Abstract
Inhalation of infectious bioaerosols has been linked to a variety of respiratory diseases. However, efficient sampling techniques to allow high temporal resolution sampling are limited to collect and study bioaerosols in the various occupational and ambient micro–environmental atmospheres. This study introduces a medium flow swirling bioaerosol sampler (SAS) approach that collects atmospheric bioaerosols at the flow rate of 167 Lpm (10 cubic meter per hour). The collection of bioaerosols is achieved through a combination of impaction and cyclonic centrifugal motion. Aerosol deposition efficiency tests were performed with monodispersive polystyrene latex (PSL) particles ranging from 0.1 to 10 μm. Results have shown that the sampler has cut–off size of 0.7 μm and 1.5 μm, with and without the assistance of added water vapor, respectively. The bioaerosol collection and viability tests were performed with comparison to the commercially–available BioSampler, and the results show that the collection efficiency of the SAS is 97% at the designed flow rate, while the higher flow of the new system yields more than 13 times of the collection rate compared to the BioSampler. The high collection efficiency and observed viability preservation of the SAS make it an attractive alternative for high time resolution bioaerosol sampling for atmospheric, occupational and indoor air quality monitoring.
Keywords: Bioaerosol, sampler, impaction and centrifugal motion
1. Introduction

Exposure to both infectious and non–infectious bioaerosols has been reported to be associated with various respiratory and other health impacts in a variety of residential and occupational environments (Jacobs, 1994; Popendorf et al., 1996). Public awareness about the adverse health effects of bioaerosols has increased since the outbreaks of swine flu, severe acute respiratory syndrome (SARS) and avian flu (Oh et al., 2010). Bioaerosol particles have relatively low number concentrations in ambient air and their small sizes make them difficult to capture for study and identification. Thus, understanding bioaerosols to allow identification and control of their adverse health effects requires development of better sampling techniques with higher collection rates.
There are different methods for bioaerosol sampling, including impactors, impingers and filtration (Reponen et al., 1998; Li et al., 1999). Impactors are among the most widely used sampler types, due to their ability to directly collect infectious agents on the agar plate for incubation. However, impaction processes may induce structural damage to microorganisms, resulting in underestimation of exposure (Park et al., 2011). Bioaerosols are also commonly collected with impingers in which the inertial impaction and diffusion of particles into the liquid collection medium are the major mechanism for collection (Lin et al., 2000; Miljevic et al., 2009). Collected liquid samples can be diluted, thus sampling with impingers is especially suitable for heavily contaminated air. Nevertheless, the recovery of particles by liquid collection can be affected by operational parameters such as sampling duration and type of sampler (Chang and Chou, 2011). Also, the bubbling of liquid produced from the airflow transforms the already collected particles to an aerosol phase that easily escapes from sampler, thus reducing overall collection efficiency (Lin et al., 1997). Filter sampling is a conventional technique of aerosol collection for toxicological and chemical studies. However, the processing and storage of filters before analysis may affect collected sample viability in bioaerosol sampling (Henningson and Ahlberg, 1994). Different types of samplers have been developed to overcome the limitations of previous systems, such as the 3–nozzle swirling aerosol collector which collects particles at 12.5 Lpm flow rate by combining impaction and centrifugal motion, called the SKC BioSampler (Willeke et al., 1998). Roux et al. (2013) reported the design of an electrostatic sampler to concentrate biosample for more efficient collection. McFarland et al. (2010) designed a high flow rate sampler of 1 250 L/min with wetted cyclone. Because of the low ambient concentration of bioaerosols, samplers need to process large volume of air for efficient monitoring while size of sampler is also important consideration for convenient field deployment. King et al. (2009) developed the batch–type wetted wall bioaerosol sampler, however long term operation (>8 hours) decreases the collection efficiency of 1 μm bioaerosols down to 10% only. The cyclone–based aerosol sampler (Sigaev et al., 2006; Tolchinsky et al., 2010) was developed with recirculating liquid film. It has collection efficiency of only 7–30% for 500 nm bioaerosols.
We introduce a new sampler design that integrates the advantages of different particle collection mechanisms: medium flow impaction and cyclonic centrifugal motion. The sampler has 4 tapered nozzles at an angle directed towards the collector's walls. The airstream carrying particles passes through the nozzles at high speed and impacts on the surface wall of the collector, followed by flowing into a cone shape bottom and forming a swirling flow for cyclonic impaction enhancement. The centrifugal motion may help to reduce particle bounce from the impaction surface, and also it lessens the impact force of the microorganisms laden air onto the walls. So the combined effect of impaction and centrifugal motion is the increased collection efficiency and sample viability. In this method, the collection can be further facilitated by mixing the incoming aerosol with water vapor and continuously moisturizing the sampler wall to improve the collection. We describe the prototype design and demonstrated the size dependent efficiency of the sampler, as well as the preservation efficiency of bioaerosol viability compared with BioSampler.
2. Experimental Approach
2.1. Design of the medium flow Swirling Aerosol Sampler (SAS)
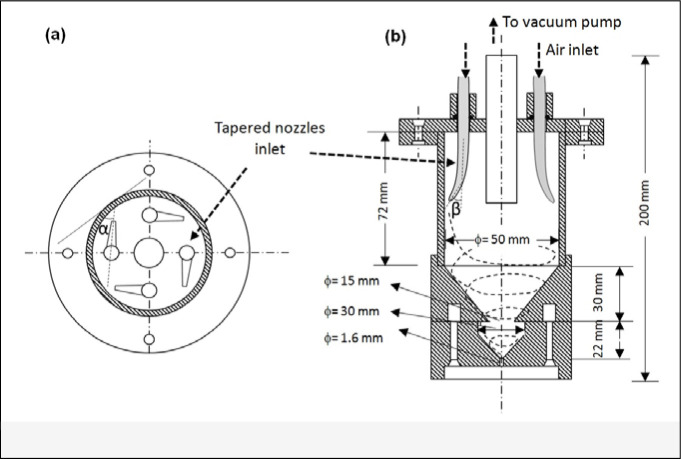
The objective of the present study is to design, construct and evaluate a medium flow sampler for bioaerosol collection. We considered the design criteria including compact size and easy operation for field deployment, equivalent or higher bioaerosol collection efficiency than the commercially available SKC BioSampler but with increased flow up to 10 m3/h. Figure 1 shows the design diagram of the sampler with a view from the top (Figure 1a) and side (Figure 1b). The prototype of the sampler body was constructed using polycarbonate material. The sample air flow enters the sampler through 4 nozzles with tapered tips made of polyethylene material, which are mounted at the top of the sampler at 90° equal spacing. The air leaves the sampler through a stainless steel tubing (3/8” O.D.) in the center and connects to a vacuum pump. Each of the four nozzles is aligned at an angle a to the horizontal tangent at an intersection point of the nozzle axis with an inner surface and angle β to the vertical axis of the sampler. The total flow rate through SAS is 167 Lpm (10 m3/h), α=60° and β=30°. Both angles were optimized and determined empirically based on the formation of swirling flow inside sampler and resulting collection efficiency. Only the results of these optimized angles are shown in the study. The total height of the sampler is 200 mm with an inner cylindrical diameter of 50.0 mm; the height of cone is 50.0 mm; distance between the nozzle bottom and cone top is 50.0 mm; distance between the center of the nozzle outlet and cylinder wall is 3 mm. The lower part of sampler has a smaller cone shape with half the size of larger cone shape. Initial design didn’t include the small cone shape, but it resulted in high evaporation rate for collection liquid. Thus the small cone shape was added so that the collection liquid can be trapped to reduce evaporation. The tapered nozzle has an outlet tip diameter of 3.0 mm to realize an ideal impaction cut–off diameter of 1.5 μm following the classic impaction theory:
| (1) |
Figure 1.
Schematic diagram of the Swirling Aerosol Sampler (SAS): (a) top view; (b) side view.
Where, ρ p is the particle density assumed as 1.2 g/cm3, d 50 is the particle diameter, C c is the Cunningham slip factor, U is the mean jet velocity and μ is the air viscosity as 1.8x10–5 Pa s, while W is the jet diameter.
The air that passes through the nozzle tips accelerates aerosols then impacts on the wall of the cylinder followed by a swirling flow, as exhibited by the dashed line in Figure 1b, to realize combined effects of impaction and centrifugal motion. The use of multiple nozzles for medium flow rate sampling yields a pressure drop through the sampler of 20 kPa at 167 Lpm.
2.2. The PSL experimental setup
Figure 2 shows the experimental setup diagram for the size dependent collection efficiency of the sampler by using mono-dispersive polystyrene latex (PSL, Fluoro–max, Thermo Scientific) particles covering the size range from 0.1 μm to 10 μm (e.g., 0.1 μm, 0.5 μm, 1 μm, 2 μm, 5 μm, 10 μm). Two configurations were tested with and without the vapor assistance as shown in Figure 2. For the dry aerosol collection efficiency test, additional backup filter connected at downstream of the sampler to inspect the penetration efficiency. Each test lasted for 30 minutes and three repeated tests were carried out (for reproducibility). The vapor was generated in a sealed stainless steel chamber with a PID-controlled heater sitting underneath. The chamber was loaded with Milli-Q water and a mild temperature of 30 °C was set to generate the vapor while incoming air remained at 25 °C. Aerosol laden air passed through the chamber mixing with the vapor and humidified up to 95% relative humidity as measured by Vaisala HMP60 probe. PSL particles were generated by a six–jet collision nebulizer (BGI CN25, BGI Inc., Waltham MA) and passed through a mixing chamber in which they are neutralized by an array of 4 ionizers (Staticmaster Ionizers 2U500, Amstat, Glenview, IL) to remove the particle static charges, followed by drying and dilution by particle-free air filtered using a HEPA (High Efficiency Particulate Air) filter. The outlet air humidity was measured by the Vaisala HMP60 probe and remained relatively consistent as room condition of 45% in the ests. Outlet aerosol concentration was measured using Condensational Particle Counter (CPC 3025a, TSI) and controlled by changing nebulizer flowrate, so that it is around 3X104 particles/ cm3 to simulate typical ambient aerosol concentration. Then, the air flow was divided into two aerosol laden streams, one of which passed through a 37 mm Teflon filter with a set flow rate at 30 Lpm as a reference sample and the other air stream was used as a sampling line at 167 Lpm for the developed sampler. Concentration of particles at both reference and sampler line were checked with CPC to make sure of uniform distribution of concentration between the two sampling lines. The pressure drop was measured with Magnehelic differential pressure gage while the vacuum pump (KRX3-P, Orion, Japan) generated the designed flow through the sampler. Initial 20.0 mL of Milli-Q (Millipore, ELGA®, VWS Ltd., England & Wales) water in the sampler was used as collection liquid. After each test, the volume of remaining liquid sample was measured to determine the evaporation rate in the sampler. That's because too high evaporation rate means reduced collection efficiency through escape of particles with effluent airflow.
Figure 2.
Schematic diagram of the PSL experimental setup.
Upon the completion of each test, the liquid sample in the SAS was measured for its volume and transferred to a 1–cm quartz cuvette for fluorescence measurement. Collected fluorescent particles on the reference Teflon filter were extracted with 6.0 mL ethyl acetate, which is approximately zero fluorescence intensity sample same as Milli–Q water sample. The fluorescence intensity of both the liquid sample and filter rinsed solutions were measured with a multi-mode microplate (SpectraMax M5e, Sunnyvale, CA) at excitation and emission wavelength of 468 and 508 nm following the PSL manufacturer's instruction. The collection efficiency, Ec, was calculated as:
| (2) |
In which, f SAS, VSAS are fluorescence intensity and volume of remaining liquid in SAS. f REF, VREF are fluorescence intensity and volume of reference filter rinsed solution measured by the fluorimeter.
2.3. Bioaerosol testing
Following PSL particle tests for size dependent collection efficiency evaluation, a separate set of bioaerosol tests was carried out to determine the viability preservation and collection efficiency of the bioaerosols by the SAS with and without vapor assistance. Figure 3 shows the experimental set up for comparisons in which a SKC BioSampler was used as a reference sampler at 12.5 Lpm while the SAS was operated at 167 Lpm. The BioSampler was chosen as a reference sampler due to its well-established performance characteristics (Cage et al., 1996; Willeke et al., 1998; Lin et al., 2000) and it has been shown to well preserve the viability of the airborne microorganisms (Lin et al., 2000). Eschehchia coli (Invitrogen Life Technologies) was used as a test bioaerosol and first incubated in a LB (lysogeny broth) broth (Tissue culture grade, Amresco) at 37 °C in a rotary shaking incubator overnight. The stationary phase of the bacterial growth yielded a concentration of 108 bacterial cells/mL. Twenty milliliters of the bacteria suspension was used to generate the bioaerosol and the air passed through the same ionizer equipped mixing chamber followed by drying and dilution by particle free air. Outlet humidity was assessed using the humidity probe as in PSL tests and it remained consistent as room condition of 45%±5% in the bioaerosol tests. An Aerodynamic Particle Sizer (APS 3321, TSI) was used to measure the nebulized bioaerosol size distribution and the mode diameter was constant at 1 μm. A HEPA filter was connected to the downstream end of the samplers to prevent the release of bioaerosol into indoor air. Bioaerosol concentration at the points of BioSampler and SAS were checked with CPC (3025a, TSI) to make sure of uniform concentrations in different lines. The BioSampler and SAS were preloaded with 15.0 mL and 20.0 mL of Milli–Q water, respectively. Each test lasted for 30 minutes and three repeated tests were carried out for reproducibility. The fluids collected by each of the samplers (SAS and BioSampler) were diluted 104 times and spread into three identical LB Agar plates (in a triplicate). The number of colonies formed on each plate was manually counted to determine the average colony number after incubation at 37 °C for 24 hours. The data were further converted to the bioaerosol concentration expressed a colony–forming units (CFU)/m3.
Figure 3.
Schematic diagram of the bioaerosol experimental setup.
3. Results and Discussion
Figure 4a shows the collection efficiency of SAS with and without the assistance of water vapor for test particle sizes ranging from 0.1 μm to 10 μm at a flow of 167 Lpm. Error bars represent the standard deviation from the three repeated runs. Figure 4b shows the comparison of dry collection and penetration efficiency. For tests without vapor assistance, the efficiency curve follows classic impaction theory in which a 50% cut point of approximately 1.5 μm was achieved by the sampler with 60-80% collection efficiency for aerosol size larger than 2 μm. Different from the impactor, inside SAS, four tapered nozzles are directed at an angle towards the cylinder wall. The air stream from the nozzles has tangential components at the inner surface of the sampler that creates a swirling motion of air. This enables collected aerosol-containing liquids to swirl along the wall of the cylinder. The continuous swirling liquid washes away collected particles preventing them from sticking to the wall. Therefore, the result is a combined effect of aerosol collection. The swirling liquid level was observed to be at same level as nozzle outlet and gradually decrease with evaporation of liquid. The swirling motion also is likely to reduce the intensity of impaction between the cylinder wall and incoming aerosols. Without assistance of water vapor, larger particles tend to bounce back from the cylinder wall and exit directly from the sampler. Thus, collection efficiency for larger particles is only around 80%. In addition to the particle bouncing issue, this experiment also showed the high evaporation rate of the collection water inside SAS. During 30-minute continuous collection in the test, 7.0 mL out of 20.0 mL water sample evaporated posing a concern for continuous sampling while 10.0 mL out of 15.0 mL evaporated for the BioSampler. Sum of sampler collection efficiency and backup filter collection, compared with reference filter, indeed proves there are particles loss in the system, exactly as predicted by particle impaction theory with particle loss as function of particle size.
Figure 4.
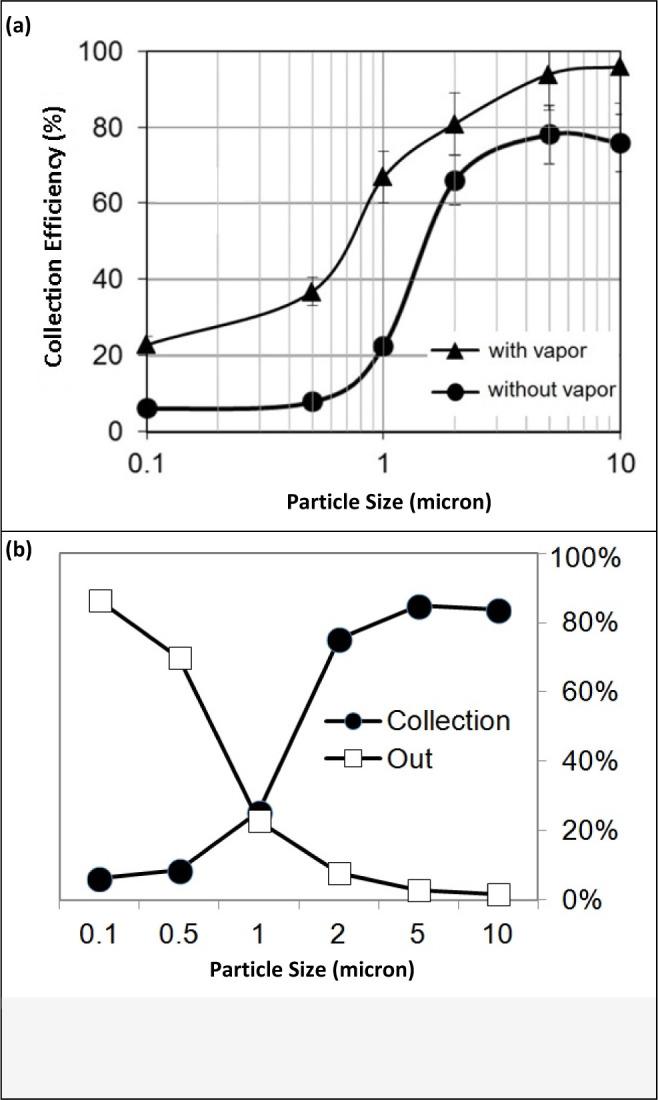
(a) Comparison for collection efficiency of SAS with vapor assistance and without vapor assistance; (b) Comparison of dry collection and penetration efficiency.
With water vapor assistance, the sampler demonstrated a much smaller cut point diameter with over 50% collection efficiency for particles larger than 0.7 μm and over 80% collection efficiency for particles larger than 2 μm, compared to 60-80% for the non-vapor assisted configuration. Also, the non-vapor assisted test showed the swirling liquid level along the cylinder wall decreases with the increasing collection time due to evaporation which lowers collection efficiency for prolonged sampling, while the presence of vapor in the vapor–assisted experiment alleviates the degree of bouncing of large particles from the impaction surface wall and exiting from the sampler. Continuous addition of water vapor also helps maintain the swirling liquid to an adequate level necessary for particle collection inside the sampler, thus greatly increasing the collection efficiency. Due to additional water vapor, only 2.5 mL out of 20.0 mL water sample evaporated after 30-minute continuous collection at the humidifier temperature setting of 30 °C.
Figure 5 presents the comparison of the bioaerosol collection efficiency using the BioSampler and the SAS, in which Figure 5a shows the agar plates of the two samples from one of the tests after incubation and Figure 5b shows the CFU concentrations obtained by the two samplers. Error bar represents the standard deviation of the results from the three repeated runs. Figure 5c shows the size distribution of the bioaerosol used in the study. The bioaerosol concentrations determined by the SAS and reference BioSampler were 105±6 and 108±40 CFU/m3, respectively, indicating a high collection efficiency and viability preservation of 97.2% for the SAS at flow rate of 167 Lpm compared with BioSampler at flow rate of 12.5 Lpm. This indicates the preference of medium to high flow sampling for ambient bioaerosol collection, especially at conditions of clean environment with low bioaerosol concentrations which requires much longer sampling time with less temporal resolution. Given the much higher (13.4 times) sampled volume per unit of time by SAS and the good agreement of the collection performance with the reference sampler, the new design provides an alternative option for high temporal resolution sampling of bioaerosol.
Figure 5.
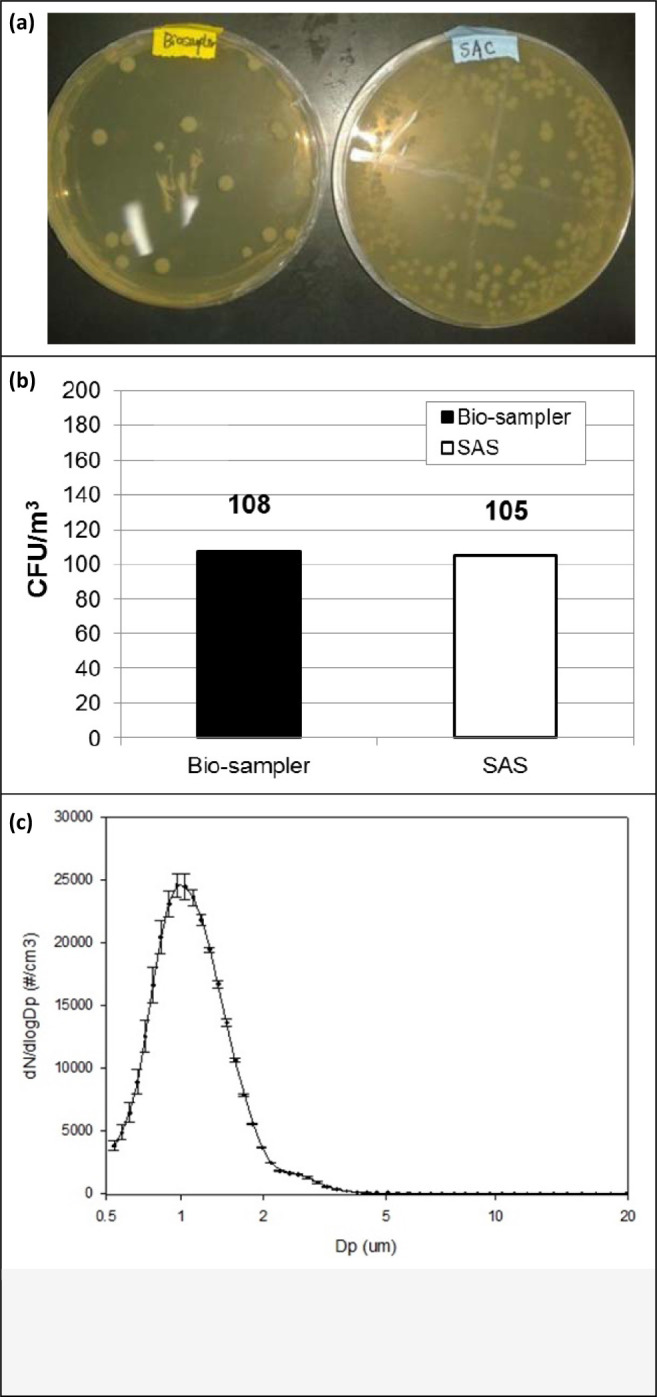
(a) The comparison of the agar plates with samples from BioSampler (Left) and SAS (Right); (b) Comparison of the CFU concentrations from SAS and reference BioSampler; (c) Size distribution of the Bioaerosol.
4. Conclusions
This study presents the development and evaluation of a medium flow swirling aerosol sampler for efficient collection of bioaerosols. The sampler collects atmospheric aerosol at the flow rate of 167 Lpm by a combined effect of impaction and cyclone based centrifugal motion. Results from the size dependent efficiency tests shows that without assistance of water vapor, the sampler has cut-off size of 1.5 μm with 50% collection efficiency. Due to bouncing of particles off the walls, collection efficiency was consistently low at 80% for larger particles and the evaporation of collection liquid also creates problems for continuous sampling. Indeed the cut point of dry sampling could be lowered by change of sampler design. A more comprehensive investigation would be needed to fully characterize the system performance and lowering the cut point. This note is intended to demonstrate improved sampler performance through added water vapor or water coatings. With the addition of water vapor, the collection efficiency was improved to a 50% cutoff size of 0.7 μm with much less bouncing for larger aerosols. Viability tests performed with E. Coli proved the sampler was shown to have the viability preservation and collection efficiency that is equivalent to the commercially available BioSampier but with more than 13 times higher collection rate. This makes it an attractive alternative for environmental bioaerosol sampling when high temporal resolution is useful. The SKC BioSampier is a refined, production-level unit while our sampler is a demonstration unit. If our sampler was moved to commercial production, it would be possible to make cut-point reductions, but the overall flow and water vapor addition we employ will remain a major design forcing factor. The compact size of the sampler with integrated humidifier also makes it an ideal device for field operations. In highly polluted areas, filters could be overloaded but from our work to date this does not seem to be a problem for this sampler. There is sufficient liquid to suspend and capture PM for several hours. Future investigations will evaluate and characterize the operating parameters to optimize the bioaerosol collection efficiency at varying ambient conditions.
Acknowledgment
The authors would like to thank Mr. Fenhuan Yang for his assistance in the experiments, and also Mr. Zhiquan Lin for his assistance in the prototype development. The work described in this paper was supported by the grants from City University of Hong Kong (Project No. 7200251, 7002918 and 6351016). The authors would also like to thank the Innovation and Technology Fund (Project No. ITS/324/13FP) from the Innovation and Technology Commission. Xiaoying Lu and Patrick K.H. Lee thank the financial supports from the Research Grants Council of Hong Kong through project 124412. Any opinions, findings, conclusions or recommendations expressed in this study do not reflect the views of the Government of the Hong Kong Special Administrative Region, the Innovation and Technology Commission or the Vetting Committee of General Support Programme of the Innovation and Technology Fund.
References
- Cage B.R., Schreiber K., Barnes C., Portnoy J. Evaluation of four bioaerosol samplers in the outdoor environment. Annals of Allergy, Asthma & Immunology. 1996;77:401–406. doi: 10.1016/S1081-1206(10)63339-X. [DOI] [PubMed] [Google Scholar]
- Chang C.W., Chou F.C. Assessment of bioaerosol sampling techniques for viable Legionella pneumophila by ethidium monoazide quantitative PCR. Aerosol Science and Technology. 2011;45:343–351. [Google Scholar]
- Henningson E.W., Ahlberg M.S. Evaluation of microbiological aerosol samplers - A review. Journal of Aerosol Science. 1994;25:1459–1492. [Google Scholar]
- Jacobs R.R. Risk environments. In: Rylander R., Jacobs R.R., editors. Organic Dusts, Exposure, Effects, and Prevention. CRC Press Inc; Boca Raton, FL: 1994. pp. 3–16. [Google Scholar]
- King M.D., Thien B.F., Tiirikainen S., McFarland A.R. Collection characteristics of a batch-type wetted wall bioaerosol sampling cyclone. Aerobiologia. 2009;25:239–247. [Google Scholar]
- Li C.S., Hao M.L., Lin W.H., Chang C.W., Wang C.S. Evaluation of microbial samplers for bacterial microorganisms. Aerosol Science and Technology. 1999;30:100–108. [Google Scholar]
- Lin X.J., Reponen T., Willeke K., Wang Z., Grinshpun S.A., Trunov M. Survival of airborne microorganisms during swirling aerosol collection. Aerosol Science and Technology. 2000;32:184–196. [Google Scholar]
- Lin X.J., Willeke K., Ulevicius V., Grinshpun S.A. Effect of sampling time on the collection efficiency of all-glass impingers. American Industrial Hygiene Association Journal. 1997;58:480–488. [Google Scholar]
- McFarland A.R., Haglund J.S., King M.D., Hu S.S., Phull M.S., Moncla B.W., Seo Y. Wetted wall cyclones for bioaerosol sampling. Aerosol Science and Technology. 2010;44:241–252. [Google Scholar]
- Miljevic B., Modini R.L., Bottle S.E., Ristovski Z.D. On the efficiency of impingers with fritted nozzle tip for collection of ultrafine particles. Atmospheric Environment. 2009;43:1372–1376. [Google Scholar]
- Oh S., Anwar D., Theodore A., Lee J.H., Wu C.Y., Wander J. Development and evaluation of a novel bioaerosol amplification unit (BAU) for improved viral aerosol collection. Journal of Aerosol Science. 2010;41:889–894. doi: 10.1016/j.jaerosci.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.W., Yoon K.Y., Do Kim Y., Park J.H., Hwang J. Effects of condensational growth on culturability of airborne bacteria: Implications for sampling and control of bioaerosols. Journal of Aerosol Science. 2011;42:213–223. [Google Scholar]
- Popendorf W., Miller E.R., Sprince N.L., Selim M.S., Thorne P.S., Davis C.S., Jones M.L. The utility of preliminary surveys to detect the cause of acute metalworking fluid hazards. American Journal of Industrial Medicine. 1996;30:744–749. doi: 10.1002/(SICI)1097-0274(199612)30:6<744::AID-AJIM11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Reponen T.A., Gazenko S.V., Grinshpun S.A., Willeke K., Cole E.C. Characteristics of airborne actinomycete spores. Applied and Environmental Microbiology. 1998;64:3807–3812. doi: 10.1128/aem.64.10.3807-3812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux J.M., Kaspari O., Heinrich R., Hanschmann N., Grunow R. Investigation of a new electrostatic sampler for concentrating biological and non–biological aerosol particles. Aerosol Science and Technology. 2013;47:463–471. [Google Scholar]
- Sigaev G.I., Tolchinsky A.D., Sigaev V.I., Soloviev K.G., Varfolomeev A.N., Chen B.T. Development of a cyclone–based aerosol sampler with recirculating liquid film: Theory and experiment. Aerosol Science and Technology. 2006;40:293–308. [Google Scholar]
- Tolchinsky A.D., Sigaev V.I., Sigaev G.I., Varfolomeev A.N., Zvyagina E.V., Brasel T., Cheng Y.S. Development of a personal bioaerosol sampler based on a conical cyclone with recirculating liquid film. Journal of Occupational and Environmental Hygiene. 2010;7:156–162. doi: 10.1080/15459620903486768. [DOI] [PubMed] [Google Scholar]
- Willeke K., Lin X.J., Grinshpun S.A. Improved aerosol collection by combined impaction and centrifugal motion. Aerosol Science and Technology. 1998;28:439–456. [Google Scholar]