence towards a camel reservoir [9], [10], [11], [12], [13], [14], major outbreaks are believed to be nosocomial involving transmission within health care facilities [2], [15], [16], [17], [18].
Early after its discovery, MERS-CoV infection screening was limited to those critically ill with severe acute respiratory illness [17], [19], [20], [21]. Later on the Saudi Arabian Ministry of Health issued guidelines for MERS-CoV screening in which they limited suspect cases to patients with clinical or radiological evidence of pneumonia, patients with respiratory illness along with a history of possible exposures to a MERS-CoV patient, and patients with unexplained acute febrile illness with hematological laboratory abnormalities (leukopenia or thrombocytopenia) and gastrointestinal or respiratory symptoms [22].
Many studies were conducted in Saudi Arabia to characterize the illness associated with MERS-CoV [17], [19], [20], [21], but they were limited by the small sample size. Studies done elsewhere were limited as well by the extremely small sample size [3], [4], [5], [6], [7], except in South Korea where a large outbreak occurred between May and July of 2015 due to an imported case from the Arabian Peninsula [2].
Travel associated cases have been observed in Europe, notably in UK, France, Germany, Austria and Italy with secondary cases in close contacts of index cases without a travel history suggesting person-to-person transmission [22], [23], [24], [25].
In this study we tried to overcome the previous limitation by studying a larger cohort of MERS-CoV patients at our facility and comparing it to a control group in an attempt to look for predictors of MERS-CoV infection.
2. Methods
2.1. Study design and population
In this study we followed a cohort of patients tested for MERS-CoV infection at Prince Mohammed bin Abdulaziz Hospital (PMAH) Emergency Department, a governmental hospital in Riyadh, Saudi Arabia, between April 1st 2014 and September 30th 2015. Patients who were felt to have Influenza Like Illness (ILI) were screened for MERS-CoV and all patients who required admission and had any respiratory symptom (cough, shortness of breath, sore throat) were screened for MERS-CoV infection and followed up until discharged. We also included patients who were called for admission after a positive MERS-CoV test that was done at an earlier visit to our emergency department and individuals who were hospital quarantined due to a significant exposure history to a MERS-CoV patient.
As PMAH is the MERS-CoV referral center for governmental (public) and private hospitals in Riyadh, Saudi Arabia, we excluded patients who were already diagnosed with MERS-CoV at other facilities and referred to our hospital for isolation and further management.
Due to the great heterogeneity of this cohort, and in an attempt to look for predictors of MERS-CoV infection, we matched the patients who tested positive for MERS-CoV (cases) to a control group from the rest of the study population who were admitted and repeatedly tested negative for MERS-CoV. We extracted a matched control for the positive cases based on age (within 5 years of age), gender, and presence of any comorbidity. The attempted matching was 1 case to 2 controls.
2.2. MERS-CoV testing
Respiratory samples (Nasopharyngeal swapping or tracheal aspirates) were obtained from all patients and tested for MERS-CoV infection using real-time reverse-transcription polymerase chain reaction (RT-PCR). The specimens were submitted to and testing was carried out at the Saudi Ministry of Health MERS-CoV regional laboratory. The test amplified both the upstream E protein (upE gene) and ORF1a for MERS-CoV. A positive case was determined if both assays were positive. Each patient was tested at least twice, each on a different day.
2.3. Patient data
Reporting suspected and confirmed cases was the original purpose of data collection which was initially limited to date of admission, sex, age, nationality, designated hospital ward, results/dates of MERS-CoV testing, and patients' outcomes. Data concerning presenting symptoms: fever, sore throat, cough, shortness of breath, and gastrointestinal symptoms (nausea, vomiting or diarrhea), as well as the initial laboratory work up: White Blood Cell count (WBC) 109 per liter, Hemoglobin (Hgb) grams per deciliter, Platelets 109 per liter, Creatinine micromoles per liter, Albumin gram per liter, Aspartate Aminotransferase (AST) units per liter, Alanine Aminotransferase (ALT) units per liter, and initial Chest-X-Ray (CXR) results were later extracted from the electronic medical records.
2.4. Statistical analysis
We summarized the data by descriptive statistics. Frequencies and percentages were calculated for categorical variables. Continuous variables (mainly laboratory values) tended to have skewed distributions; thus we used medians rather than means. A conditional logistic regression analysis was conducted to identify the variables that are independently associated with MERS-CoV infection. The magnitude of association, presented as the odds ratio and 95% CI was also determined this way. All reported P values in this article are also based on the logistic regression.
The laboratory measures were found to not be linearly related to the logit of MERS-CoV infection, so the values were broken into 4 approximately equally sized groups (via quartiles, referred to as quarters) and odds ratios estimated for each as compared to the first quartile as reference. Note that for analysis of laboratory measures, a few people had to be excluded due to missing values.
We used SAS version 9.4 (SAS Institute, Cary NC) to perform all of the analysis. The significance level for all of the statistical tests was set at 0.05.
3. Results
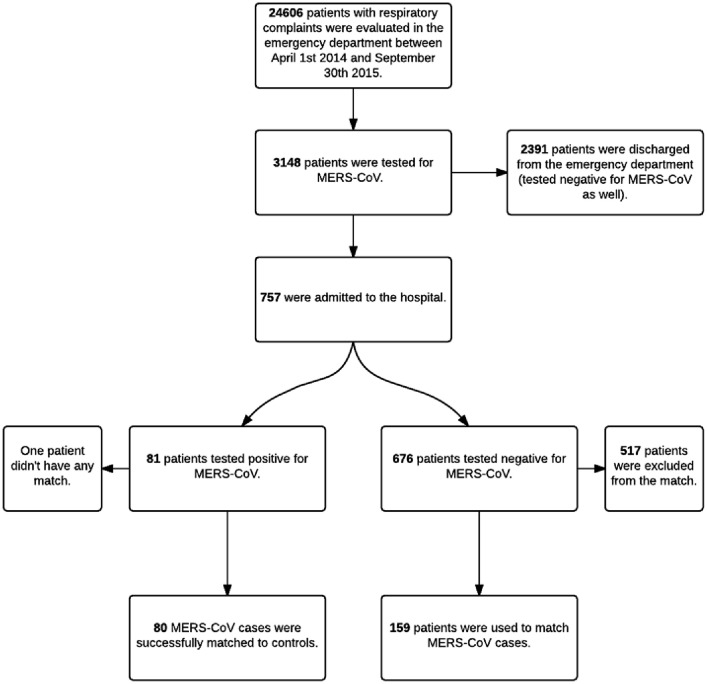
From 24,606 patients who presented to our Emergency Department between April 1st 2014 and September 30th 2015 with respiratory complaints, 3148 were tested for MERS-CoV. From those who were screened, only 757 patients were admitted. Eighty one patients tested positive for MERS-CoV and the rest, 676 patients, repeatedly tested negative and were used to find matches to MERS-CoV cases. See Fig. 1 .
Figure 1.
Study sample flow chart.
We were able to match all but two of the confirmed MERS patients. For one of them we were only able to find one control, and for the other no match was found. This resulted in a total of 239 patients for analysis. Table 1 shows the characteristics of both the MERS-CoV infected group and the control group.
Table 1.
Characteristics of the study population.
| Confirmed (n = 80) | Control (n = 159) | OR | 95% CI | Pa | |
|---|---|---|---|---|---|
| Age (median)b | 40 | 40 | – | – | – |
| Female sexb | 32 (40%) | 64 (40.2%) | – | – | – |
| Saudi Nationality | 53 (66.2%) | 103 (64.8%) | 1.07 | 0.61–1.88 | 0.822 |
| HCW | 13 (16.2%) | 18 (11.3%) | 2.20 | 0.72–6.69 | 0.164 |
| Co-morbiditiesb | |||||
| Any | 37 (46.2%) | 74 (46.5%) | – | – | – |
| Asthma | 7 (8.7%) | 16 (10.1%) | – | – | – |
| Cancer | 0 (0%) | 1 (0.6%) | – | – | – |
| Cardiac | 5 (6.2%) | 11 (6.9%) | – | – | – |
| ESRD | 1 (1.2%) | 5 (3.1%) | – | – | – |
| HTN | 18 (22.5%) | 41 (25.8%) | – | – | – |
| Diabetes | 25 (31.2%) | 33 (20.7%) | – | – | – |
| Others | 7 (8.7%) | 10 (6.3%) | – | – | – |
| Symptoms | |||||
| Any | 64 (80%) | 151 (95%) | 0.21 | 0.08–0.54 | 0.001 |
| GI | 14 (17.5%) | 22 (13.8%) | 1.37 | 0.63–2.94 | 0.424 |
| Sore throat | 11 (13.7%) | 23 (14.5%) | 0.95 | 0.43–2.09 | 0.894 |
| Fever | 47 (58.7%) | 102 (64.1%) | 0.80 | 0.46–1.39 | 0.427 |
| Cough | 42 (52.5%) | 94 (59.1%) | 0.77 | 0.44–1.33 | 0.35 |
| SOB | 30 (37.5%) | 56 (35.2%) | 1.10 | 0.63–1.90 | 0.743 |
| Abnormal CXR | 38 (50.7%) | 88 (57.8%) | 0.72 | 0.40–1.30 | 0.271 |
| Labs (median) | |||||
| WBCs | 5.7 | 9.3 | – | – | 0.0004 |
| Neutrophils | 3.6 | 6.7 | – | – | 0.0001 |
| Hgb | 145 | 138 | – | – | 0.07 |
| Platelets | 205 | 238 | – | – | 0.223 |
| AST | 42 | 36 | – | – | 0.031 |
| ALT | 33 | 28 | – | – | 0.003 |
| Albumin | 37 | 36 | – | – | 0.844 |
| Creatinine | 68.1 | 70.1 | – | – | 0.579 |
| ICU on admission | 15 (18.7%) | 26 (16.3%) | 2.29 | 0.57–2.39 | 0.667 |
| Mortality | 8 (10%) | 7 (4.4%) | 2.29 | 0.83–6.30 | 0.11 |
Abbreviations: ALT, alanine aminotransferase, AST: aspartate aminotransferase, CI: confidence interval, CXR: chest-X-ray, ESRD: End Stage Renal Disease, Hgb: hemoglobin, ICU: intensive care unit, OR: odds ratio, WBCs: white blood cells.
p-values based on logistic regression.
Matched variables.
3.1. MERS-CoV infected group
The median age for MERS-CoV patients was 40 years; 60% were males, 66.2% were Saudi, and 16.2% were health care workers. 46% had at least one co morbidity; hypertension and diabetes were the most common. 80% of MERS-CoV patients were symptomatic; fever was the most common symptom among MERS-CoV infected group 58.7% (73% of the symptomatic patients), followed by cough 52.5% (73% of symptomatic patients). Shortness of breath was the third common symptom that was reported by 37.5% of MERS-CoV infected patients (47% of the symptomatic patients). Around 17.5% of MERS-CoV infected patients reported GI symptoms (22% of the symptomatic patients). Sore throat was reported in 13.7% (17% of the symptomatic patients).
In MERS-CoV infected group the median value for WBCs was 5.7 (IQR: 4.1–8.6), neutrophils 3.6 (IQR: 2.2–5.3), Hgb 145 (IQR: 128–157), platelets 205 (IQR: 148–270), albumin 37 (IQR: 34–42), AST 42 (IQR: 26–105), ALT 33 (IQR: 21–93), and creatinine 68.1 (IQR: 60.8–81.9). All of the above mentioned medians were within the normal ranges. Almost 51% of MERS-CoV patients had chest X-ray findings upon admission. Around 19% were sick enough to be admitted directly to the ICU, and around 10% of MERS-CoV infected patients expired.
3.2. Matched control group
As discussed above, matching was based on age (within 5 years of age), gender, and presence of any comorbidity. 64.8% of the control group were Saudi and11.3% were health care workers. Hypertension and diabetes were the most common comorbidities. 95% of the control group patients were symptomatic; fever 64.1% and cough 59.1% were the most commonly reported symptoms in the control group. Around 35% complained of shortness of breath, and 14.5% had a sore throat. 13.8% of the control group reported GI symptoms.
Laboratory analysis showed a median WBCs value of 9.3 (IQR: 6.0–14.2), neutrophils 6.7 (IQR: 3.7–11.1), Hgb 138 (IQR: 114–153), platelets 238 (IQR 170–338), albumin 36 (IQR: 32–41), AST 36 (IQR: 23–62), ALT 28 (IQR: 14.5–48.5), and creatinine 70.1 (IQR: 61.4–86.9). Almost 58% of the control group patients had chest X-ray findings upon admission. Around 16.3% of the control group patients were sick enough to be admitted directly to the ICU on presentation, and 4.4% of the control group patients expired during their hospital stay.
3.3. Predictors of MERS-CoV infection
There was no statistical difference in the proportion of Saudi Nationals (66.2% vs 64.8% OR, 1.07; P = 0.82) as well as health care workers (16.2% vs11.3% OR, 2.20; P = 0.16) between the confirmed and matched groups. Though confirmed MERS-CoV patients were statistically less likely to be symptomatic (80% vs 95% OR: 0.21; p: 0.001), no statistically significant differences between the two groups were found in regards to frequency of a specific symptom (fever, cough, shortness of breath, gastrointestinal symptoms or sore throat with P values of 0.43, 0.35, 0.74, 0.42, and 0.89 respectively). This was as well the case in regards to the presence of chest-X-ray findings upon admission (50.7% vs 57.8%, OR: 0.72; P: 0.27). No significant statistical difference was observed between the MERS-CoV confirmed group and the control group in regards to intensive care unit need upon admission (18.7% vs 16.3%, OR: 2.29; P: 0.67). Though mortality rate seemed to be higher among MERS-CoV infected group, this was not statistically significant (10% vs 4.4%, OR: 2.29; P: 0.11). The median WBCs counts (5.7 vs 9.3), as well as the median neutrophil counts (3.6 vs 6.7) were both lower in the infected group, and that was statistically significant (P values 0.0004 and 0.0001 respectively). The median alanine aminotransferase (ALT) value was higher among MERS-CoV infected group compared to the control group (33 vs 28, P: 0.003). That was as well the case with aspartate aminotransferase (AST) (42 vs 36, P: 0.031). There was no statistical difference in the median values for Hgb, platelets, albumin, and creatinine between the two groups, although the relationship for Hgb was marginally significant (P values of 0.07, 0.22, 0.84, and 0.58 respectively). Table 2 displays comparative analysis of significant laboratory values between confirmed and suspected cases.
Table 2.
Laboratory values – odds ratios (95% CI) compared to 1st (lowest) quarter.a
| 2nd quarter | 3rd quarter | 4th quarter | |
|---|---|---|---|
| WBCs | 0.76 (0.35–1.62) | 0.24 (0.10–0.57) | 0.21 (0.08–0.52) |
| Neutrophils | 0.76 (0.37–1.56) | 0.22 (0.09–0.53) | 0.18 (0.17–0.44) |
| Hgb | 2.49 (1.15–5.56) | 2.06 (0.89–4.77) | 3.24 (1.25–8.38) |
| AST | 2.56 (0.64–3.77) | 1.16 (0.41–3.32) | 3.31 (1.24–8.86) |
| ALT | 3.72 (1.39–9.94) | 1.48 (0.52–4.32) | 5.94 (2.01–17.55) |
Quarters (25th, 50th, 75th percentiles): WBC: 4.8, 8.2, 12.7; Neutrophil: 2.93, 5.06, 9.28; Hgb: 118, 151, 154; AST: 24.37.5, 76.5; ALT: 17, 29, 60.
The odds of being a confirmed case was significantly lower for those with WBC in the 3rd and 4th quarters (WBCs ≥ 8.2) as compared to the reference category of patients with WBC less than the 25th percentile (WBCS < 4.8). The odds of being a confirmed case was significantly lower for those with neutrophil values in the 3rd and 4th quarters as compared to the reference category of patients with neutrophil less than the 25th percentile, with odds ratios 0.22 and 0.18 respectively (quartiles 2.93, 5.06, 9,38) for neutrophil.
The median ALT values were higher for those with confirmed MERS-CoV infection as compared to those without (median 33 vs 28 respectively). The odds ratios were significantly increased for patients with confirmed MERS when ALT values were in the 2nd and 4th quarters (ALT 17–29, and ALT > 60) as compared to patients with ALT values in the lowest quarter (ALT < 17); OR = 3.72 and 5.94 respectively. Patients with values between 29 and 60 did not have statistically significantly increased odds of confirmed MERS. Patients with confirmed MERS had higher median values of AST (42 vs 36). Patients with AST greater than 76.5 had statistically significant higher odds of having confirmed MERS than those in the lowest quarter (values less than 24) with an odds ratio of 3.31, 95% CI: 1.24, 8.86.
4. Discussion
As observed in previous studies [17], [19], [20], [21], we found that having WBCs and neutrophil counts within the normal range is more likely to be associated with MERS-CoV. By comparing our cohort of MERS-CoV patients to another cohort of 47 patients diagnosed with MERS-CoV between September 2012 and June of 2013 described by Assiri et al. [21] we can notice that both cohorts were predominated by male sex (60% vs 77%), though ours had a lower male proportion. Male predominance, which was observed in almost every surveillance study [17], [19], [20], [21] could be related to the culture in Saudi Arabia, where women wear veils that cover both the nose and mouth and may help protect from exposure, along with decreased outdoor activities compared to men. Our patients' median age was 40 years compared to a median age in the range of 50–59 in the previous cohort. Fever (59% vs 98%), cough (53% vs 83%), and shortness of breath (38% vs 72%) were the main symptoms, though our patients were less likely to be symptomatic. We also noticed that our patients were less likely to have co morbid conditions (46% vs 96%), less likely to have chest-X-ray abnormalities (51% vs. 100%) and had a significantly lower mortality (10% vs 60%). All this implies that, earlier in the outbreak, screening and diagnoses were limited to the very sick population who subsequently had a high mortality.
In our patient population we were liberal in screening any potential admission who complained of respiratory symptoms and due to very strict infection control program we included individuals who were quarantined due to a significant exposure history to a MERS-CoV patient. By doing so we were aiming at preventing a possible MERS-CoV outbreak related to inadequate infection control measures. This helped uncover many asymptomatic or mildly symptomatic cases. This might also imply that the true burden of the disease in the kingdom is still uncovered and that we might be just seeing the tip of the iceberg. This theory was first brought up after a nationwide, cross-sectional, serological study done between December 1st 2012 and December 1st 2013 in which serum samples from just over ten thousand individuals, whom age and sex distribution largely matched the general population [26].
This report has far reaching implications. In this study we found that none of the presenting symptoms helped distinguish those with MERS-CoV infection from the matched control group presenting with ILI symptoms. Almost half of MERS-CoV patients had no CXR abnormalities on presentation. In addition to raising significant questions on the validity of the current MOH suspect case definition, this will challenge the practicing physicians in the emergency room in endemic and non-endemic countries on how to deal with patients presenting with ILI symptoms [27], [28]. Even with access to full viral panel on all ILI patients and with evidence of influenza virus as the etiology, MERS-CoV can't be ruled out. This is based on data from Iran where 3/5 MERS cases had concomitant influenza infection [29], [30].
Our study has a few limitations, the main being the lack of comprehensive testing for viral respiratory panels for patients admitted with ILI symptoms (cases and matched controls). Recently this has been added to the testing of all patients admitted with ILI. A larger, prospective, multicenter study in the endemic areas is needed to better characterize the illness associated with MERS-CoV infection and specify its predictors.
Funding source
None.
Conflict of interest
None of the authors declared COI.
Contributor Information
Hamzah A. Mohd, Email: dr.hmohd@gmail.com.
Ziad A. Memish, Email: zmemish@yahoo.com.
Sarah H. Alfaraj, Email: alfarajsa@pmah.med.sa.
Donna McClish, Email: donna.mcclish@vcuhealth.org.
Talal Altuwaijri, Email: altuwaijrit@pmah.med.sa.
Marzouqah S. Alanazi, Email: Alanazima@pmah.med.sa.
Saleh A. Aloqiel, Email: aloqiels@pmah.med.sa.
Ahmed M. Alenzi, Email: alenezia@pmah.med.sa.
Fahad Bafaqeeh, Email: bafaqeehf@pmah.med.sa.
Amal M. Mohamed, Email: mohameda@pmah.med.sa.
Kamel Aldosari, Email: aldosaryk@pmah.med.sa.
Sameeh Ghazal, Email: ghazals@pmah.med.sa.
References
- 1.Zaki A.M., Boheemena S., Bestebroer T.I.M., Osterhaus A., Fouchier R. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Korea Centers for Disease Control and Prevention Middle East Respiratory Syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015 Aug;6(4):269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epidemiological update . European Centre for Disease Prevention and Control; 22 Jul 2015. Middle East Respiratory Syndrome Coronavirus (MERS-CoV)http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1256&List=8db7286c-fe2d-476c-913318ff4cb1b568&Source=http%253A%252F%252Fecdc.europa.eu%252Fen%252Fhealthtopics%252Fcoronavirus-infections%252FPages%252Fnews_and_epidemiological_updates.aspx [Google Scholar]
- 4.Mailles A., Blanckaert K., Chaud P., van der Werf S., Lina B., Caro V. The investigation team. First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18(24) pii=20502. [PubMed] [Google Scholar]
- 5.Kraaij – Dirkzwager M., Timen A., Dirksen K., Gelinck L., Leyten E., Groeneveld P., on behalf of the MERS-CoV outbreak investigation team of the Netherlands Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in two returning travellers in The Netherlands, May 2014. Euro Surveill. 2014;19(21) doi: 10.2807/1560-7917.ES2014.19.21.20817. pii=20817. [DOI] [PubMed] [Google Scholar]
- 6.Premila Devi J., Noraini W., Norhayati R., CheeKheong C., Badrul A.S., Zainah S. Laboratory-confirmed case of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection in Malaysia: preparedness and response, April 2014. Euro Surveill. 2014;19(18) doi: 10.2807/1560-7917.ES2014.19.18.20797. pii=20797. [DOI] [PubMed] [Google Scholar]
- 7.Bialek SR, Allen D, Alvarado-Ramy F, Arthur R, Balajee A, Bell D, et al. First confirmed cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities - May 2014. Am J Transplant; 14(7):1693–1699. http://dx.doi.org/10.1111/ajt.12841. [PMC free article] [PubMed]
- 8.Command and control center (CCC) statistics. Ministry of Health (MOH); Kingdom of Saudi Arabia: April 2016. http://www.moh.gov.sa/en/CCC/PressReleases/Pages/default.aspx [Google Scholar]
- 9.Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 10.Adney D.R., van Doremalen N., Brown V.R., Bushmaker T., Scott D., de Wit E. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis. 2014 Dec;20(12):1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reusken C.B., Haagmans B.L., Muller M.A., Gutierrez C., Godeke G.J., Meyer B. Middle East Respiratory Syndrome Coronavirus neutralizing serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer B., Müller M.A., Corman V.M., Reusken C., Ritz D., Godeke G. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014 Apr;20(4):552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Wit E.D. Middle East Respiratory Syndrome Coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5:e00884–e00914. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemida M.G., Perera R.A., Wang P., Alhammadi M.A., Siu L.Y., Li M. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013 Dec 12;18(50):20659. doi: 10.2807/1560-7917.ES2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- 15.Drosten C., Muth D., Corman V.M., Hussain R., Al Masri M., HajOmar W. An observational, laboratory-based study of outbreaks of Middle East Respiratory Syndrome coronavirus in Jeddah and Riyadh, kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60(3):369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R. Hospital-associated outbreak of Middle East Respiratory Syndrome Coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59(9):1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T. Hospital outbreak of Middle East Respiratory Syndrome Coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. August 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowell G., Blumberg S., Simonsen L., Miller M.A., Viboud C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics. 2014 Dec;9:40–51. doi: 10.1016/j.epidem.2014.09.011. Epub 2014 Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Tawfiq Jaffar A., Hinedi Kareem, Ghandour Jihad, Khairalla Hanan, Musleh Samir, Ujayli Alaa. Middle East Respiratory Syndrome Coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Abdul Matin M, et al. Clinical aspects and outcomes of 70 patients with Middle East Respiratory Syndrome Coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis; 29:301–306. http://dx.doi.org/10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed]
- 21.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East Respiratory Syndrome Coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Command and Control Center . 3rd ed. Ministry of Health (MOH); Kingdom of Saudi Arabia: June 2015. Infection prevention and control guidelines for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection.http://www.moh.gov.sa/Documents/2015%20update.pdf [Google Scholar]
- 23.Sridhar S., Brouqui P., Parola P., Gautret P. Imported cases of Middle East Respiratory Syndrome: an update. Travel Med Infect Dis. 2015 Jan-Feb;13(1):106–109. doi: 10.1016/j.tmaid.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Tawfiq J.A., Zumla A., Memish Z.A. Travel implications of emerging coronaviruses: SARS and MERS-CoV. Travel Med Infect Dis. 2014 Sep-Oct;12(5):422–428. doi: 10.1016/j.tmaid.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Tawfiq J.A., Omrani A.S., Memish Z.A. Middle East Respiratory Syndrome Coronavirus: current situation and travel-associated concerns. Front Med. 2016 Jun;10(2):111–119. doi: 10.1007/s11684-016-0446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D. Presence of Middle East Respiratory Syndrome Coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15:559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunha Burke A., Dunont Marie, Abruzzo Eileen. An adult returned traveler from Dubai hospitalized with an influenza-like illness (ILI): Middle East Respiratory Syndrome (MERS) or influenza? Infection control implications from a Near MERS case. Infect Control Hosp Epidemiol. 2015;36(7):858–860. doi: 10.1017/ice.2015.91. [DOI] [PubMed] [Google Scholar]
- 28.Cunha C.B., Opal S.M. Middle East Respiratory Syndrome (MERS): a zoonotic viral pneumonia. Virulence. 2014;5:650–654. doi: 10.4161/viru.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vink M.A., Bootsma M.C., Wallinga J. Serial intervals of respiratory infectious diseases: a systematic review and analysis. Am J Epidemiol. 2014;180:865–875. doi: 10.1093/aje/kwu209. [DOI] [PubMed] [Google Scholar]
- 30.Yavarian J., Rezaei F., Shadab A., Soroush M., Gooya M.M., Azad T.M. Cluster of Middle East Respiratory Syndrome coronavirus infections in Iran, 2014. Emerg Infect Dis. 2015;21:362–364. doi: 10.3201/eid2102.141405. [DOI] [PMC free article] [PubMed] [Google Scholar]