Highlights
-
•
Multiyear studies are preferred for estimating robust influenza vaccine effectiveness over time.
-
•
An efficient way to evaluate the influenza vaccine effectiveness was used, through data linkage of two already established systems in the public health sector.
-
•
We applied both fixed-effects and random-effects meta-analysis of case–control studies to estimate the pooled vaccine effectiveness for children aged 6–59 months across five influenza seasons and considered the variation in antigenic match and epidemics year by year as the heterogeneity between studies.
Keywords: Influenza vaccine effectiveness, Case–control studies, Influenza surveillance
Abstract
Objectives
We aimed to estimate the pooled vaccine effectiveness (VE) in children over five winters through data linkage of two existing surveillance systems.
Methods
Five test-negative case–control studies were conducted from November to February during the 2004/2005 to 2008/2009 seasons. Sentinel physicians from the Viral Surveillance Network enrolled children aged 6–59 months with influenza-like illness to collect throat swabs. Through linking with a nationwide vaccination registry, we measured the VE with a logistic regression model adjusting for age, gender, and week of symptom onset. Both fixed-effects and random-effects models were used in the meta-analysis.
Results
Four thousand four hundred and ninety-four subjects were included. The proportion of influenza test-positive subjects across the five seasons was 11.5% (132/1151), 7.2% (41/572), 23.9% (189/791), 6.6% (75/1135), and 11.2% (95/845), respectively. The pooled VE was 62% (95% confidence interval (CI) 48–83%) in both meta-analysis models. By age category, VE was 51% (95% CI 23–68%) for those aged 6–23 months and 75% (95% CI 60–84%) for those aged 24–59 months.
Conclusions
Influenza vaccination provided measurable protection against laboratory-confirmed influenza among children aged 6–59 months despite variations in the vaccine match during the 2004/2005 to 2008/2009 influenza seasons in Taiwan.
1. Introduction
Influenza viruses cause annual epidemics and the occasional pandemic of acute respiratory disease, which pose a threat to the health of the population.1 Vaccination is considered a priority in public health departments and is an effective way to prevent influenza-associated morbidity, mortality, and expense.2 Since 1998, the Department of Health in Taiwan has gradually endorsed annual influenza vaccination campaigns to encourage susceptible subjects, including the elderly, healthcare workers, poultry workers, and young children, to receive free influenza immunization, based on the recommendations of the Advisory Committee on Immunization Practices in Taiwan.
The recommendation of universal influenza vaccination of young children was not popular in many countries initially, probably because of the absence of studies providing solid evidence of effectiveness in the targeted population.3, 4 During the 2004/2005 influenza season, the Centers for Disease Control in Taiwan (Taiwan CDC) started to vaccinate groups of children aged 6–23 months; this program was extended to those aged 24–35 months starting in 2008/2009. All vaccination target groups had the same opportunity to receive free influenza shots beginning in October each year. In addition to the recommended groups, all people could receive free influenza vaccination after December 1 each season in order to best utilize the influenza vaccine resources and to increase the vaccine coverage of the entire population.
It is important to determine the influenza vaccine effectiveness (VE) after the implementation of such a program. Previous studies have encouraged large studies to assess the impact of influenza vaccination on children in terms of specific outcome measurements.5, 6 Furthermore, multiyear studies are preferred for estimating robust influenza VE over time through a meta-analysis methodology.7, 8 The Taiwan CDC has successfully coordinated a laboratory-based surveillance network for influenza virus for all ages since 2000 and established the National Immunization Information System (NIIS) for children aged <6 years in 2003.9 By using the retrospective laboratory-confirmed influenza surveillance data and linking these to individual vaccination records, we were able to rapidly and efficiently demonstrate the influenza VE in children for the 2004/2005 to 2008/2009 seasons.
Previous reports have demonstrated influenza VE using routinely collected laboratory and/or surveillance data and directly pooling results from multiple years to provide the overall VE.10, 11, 12 In this study, we implemented a fixed-effects and a random-effects meta-analysis of case–control studies to estimate the pooled VE for children aged 6–59 months across the five consecutive influenza seasons, and considered the variation in antigenic match across seasons and epidemics year by year as the heterogeneity between studies. Such effectiveness studies of inactivated influenza vaccine among young children could assist public health sectors in reassessing the current national influenza vaccination strategy for the target groups, especially when vaccine match varies year to year.
2. Subjects and methods
2.1. Study population
Children aged 6–59 months with an influenza-like illness (ILI) during the November to February winter epidemics over five seasons from 2004/2005 to 2008/2009 were investigated. The Viral Surveillance Network required sentinel physicians to collect throat or nasal swabs among verbally consenting ILI patients regardless of the patient's influenza vaccination status and underlying medical conditions. ILI was defined as a body temperature ≥38 °C plus one of the following four clinical manifestations: cough, sore throat, hoarseness and running nose, or headache and myalgia/fatigue. This study was initiated as a public health response and used routinely collected surveillance data and vaccination records to assess influenza VE. The Taiwan CDC determined these activities to be non-research and thus the study did not require review by an institutional review board.
2.2. Viral surveillance network and virological testing
The Viral Surveillance Network coordinated by the Taiwan CDC was started in October 2000; it comprises 10–13 collaborating laboratories (the number is affected by the annual budget) and aims to survey and isolate nationwide circulating viruses related to respiratory tract infections year-round.9 Clinical specimens obtained from nasal or throat swabs were collected by the sentinel physicians and sent to the local collaborating laboratories for virus identification using viral culture and/or reverse transcriptase PCR. Methods of virus isolation have been described previously.13 The Taiwan CDC collected and analyzed these results on a weekly basis and posted this information on their website. The antigenic match between vaccine and circulating strains in each season was evaluated by hemagglutination inhibition (HAI) assay.
2.3. Determination of case and control subjects
Children whose specimens tested positive for laboratory-confirmed influenza infection during the study periods were defined as case subjects. Control subjects were those with the same symptoms but who were negative for influenza. For cases and controls, information about age, sex, week of symptom onset, and personal identifiers were obtained from the reports submitted by the sentinel physicians.
2.4. Influenza vaccination status
Information on the influenza vaccination status of the subjects was obtained from the NIIS, which was established by the Taiwan CDC to collect vaccination records for children at a national level. Children were classified as vaccinated if they had received one or more vaccine doses in the current influenza season and it was administered ≥14 days before the onset of ILI. Children were classified as unvaccinated for the given season if they were not vaccinated in that study season or if they had received the first vaccine dose within 14 days before respiratory tract infection. In this study, we did not define the status of partially vaccinated children because many influenza epidemic strains in Taiwan become the vaccine strains 2–3 years later, as shown by hemagglutination sequence comparisons.9, 14 Therefore, children aged 6–59 months were considered immunized if they had received one or more vaccine doses in the current influenza season regardless of previous influenza immunization history.
2.5. Statistical analysis
We linked the NIIS and National Viral Surveillance System using the personal identifier. We used a logistic regression model to adjust for age, gender, and week of symptom onset, with the first week including November 1 and the last week including February 28 in the five different epidemic seasons.15, 16 The adjusted odds ratio (aOR) was used to model the association between influenza vaccination and laboratory-confirmed influenza-related medical visits in each season. VE and 95% confidence intervals (CI) were estimated using the formula VE = (1 − OR) × 100%. Stratified VE estimates were calculated according to age (6–23 months or 24–59 months) and adjusted for gender and week of symptom onset. We used both a fixed-effects model with inverse variance method and a random-effects model with DerSimonian–Laird weighting method17 to run the synthesis results. A forest plot was used to display the estimated overall ORs and separate ORs in the five epidemic seasons according to the two age groups.18 We used the ‘meta’ package for the R system for statistical computing to implement the meta-analysis.19, 20 Annual vaccination rates among control groups were examined using the Cochran–Armitage test for trend in SAS, version 9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
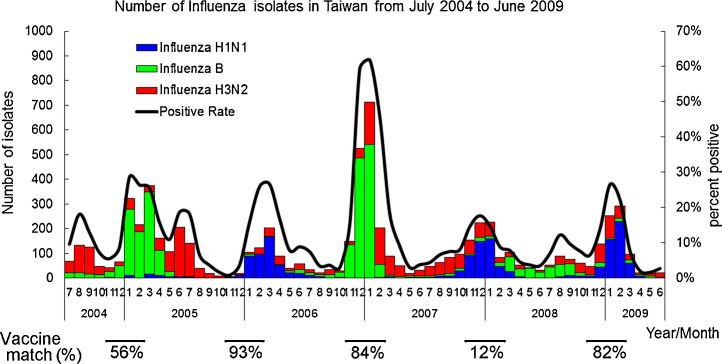
In Taiwan, five winter epidemics occurred between November 2004 and February 2009, which were dominated by influenza A H1N1 in 2005/2006, 2007/2008, and 2008/2009, influenza B in 2004/2005, and influenza B followed by influenza A H3N2 in 2006/2007 (Figure 1 , Table 1 ). Information on influenza activity obtained from the Viral Surveillance Network demonstrated that positive rates of influenza isolates for 6–59-month-old children varied each winter; from high to low, these rates were 23.9% (189/791) in the 2006/2007 season, 11.5% (132/1151) in the 2004/2005 season, 11.2% (95/845) in the 2008/2009 season, 7.2% (41/572) in the 2005/2006 season, and 6.6% (75/1135) in the 2007/2008 season (Table 2 ). Furthermore, matches between circulating and vaccine influenza strains were analyzed based on HAI assays, and a range of 12–93% of influenza virus isolates were antigenically similar to influenza vaccine strains during 2004/2005 to 2008/2009 (Figure 1).
Figure 1.
Nationwide laboratory-based influenza surveillance in Taiwan from July 2004 to June 2009 is illustrated. Five winter epidemics occurred between November 2004 and February 2009. Vaccine match: The antigenic match between recommended vaccines and circulating viruses in each season was evaluated using the hemagglutination inhibition (HAI) assay. The vaccine match (%) was calculated through the ratios of collected influenza A H1N1, influenza A H3N2, and influenza B viruses and the respective antigenic match of three tested type/subtype viruses.
Table 1.
Antigenic characteristics of influenza viruses and vaccine strains from the 2004/2005 to 2008/2009 seasons
| Season and type or subtype | Vaccine component | Circulating strains |
|---|---|---|
| 2004/2005 | ||
| H1N1 | A/New Caledonia/20/99-like | - |
| H3N2 | A/Fujian/411/2002-like | A/California/7/2004(H3N2)-like |
| B | B/Shanghai/361/2002-like | B/Malaysia/2506/2004-like* B/Shanghai/361/2002-like* |
| 2005/2006 | ||
| H1N1 | A/New Caledonia/20/99-like | A/New Caledonia/20/99* |
| H3N2 | A/California/7/2004-like | A/Wisconsin/67/2005-like |
| B | B/Shanghai/361/2002-like | - |
| 2006/2007 | ||
| H1N1 | A/New Caledonia/20/99 | - |
| H3N2 | A/Wisconsin/67/2005 or A/Hiroshima/52/2005 | A/Wisconsin/67/2005-like |
| B | B/Malaysia/2506/2004 | B/Malaysia/2506/2004-like* |
| 2007/2008 | ||
| H1N1 | A/Solomon Islands/3/2006 | A/Brisbane/59/2007-like* |
| H3N2 | A/Wisconsin/67/2005 or A/Hiroshima/52/2005 | A/Brisbane/10/2007-like |
| B | B/Malaysia/2506/2004 | B/Florida/4/2006-like |
| 2008/2009 | ||
| H1N1 | A/Brisbane/59/2007 | A/Brisbane/59/2007-like* |
| H3N2 | A/Brisbane/10/2007 | A/Brisbane/10/2007-like |
| B | B/Florida/4/2006-like | B/Florida/4/2006-like |
Dominant types/subtypes during the given season.
Table 2.
Characteristics of influenza-positive case subjects and influenza-negative control subjects according to influenza season, 2004/2005 through 2008/2009
| Characteristics | Cases |
Controls |
p-Value | ||
|---|---|---|---|---|---|
| 2004/2005 | (n = 132) | (n = 1019) | |||
| Gender | 0.33 | ||||
| Male | 71 | 53.8% | 488 | 47.9% | |
| Female | 54 | 40.9% | 449 | 44.1% | |
| Unknown | 7 | 5.3% | 82 | 8.0% | |
| Age group | <0.01 | ||||
| 6–23 months | 11 | 8.3% | 320 | 31.4% | |
| 24–59 months | 121 | 91.7% | 699 | 68.6% | |
| Vaccination status | <0.01 | ||||
| Vaccinated | 12 | 9.1% | 288 | 28.3% | |
| Unvaccinated | 120 | 90.9% | 731 | 71.7% | |
| 2005/2006 | (n = 41) | (n = 531) | |||
|---|---|---|---|---|---|
| Gender | 0.85 | ||||
| Male | 21 | 51.2% | 295 | 55.6% | |
| Female | 19 | 46.3% | 226 | 42.6% | |
| Unknown | 1 | 2.4% | 10 | 1.9% | |
| Age group | <0.01 | ||||
| 6–23 months | 4 | 9.8% | 225 | 42.4% | |
| 24–59 months | 37 | 90.2% | 306 | 57.6% | |
| Vaccination status | <0.01 | ||||
| Vaccinated | 3 | 7.3% | 204 | 38.4% | |
| Unvaccinated | 38 | 92.7% | 327 | 61.6% | |
| 2006/2007 | (n = 189) | (n = 602) | |||
|---|---|---|---|---|---|
| Gender | 0.29 | ||||
| Male | 109 | 57.7% | 355 | 59.0% | |
| Female | 80 | 42.3% | 240 | 39.9% | |
| Unknown | 0 | 0.0% | 7 | 1.2% | |
| Age group | <0.01 | ||||
| 6–23 months | 45 | 23.8% | 226 | 37.5% | |
| 24–59 months | 144 | 76.2% | 376 | 62.5% | |
| Vaccination status | <0.01 | ||||
| Vaccinated | 25 | 13.2% | 199 | 33.1% | |
| Unvaccinated | 164 | 86.8% | 403 | 66.9% | |
| 2007/2008 | (n = 75) | (n = 1060) | |||
|---|---|---|---|---|---|
| Gender | 0.40 | ||||
| Male | 46 | 61.3% | 580 | 54.7% | |
| Female | 28 | 37.3% | 473 | 44.6% | |
| Unknown | 1 | 1.3% | 7 | 0.7% | |
| Age group | <0.01 | ||||
| 6–23 months | 28 | 37.3% | 575 | 54.2% | |
| 24–59 months | 47 | 62.7% | 485 | 45.8% | |
| Vaccination status | <0.01 | ||||
| Vaccinated | 8 | 10.7% | 330 | 31.1% | |
| Unvaccinated | 67 | 89.3% | 730 | 68.9% | |
| 2008/2009 | (n = 95) | (n = 750) | |||
|---|---|---|---|---|---|
| Gender | 0.74 | ||||
| Male | 52 | 54.7% | 397 | 52.9% | |
| Female | 43 | 45.3% | 353 | 47.1% | |
| Unknown | 0 | 0.0% | 0 | 0.0% | |
| Age group | <0.01 | ||||
| 6–23 months | 16 | 16.8% | 244 | 32.5% | |
| 24–59 months | 79 | 83.2% | 506 | 67.5% | |
| Vaccination status | <0.01 | ||||
| Vaccinated | 11 | 11.6% | 211 | 28.1% | |
| Unvaccinated | 84 | 88.4% | 539 | 71.9% | |
VE was estimated in a total of 4494 children aged 6–59 months for whom laboratory results and vaccination status were available for the five winter epidemics. Table 2 shows the demographic distribution of the cases and controls in each season. Categorized according to epidemic, the sex distribution was similar among case and control subjects; however, the proportion of subjects who tested positive was significantly higher in children aged 24–59 months (15.2%) than in those aged 6–23 months (6.1%). From 2004/2005 through 2008/2009 seasons, annual vaccination rates among control groups in children aged 6–23 months were 66.6% (213/320), 61.8% (139/225), 57.1% (129/226), 41.0% (236/575), and 47.1% (115/244), which were higher than the rates in those aged 24–59 months (10.7% (75/699), 21.2% (65/306), 18.6% (70/376), 19.4% (94/485), and 19.0% (96/506)) (test for trend, p < 0.0001 and p < 0.001 for the two age groups, respectively). Vaccine coverage rates among the control groups were close to those in the corresponding population nationwide. The national influenza vaccine coverage available for children aged 6–23 months was 68.9% (260 499/377 933), 66.6% (243 149/365 335), 60.3% (212 605/352 502), and 44.8% (150 675/335 972) for seasons 2004/2005 to 2007/2008, respectively, which were estimated through the NIIS database. The national vaccine coverage changed to 48.2% (255 565/530 561) for children aged 6–35 months in the 2008/2009 season when the influenza vaccination program was extended to those aged 24–35 months. Otherwise, influenza was detected in 532 (11.8%) enrollees. Approximately 11.1% (59/532) of those who tested positive and 31.1% (1232/3962) of those who tested negative had been vaccinated.
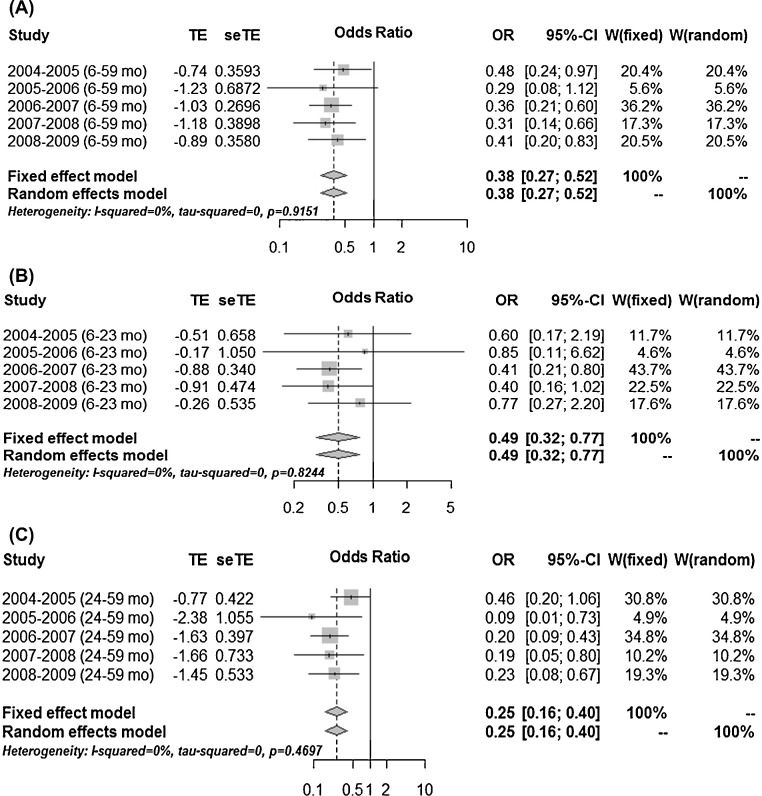
The pooled estimate of VE for children aged 6–59 months during 2004/2005 to 2008/2009 was 62% (95% CI 48–83%) using the meta-analysis method (Figure 2 ). By age category (6–23 months and 24–59 months), the VE estimates were 51% (95% CI 23–68%) and 75% (95% CI 60–84%) for those aged 6–23 months and 24–59 months, respectively. The I 2 value of 0% possibly indicates that statistical heterogeneity was not observed across the five winter epidemics, and across the age ranges of 6–23 months and 24–59 months (Figure 2). The VE estimates were higher among those aged 24–59 months than among those aged 6–23 months across the five seasons.
Figure 2.
A fixed-effects model with inverse variance method and a random-effects model with DerSimonian–Laird weighting method were applied to illustrate the vaccine effectiveness by meta-analysis. Forest plots are used to display the estimated overall and separate adjusted odds ratios (aORs) and 95% confidence interval (CI) in the five epidemic seasons among children aged 6–59 months, stratified by age (6–23 months and 24–59 months). TE: log odds ratio; seTE: standard error of the log odds ratio; w: weight.
4. Discussion
In this study, we found clear evidence that the current public health policy to reduce laboratory-confirmed influenza among young children through immunization is effective. The linkage of routinely collected data is considered an efficient method for estimating influenza vaccine effectiveness more accurately.21, 22 We successfully demonstrated an efficient way to evaluate the influenza VE for each winter epidemic through data linkage of two already established systems in the public health sector and used meta-analysis to estimate the pooled VE for children aged 6–59 months over consecutive seasons.
Meta-analysis could be appropriate when a group of studies is sufficiently homogeneous in terms of participants, interventions, and outcomes to provide a meaningful summary.23 The reason we did not simply add up the subjects in each season to summarize the influenza VE across the five seasons of test-negative case–control studies was to consider heterogeneity such as variation in the antigenic match among years, influenza activity year by year, vaccine policy changes over time with expansion of the age groups targeted, and changes in the dominant circulating subtypes of influenza virus, etc. Although statistical heterogeneity between studies was not found in our study, to estimate VE across years using a meta-analysis methodology might be a feasible and applicable approach. Further stratified analysis of influenza type-specific effectiveness was not possible due to the small sample of laboratory-confirmed cases with virus type categories.
We estimated VE against laboratory-confirmed influenza infection using the test-negative case–control study design, which is less susceptible to bias due to misclassification of infection and to confounding by health-seeking behavior, relative to traditional case–control or cohort studies.15 Virological swab tests for influenza as part of routine influenza surveillance to estimate influenza VE more specifically in time and to compare VEs internationally is encouraged and practiced in many countries.24 Recent studies have suggested that vaccine-induced protection against influenza may decline over time among young children and older adults in the test-negative case–control study design.25, 26 Nunes et al. raised the issue of the best influenza-negative control group to use in the test-negative study design.27 They observed that a VE difference existed when choosing a non-influenza virus control group and a pan-negative control group. Further studies should be conducted to clarify such important issues. Another challenge exists in establishing and maintaining the quality of the vaccination register for vaccination programs, especially for annual influenza vaccinations; such a register will provide information for resource allocation, comprehensive evaluation, and a timely response to all vaccine-preventable diseases.28
Our findings demonstrated a significant pooled VE of 51% (95% CI 23–68%) in 6–23-month-old children over five seasons. Previous reports have shown that influenza vaccines are effective in healthy children against laboratory-confirmed influenza, serologically confirmed influenza, and clinical illness by systematic meta-analysis.29, 30 A recent study reported by Yang et al. with a study design similar to ours found an influenza VE against medically-attended influenza illness of 16% for those aged 6–35 months in the 2012/2013 season.31 Evidence for VE against outcomes other than laboratory-confirmed influenza infection such as preventing emergency department visits and hospitalizations for ILI in children has been evaluated, although more robust evidence is needed in the future.32 Nevertheless, few of these studies have provided adequate evidence of influenza VE among children younger than 2 years of age, who, without chronic or serious medical conditions, are still at increased risk of hospitalization during the influenza season.3, 4, 33, 34, 35 Findings regarding the efficacy and effectiveness of inactivated influenza vaccines in children younger than 2 years are inconsistent.24 Although a smaller reduction in laboratory-confirmed influenza infection was shown in this age group compared to those older than 2 years of age, the clinical relevance and public health implications of routine influenza vaccination for both age groups has been confirmed.3, 30, 36
Several factors may affect the efficacy and effectiveness of influenza vaccine, including (1) antigenic similarity between the circulating and vaccine types or strains of influenza virus; (2) specificity of the outcome measurement of VE; (3) yearly variability in influenza illness rates; (4) host characteristics (e.g., age and underlying medical conditions) in relation to immune responses; (5) vaccine coverage and herd immunity; and (6) relatively small sample sizes for influenza-positive cases within each stratum evaluated.7, 30, 33, 37, 38, 39, 40, 41
Annual vaccination rates in children aged 6–23 months appeared to decline over the study period. This might be explained by the severe acute respiratory syndrome (SARS) outbreaks in Taiwan in 2003 and the introduction of the influenza vaccination program in the 2004/2005 season for children aged 6–23 months. Both of these events were an incentive to parents to have their young children vaccinated with the influenza vaccine during the early part of the study.
The possibility of cross-protection by influenza vaccine with a suboptimal match has been debated.42 In years with a suboptimal match, the VE has typically been lower or even not clearly demonstrated.40, 41 However, we found that only 12% of circulating strains were similar to the vaccine strains in the 2007/2008 season, but the VE was not as low as expected. The lack of significant VE in 6–23-month-old children despite a high vaccine match in the 2005/2006 and 2008/2009 seasons might be related to the low ILI rates, which were associated with the relatively small sample size of children who tested positive for influenza.40 The seasonal variations in terms of the proportions of case subjects in our data obtained from the Viral Surveillance Network may reflect the variable nature of influenza epidemics.38 The effect measure modification of age might be a concern in the analysis of vaccine effectiveness; therefore it was appropriate to demonstrate both age strata and age adjustment in the statistical models to estimate vaccine effectiveness.43
Children aged 6 months to 8 years who have never received seasonal influenza vaccines previously or who have received only one dose in their first year of vaccination should receive two doses of seasonal influenza vaccine to be categorized as fully immunized according to the recommendations of the US CDC.38, 44 However, influenza epidemic strains in Taiwan often circulate earlier than vaccine strains recommended by the World Health Organization.9 Therefore, in this study, we defined children aged 6–59 months who had received at least one dose of seasonal influenza vaccine as immunized regardless of their previous influenza vaccination history. This may not be appropriate for infants aged 6–23 months.45 We speculate that the younger the children were, the less chance they had had to experience the circulating local strains in previous seasons for immune priming before vaccination, which could have led to an underestimation of VE among younger children if they were only partially immunized. Besides, high vaccine coverage (50–70%) in children could possibly have an impact on reducing influenza-related morbidity and mortality, not only among the vaccinated children, but also in other age groups.30, 37 The challenge of increasing vaccine coverage still exists.
The findings of this study are subject to several limitations. First, children with underlying medical conditions are at even greater risk of an adverse outcome related to influenza than healthy children.33, 39 Data linkage of the Viral Surveillance Network and the NIIS did not provide personal medical information, so we could not examine this issue. Second, only two categories of vaccination status were defined in this study. To resolve this problem, a validation study to confirm the serology indicator of an HAI antibody titer ≥1:40 after one dose of trivalent influenza vaccine between two age strata might assist in confirming the immunization status in different geographic areas.46 Third, there might have been selection bias with regard to the vaccination status of subjects who were enrolled for laboratory testing. It is possible that the testing of samples was biased as this was done by the clinicians who provided the influenza shot to the patient. However, in Taiwan, most patients can see the doctor of their choice, therefore, most clinicians would not know the patient's influenza vaccination status. If sampling was done on an informed basis such that the sentinel physician collected fewer swabs from vaccinated ILI patients, a potential biasing of estimates of effectiveness upwards might have occurred.47 In the test-negative design for estimating influenza VE, effectiveness does not vary by health-seeking behavior with the assumption that the distribution of non-influenza causes of acute respiratory infection does not vary by influenza vaccination status.15 Therefore, cases and controls in our study probably had similar characteristics with regard to their willingness to seek medical care and their willingness to be swabbed, which could eliminate the uncontrolled confounding between cases and controls. The Viral Surveillance Network did not include all ILI patients (those willing to be swabbed and those not willing to be swabbed), thus it was not possible to determine the characteristics of the children who were swabbed and those who were not. The voluntary enrolment of swabbed participants in our study raises the question of potential selection bias, which might bias the VE estimation and limit the generalizability.3 Fourth, the impact of repeated seasonal influenza vaccination on vaccine effectiveness against influenza A and B virus has been debated, and further studies are needed to provide more clear evidence.48, 49 As this was an observational study, residual confounding may still be present despite different statistical models. We plan to investigate this further in the future.50
In conclusion, our study rapidly and efficiently determined VE across five influenza seasons using data linkage of immunization records and viral surveillance data at the national level. Because the annual burden of influenza illness and vaccine match could influence VE, we combined studies using a case–control design across consecutive influenza seasons using a novel method of meta-analysis. Influenza vaccination provided measurable protection against laboratory-confirmed influenza among children aged 6–23 months, 24–59 months, and the entire range of 6–59 months, despite variation in vaccine match during the 2004/2005 to 2008/2009 influenza seasons in Taiwan.
Acknowledgements
The authors would like to thank Mr Yung-Tai Tsai, Mr Kun-Ju Tsai, and Mr Hsu-Wen Yang for their excellent technical assistance in data collection and extraction.
Conflict of interest: The authors declare that they have no conflicts of interest and the study was not supported by any funding sources.
References
- 1.Cromer D., van Hoek A.J., Jit M., Edmunds W.J., Fleming D., Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014;68:363–371. doi: 10.1016/j.jinf.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Fiore A.E., Shay D.K., Broder K., Iskander J.K., Uyeki T.M., Mootrey G. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 3.Heinonen S., Silvennoinen H., Lehtinen P., Vainionpaa R., Ziegler T., Heikkinen T. Effectiveness of inactivated influenza vaccine in children aged 9 months to 3 years: an observational cohort study. Lancet Infect Dis. 2011;11:23–29. doi: 10.1016/S1473-3099(10)70255-3. [DOI] [PubMed] [Google Scholar]
- 4.Cowling B.J., Chan K.H., Feng S., Chan E.L., Lo J.Y., Peiris J.S. The effectiveness of influenza vaccination in preventing hospitalizations in children in Hong Kong, 2009–2013. Vaccine. 2014;32:5278–5284. doi: 10.1016/j.vaccine.2014.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jefferson T., Smith S., Demicheli V., Harnden A., Rivetti A., Di Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet. 2005;365:773–780. doi: 10.1016/S0140-6736(05)17984-7. [DOI] [PubMed] [Google Scholar]
- 6.Orenstein E.W., De Serres G., Haber M.J., Shay D.K., Bridges C.B., Gargiullo P. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–631. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 7.Bridges C., Katz J.M., Levandowski R.A., Cox N.J. Inactivated influenza vaccine. In: Plotkin S.A., Orenstein W.A., Offit P.A., editors. Vaccines. 5th Expert Consult edition. Saunders Elsevier; 2008. pp. 259–290. [Google Scholar]
- 8.Smith S., Demicheli V., Di Pietrantonj C., Harnden A.R., Jefferson T., Matheson N.J. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2006:CD004879. doi: 10.1002/14651858.CD004879.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Shih S.R., Chen G.W., Yang C.C., Yang W.Z., Liu D.P., Lin J.H. Laboratory-based surveillance and molecular epidemiology of influenza virus in Taiwan. J Clin Microbiol. 2005;43:1651–1661. doi: 10.1128/JCM.43.4.1651-1661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly H., Carville K., Grant K., Jacoby P., Tran T., Barr I. Estimation of influenza vaccine effectiveness from routine surveillance data. PLoS One. 2009;4:e5079. doi: 10.1371/journal.pone.0005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi A.Y., Iyer V.N., St Sauver J.L., Jacobson R.M., Boyce T.G. Effectiveness of inactivated influenza vaccine in children less than 5 years of age over multiple influenza seasons: a case–control study. Vaccine. 2009;27:4457–4461. doi: 10.1016/j.vaccine.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Fleming D.M., Andrews N.J., Ellis J.S., Bermingham A., Sebastianpillai P., Elliot A.J. Estimating influenza vaccine effectiveness using routinely collected laboratory data. J Epidemiol Community Health. 2010;64:1062–1067. doi: 10.1136/jech.2009.093450. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh Y.C., Chen H.Y., Yen J.J., Liu D.P., Chang L.Y., Lu C.Y. Influenza in Taiwan: seasonality and vaccine strain match. J Microbiol Immunol Infect. 2005;38:238–243. [PubMed] [Google Scholar]
- 14.Wang S.F., Lee Y.M., Chan Y.J., Liu H.F., Yen Y.F., Liu W.T. Influenza A virus in Taiwan, 1980–2006: phylogenetic and antigenic characteristics of the hemagglutinin gene. J Med Virol. 2009;81:1457–1470. doi: 10.1002/jmv.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 16.Jacoby P, Kelly H. Is it necessary to adjust for calendar time in a test negative design? Responding to: Jackson ML, Nelson JC. The test negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. Vaccine 2014; 32:2942. [DOI] [PubMed]
- 17.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Lewis S., Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322:1479–1480. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010.
- 20.Schwarzer G. R Foundation for Statistical Computing; Vienna, Austria: 2010. meta: Meta-Analysis with R. R package version 1. 6–1 ed. [Google Scholar]
- 21.Kavanagh K., Robertson C., McMenamin J. Assessment of the variability in influenza A(H1N1) vaccine effectiveness estimates dependent on outcome and methodological approach. PLoS One. 2011;6:e28743. doi: 10.1371/journal.pone.0028743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwong J.C., Campitelli M.A., Gubbay J.B., Peci A., Winter A.L., Olsha R. Vaccine effectiveness against laboratory-confirmed influenza hospitalizations among elderly adults during the 2010–2011 season. Clin Infect Dis. 2013;57:820–827. doi: 10.1093/cid/cit404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available at: http://www.cochrane-handbook.org (accessed October 18, 2014).
- 24.The compelling need for game-changing influenza vaccines: an analysis of the influenza vaccine enterprise and recommendations for the future. Center for Infectious Disease Research and Policy; 2012. Available at: http://www.cidrap.umn.edu/sites/default/files/public/downloads/ccivi_report.pdf (accessed October 19, 2014).
- 25.Jimenez-Jorge S., de Mateo S., Delgado-Sanz C., Pozo F., Casas I., Garcia-Cenoz M. Effectiveness of influenza vaccine against laboratory-confirmed influenza, in the late 2011–2012 season in Spain, among population targeted for vaccination. BMC Infect Dis. 2013;13:441. doi: 10.1186/1471-2334-13-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belongia E.A., Sundaram M.E., McClure D.L., Meece J.K., Ferdinands J., VanWormer J.J. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine. 2014 Jun 21 doi: 10.1016/j.vaccine.2014.06.052. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunes B., Machado A., Guiomar R., Pechirra P., Conde P., Cristovao P. Estimates of 2012/13 influenza vaccine effectiveness using the case test-negative control design with different influenza negative control groups. Vaccine. 2014;32:4443–4449. doi: 10.1016/j.vaccine.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar I., Reyes M., Martinez-Baz I., Guevara M., Albeniz E., Belza M. Use of the vaccination register to evaluate influenza vaccine coverage in seniors in the 2010/11 influenza season, Navarre, Spain. Euro Surveill. 2012;17 doi: 10.2807/ese.17.17.20154-en. pii:20154. [DOI] [PubMed] [Google Scholar]
- 29.Jefferson T., Rivetti A., Harnden A., Di Pietrantonj C., Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008:CD004879. doi: 10.1002/14651858.CD004879.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Negri E., Colombo C., Giordano L., Groth N., Apolone G., La Vecchia C. Influenza vaccine in healthy children: a meta-analysis. Vaccine. 2005;23:2851–2861. doi: 10.1016/j.vaccine.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 31.Yang P., Thompson M.G., Ma C., Shi W., Wu S., Zhang D. Influenza vaccine effectiveness against medically-attended influenza illness during the 2012–2013 season in Beijing, China. Vaccine. 2014;32:5285–5289. doi: 10.1016/j.vaccine.2014.07.083. [DOI] [PubMed] [Google Scholar]
- 32.Menniti-Ippolito F., Da Cas R., Traversa G., Santuccio C., Felicetti P., Tartaglia L. Vaccine effectiveness against severe laboratory-confirmed influenza in children: results of two consecutive seasons in Italy. Vaccine. 2014;32:4466–4470. doi: 10.1016/j.vaccine.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 33.Izurieta H.S., Thompson W.W., Kramarz P., Shay D.K., Davis R.L., DeStefano F. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 34.Neuzil K.M., Mellen B.G., Wright P.F., Mitchel E.F., Jr., Griffin M.R. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 35.Fujieda M., Maeda A., Kondo K., Fukushima W., Ohfuji S., Kaji M. Influenza vaccine effectiveness and confounding factors among young children. Vaccine. 2008;26:6481–6485. doi: 10.1016/j.vaccine.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 36.Zangwill K.M., Belshe R.B. Safety and efficacy of trivalent inactivated influenza vaccine in young children: a summary for the new era of routine vaccination. Pediatr Infect Dis J. 2004;23:189–197. doi: 10.1097/01.inf.0000116292.46143.d6. [DOI] [PubMed] [Google Scholar]
- 37.Reichert T.A., Sugaya N., Fedson D.S., Glezen W.P., Simonsen L., Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344:889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg K.W., Szilagyi P.G., Fairbrother G., Griffin M.R., Staat M., Shone L.P. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics. 2008;122:911–919. doi: 10.1542/peds.2007-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuzil K.M., Wright P.F., Mitchel E.F., Jr., Griffin M.R. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000;137:856–864. doi: 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- 40.Szilagyi P.G., Fairbrother G., Griffin M.R., Hornung R.W., Donauer S., Morrow A. Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case–cohort study. Arch Pediatr Adolesc Med. 2008;162:943–951. doi: 10.1001/archpedi.162.10.943. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine—Marshfield, Wisconsin, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep. 2008;57:393–398. [PubMed] [Google Scholar]
- 42.Tricco A.C., Chit A., Soobiah C., Hallett D., Meier G., Chen M.H. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangtani P., Cumberland P., Hodgson C.R., Roberts J.A., Cutts F.T., Hall A.J. A cohort study of the effectiveness of influenza vaccine in older people, performed using the United Kingdom general practice research database. J Infect Dis. 2004;190:1–10. doi: 10.1086/421274. [DOI] [PubMed] [Google Scholar]
- 44.Fiore A.E., Uyeki T.M., Broder K., Finelli L., Euler G.L., Singleton J.A. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 45.Englund J.A., Walter E.B., Gbadebo A., Monto A.S., Zhu Y., Neuzil K.M. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics. 2006;118:e579–e585. doi: 10.1542/peds.2006-0201. [DOI] [PubMed] [Google Scholar]
- 46.Neuzil K.M., Jackson L.A., Nelson J., Klimov A., Cox N., Bridges C.B. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8-year-old children. J Infect Dis. 2006;194:1032–1039. doi: 10.1086/507309. [DOI] [PubMed] [Google Scholar]
- 47.Halloran M.E., Longini I.M., Jr., Gaglani M.J., Piedra P.A., Chu H., Herschler G.B. Estimating efficacy of trivalent, cold-adapted, influenza virus vaccine (CAIV-T) against influenza A (H1N1) and B using surveillance cultures. Am J Epidemiol. 2003;158:305–311. doi: 10.1093/aje/kwg163. [DOI] [PubMed] [Google Scholar]
- 48.Houser K.V., Pearce M.B., Katz J.M., Tumpey T.M. Impact of prior seasonal H3N2 influenza vaccination or infection on protection and transmission of emerging variants of influenza A(H3N2)v virus in ferrets. J Virol. 2013;87:13480–13489. doi: 10.1128/JVI.02434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLean H.Q., Thompson M.G., Sundaram M.E., Meece J.K., McClure D.L., Friedrich T.C. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014 Sep 29 doi: 10.1093/cid/ciu680. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lone N.I., Simpson C., Kavanagh K., Robertson C., McMenamin J., Ritchie L. Seasonal influenza vaccine effectiveness in the community (SIVE): protocol for a cohort study exploiting a unique national linked data set. BMJ Open. 2012;2:e001019. doi: 10.1136/bmjopen-2012-001019. [DOI] [PMC free article] [PubMed] [Google Scholar]