Abstract
Background
Klebsiella pneumoniae is a public health concern because of its ability to develop multidrug resistance and hypervirulent genotypes, of those capsular types K1 and K2 cause community and nosocomial life-threatening infections. This study aimed to determine the antibiotic susceptibility patterns and genotypic traits of a collection of Klebsiella spp. isolates. Furthermore, the clonal relatedness of blaNDM producing strains was investigated.
Methods
During a 19-months surveillance study, 122 Klebsiella spp. isolates were cultured from extraintestinal specimens of patients admitted to the tertiary referral hospital in Semnan, Iran. Isolates were identified using biochemical tests and subjected to determination of phylogroups, capsular types and virulence/resistance genes content. Hypervirulent K. pneumoniae (hvKp) strains were detected genotypically, and Enterobacterial Repetitive Intergenic Consensus (ERIC)-PCR fingerprinting was used to determine the clonality of blaNDM producing strains.
Results
Multidrug resistant phenotype was detected in 75 (61.5%) isolates and amikacin was found as the most potent antibiotic with the susceptibility rate of 85.2%. The carbapenemase genes were detected in 45 (36.8%) strains, including 21 (17.2%) blaOXA-48, 7 (5.6%) blaNDM-1, 14 (11.4%) blaNDM-1/OXA-48 and 3 (2.4%) blaIMP- carrying strains, while 55 (45.08%) isolates showed carbapenem resistant phenotype. The first blaNDM-1 carrying strain was cultured from a sputum specimen on March 2015, while the last positive one was recovered from blood culture on September 2016. Most of the isolates (80.3%) belonged to phylogroup I, and blaNDM-1 was identified among all three phylogroups. The ERIC-PCR clustered the 101 blaNDM negative and 21 blaNDM-1 positive isolates into 25 and five clusters, respectively, and the latter group belonged to clonal complex 147 (CC147). One K1 and 15 K2 blaNDM-1 negative isolates were detected, of those three strains were identified as hvKp. Five K2 positive strains, including four blaOXA-48 producer and one hvKp sequence type 86 (ST86) were carbapenem resistant. Among carbapenem resistant isolates, CC147 strains harboured higher rates of siderophores iutA and ybtS.
Conclusion
The present findings showed a hospital circulation of CC147 blaNDM-1 or blaNDM-1/OXA-48 producing strains, disseminated in different wards. The hvKp/ST86 strain expressing K2 capsular type and carbapenem resistant phenotype wasn’t reported from Iran so far. So, it seems that we must be aware of the emergence and spread of new K. pneumoniae clones associated with resistant and hypermucoviscous phenotypes.
Keywords: hvKp, NDM, OXA-48, rmtC, Phylogroup, Siderophore, Capsular type
Background
Klebsiella pneumoniae, one of the most important members of the Enterobacteriaceae family, is the leading cause of both of the community and healthcare-associated infections. The capsule, as a critical virulence factor in this organism, plays an important role in its pathogenesis and avoids phagocytosis [1]. Over the past decade, the emergence of hypervirulent variants of K. pneumoniae (hvKp) which characteristically express hypermucoviscosity phenotype caused serious concerns [2]. These strains carry virulence genes associated with invasive disease and may cause severe infections such as pyogenic liver abscesses and endophthalmitis, in immunocompetent, healthy individuals [2]. While the hvKp strains were rarely resistant to commonly used antibiotics when firstly described, the emergence of multidrug resistant (MDR) phenotypes among these strains are increasingly reported in recent years due to the dissemination of mobile genetic elements encoding drug resistance [2]. Of special concern is the acquisition of carbapenem resistance genes and development of carbapenem resistant phenotype, since these agents are the last resort of antibiotics for treatment of infections caused by multidrug resistant organisms [2].
Increasing recognition of carbapenemase producing K. pneumoniae, including class A (KPC), class B (IMP, VIM and NDM) and class D (OXA-48-like enzymes) carbapenemases has led to international concern, as they are carried on transposable elements in association with other resistance determinants, such as Extended spectrum β-lactamases (ESBLs), ampC cephalosporinases and 16S rRNA methyltransferases [3]. Indeed, the convergence of carbapenemase production and hypermucoviscosity phenotype in this organism poses an important threat to public health.
The blaNDM, a relatively newly described Metallo-β-lactamase (MBL), was first identified in K. pneumoniae and E. coli isolated from a Swedish patient who was hospitalized in India in 2008 [4]. Since then, it has spread worldwide and now NDM producing Gram-negative bacilli have been reported from more than 40 countries, and the Indian subcontinent and the Middle East are considered as the main reservoirs for blaNDM producing bacteria [5]. Iran, as one of the Middle East countries, neighbor countries where the NDM and OXA-48 producing bacteria are endemic [6]. While blaNDM producing K. pneumoniae isolates have been reported from different cities of Iran in recent years, sequence types of these isolates are determined and published from three cities located in the center, south, and south-east of this country [6–8]. In our previous study, we reported a relatively high prevalence of blaOXA-48/blaNDM-1 producing Enterobacteriaceae isolates collected from the large tertiary hospital of Semnan [9], an important city along the historical Silk Road. So in this survey, our goal was first to determine the phylogenetic groups, capsular genotypes, hypermucoviscosity biomarkers, and resistance determinants of K. pneumoniae isolates collected during 19-months surveillance study and second, to carry out sequence typing of representatives of NDM producing isolates based on the Enterobacterial Repetitive Intergenic Consensus (ERIC) fingerprinting.
Methods
Sample collection
A 19-months surveillance study was conducted in the main tertiary teaching hospital of Semnan, Iran (Kosar hospital). During March 2015 to September 2016, 122 non-duplicate K. pneumoniae isolates were recovered from clinical specimens of patients admitted to the hospital. The isolates were cultured from different extraintestinal specimens including urine, wound, sputum, blood and tracheal aspirate. Specimens were collected as the routine diagnostic purposes. K. pneumoniae isolates were identified based on the biochemical reactions, including reaction on Triple Sugar Iron (TSI) agar, SH2/Indole/Motility (SIM) pattern, growth on Simmon-citrate agar medium, urease production on urea agar, Methyl Red/Vogues Proskauer (MR/VP), and Ornithine decarboxylase (OD) test. Isolates were confirmed by PCR in which both k. pneumoniae subsp. Pneumoniae and subsp. ozaenae give a 130 bp band, and subsp. rhinoscleromatis is negative [10]. K. oxytoca species were identified based on the VP +/Indole +/OD negative, tests results [11].
Antimicrobial susceptibility testing
Antibiotic susceptibility patterns for 16 antibiotics were obtained using standard disc diffusion test. For carbapenem non-susceptible isolates (resistant to either of the imipenem, meropenem, and ertapenem) carbapenem MICs were determined using gradient E-test strips (Liofilchem, Italy). Susceptibility testing results were interpreted according to the Clinical and Laboratory Science Institute (CLSI) recommendations. The halo zones of ≥ 23 mm and ≥ 22 mm were considered as susceptible breakpoints for imipenem/meropenem and ertapenem, respectively [12]. Multidrug-resistant (MDR) isolates were those resistant to at least one representative of ≥ 3 antimicrobial classes—that is, penicillins (ampicillin/sulbactam, piperacillin-tazobactam), Extended Spectrum Cephalosporins (ESCs)/monobactams (cefotaxime, ceftazidime, cefepime/aztreonam), aminoglycosides (gentamicin), fluoroquinolones (ciprofloxacin), and antifolate agents (trimethoprim-sulfamethoxazole) [13]. Extended spectrum β-lactamase (ESBL) producing strains were identified using phenotypic combined disk test as recommended by CLSI [12].
Detection of resistancegenes and capsular typing
Genomic DNA of collected isolates was extracted using Cetyl trimethylammonium bromide (CTAB) method [14, 15]. The isolates carrying carbapenemases (blaNDM-, blaIMP-, blaVIM-, blaOXA-48, blaKPC) [16], extended spectrum β-lactamases (blaTEM-, blaSHV-, blaOXA-1, and CTX-M clusters including CTX-M-G1, G2, G8, G9 and G25) [16], plasmid mediated quinolone resistance (PMQR) (qnrA, qnrB, qnrS, aac-6Ib-cr) [17], aminoglycoside resistance determinants (ARD) aac-6Ib, aac3IIa and 16S rRNA methyltrasfrases (armA, rmtB, rmtC) were detected as described previously [16]. The capsular genotypes, including K1 (using magA primers), K2, K5, K20, K54 and K57, which are strongly associated with invasive disease, were determined by multiplex PCR as described previously [10]. The presence of plasmid-encoding virulence genes, including iucA (aerobactin siderophore biosynthesis), the plasmid-borne rmpA gene (prmpA), prmpA2, (regulators of the mucoid phenotype via increased capsule production), and peg-344 (putative transporter) which have been shown experimentally to contribute to hypervirulence in in vivo infection models, was assessed as described by Russo et al. [18]. Of the virulence genes which are associated epidemiologically with putative hvKp strains, carriage of the iroB (salmochelin siderophore biosynthesis), iutA (receptor for hydroxamate siderophore), allS (associated with allantoin metabolism), mrkD (type 3 fimbrial adhesion) and ybtS (yersiniabactin) genes was investigated by multiplex PCR [18, 19]. Furthermore, the K2 positive strains were subjected to a multiplex PCR to identify the main hvKp clones, including clonal group (CG) 380, sequence type (ST) ST86, ST65 and ST375, as described by Davenet et al. [20].
Phylogenetic determination and clonal relatedness
For phylogenetic analysis, gyrA PCR–RFLP using restriction enzymes TaqI and HaeIII was performed as described by Brisse et al. [21]. The clonality of strains was determined by ERIC-PCR fingerprinting and the obtained dendograms were analyzed with Bionumerics software, version 6.1 (Applied Maths, Sint-Martens-Laten, Belgium). The similarities in amplicon profiles were compared using a Dice coefficient at 1% tolerance and 0.5% optimization, and a dendogram was constructed using the unweighted-pair group method, with a cutoff of 80% similarity [22]. Sequence type of isolates was determined for representatives of NDM positive strains of each cluster of ERIC dendogram according to the K. pneumoniae MLST website (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Statistical analysis
Dichotomous variables were described using frequencies and percentages, and they were compared using Chi square test, as appropriate. The criterion for statistical significance was P < 0.05. Data were analyzed with software SPSS version 16.
Results
Patients and isolates
In this study, a total of 122 Klebsiella spp. isolates were recovered from all clinical specimens except for stool. Most of the isolates were cultured from urine (80, 65%), followed by sputum (33, 26.8%), wound (5, 4.1%), blood (2, 1.6%) and chest tube (1, 0.8%). Four strains, including two Klebsiella subsp. ozaenae (one cultured from urine, other one from sputum), one Klebsiella subsp. rhinoscleromatis (cultured from urine) and one Klebsiella oxytoca (cultured from urine) were identified among collected isolates.
Antibiotic susceptibility patterns and frequency of resistance genes
The highest susceptibility rate was obtained against amikacin (104, 85.2%), followed by meropenem (81, 66.4%), imipenem (77, 63.1%) and gentamicin (74, 60.7%). The presence of blaOXA-48, blaNDM-, both of blaOXA-48 and blaNDM- (blaOXA-48/NDM) and blaIMP- genes was detected among 21 (17%), 7 (5.6%), 14 (11.3%) and 3 (2.4%) of isolates, respectively. The NDM PCR products from all positive strains were subjected to sequencing and identified as NDM-1 variant [9]. The most active agents against blaNDM-1 producers were amikacin (13, 61.9%) and trimethoprim/sulfamethoxazole (3, 14.3%) (Table 1).
Table 1.
Antibiotic resistance rates and frequency of resistance genes among NDM positive and negative isolates
| NDM positive (n = 21) | NDM negative (n = 101) | NDM positive vs. NDM negative P value |
|
|---|---|---|---|
| Resistance rates n (%) | |||
| Imipenem | 21 (100) | 24 (23.8%) | 0.001 |
| Meropenem | 21 (100) | 20 (19.8%) | 0.001 |
| Ertapenem | 21 (100) | 30 (29.7%) | 0.001 |
| Cefepime | 20 (95.2) | 39 (38.6%) | 0.001 |
| Ceftazidime | 21 (100) | 52 (51.5%) | 0.001 |
| Cefotaxime | 21 (100) | 66 (65.3%) | 0.001 |
| Aztreonam | 21 (100) | 51 (50.5%) | 0.001 |
| Ampicillin/sulbactam | 21 (100) | 54 (53.5%) | 0.001 |
| Piperacillin/tazobactam | 21 (100) | 36 (35.3%) | 0.001 |
| Amoxicillin/clavulanate | 21 (100) | 53 (52.5%) | 0.001 |
| Ciprofloxacin | 20 (95.2) | 48 (47.5%) | 0.001 |
| Levofloxacin | 20 (95.2) | 37 (36.6%) | 0.001 |
| Gentamicin | 20 (95.2) | 28 (27.7%) | 0.001 |
| Tobramycin | 20 (95.2) | 33 (32.7%) | 0.001 |
| Amikacin | 8 (38.1) | 10 (9.9%) | 0.003 |
| Trimethoprim/sulphamethoxazole | 18 (85.7) | 46 (45.5%) | 0.001 |
| MDR | 21 (100) | 54 (53.5) | 0.001 |
| Presence of resistance and capsular markers n (%) | |||
| armA | 10 (47.6) | 16 (15.8%) | 0.003 |
| rmtC | 3 (14.3) | 3 (3%) | 0.06* |
| aac6Ib | 14 (66.7) | 35 (34.7%) | 0.01 |
| aac3IIa | 19 (90.5) | 28 (27.5%) | 0.001 |
| qnrB | 1 (4.8) | 22 (21.8%) | 0.1 |
| qnrS | 18 (85.7) | 17 (16.8%) | 0.001 |
| aac6Ib-cr | 19 (90.5) | 47 (47%) | 0.001 |
| blaOXA-48 | 14 (66.7) | 21 (20.8%) | 0.001 |
| blaIMP- | 0 | 3 (3%) | 1 |
| CTX-M-G1 | 21 (100) | 53 (52.5%) | 0.001 |
| CTX-M-G2 | 0 | 3 (3%) | 0.5 |
| CTX-M-G8 | 0 | 2 (2.0%) | 0.6 |
| CTX-M-G9 | 0 | 2 (2.0%) | 0.6 |
| CTX-M-G25 | 0 | 7 (7%) | 0.2 |
| blaTEM- | 12 (57.1) | 29 (28.7%) | 0.02 |
| blaSHV- | 19 (90.5) | 67 (66.3%) | 0.03 |
| blaOXA-1 | 15 (71.4) | 25 (24.8) | 0.001 |
| K1 | 0 | 1 (1%) | 1 |
| K2 | 0 | 15 (14.8) | 0.07 |
Italic values indicate the significance of P value (P < 0.05)
MDR multidrug resistance
*P value was borderline
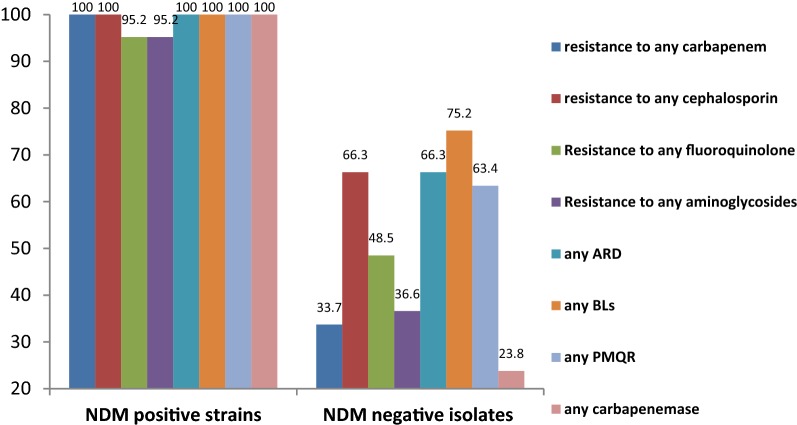
One of the 21 blaNDM-1 producing isolates was identified as subsp. ozaenae (isolate no. 500A, cultured from sputum). The blaNDM-1 producers were mostly cultured from urine (10, 47.5%), followed by sputum (9, 42.9%), wound and blood (one, 4.8% for each of them). Carbapenemase encoding gene, including either of the blaOXA-48, blaNDM-1, or blaIMP-was detected in 43 strains out of 55 carbapenem resistant (resistant to at least one of the three carbapenems) isolates (78.2%, P < 0.001), and the two blaIMP- carrying strains were found susceptible to carbapenems. All of the blaNDM-1 carrying strains were found non-susceptible to carbapenems, with the MICs ranging from 1.5 to > 32 µg/ml [9]. As compared to blaNDM negative isolates, blaNDM-1 carrying strains showed significantly higher carriage rates of any resistance determinants (PMQR, ARD or β-lactamases) and higher resistance rates against any studied antibiotics (P < 0.001) (Fig. 1). Of the ARD studied, the presence of armA (P: 0.01), aac6Ib (P < 0.001), and aac3IIa (P < 0.001) was associated with resistant phenotype to any of the aminoglycoside antibiotics. Of the PMQR determinants, the carriage of qnrS (P < 0.001) and/or aac6-Ib-cr (P: 0.03) genes was associated with resistance to any of the fluoroquinolones. For β-lactamase genes, isolates harbouring the blaTEM-, blaSHV-, blaOXA-1 and/or CTX-M-G1 were resistant to any of the three studied cephalosporins (P < 0.001).
Fig. 1.
Frequency (percent) of any resistance determinants, and resistant phenotype to any carbapenems, any cephalosporins, fluoroquinolones and any aminoglycosides in two groups of NDM positive and negative isolates. Any carbapenemase: blaOXA-48, blaNDM-1, blaIMP-; any PMQR: qnrB, qnrS, aac6Ib-cr; any β-lactamase (BL): blaTEM-, blaSHV-, CTX-M, blaOXA-1; any ARD: aac3IIa, aac6Ib, armA, rmtC
The resistance determinants which detected significantly among NDM producers were blaOXA-48, blaTEM-,blaSHV-, blaOXA-1, armA, aac3IIa, aac-6Ib, aac6-Ib-cr, qnrS, and CTX-M-G1. The co-carriage of rmtC and blaNDM-1 was not found statistically significant, however this association was on the border of significance (P: 0.06).
Genetic relatedness and sequence typing of blaNDM producers
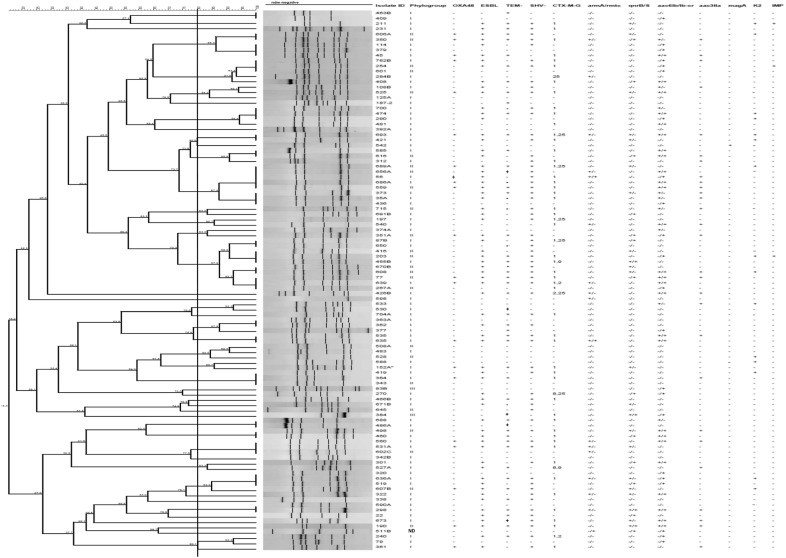
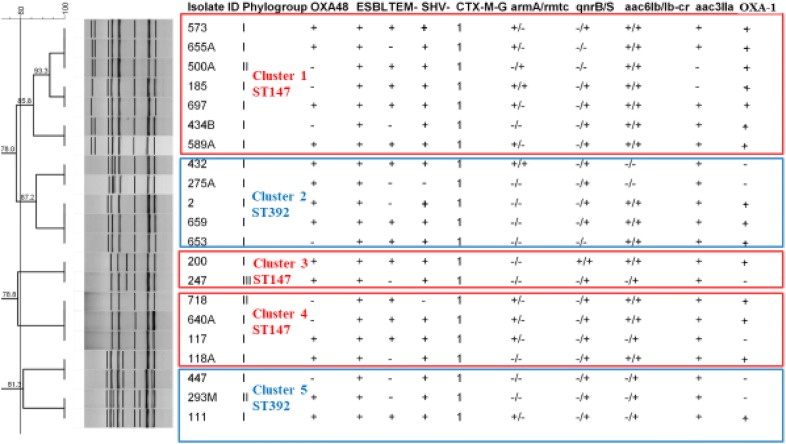
Based on the ERIC-fingerprinting of blaNDM negative isolates, 25 clusters were detected, including of two to 10 isolates in each cluster. Twenty-six isolates were also found as singletons (Fig. 2). In contrast, NDM carrying strains were grouped into five clusters (Fig. 3). Consequently, as the representatives of NDM producers, one strain was selected from each of the cluster and subjected to MLST assay. Two sequence types, ST392 (n = 9) and ST147 (n = 12) were identified among NDM positive isolates. Noted that the ST392 is the clonal complex (CC) of ST147. The first blaNDM-1 strain was obtained on March 2015 from internal intensive care unit (ICU) followed by the couple of isolates retrieved on May from surgical ICU. On June, there was only one strain isolated from a patient hospitalized in internal ward. On July, we obtained one blaNDM-1 isolate from each of the internal ICU and surgical ICU sites. On August, there were three isolates; two from internal ICU and one from internal ward. On the following months from October 2015 to February 2016, nine strains were isolated from patients hospitalized at internal ICU. On March, we obtained one isolate from internal ward and afterward we received two other isolates from coronary care unit (CCU) and surgery wards on April. The last positive strain recovered from a dialysis patient whom was initially hospitalized in internal ICU on September 2016.
Fig. 2.
ERIC-PCR based dendogram of blaNDM negative isolates. Cluster analysis of the Dice similarity indices based on the unweighted pair group method using average linkages (UPGMA) was done to generate a dendogram describing the relationship among the ERIC profiles. ESBL: indicate the phenotypic results, IMP: imipenemase
Fig. 3.
ERIC-PCR based dendogram of blaNDM producers. Five representatives were subjected to MLST and two STs, including ST147 and ST392 were identified. ESBL: indicate the phenotypic results
Phylogroups, capsular genotypes and hypervirulent clones
Except for Klebsiella oxytoca strain (strain no. 511b) which showed a different RFLP-pattern, all the remaining 121 studied isolates were grouped in three phylogroups, including 98 (80.3%) in group I, 20 (16.4%) in group II and three (2.5%) in group III. Most of the NDM-1 producing isolates, including 17 (81%) strains, belonged to the group I, and the remaining four strains belonged to group II and III. Of the CTX-M genes, the CTX-M-G2, G8 and G9 were detected exclusively among isolates of phylogroup I. Of the six capsular types studied; K1 and K2 genotypes were identified among one and 15 isolates, respectively. The K1 positive strain was cultured from urine specimen, while 12 (80%) and three (20%) of K2 strains were recovered from urine and sputum cultures, respectively. Carbapenem resistant and MDR phenotypes were detected among five (33.3%) and 11 (73.3%) K2 positive strains, respectively. The K1 strain detected in phylogroup I, while the K2 isolates belonged to both phylogroup I (14 [93.3%]) and II (1 [6.7%]) (P > 0.05).
Based on the carriage of peg344, iucA, iroB, rmpA and rmpA2 biomarkers, three strains, including K1 (strain 542, carbapenem susceptible) and two K2 (strain 290, carbapenem resistant and 533, carbapenem susceptible) isolates were identified as hvKp. Virulence genes iutA, mrkD and ybtS were detected in these three hvKp strains, except for K1 isolate which was negative for the latter gene. The two K2+/hvkp strains (strains 533 and 290) were identified as ST86 using the hvKP-K2 multiplex PCR. As expected, this multiplex PCR was completely negative for the only K2+/phylogroup II strain (strain 608). The alls gene was not detected among study isolates. None of the blaNDM-1 producers were positive for K1/K2 capsular types and were not identified as hvkp.
We also studied the presence of virulence genes in all carbapenem resistant isolates and peg344, iucA, rmpA, rmpA2, iroB1, iroB2, iutA, mrkD and ybtS were detected in 1.8%, 1.8%, 1.8%, 1.8%, 7.3%, 12.7%, 18.2%, 89.1% and 67.3%, of strains, respectively. Higher rates of iutA and ybtS carriage were detected among blaNDM-1 positive (19% and 71.4%) strains as compared to negative (17.6% and 64.7%) ones, while the differences were not statistically significant (P > 0.05). Of the resistance genes studied, the carriage of qnrB and qnrS had positive and negative association with K2 capsular type, respectively.
Discussion
The first report of NDM producing Gram–negative bacilli from Iran was described in 2013 in a K. pneumoniae strain, which was cultured from the urine specimen of a patient with kidney transplant rejection history [23]. In the current study, we investigated the molecular epidemiology of a group of blaNDM-1 producing isolates cultured from various extraintestinal specimens collected from Kosar hospital, Semnan, Iran. In our study, NDM-1 producing K. pneumoniae strains were mostly derived from urine specimens, followed by sputum of patients admitted to different hospital wards.
In our study and comparison to NDM negative isolates, blaNDM-1 carrying strains were much resistant to different classes of β-lactam and non β-lactam antibiotics by disk diffusion assay. It has been shown that tigecycline, fosfomycin and colistin are the most potent agents against NDM producing isolates [8]. While we didn’t determine the susceptibility patterns of NDM producing strains against these antibiotics, amikacin which found with the highest susceptibility rates may be considered as a treatment option due to some limitation of the extensive clinical usage of the three aforementioned antibiotics.
Phylogrouping of study Klebsiella isolates identified three groups, with the phylogroup I as the dominant group, followed by group II and III. It has been shown that the level of resistance for most of the antibiotics is highest among phylogroup I, intermediate in group II and lowest in group III, with the highest number of normal microbiota among the latter group [24]. Accordingly, most of the blaNDM-1 producing strains (81%) belonged to group I and co-harbored different resistance genes, including blaSHV-, CTX-M-G1, qnrS, aac6-Ib-cr and aac3IIa. While the resistance is a critical parameter for the transmission of phylogroup I K. pneumoniae strains, detection of NDM/OXA-48 carbapenemases and consequently carbapenem resistant phenotype among phylogroup II (strains: 500A, 718, 293 M) and III (isolate: 247) strains, confirm the role of this mainly nosocomial and opportunistic pathogen in the dissemination of resistance elements.
Among the blaNDM-1 producers, 11 (52.3%) strains co-harboured any β-lactamase gene, CTX-M; any PMQR; and 16S rRNA methylase gene armA or rmtC. PMQR coexisting with any β-lactamase gene was also high (21 [100%]). A strong association between the carriage of NDM and 16S rRNA methylase encoding gene, specifically rmtC methylase has been shown [25]. While the prevalence of rmtC among blaNDM-1 producing strains was higher than the negative ones, this association was found borderline. In contrast, the co-occurrence of armA or the other aminoglycoside resistance genes with NDM was found significant. So our results indicate that NDM producing Klebsiella isolates, have acquired a broad spectrum of singular and different resistance determinants.
It has been reported that carbapenemase producing K. pneumoniae strains have a different population and less genetic diversity as compared to carbapenem susceptible isolates [26]. In our study, ERIC-PCR showed five clusters and higher genetic homogeneity among blaNDM-1 producers as compared to negative ones. Accordingly, the MLST assay identified two STs among NDM producing isolates. ST392, which is an SLV of ST147, has been associated with the carriage of blaKPC, blaNDM and blaOXA-48 [27]. In the current study, ST392 has detected among NDM positive isolates with different ERIC patterns. Another detected ST, ST147 which is an international clone, has been reported from center, south and south east of Iran [6–8]. In our study, ST147 has been associated with CTX-M-G1, blaSHV-, aac6Ib-cr and armA. This ST has been recognized as a pandemic clone and is considered as a threat to public health worldwide [28]. ST147 was firstly observed by Papagiannitsis et al., as hosting the blaVIM gene, and then Samuelson et al. reported this clone among K. pneumoniae isolates imported to Scandinavia, mostly from Greece [28]. So, these findings imply that ST147 has the potential to acquire different resistance elements, of note, blaNDM/OXA-48 carbapenemases and facilitate their rapid spread into the other pandemic clones of K. pneumoniae.
String test, a phenotypic assay that is commonly used to identify the hvKp strains, has been shown to performed suboptimally, particularly in low prevalence areas [18]. So, the identification of some genetic markers including peg344, irob, rmpA, rmpA2 and iucA has been suggested for accurate differentiation between hvKp and classical K. pneumoniae strains [18]. Accordingly, one K1 and two K2 ST86 strains were identified as hvKp, of those one ST86 strain was MDR and carbapenem resistant. While these capsular types have not generally been associated with acquired resistance genes at the time which were identified, in the last few years increasing reports of resistant strains were observed among these genotypes [29]. In agreement with these new reports, four out of five carbapenem resistant K2 strains harboured the blaOXA-48 gene. It has been reported by Turton et al., that isolates of CC147 carrying blaOXA-48 or blaNDM which was resistant to colistin, harboured many antibiotic resistance determinants, and contained a quarter of the virulence genes which were found in the K1-ST23/OXA-48+ isolates [30]. While our CC147 strains (all carrying NDM) were K1/K2/hvkp negative, they harboured relatively higher rates of siderophores iutA and ybtS as compared to another carbapenem resistant NDM negative isolates. So, concerning this finding that the acquisition of any one of the siderophore clusters increases the risk of complicated infections [2, 30], the combination of virulence and antibiotic resistance in this pandemic clone is extremely worrying.
Our study had some limitations. The blaOXA-48 producers and K1/K2 strains were not subjected to sequence type determination. Furthermore, the TEM- and SHV- variants of positive isolates were not determined. We investigated the carriage of some limited virulence factors among carbapenem resistant strains, while the other important virulence determinants remained to be characterized among both of carbapenem susceptible and resistant isolates.
Conclusions
In summary, clonal dissemination of blaNDM-1 carrying K. pneumoniae that co-harbour different β-lactamases, aminoglycoside modifying enzymes, and PMQR determinants have been observed. Isolation of carbapenem resistant K. pneumoniae strains from clinical sources has been reported from Iran, previously. However, their association with K1 or K2 hypervirulent capsular types has never been reported. The emergence of the CC147 carbapenem resistant K. pneumoniae strains warrants urgent surveillance because not only are they considered as international clone, but also they simultaneously have higher rates of siderophores in a pandrug resistance profile.
Acknowledgements
The authors would like to appreciate Prof. Thomas A. Russo for his helpful comments.
Authors’ contributions
OP designed the proposal of the project, ND, AB and MA carried out the laboratory work. RGH participated in statistical analysis. ZB and ZH participated in drafting the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported fully by Semnan University of Medical Sciences (Grants No. 1362, 871, 898, 918).
Availability of data and materials
The data can be accessible to the interested researchers by the corresponding author on behalf of all authors on reasonable request.
Ethics approval and consent to participate
The ethical clearance and consent to participate were approved by the Iranian Health Research Council.
Consent for publication
The clinical isolate samples used in this research were part of the routine hospital laboratory procedure. We do not use patients’ names or personal information so no need to take writing consent.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heydari M, Nasiri MJ, Dabiri H, Tarashi S. Prevalence of drug-resistant Klebsiella pneumoniae in Iran: a review article. Iran J Public Health. 2018;47:317–326. [PMC free article] [PubMed] [Google Scholar]
- 2.Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32(3):e00001-19. doi: 10.1128/CMR.00001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan Latania K, Weinstein Robert A. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(Suppl 1):S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;13(7):895. doi: 10.3389/fmicb.2016.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamal WY, Albert MJ, Rotimi VO. High prevalence of New delhi metallo-β-lactamase-1 (NDM-1) producers among carbapenem-resistant Enterobacteriaceae in Kuwait. PLoS ONE. 2016;31(11):e0152638. doi: 10.1371/journal.pone.0152638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solgi H, Badmasti F, Giske CG, Aghamohammad S, Shahcheraghi F. Molecular epidemiology of NDM-1- and OXA-48-producing Klebsiella pneumoniae in an Iranian hospital: clonal dissemination of ST11 and ST893. J Antimicrob Chemother. 2018;73:1517–1524. doi: 10.1093/jac/dky081. [DOI] [PubMed] [Google Scholar]
- 7.Kiaei S, Moradi M, Hosseini-Nave H, Ziasistani M, Kalantar-Neyestanaki D. Endemic dissemination of different sequence types of carbapenem-resistant Klebsiella pneumonia strains harboring blaNDM and 16S rRNA methylasegenes in Kerman hospitals, Iran, from 2015 to 2017. Infect Drug Resist. 2018;21:45–54. doi: 10.2147/IDR.S186994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoja S, Ansari M, Faridi F, Azad M, Davoodian P, Javadpour S. Identification of carbapenem-resistant Klebsiella pneumoniae with emphasis on New Delhi metallo-beta-lactamase-1 (blaNDM-1) in Bandar Abbas, South of Iran. Microb Drug Resist. 2018;24:447–454. doi: 10.1089/mdr.2017.0058. [DOI] [PubMed] [Google Scholar]
- 9.Hojabri Z, Arab M, Darabi N, Kia NS, Lopes BS, Pajand O. Evaluation of the commercial combined disk test and minimum inhibitory concentration (MIC) determination for detection of carbapenemase producers among Gram-negative bacilli isolated in a region with high prevalence of blaOXA-48 and blaNDM. Int Microbiol. 2019;22:81–89. doi: 10.1007/s10123-018-0030-1. [DOI] [PubMed] [Google Scholar]
- 10.Turton JF, Perry C, Elgohari S, Hampton CV. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J Med Microbiol. 2010;59:541–547. doi: 10.1099/jmm.0.015198-0. [DOI] [PubMed] [Google Scholar]
- 11.Hall GS. Enterobacteriaceae. In: Mahon CR, Lehman D, Manuselis G, editors. Textbook of diagnostic microbiology. 3. New York: Saunders Company; 1995. pp. 464–514. [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-second informational supplements. CLSI document M100S-S26; 2016.
- 13.Johnson JR, Porter S, Thuras P, Castanheira M. The pandemic H30 subclone of sequence type 131 (ST131) as the leading cause of multi drugresistant Escherichia coli infections in the United States (2011–2012) Open Forum Infect Dis. 2017;4:ofx089. doi: 10.1093/ofid/ofx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hojabri Z, Darabi N, Arab M, Saffari F, Pajand O. Clonal diversity, virulence genes content and subclone status of Escherichia coli sequence type 131: comparative analysis of E. coli ST131 and non-ST131 isolates from Iran. BMC Microbiol. 2019;19:117. doi: 10.1186/s12866-019-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saffari F, Widerström M, Gurram BK, Edebro H, Hojabri Z, Monsen T. Molecular and phenotypic characterization of multidrug-resistant clones of Staphylococcus epidermidis in Iranian hospitals: clonal relatedness to healthcare-associated methicillin-resistant isolates in Northern Europe. Microb Drug Resist. 2016;22(7):570–577. doi: 10.1089/mdr.2015.0283. [DOI] [PubMed] [Google Scholar]
- 16.Hojabri Z, Mirmohammadkhani M, Darabi N, Arab M, Pajand O. Characterization of antibiotic susceptibility patterns and virulence genes of five major sequence types of Escherichia coli isolates cultured from extraintestinal specimens: a one year surveillance study from Iran. Infect Drug Resist. 2019;12:893–903. doi: 10.2147/IDR.S199759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hojabri Z, Mirmohammadkhani M, Kamali F, Ghassemi K, Taghavipour S, Pajand O. Molecular epidemiology of Escherichia coli sequence type 131 and its H30/H30-Rx subclones recovered from extra-intestinal infections: first report of OXA-48 producing ST131 clone from Iran. Eur J Clin Microbiol Infect Dis. 2017;36:1859–1866. doi: 10.1007/s10096-017-3021-9. [DOI] [PubMed] [Google Scholar]
- 18.Russo TA, Olson R, Fang CT, Stoesser N, Miller M, MacDonald U, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. 2018;56:e00776-18. doi: 10.1128/JCM.00776-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compain F, Babosan A, Brisse S, Genel N, Audo J, Ailloud F, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumonia. J Clin Microbiol. 2014;52:4377–4380. doi: 10.1128/JCM.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bialek-Davenet S, Criscuolo A, Ailloud F, Passet V, Nicolas-Chanoine MH, Decré D, et al. Development of a multiplex PCR assay for identification of Klebsiella pneumonia hypervirulent clones of capsular serotype K2. J Med Microbiol. 2014;63(Pt 12):1608–1614. doi: 10.1099/jmm.0.081448-0. [DOI] [PubMed] [Google Scholar]
- 21.Brisse S, van Himbergen T, Kusters K, Verhoef J. Development of a rapid identification method for Klebsiella pneumoniae phylogenetic groups and analysis of 420 clinical isolates. Clin Microbiol Infect. 2004;10:942–945. doi: 10.1111/j.1469-0691.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 22.McLellan SL, Daniels AD, Salmore AK. Genetic characterization of Escherichia coli populations from host sources of fecal pollution by using DNA fingerprinting. Appl Environ Microbiol. 2003;69:2587–2594. doi: 10.1128/AEM.69.5.2587-2594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahcheraghi F, Nobari S, Rahmati Ghezelgeh F, Nasiri S, Owlia P, Nikbin VS. First report of New Delhi metallo-beta-lactamase-1-producing Klebsiella pneumoniae in Iran. Microb Drug Resist. 2013;19:30–36. doi: 10.1089/mdr.2012.0078. [DOI] [PubMed] [Google Scholar]
- 24.de Melo ME, Cabral AB, Maciel MA, da Silveira VM, de Souza Lopes AC. Phylogenetic Groups among Klebsiella pneumoniae isolates from Brazil: relationship with antimicrobial resistance and origin. Curr Microbiol. 2011;62:1596–1601. doi: 10.1007/s00284-011-9903-7. [DOI] [PubMed] [Google Scholar]
- 25.Sartor AL, Raza MW, Abbasi SA, Day KM, Perry JD, Paterson DL, Sidjabat HE. Molecular epidemiology of NDM-1-producing Enterobacteriaceae and Acinetobacter baumannii isolates from Pakistan. Antimicrob Agents Chemother. 2014;58:5589–5593. doi: 10.1128/AAC.02425-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esteban-Cantos A, Aracil B, Bautista V, Ortega A, Lara N, Saez D. The population of carbapenemase-producing Klebsiella pneumoniae is distinct and more clonal than the carbapenem susceptible population. Antimicrob Agents Chemother. 2017;61:e02520-16. doi: 10.1128/AAC.02520-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bocanegra-Ibarias P, Garza-González E, Morfín-Otero R, et al. Molecular and microbiological report of a hospital outbreak of NDM 1- carrying Enterobacteriaceae in Mexico. PLoS ONE. 2017;12(6):e0179651. doi: 10.1371/journal.pone.0179651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giske CG, Fröding I, Hasan CM, Turlej-Rogacka A, Toleman M, Livermore D. Diverse sequence types of Klebsiella pneumoniae contribute to the dissemination of blaNDM-1 in India, Sweden, and the United Kingdom. Antimicrob Agents Chemother. 2012;56:2735–2738. doi: 10.1128/AAC.06142-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Y, Xu M, Liu Y, Li A, Zhou J. Virulence and genomic features of a blaCTX-M-3 and blaCTX-M-14 coharboring hypermucoviscous Klebsiella pneumoniae of serotype K2 and ST65. Infect Drug Resist. 2019;12:145–159. doi: 10.2147/IDR.S187289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turton JF, Payne Z, Coward A, Hopkins KL, Turton JA, Doumith M. Virulence genes in isolates of Klebsiella pneumoniae from the UK during 2016, including among carbapenemase gene positive hypervirulent K1-ST23 and ‘non-hypervirulent’ types ST147, ST15 and ST383. J Med Microbiol. 2018;67:118–128. doi: 10.1099/jmm.0.000653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be accessible to the interested researchers by the corresponding author on behalf of all authors on reasonable request.