Abstract
The order Rodentia comprises more than 2000 species divided into 3 groups based on anatomic and functional differences of the masseter muscle. Myomorph and sciuromorph species have elodont incisors and anelodont cheek teeth, unlike hystrichomorph species which have full anelodont dentition. Diseases of incisors and cheek teeth of rat-like and squirrel-like rodents result in a wide variety of symptoms and clinical signs. Appropriate diagnostic testing and imaging techniques are required to obtain a definitive diagnosis, formulate a prognosis, and develop a treatment plan. A thorough review of elodontoma, odontoma, and pseudo-odontoma is provided, including treatment of pseudo-odontomas in prairie dogs.
Keywords: Myomorphs, Sciuromorphs, Dental disease, Pseudo-odontoma, Elodontoma
Key points
-
•
The order Rodentia is the largest and most diversified mammalian group, comprising approximately 40% of all mammalian species, differing widely in size, behavior, feeding habits, anatomy, and physiology.
-
•
All rodent species have 2 well-developed maxillary and mandibular aradicular incisor teeth, which represent the most distinguishable dental feature of this order.
-
•
Myomorphs and sciuromorphs have elodont incisors and anelodont cheek teeth, whereas hystrychomorphs have full anelodont dentition.
-
•
Odontogenic tumors and tumor-like lesions (odontomas, complex odontomas and elodontomas) affecting incisor teeth are frequently seen in sciuromorph rodents, including pseudo-odontoma in prarie dogs. Challenging surgical approaches may be required for treatment.
-
•
The use of computed tomography is particularly useful and has great advantages compared with standard radiography.
Introduction
The order Rodentia represents the largest and most diverse mammalian group, comprising approximately 40% of all mammal species. These animals differ vastly in habitat, body size, feeding habits (omnivore to specialized herbivore), dietary requirements, behavior, anatomy, and physiology. The past decade has seen an exponential increase in the number of certain rodent species, such as rats, mice, hamsters, gerbils, prairie dogs, and squirrels, being owned as pets. Rodent owners are increasingly expecting the same high-quality veterinary care to be afforded to their valued companion as is provided to dogs and cats. The approach to oral disorders in these animals can be in part extrapolated from other more common rodent species such as guinea pigs, and from rabbits; however, specific information regarding their natural feeding habits along with a knowledge of their dental and oral anatomy and physiology is essential. Appropriate diagnostic testing and imaging techniques are also necessary to obtain a definitive diagnosis, formulate a prognosis, and develop a treatment plan. In particular, pseudo-odontomas of prairie dogs require an intimate understanding of their anatomy, as they pose a unique challenge regarding anesthetic protocols and surgical treatment.
Taxonomy of rodent species
The order Rodentia (from the Latin word rodere, ie, “to gnaw”)1 is the largest and most diversified mammalian group, comprising approximately 40% of all mammalian species (more than 2000) differing widely in size, behavior, feeding habits, anatomy, and physiology.2 These hundreds of species are grouped in 3 suborders based on the anatomic and functional differences of the masseter muscles: the Caviomorpha or Hystrichomorpha (otherwise named “porcupine-like”, “cavy-like” or “guinea pig–like” rodents, such as the guinea pig, chinchilla, and degu), the Myomorpha (“rat-like” or “mouse-like” rodents), and the Sciuromorpha (“squirrel-like”rodents).3
Members of the rat-like group include many species commonly kept as pets, such as the rat (Rattus norvegicus), the Golden or Syrian hamster (Mesocricetus auratus), the Russian hamsters (Phodopus sungorus, Phodopus campbelli, Phodopus roborovskii), the Chinese hamster (Cricetulus griseus), the mouse (Mus musculus), the gerbil (Meriones unguiculatus) and the Duprasi or “fat-tailed” gerbil (Pachyuromys duprasi). The sciuromorph group includes the prairie dog (Cynomys ludovicianus), the European ground squirrel (Spermophilus citellus), the Richardson ground squirrel (Spermophilus richardsonii), and the chipmunk (Tamias striatus, Eutamias sibiricus),3 which are less commonly kept as pets.4
Anatomy and physiology of dentition and mastication
All rodent species have 2 well-developed maxillary and mandibular elodont aradicular (continually growing and erupting throughout life, which lack an anatomic root) incisor teeth, which represent the most distinguishable dental feature of this order.1, 5 They lack canine teeth, and incisors are separated from premolars by a long toothless space named diastema.3 Unlike rabbits, rodents are monophyodont (having a single set of teeth) and simplicidentata (having just 1 pair of maxillary incisors).1, 3, 5, 6 The incisor teeth have a chisel-shaped occlusal surface and are covered by enamel only over the labial surface. The enamel is characteristically pigmented yellow-orange in most rodents. In cavy-like rodents, all teeth are elodont. Also, premolar and molar teeth are anatomically similar in this group, and are commonly named “cheek teeth”.1, 3 A remarkable difference between rat-like and squirrel-like rodent species is that the first group lacks premolar teeth. This is not clinically relevant, but results in diverse dental formulas (Figs. 1 and 2 ). Different from hystrichomorphs, squirrel-like and rat-like rodents have anelodont premolar and/or molar teeth. They are truly rooted (with a limited growth period), single or multiple rooted, and are not worn during normal chewing activity. They are brachyodont (short crowned) with multiple cusps. Depending on species, premolars and molars have more or less different size and shape in the same quadrant. The premolar and molar teeth of prairie dogs and other herbivorous ground squirrels have multiple roots. Molar teeth of myomorphs also have multiple roots. The crown has cusps and ridges that create a rough occlusal surface.7 Regardless of these differences, the premolar and/or molar cheek teeth of myomorph and sciuromorph rodents are commonly named “cheek teeth” as well.
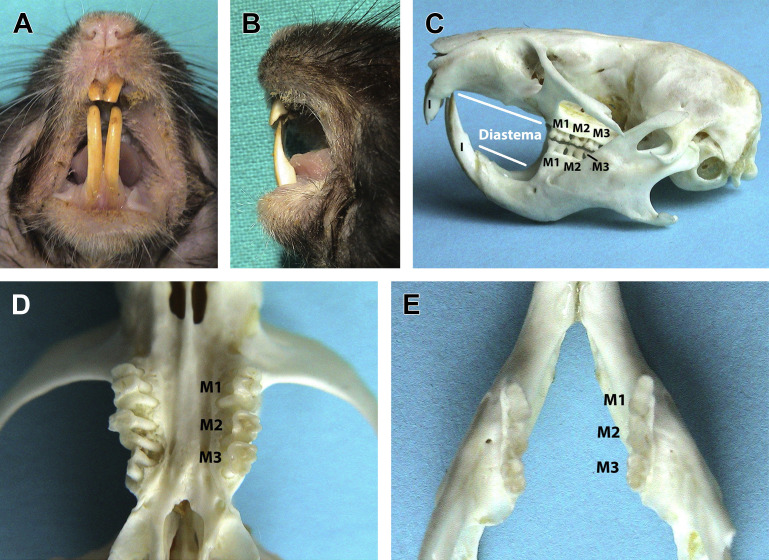
Fig. 1.
Dentition of the myomorph rodents shown in a golden hamster (Mesocricetus auratus). (A) Labial aspect of the clinical crowns of incisor teeth. The enamel is yellow-orange. Divergence between mandibular incisors is variable due to the incomplete ossification and the flexibility of the mandibular symphysis. (B) Lateral (distal) aspect of incisor teeth. Clinical crowns are white because the enamel does not cover the distal, mesial, and lingual/palatal aspects of incisor teeth. Note the chisel-shaped occlusal plane and the enognathic appearance of the mandible. (C) Lateral view of the skull and jaws shown on a bony specimen displaying the long diastema and the anelodont, multiple rooted molar teeth. Unlike squirrel-like rodents, rat-like rodents lack premolar teeth. (D) Close up of the maxilla from the ventrodorsal view and (E) of the mandible from the dorsoventral view, displaying the molar teeth. Multiple roots are also visible in (D).
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
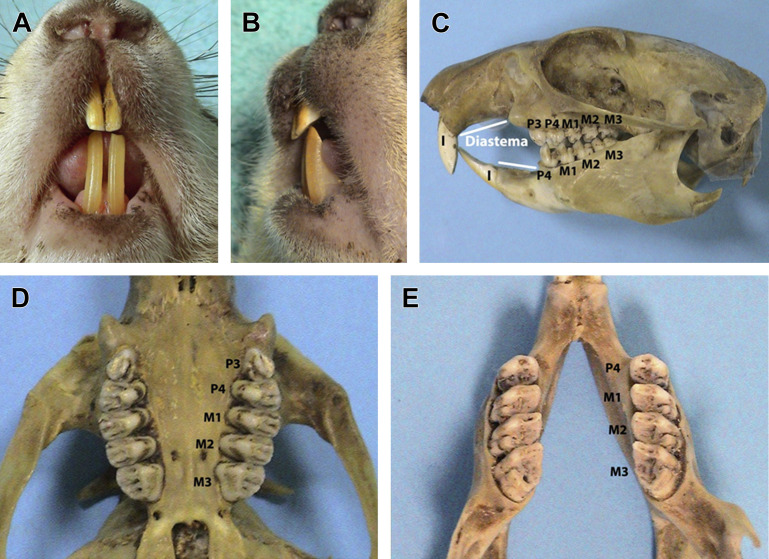
Fig. 2.
Dentition of the sciuromorph rodents shown in a prairie dog (Cynomys ludovicianus). (A) Labial aspect of the clinical crowns of incisor teeth. The enamel is yellow-orange. Divergence or slight separation between mandibular incisors is variable, and due to the incomplete ossification and the flexibility of the mandibular symphysis. (B) Lateral (distal) aspect of incisor teeth. The enamel covers the distal and mesial aspects of incisor teeth. Note the chisel-shaped occlusal plane and the enognathic appearance of the mandible. (C) Lateral view of the skull and jaws shown on a bony specimen, displaying the long diastema and the crowns with multiple cusps of anelodont premolar and molar teeth. (D) Close up of the maxilla from the ventrodorsal view and (E) of the mandible from the dorsoventral view, displaying the cheek teeth. The standard system numbering the premolars as P3 and P4 is used in this figure.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
From an anatomic, physiologic, and clinical standpoint, the rat-like and squirrel-like groups can be merged into a single group, making the 3 suborders of rodents into 2 groups: cavy-like rodents with full elodont dentition (most similar to lagomorphs), and all the other rodents with elodont incisors but anelodont cheek teeth.
The dental formulas of selected rat-like and squirrel-like rodent species are listed in Table 1 .
Table 1.
Dentition and dental formula of selected rat-like and squirrel-like rodent species
| Species | Dental Formula | Total No. Teeth |
|---|---|---|
| Rat, Golden hamster | 2(I 1/1, C 0/0, P 0/0, M 3/3) | 16 |
| Prairie dog | 2(I 1/1, C 0/0, P 2/1, M 3/3) | 22 |
| Eastern gray squirrel (Sciurus carolinensis) | 2(I 1/1, C 0/0, P 1–2/1, M 3/3) | 20–22 |
Data from Capello V, Gracis M, Lennox AM. Rabbits and rodents dentistry handbook. Lake Worth (FL): Zoological Education Network; 2005. p. 1–274; and Legendre LFJ. Oral disorders of exotic rodents. Vet Clin North Am Exot Anim Pract 2003;6:601–28.
Several rodent species, such as hamsters and squirrels, have para-oral structures between the jaws and the cheek, representing an important anatomic feature: the cheek pouches. Cheek pouches are paired, thin-walled, highly vascular, sac-like evaginations of the oral cavity. The pouch actually consists of 4 layers: keratinized stratified squamous epithelium, lamina propria, skeletal muscle, and adventitial connective tissue. The inner surface is covered by mucosa. These distensible, loose cutaneous structures can stretch dorso-caudally along the lateral aspects of the mandible from the mouth over the shoulder regions ventral to the ears.8, 9 Cheek pouches allow rapid collection and transport of food. Female hamsters have been known to hide their litter when they perceive the pups are in danger.
Rodents have a highly distinctive anatomic arrangement of the masseter muscle of the jaw (the dominant jaw-closing muscle) and the zygomatic arch of the skull, compared with that of other mammalian groups.10, 11, 12 Researchers have been able to identify 3 distinct layers of the masseter muscle (superficial masseter, deep masseter, and zygomaticomandibularis, although inconsistencies exist over nomenclature and anatomic details) in all 3 rodent groups.11
In hystrichomorphs, an enlarged (as compared with the rat and squirrel) superficial portion of the masseter muscle moves the mandible forward and backward, whereas the reduced deep portion adducts the jaw, thereby closing the mouth.1, 3, 11 This propalineal motion (combined lateral and rostrocaudal movement; ie, oblique) results in an effective grinding action by the molars and a reduced gnawing action by the incisors. In the sciuromorphs, the deep masseter expands forward onto the rostrum and it separates into 2 parts (anterior and posterior). In myomorphs, the origins of both parts of the masseter muscle have migrated forward onto the rostrum and work together to move the mandible forward and backward and are therefore equally adapted for both feeding modes (gnawing by the incisors and chewing by the molars).10, 11 In myomorphs, the mandible has a wide range of rostrocaudal movement and can even be subluxated rostrally during gnawing.1, 3 The cheek teeth are in occlusion when the jaw is at rest, giving the mandible a brachygnathic appearance.1, 3 Ultimately, when the incisors are gnawing and the cutting edges of mandibular and maxillary incisors are approximated, the molars are pulled out of occlusion. When chewing of food occurs, it is then that the mandibular incisors move posteriorly to the maxillary ones and the molars occlude again. During crushing of food, upward and forward movements of the mandible become evident.13
The dental anatomy reflects into different dietary habits and feeding strategies of the various rodent species as the anatomy of masticatory muscles leads to different stress and strains distributed across the 3 skull morphologies during chewing and gnawing.11, 14 The enlargement of the rostral portion of the deep masseter in sciuromorphs increases the efficiency of biting with the incisors. Conversely, the reduced deep masseter of the guinea pig may indicate this species mostly eats by chewing with the cheek teeth while spending less time gnawing with the incisors compared with squirrels or rats.15 Actually, rats and squirrels consume more hard food (eg, nuts and seeds) than guinea pigs, which feed primarily on vegetation. Myomorphs are the most versatile, as they merge anatomic features of sciuromorphs and hystricomorphs and are equally efficient at both gnawing and chewing. These 2 feeding modes are mutually exclusive because incisors and molars cannot be in occlusion at the same time due to the mismatch between maxillary and mandibular length. Hence propalineal action is necessary to accomplish these tasks.16, 17
In some species (eg, rats and squirrels), the cartilaginous tissue between the mandibular symphysis is not ossified, allowing a certain degree of movement between the mandibles13 visible at the lower incisor teeth.
Dental disease and other oral disorders
Incisor Teeth
Primary congenital malocclusion of incisor teeth has been described in growing rats, hamsters, and squirrels.6 However, this condition may be difficult to distinguish from acquired malocclusion of traumatic origin in adult animals, as often the initial injury is not recognized by most owners. True dwarfism, as recognized in pet rabbits, has not been documented in rodents because these species have not been selectively bred for extreme size variation.7 However, brachygnathia superior (maxillary brachygnathism and enognathism; ie, shortening of the upper jaw) has been reported in myomorphs.18
Traumatic injuries of incisor teeth may be uneventful, but severe fractures affecting the pulp canal and/or the germinative tissue are frequently followed by dental disease and malocclusion of clinical crowns (Fig. 3 ). The most common form of acquired malocclusion seen in myomorphs is overgrowth (abnormal coronal elongation of clinical crowns), often accompanied by lateral mandibular shifting (Figs. 4 and 5 ). Because of the flexible symphysis and related movement of each mandible, malocclusion may create abnormal forces capable of slight rotation of the mandibles over the long axis. This allows the mandibular incisors to bypass the maxillary incisors laterally, while the maxillary clinical crowns follow the curvature of the reserve crowns into the oral cavity. In those species with high mechanical pressure at the apical sites of the upper incisors, palatal perforation can occur.19 Malpositioning after a jaw fracture and age-related loss of clinical crowns in gerbils have also been reported as frequent causes of incisor malocclusion in myomorphs.18 Abscess formation and osteoresorptive lesions may be seen involving the incisors in these rodent species.13
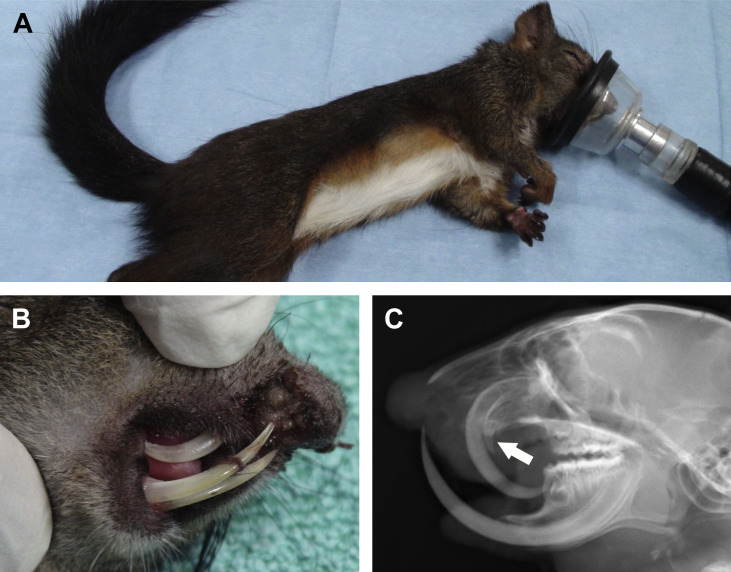
Fig. 3.
Malocclusion of incisor teeth in a European red squirrel (Sciurus vulgaris) (A). This young squirrel was rescued with a severe wound of the maxilla. This lesion cause brachignathism of the maxilla, followed by abnormal growth of maxillary incisor teeth. (B) Severe malocclusion of incisor teeth creating this lesion was not related to the initial trauma. (C) Radiograph of the head, lateral view. Note the severe brachygnathism of the maxilla causing enognathism of the maxillary cheek teeth, the abnormal curvature of the right maxillary incisor, and the hypoplastic left maxillary incisor, not erupting above the gingival level (arrow).
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Fig. 4.
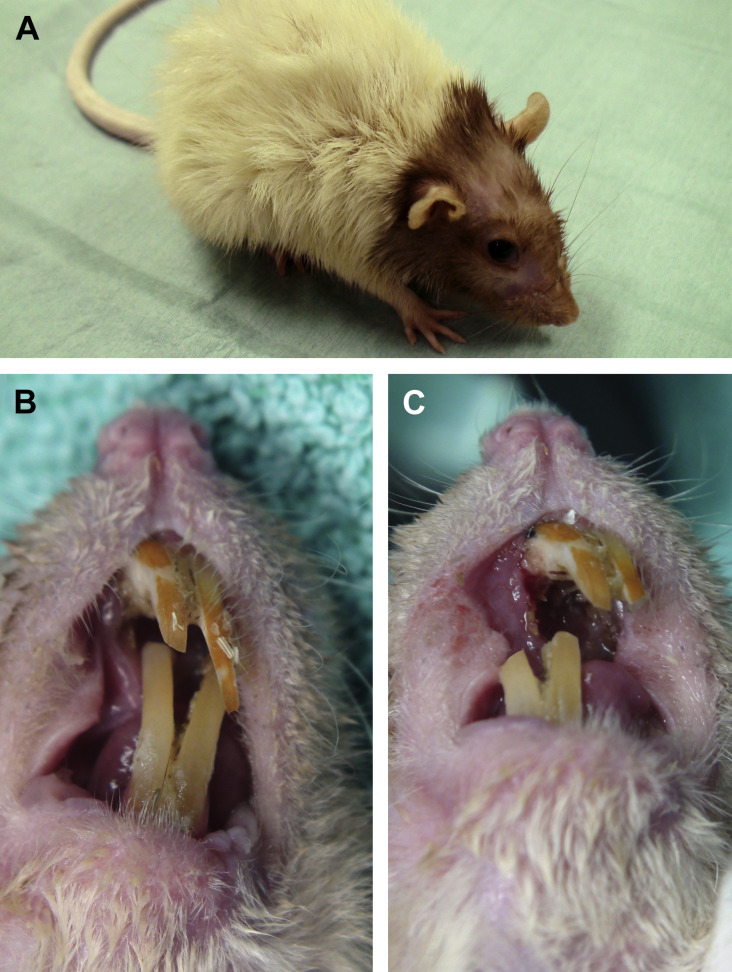
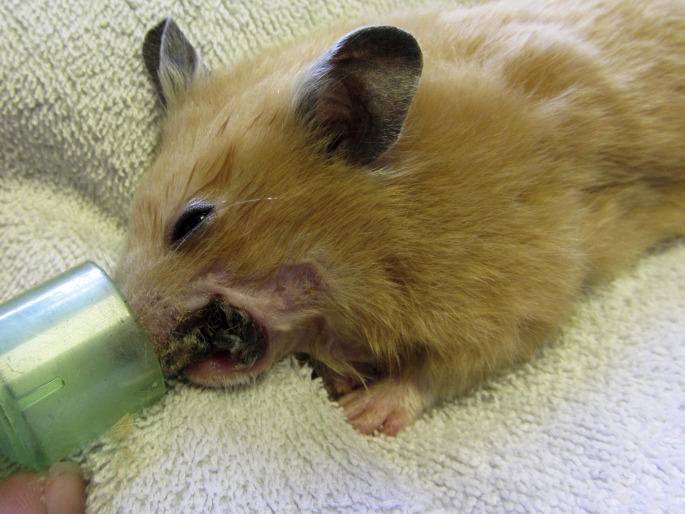
Dental disease and malocclusion of incisor teeth in golden hamsters. (A) Elongation of the right mandibular incisor, and deviation of maxillary incisors. (B) Elongation of the left mandibular incisor, and fracture of the other incisors. The clinical crowns of maxillary incisor teeth are fractured at the gingival level. (C) Severe malocclusion and elongation with lateral deviation of maxillary incisor teeth. The clinical crowns of mandibular incisors are fractured, and no longer erupting. (D) Severe overgrowth and deviation of mandibular incisor teeth, protruding outside the oral cavity and creating a wound of the right cheek pouch.
(Courtesy of [A, B] Elisabetta Mancinelli, DVM; and [C, D] Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Fig. 5.
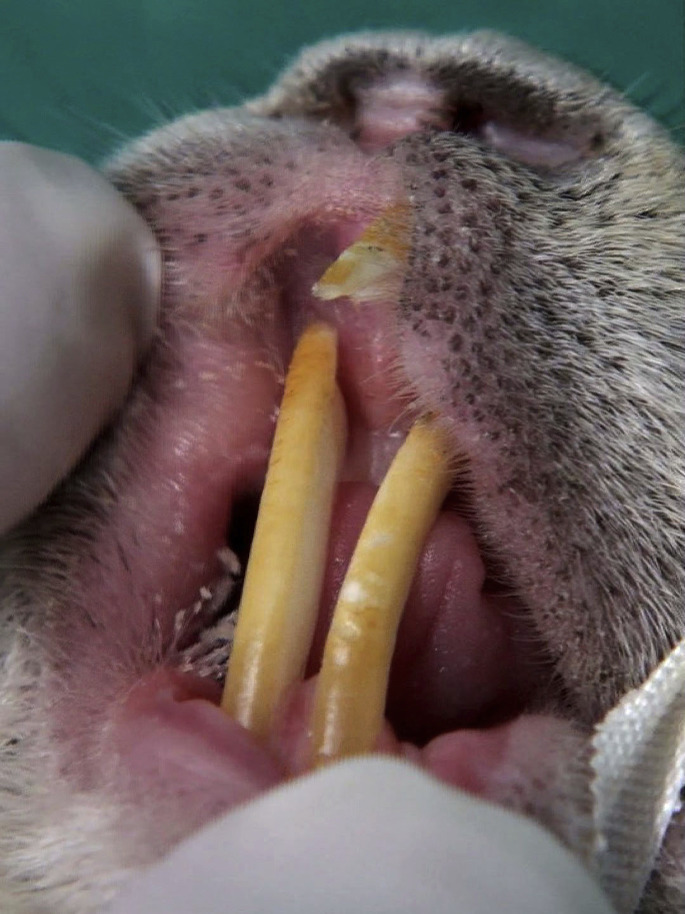
Malocclusion of incisor teeth in a female rat. (A) Weight loss and evident dehydration were associated with mild dyspnea and sneezing. (B) Malocclusion of incisor teeth. Unlike the common pattern of malocclusion in which the clinical crown of maxillary incisor teeth can penetrate the palate, this injury is due to elongation of mandibular incisors in this patient. (C) A wide oro-nasal fistula is visible after palliative coronal reduction of incisor teeth.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Other types of malocclusion and dental disease of incisor teeth are seen in sciuromorphs (odontomas, complex odontomas, and elodontomas).
Cheek Teeth
Metabolic disease as a predisposing factor for dental disease has not been fully explored or proven for rat-like and squirrel-like rodents.3, 7 Insufficient wearing of cheek teeth following inappropriate diet does not occur in species with anelodont cheek teeth. However, dental problems due to excessive wearing and severe caries can still occur in these rodents, deeply reducing the crown and resulting in teeth loss3, 7 (Fig. 6 ). Captive myomorphs and sciuromorphs can develop periodontal disease, dental caries, and tooth decay if fed an inappropriate, high-sugar diet.13, 20 Gerbils have been reported to develop spontaneous periodontal disease if fed a standard rat or mouse diet for more than 6 months.21 These rodents should therefore be fed an appropriate pelleted diet targeted to their specific requirements. Periodontal inflammation stimulates odontoclastic resorption, which creates cavities in the affected tooth. In some cases, repair takes place with granulation tissue moving in, as an attempt to contain the damage but lost dentin is not replaced. Caries lesions in rodents with brachyodont cheek teeth are similar to those seen in humans. Rats, in particular, have been used as animal models for human caries.22 Hamsters are especially prone to develop periodontal disease, dental caries, and facial abscesses caused by a tooth root abscess.3, 23
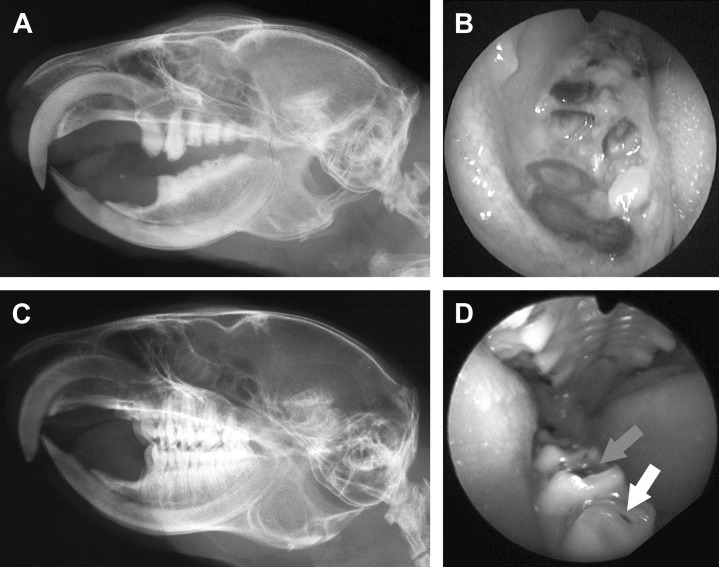
Fig. 6.
Dental disease of cheek teeth in a 7-year-old prairie dog (A, B) and comparison with a normal animal (C, D). (A) Radiography of the head (lateral view), displaying severe damage of most cheek teeth, including the maxillary molar teeth and all the mandibular cheek teeth. Severe and diffuse remodeling of the roots is also present. Note that the incisor teeth are normal. (B) Endoscopic view of the right mandibular cheek teeth arcade in the same patient. Crowns are missing, likely following severe caries, and diseased portions of roots are visible at the gingival level. (C) Radiography of the head (lateral view), in a normal prairie dog. Note the coronal cusps and the multiple roots of cheek teeth. (D) Endoscopic view of the right mandibular cheek teeth arcades in a normal prairie dog with a small caries (white arrow) and food debris (grey arrow).
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Abscesses, possibly involving the masseter muscle, may be seen in rats, mice, hamsters, and other small rodents (Fig. 7 ). They can be secondary to fracture or cavity of cheek teeth, or to endodontic or periapical infections of diseased incisors.6
Fig. 7.
Extraoral fistula following removal of a facial abscess in a rat.
(Courtesy of Elisabetta Mancinelli, DVM.)
Fractures of the jaws may occur in myomorph and sciuromorph rodents, as well as separation of the mandibular symphysis.6
Odontogenic Tumors and Tumor-like Lesions (Odontoma, Pseudo-Odontoma, and Elodontoma)
Odontogenic tumors have long been recognized as uncommon entities in humans and animals of many species.24 Because most of these tumors are not malignant,24 the distinction between neoplasms and tumor-like (non-neoplastic) lesions is debatable. Several classifications have been proposed to describe these odontogenic abnormalities. Some of them focus on tumors of odontogenic origin,25 others include hamartomas, congenital malformations, or degenerative or dysplastic lesions.25, 26, 27 Hamartomas are defined as benign tumor-like lesions composed of an overgrowth of mature tissue that normally occurs in the affected part of the body but with disorganization, and often with one element predominating.28
Tumors of odontogenic epithelium with odontogenic mesenchyma have been initially reported as odontomas.29 They have been reported in rats,29 mice,30 prairie dogs,23, 31, 32, 33, 34 and other non-rodent species.28 Odontomas are further classified as compound odontomas and complex odontomas, according to their histologic features and degree of organization.25 All features of normal odontogenesis (ameloblastic epithelium, organized dentin matrix, and enamel matrix formation) are found in these tumors. The compound odontoma (not reported in rodents) is a mass lesion characterized by the presence of numerous fully differentiated, although abnormally shaped, toothlike structures (denticles) originating from within the mass. It is a locally destructive tumor with no metastatic potential.25 Some consider this lesion more as a hamartoma and not a neoplasia.25, 28
The complex odontoma (reported in rodents) is a conglomerate mass lesion with fully differentiated dental components that do not form tooth-like structures.25, 28 Histologically, well-differentiated dentinal tissue, enamel matrix, odontogenic epithelium, and cementum are present. Similar to the compound odontoma, it is locally destructive, has no metastatic potential, and may be considered as a hamartoma rather than a neoplasm.25, 28
An important contribution to the classification and definition of these lesions has been proposed by Boy and Steenkamp.28 Considering the elodont nature of rodent incisor teeth and the debatable hamartomous versus neoplastic nature of odontomas in brachyodont (and anelodont) teeth, they proposed the term elodontoma to replace the term odontoma with respect to hamartomatous jaw lesions in squirrels and similar species with elodont teeth. Elodontoma would therefore be described as a hamartoma of continuously developing odontogenic tissue and alveolar bone at the periapical bud of elodont teeth.28
The odontogenic dysplasia in aging rodents and lagomorphs has been classified as a tumor-like (non-neoplastic) lesion aside tumors of odontogenic origin. It defines the disorganized development and tumor-like dysplasia at the apex of the continuously erupting incisor teeth of rodents and lagomorphs, following inflammation, trauma, toxicosis, or age alone.25 Histology reveals disorganized tissue proliferation and dysplastic differentiation of odontogenic elements, including odontogenic epithelium, enamel matrix, or fully mineralized enamel, dentin, cementum, and dental pulp.
Mature prairie dogs, between 2 and 6 years old, are commonly affected by odontoma-like lesions, usually involving the apex and the reserve crown of incisors, particularly the maxillary incisors.3, 5, 7, 31, 32, 33, 34, 35
This dental abnormality is characterized by abnormal elongation of the reserve crown and the apex. The apical growth causes increased pressure and deformation of the germinal tissue. Apical tooth growth continues, causing primary deformation of the apex and of the reserve crown.3, 6, 7, 35 This is followed by reduced growth rate, and eventually by arrested eruption, whereas irregular production of new dentine continues.3, 7, 35 This results in apical deformity of the apex and part of the reserve crown, and in secondary abnormalities of the surrounding structures (eg, incisive bones, hard palate, and nasal cavities in the case of maxillary incisors). The dental deformity acts as a locally compressive and space-occupying mass, especially with regard to the nasal cavity. Prairie dogs are typically presented with respiratory signs and symptoms.3, 6, 7, 35 Nasal obstruction at different anatomic levels occurs, often resulting in severe respiratory compromise to death in this obligate nasal breathing species.36 Gross anatomy and the longitudinal section of an histology specimen demonstrate folding of new dentine and apex deformity.3, 35 Radiographs show abnormalities consistent with apical dysplasia and display walls of the reserve crowns plicating. Severity can range from mild irregularities due to newly formed folds of dentine (visible in particular at the dorsal border of maxillary incisor teeth), to severe deformity of the reserve crown and apex, with concurrent obstruction of the nasal cavities.3, 5, 7, 35 This dental abnormality may affect 1 or more incisor teeth, even if the clinical impact is much more severe in the case of diseased maxillary incisor teeth, because of the adjacent nasal passages. If the lesions affect the maxillary incisor teeth, they are usually bilateral, even if their severity can be different between the 2 quadrants.
David Crossley,35 therefore, proposed the more appropriate term pseudo-odontoma for this particular dysplastic, non-neoplastic dental disease of prairie dogs.3
The term elodontoma is therefore more specifically a synonym of complex odontoma (locally invasive and destructive lesion with no metastatic potential), not of pseudo-odontoma (locally compressive dysplastic lesion) nor of odontogenic dysplasia, because it does not include dysplastic changes.37 However, the term elodontoma is sometimes used with a broader meaning (either with regard to complex odontoma and pseudo-odontoma/odontogenic dysplasia) rather than as indication of a specific histologic type.37 However, the difference carries important clinical implications when formulating a diagnosis, prognosis, and possible treatment plan.
Elodontomas and pseudo-odontomas have been reported affecting elodont incisor teeth of several rodent species. Dental disease with histologic, clinical, and radiological elements consistent with elodontomas or former odontomas have been reported in the guinea pig,38 degu,39 pet tree squirrels (Paraxerus cepapi),28 rats,29, 40, 41, 42 and mice.30, 43, 44, 45
Dental disease with histologic, clinical, and radiological elements consistent with pseudo-odontomas have been reported in the prairie dog3, 5, 7, 31, 32, 33, 34, 35, 46 (reported also as elodontomas),47 eastern gray squirrel,48 and anecdotally in a chinchilla, a chipmunk,3 and a citellus.37 Apart from Smith and colleagues47 and Capello (when those different abnormalities had not yet been identified and classified),33 elodontoma has not been reported in the prairie dog.
Various etiopathogenetic theories have been proposed for both spontaneously developing and experimentally induced lesions consistent with complex odontomas/elodontomas and pseudo-odontomas. Osteopetrosis is an autosomal recessive disease characterized by abnormal recruitment of osteoclasts. Affected patients may suffer from altered tooth eruption and the formation of odontoma-like masses.29, 30, 42, 45 Repeated trauma or fractures (from chewing on cage bars, falls from a height, or improper trimming of maloccluded incisors) with resultant acquired dental disease, localized infection near the apical tissue of incisors with damage to the continuously developing odontogenic tissue, its follicle, and to the surrounding alveolar bone have also been suggested as a cause of odontomas, pseudo-odontomas, and elodontomas in prairie dogs and other squirrels.3, 7, 28, 32 Traumatic damage to the odontogenic tissue may be so severe that it completely disrupts the epithelial cords creating daughter germ cells, each being capable of continuous development forming its own hard and soft tissue components but in a haphazard manner, leading to the formation of hamartomatous masses.28, 30 Whether pseudo-odontomas represent an early stage potentially progressing to elodontoma or the fact that trauma could potentially result in 2 different pathologic processes cannot be determined at this stage.49
Other proposed causes considered in laboratory rodents include acquired dental disease, aging changes, hypovitaminosis A, hypomagnesemia, hypophysectomy, and toxin exposure.23, 35, 39, 50, 51
Other Oral Disorders
Lesions of oral soft tissues with various degrees of inflammation and infection may be seen when seeds, grass, or hay becomes impacted around teeth or in the cheek pouches.13
Glossitis (“wooden tongue”) due to Actinobacillus israeli infection has been diagnosed in rats. The tongue becomes swollen, hard, and painful. Affected animals deteriorate rapidly because they are unable to eat and drink.13
Sialodacryoadenitis, caused by a highly contagious rat coronavirus, can result in visible swelling under the mandible and neck due to inflammation and edema of the cervical salivary glands. Concurrent lacrimal gland dysfunction may occur, leading to conjunctivitis, keratitis, corneal ulcers, and hyphema. Cervical lymph nodes also may be enlarged, and rhinitis may be present.6
The Syrian hamster is often the preferred species for carcinogenic studies.36, 52, 53 This species can be affected by a broad range of tumors, as frequently reported in the literature and many histologic types have been described. However, spontaneous neoplasia of the oral cavity is rare in this and other rodent species.
Impaction, prolapse, abscessation, and neoplasia of cheek pouches have been described in the golden hamster54 (Fig. 8 ). A spontaneous cheek pouch fibroma has been reported in a Syrian hamster.55
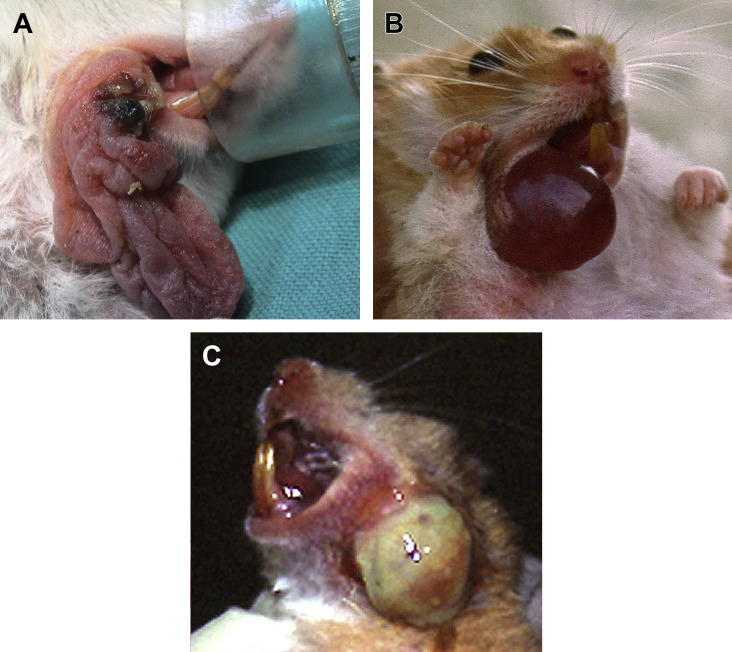
Fig. 8.
Diseases of the cheek pouches. (A) Prolapse of the right cheek pouch in a Russian hamster (Phodopus campbelli). A small area of necrotic tissue is present. (B) Neoplasia of the right cheek pouch in a golden hamster (Mesocricetus auratus) after eversion of the mass. Differential diagnosis with impaction (before eversion, or before emptying the food material) and with edema of the mucosa concurrent with prolapse should be considered. (C) Abundant pus after rupture of an abscess involving the left cheek pouch in a golden hamster.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Adenomas or adenocarcinomas of the Zymbal gland located at the base of the ear and surrounded by the parotid gland in rats may be locally invasive and should be taken into consideration when assessing skull and facial structures in this species.52 Tumors of the Harderian lacrimal gland can result in exophthalmos and porphyrin staining of periocular hair. They tend to be highly invasive in mice, leading to infiltration of bone and other structures of the head and should therefore be taken into consideration in the list of differentials. A maxillary osteosarcoma has been reported in a prairie dog56 and an adenocarcinoma of the buccal salivary gland has been described in a Richardson ground squirrel.57
Electrical injuries are not uncommonly seen in domestic rodents, as a result of chewing on power cables and subsequent direct body contact with an electrical source. The hallmark of electrocution is burn marks generally confined to the sites of body contact with the electrical source, especially the lips, oral commissure, and tongue (Fig. 9 ). Paresthesia and drooling may result.13
Fig. 9.
Skin lesion of the left lips and the labial commissure following an electrocution in a golden hamster.
(Courtesy of Joanna Hedley, DVM, London, UK; with permission.)
Clinical presentation
The acquired dental disease syndrome in pet rodents often results in a complex of clinical signs and symptoms, both primarily related to dental and/or oral problems and/or secondary to complications affecting other organs. Clinical presentation may therefore vary greatly among individual patients depending on concurrent disease.7 However, myomorph and sciuromorph rodent species are rarely presented for symptoms and signs specifically related to dental disease.
Patients are frequently presented for reduced activity and reduced food intake, weight loss, emaciation, and ptyalism often accompanied by moist dermatitis. Grooming difficulties and poor coat quality also may be seen. Change of feeding habits or food preferences may be sometimes noted by the owners.
Overgrowth, malocclusion, or other abnormalities of incisor teeth may be immediately evident on visual inspection in rat-like rodents, but often missed by the owners. Mild to severe malocclusion may be concurrent with slightly elongated or fractured incisors, and maxillary incisors may be curved toward the palate. Secondary lesions of the tongue, lips, and palate may result. A severe complication is represented by perforation of the palate and oro-nasal fistula.3, 19 Facial swelling or ocular or periocular signs may be seen secondary to periapical infections and abscessation of cheek teeth. In rats, infection with sialodacryoadenitis virus should be considered in case of mandibular and neck swellings. Ocular and periocular structures also may be secondarily involved in both situations.
Hamsters may be presented with a large, persistent unilateral or bilateral swelling of the face. This may be seen when the cheek pouch cannot be emptied by the animal, and food remains impacted. The prolapsed mucosa of a cheek pouch, usually unilateral, presents as a pink mass protruding from the mouth and resembling a tumor. Following self-trauma, the edematous everted mucosa may get dry, inflamed, infected, ulcerated, and necrotic. Abscesses of the cheek pouches may be secondary to lesions and infections of soft tissues, or to dental disease.3, 6, 7, 54
Signs and symptoms of dental and oral disorders in captive and free-living squirrels are similar to those encountered in other rodent species6, 58 and may include malocclusion with different types of fractures (especially affecting the incisors); variations in the number, size and shape of teeth; presence of supernumerary teeth; caries; periodontal disease; excessive wearing of cheek teeth in aging patients; periapical infections and abscesses (possibly complicated by osteomyelitis); and traumatic lesions of the oral soft tissues.58, 59, 60
In captive prairie dogs, fractures of incisor teeth are so frequent following repeated trauma due to poor husbandry, that owners may report “regularly shedding teeth.” Fractures may occur either along the clinical crown resulting in visibly shortened tooth or teeth, or along the reserve crown (under the gum line) (Fig. 10 ). In the latter case, the clinical crown may either appear mobile but still attached to the gingiva, or missing. Both lesions are frequently overlooked by owners. In prairie dogs, respiratory signs and symptoms are most common, and are frequently associated with reduced activity, reduced food intake, weight loss, and emaciation.3, 7 Respiratory symptoms may include sneezing, a particular type of snoring (often referred to as “reverse sneezing”), pronounced inspiratory effort, or true dyspnea. They are secondary to the dysplastic changes and apical deformities of diseased maxillary incisors. Because prairie dogs are obligate nasal breathers, these changes may result in reduced nasal air passage to obstruction. A firm swelling of the hard palate caused by the deformed apices may be visible on intraoral inspection of the anesthetized patient3, 7; however, the size of the lesion above the hard palate does not seem to correlate with the degree of respiratory distress.23, 31 Aging patients, usually older than 5 years, also may be presented for reduced food intake, reduced fecal output, and emaciation in the absence of respiratory signs. Underlying medical issues should be ruled out in these cases, but end-stage dental disease with excessive wearing, caries, and fractures causing flattening of cheek teeth crowns, is usually the primary cause of these presenting signs.3, 5, 7
Fig. 10.
Malocclusion of incisor teeth in a prairie dog affected by pseudo-odontoma of the right maxillary incisor tooth. History reported several fractures of the clinical crowns of maxillary cheek teeth. The clinical crown of the right maxillary incisor is missing, and the left is shorter than normal. The clinical crown of the right mandibular incisor is elongated; the left is curved, out of occlusion with the opposite maxillary tooth.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Mandibular odontomas also have been reported causing dysfunction but not respiratory obstruction, as a space-occupying lesion of the oropharynx, which can lead to starvation and death of the affected animal.23, 47
Diagnosis of dental disease and diagnostic imaging
Physical Examination
Patients may be presented with a wide variety of symptoms, either specifically related to dental problems and associated complications, or secondary to involvement of other organ systems. Therefore, a systematic approach is required. Thorough history, including dietary details, feeding habits, and husbandry with regard to cage construction and design, is of utmost importance. However, the absence of a clear history does not rule out underlying dental problems. In selected rodent species or in calm and adequately restrained individuals, a partial physical and dental examination may be possible without anesthesia. Inspection of incisors may be possible in some prairie dogs. The incisor teeth should be examined from the labial and lateral aspect. Evaluation of the facial symmetry and palpation (including the ventral mandibular borders, and the zygomatic and temporomandibular joint areas) may allow identification of irregularities and swellings. When feasible in the awake patient, lateral and horizontal jaw movement should be assessed. Any discomfort or pain should be noted.13 Gentle expression of the cheek pouches is necessary to remove stored food, which may hinder examination. They should be examined fully and not mistaken for a pathologic swelling secondary to impaction, prolapse, or neoplasia. An otoscope with appropriately sized plastic cone, or alternatively nasal or vaginal speculum may be used to examine the oral cavity in rodents. The use of a pediatric laryngoscope for examination of the oral cavity of manually restrained small mammals, including prairie dogs, is reported.61 Eyes, periocular structures, and cheek teeth should be examined as well. Despite the large mouth opening, the oral cavity is small, and the cheek teeth are positioned caudally to the long diastema. Therefore, a complete oral examination may be effectively and safely performed only with the patient under general anesthesia.3
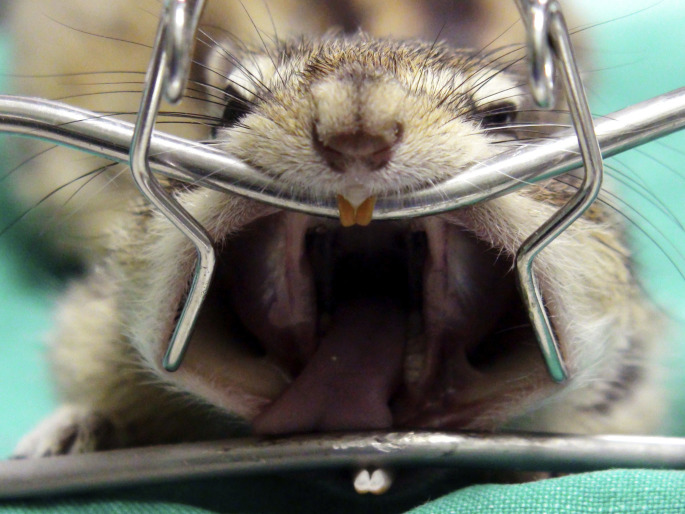
The use of specific dental equipment, such as the rabbit and rodent table retractor/restrainer, mouth gags, cheek dilators, adequate lighting, and magnification, greatly enhance a thorough oral cavity examination3, 5 (Fig. 11 ). Gentle and appropriate manipulation is required during the examination. For example, it is imperative to avoid excessive opening of the oral cavity to prevent damage to the temporomandibular joints.13
Fig. 11.
The rabbit and rodent table retractor/restrainer is a special platform acting as a combined mouth gag and patient positioner. It can be effectively used also for small rodents, such as a chipmunk. In this patient, the cheek dilator is positioned out of the oral cavity to dilate the opening of cheek pouches. The face mask has been temporarily removed for demonstration purpose.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Oral Endoscopy
Oral endoscopy (stomatoscopy) is a relatively simple, noninvasive diagnostic procedure, and should be part of a complete oral examination in rodent species.3, 61, 62, 63 Magnification of dental structures allows a more thorough inspection of the oral cavity, facilitating detection of subtle lesions64 and evaluation of the occlusal surface of the teeth, the mucosal surface of the tongue and hard palate, and the caudal part of the oropharynx. Dental abrasions, fractures, and caries may have fewer symptoms and may be more readily identified on stomatoscopic examination (see Fig. 6). A 1.9-mm or 2.7-mm rigid telescope with a 30° viewing angle are generally used for these procedures, because the 30° angle provides a better view of the occlusal surfaces. Each tooth should be examined individually with the use of a periodontal probe. The gingival sulcus should be probed circumferentially to identify the presence of pockets and their depth; purulent discharge; food or hair impaction; and evidence of decay, caries, and any other abnormality.13 The telescope also can be used periodically to evaluate the intraoperative progress of premolar or molar extractions or to examine the cavity left following extraction.63
Endoscopic inspection of the cheek pouches also should be performed.3
Radiography
A full radiographic assessment should be done in every rodent species to identify dental abnormalities.3 General anesthesia and optimal positioning are necessary to obtain good-quality images of diagnostic value. This may be particularly challenging in very small species, such as myomorphs smaller than rats. Multiple views are necessary for full assessment.65 At least 4 standard views (latero-lateral, 2 obliques, ventrodorsal, or dorsoventral) are recommended.3, 66 Intraoral views may be useful in larger species, such as the prairie dog, to visualize cheek teeth root tips and the apical incisor area.3 Among the many dental abnormalities, it is important to discuss those affecting the incisor teeth in case of pseudo-odontomas of prairie dogs and elodontomas of other squirrels. In case of dysplastic changes, the apex of maxillary incisor teeth appears enlarged. Both the apex and most of the reserve crown have a dorsal irregular margin, displaying newly formed folds of dentine.3, 5, 6, 35, 66 These radiographic findings are best evaluated at lateral or slightly oblique views. Thirty-degree oblique, ventrodorsal, and rostrocaudal views help to assess if the pseudo-odontoma is unilateral or bilateral, and the stage of severity (Fig. 12 ). Detailed diagnosis is critical for prognosis and the treatment plan. The reserve and/or clinical crown is usually shorter than normal, and the occlusal plane is abnormal as well. Pseudo-odontomas affecting the mandibular incisors show similar radiographic findings, although usually less evident. Similar to radiographic findings in cavy-like rodents affected by elodontoma, such as the degu,39 radiography of squirrels shows a large radiopaque mass with increased mineral opacity well beyond the apex and the reserve crown, locally invasive and destructive.28
Fig. 12.
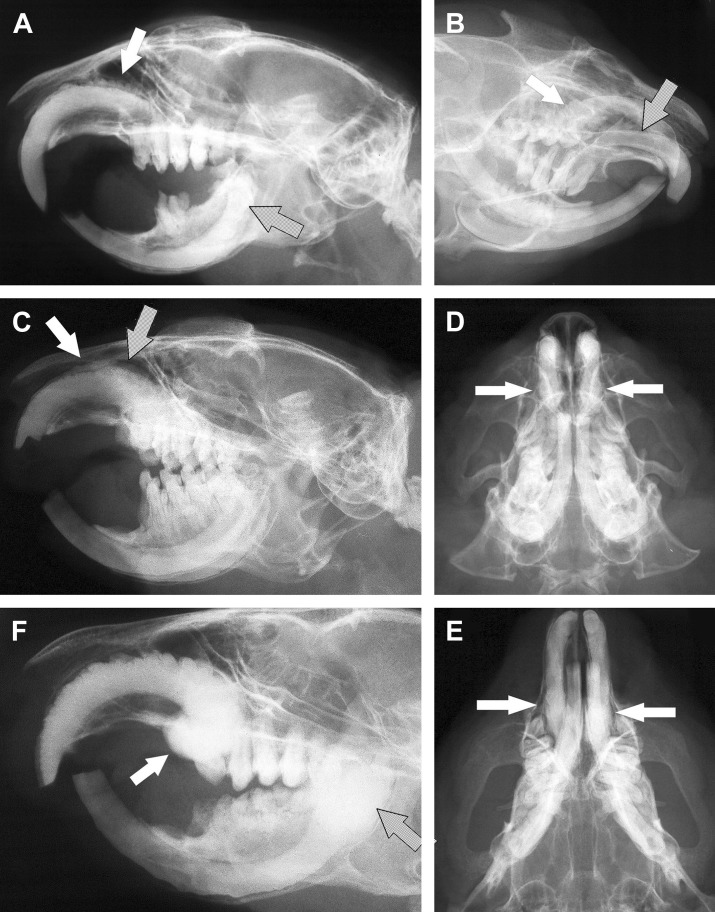
Standard radiography for diagnosis of a maxillary pseudo-odontomas in prairie dogs. (A) Lateral view displaying the irregular dorsal margin of a maxillary incisor tooth (white arrow). Apical deformity is minimal in this patient. The mandibular incisors are affected by similar radiographic abnormalities consistent with pseudo-odontoma (dotted arrow). Abnormal and missing crowns of mandibular cheek teeth are also present. (B) A 30° oblique view is useful to evacuate asymmetric abnormalities. In this patient, the apex of the right maxillary incisor is enlarged (white arrow), whereas the left is normal (dotted arrow). (C) Slight oblique view in a prairie dog affected by bilateral pseudo-odontoma. Obstruction of the dorsal nasal passage (white arrow) and reduced nasal cavity (dotted arrow) are visible. Evident malocclusion of incisor teeth is also present. Rostrocaudal (D) and ventrodorsal (E) views in the same patient displaying bilateral deformity of the apices of maxillary incisor teeth (arrows). (F) Large maxillary pseudo-odontoma in a prairie dog with enlarged and deformed apex (white arrow) and typical folds of dentine visible on the dorsal aspect of the clinical and reserve crown. Severe deformity of the mandibular apices is also present (dotted arrow).
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Computed Tomography
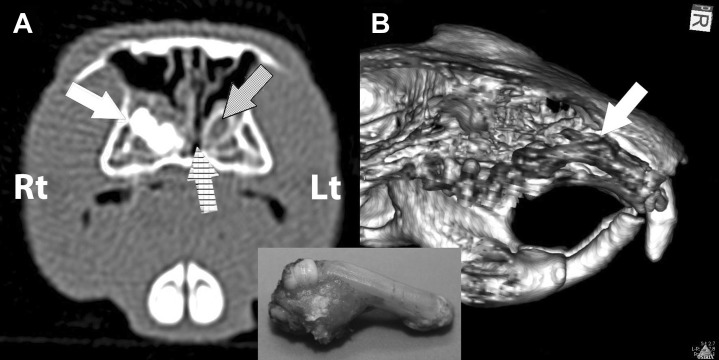
The use of computed tomography (CT) is particularly useful and has great advantages compared with standard radiography. CT overcomes superimposition on a single plane of adjacent and overlying anatomic structures, and 3-dimensional renderings provide excellent details (even in small mammal species) of bone structures in particular. Superior details and dynamic views allow detection of early changes in tooth structure, evaluation of the involvement of surrounding soft tissue, or extension of osteomyelitic foci. CT therefore enhances the level of diagnostic accuracy, providing more accurate and detailed information than traditional radiographic images. These are critical for accurate prognosis, more tailored surgical planning, and successful treatment.67 In prairie dogs affected by pseudo-odontomas, CT is particularly useful to assess the extension of the hard mass, the degree of compression of the nasal cavity, the involvement of surrounding tissues, and the most appropriate surgical approach37 (Fig. 13 ). CT is also particularly useful for assessment of the temporomandibular joint.
Fig. 13.
CT for diagnosis of a maxillary pseudo-odontoma in a prairie dog. (A) The axial view depicts the deformity of the reserve crown of the right maxillary incisor tooth (white arrow), and the compression of the common nasal meatus (chiseled arrow). The pseudo-odontoma is unilateral, facilitating comparison with the transverse section of the normal left maxillary incisor tooth (dotted arrow), and with the left, patent, common nasal meatus. (B) The 3-dimensional volume reconstruction, after cropping part of the right incisive bone, displays the actual shape of the diseased reserve crown and apex (the grey anatomic structure pointed by the white arrow). The CT image compared with the pseudo-odontoma after extraction (inset) is very accurate.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Other Diagnostic Testing
A complete blood count and biochemical panel allows evaluation of the general health status of a patient. Bacterial culture and sensitivity is important in cases of periapical infections and abscessation to allow a more targeted antibiotic treatment. Histopathology can be useful when neoplasia or pseudo-odontoma is suspected.3
Treatment of dental disease and other oral disorders
Prognosis
The prognosis for dental disease in rodents is generally more guarded than in other small mammal species, such as rabbits7; however, it should be formulated for each individual species and case depending on different pathologic patterns. Noncomplicated incisor malocclusion in myomorphs or sciuromorphs usually carries a fair prognosis. When cheek teeth are involved (in particular with the complication of a facial abscess), prognosis is guarded to poor, considering the difficulty carried by any surgical approach in such small patients and the extensive involvement of surrounding anatomic structures.7
Prognosis for pseudo-odontomas in prairie dogs is strictly related to the presence of unilateral or bilateral disease, the stage of disease at the time of diagnosis, and the degree of compromise of the respiratory system. The overall clinical condition needs to be considered, as well as the feasibility to surgically access and remove the compressive mass. Locally destructive elodontomas in other squirrels carry a poor prognosis, and successful treatment, even if conservative, is not reported. End-stage dental disease of cheek teeth also carries a poor medium to long-term prognosis in prairie dogs.
Treatment of Incisor Teeth
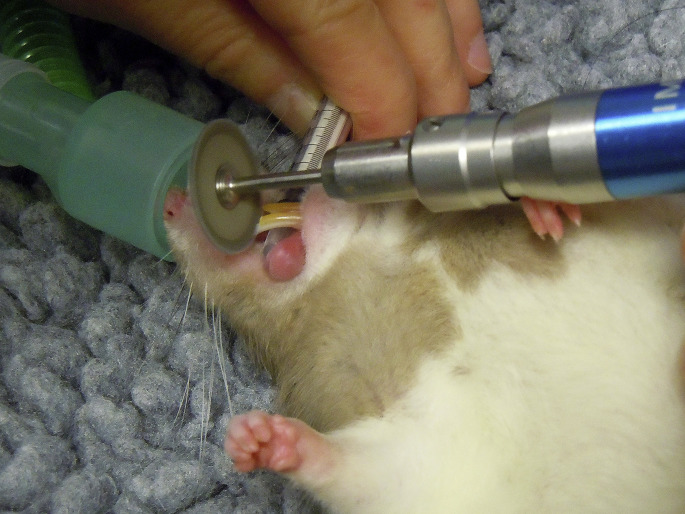
Overgrowth and malocclusion of the incisor teeth is the most common dental disorder of rat-like rodents.6 Regular coronal reduction may be sufficient to restore an adequate occlusal plane and manage early, uncomplicated cases. However, in most cases, this dental procedure must be repeated for the remainder of the patient’s life, unless complications, such as pulp infection or periapical abscessation occur. A cutting burr mounted on a high-speed angled handpiece is used for this procedure. Alternatively, diamond discs mounted on low-speed handpieces are available as well.6 The soft tissues are protected by placing a tongue depressor or a syringe case caudal to the incisors (Fig. 14 ). Nail clippers or cutters are strongly contraindicated, even under anesthesia, because they can create oblique fractures, lead to pulp exposure, damage the periodontal ligament, and cause soft tissue injuries.
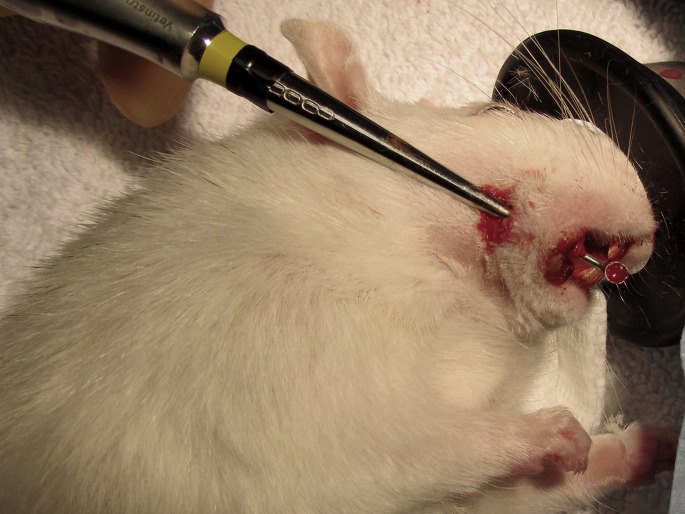
Fig. 14.
Coronal reduction of the right mandibular incisor tooth in a golden hamster, using a diamond cutter disc mounted on a straight handpiece. The tongue and other soft tissues are protected using a plastic syringe. The chisel-shaped occlusal plane may be refined after coronal reduction.
(Courtesy of Elisabetta Mancinelli, DVM.)
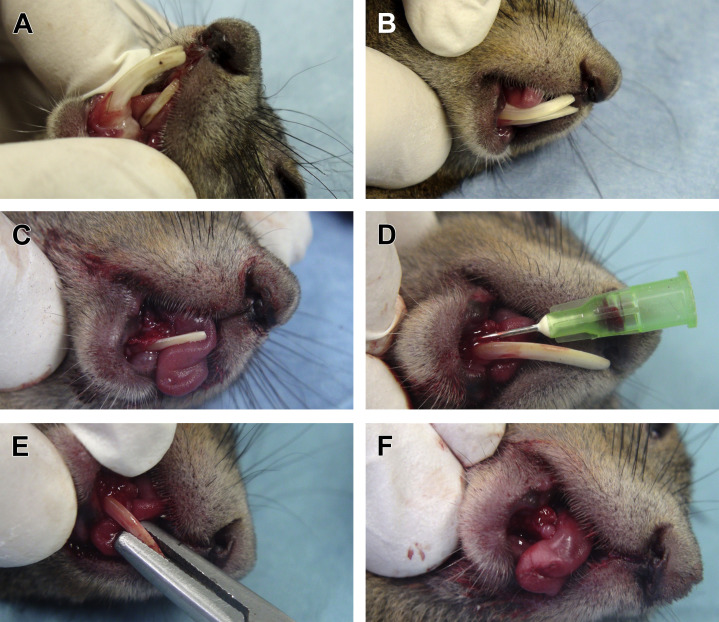
Extraction of incisor teeth can be considered in myomorph and sciuromorph rodents when overgrowth is recurrent and persistent due to severe deviation. Extraction is also an indication for treatment of pseudo-odontomas in prairie dogs. Extraction of incisors does not seem to affect the feeding behavior in captive pets,6 as they can manipulate food with their forearms. However, diet modification by eliminating hard food items should be considered. The procedure can be performed with careful dissection of the periodontal ligament with the same technique described in rabbits by using small dental luxators6 or appropriately contoured hypodermic needles3, 7 (Fig. 15 ). However, this is more intricate and difficult due to the small size of the patient, the dental anatomy of these species (mandibular incisors extend within the mandible below the cheek teeth and the apex is located distal to the last molar in hamsters, rats, and squirrels), and possible complications (namely, fracture of the maxillary incisors, fracture of the mandible, and diastasis of the mobile mandibular synchondrosis/symphysis).7 Radiographs before extraction are recommended to evaluate tooth morphology, curvature, and fracture type, as well as for assessing presence and extension of injuries to the surrounding soft tissues.13 Post-extraction control radiographs are also important to verify complete extraction. Jekl13 recommends coronal reduction of the incisors followed by extraction 5 to 7 days later, in cases of failure of correction and excessive regrowth. Regrowth and eruption of a new tooth is a possible complication; therefore, it is recommended to damage the germinative tissue following extraction.
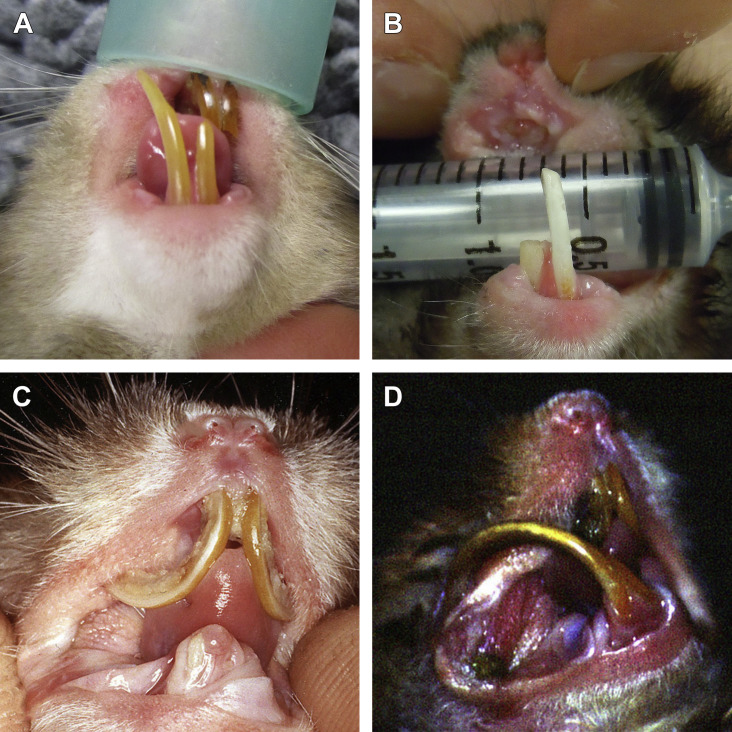
Fig. 15.
Extraction of incisor teeth in a European red squirrel (Sciurus vulgaris), same case as in Fig. 3. (A) Overgrowth and malocclusion of incisor teeth. The nasal wound shown in Fig. 3B healed after conservative coronal reduction of mandibular incisor teeth. (B) The maxillary incisor tooth has been extracted. (C) Appearance of the gingiva after extraction of the right mandibular incisor. (D) Luxation of the left mandibular incisor. A contoured 22-G needle is used on the mesial aspect of the tooth as an elevator. Luxation is performed also on the distal, labial, and lingual aspects of the tooth. (E) When the tooth is after luxation, the clinical crown is grasped with a small needle holder used as an extraction forceps. (F) Appearance of the gingiva after extraction. It may be sutured or left to heal by second intention, in this case.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Treatment of Cheek Teeth
Coronal reduction of anelodont cheek teeth in rat-like and squirrel-like rodents must never be performed, as excessive elongation and malocclusion do not occur in these species.3 Conservative treatment for dental caries and periodontal disease is rarely undertaken in rodent species because of the small size of these patients. When lesions affecting anelodont teeth are extensive or symptomatic, extraction is more appropriate.6
Facial abscesses often cause extensive involvement of surrounding anatomic structures. Treatment is usually very challenging and unrewarding; however, the same surgical principles as in other species (eg, thorough debridement, tooth extraction, marsupialization, daily wound care, antibiotic treatment, analgesia, and supportive care) should be followed. Abscesses will likely recur if the infected tooth is not addressed and removed.68
Treatment of Diseased Cheek Pouches
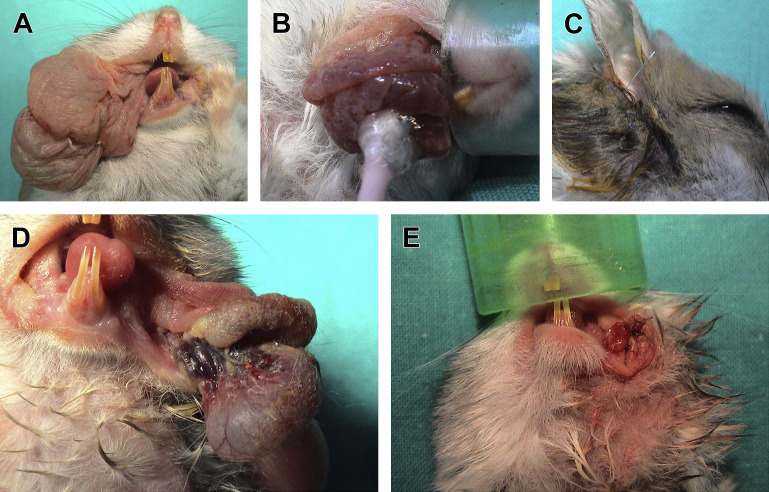
Impaction of cheek pouches should be treated by emptying the contents adhered to the mucosa, and topical application of antiseptic solutions. If the prolapse of a cheek pouch is recent and the mucosal surface is intact, repositioning can be attempted with the patient under general anesthesia (Fig. 16A–C). The mucosa should be rehydrated and lubricated before being replaced. The pouch is repositioned using cotton-tip applicators.69 A single full-thickness percutaneous mattress suture can be placed into the cheek pouch using 4:0 or 5:0 monofilament nonabsorbable suture material to maintain the pouch in its normal position and prevent reoccurrence.54, 68, 69 Amputation of the cheek pouch is indicated in cases of severe mucosal lesions following prolapse, recurrent prolapse, or neoplasia.54, 69 A hemostat is placed at the base of the prolapsed tissue, and the necrotic pouch is resected. The base of the everted pouch is sutured with absorbable material in a simple interrupted pattern (Fig. 16D, E).
Fig. 16.
Treatment of prolapse of cheek pouches in 2 Russian hamsters. Repositioning of the prolapse in a Phodopus campbelli (A–C) and amputation in a Phodopus sungorus (D, E). (A) Prolapse of the left cheek pouch. (B) The prolapse is reduced using a cotton-tip applicator lubricated with lidocaine gel. (C) A percutaneous, nonabsorbable suture prevents the relapse. (D) Amputation of the cheek pouch is indicated. (E) Suture of the mucosa with absorbable suture.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Abscesses are best treated with surgical removal of the infected and/or necrotic tissue followed by thorough flushing, marsupialization, and healing by second intention, rather than with a simple incision.
Treatment of Pseudo-Odontoma
Medical, symptomatic treatment (antibiotics, corticosteroids, and decongestants) aimed at relieving respiratory signs and symptoms, as well as providing supportive feeding for these herbivorous species, is considered of little value for the management of patients with pseudo-odontomas. Instead, it is of paramount importance as adjunctive therapy to dental or surgical treatment.
Various dental and/or surgical approaches and techniques have been described for treatment of this condition in prairie dogs.7, 69 These procedures can be classified into symptomatic or palliative (aimed at restoring patency of the upper airways) and causative or primary (aimed at extracting the diseased teeth). Both groups carry advantages and disadvantages, and at this stage none of them can be considered objectively superior to the other. The choice of causative versus palliative treatment should be based on many individual clinical and practical elements, including detailed diagnosis and degree of severity, unilaterality or bilaterality with regard to the diseased maxillary incisors, patient’s age and overall clinical conditions, patient’s behavior, surgeon’s experience and skills, and owner’s expectations. Palliative treatment even may be considered as a preliminary surgical step to improve the overall condition of the patient before extraction of diseased teeth.
Anesthesia
The anesthetic procedure for treatment of pseudo-odontoma is challenging, especially in patients in which compression and subsequent obstruction of the nasal passages are severe, and respiratory function is compromised. Two surgical aspects to consider before planning an anesthetic protocol are the surgery time (which may vary significantly depending on the kind of treatment and the surgical approach/technique) and anticipated hemorrhage at the maxillary and nasal surgical sites.
The gold standard of anesthetic maintenance for this surgery is orotracheal intubation. The procedure for the orotracheal intubation in the prairie dog has been reported.70, 71 It is challenging due to anatomic features, such as the fleshy tongue and the presence of the oropharyngeal ostium, but may be less difficult with specialized equipment. Alternative techniques to assess patency of upper or lower airways during surgery are nasal intubation via rhinotomy/rhinostomy (which is actually part of the palliative technique), and direct tracheal intubation via tracheotomy (feasible, but more invasive).37, 71 In selected patients with milder respiratory distress, oxygen and inhalant anesthetics delivered via a face mask, preferably coupled with an extranasal approach to the diseased tooth, may be sufficient in cases in which intubation attempts fail. In these circumstances, the surgical procedure time should be as short as possible, and special attention should be paid to hemostasis at the surgical site. Monitoring oxygen saturation via pulse oximetry is mandatory for these surgical procedures.
Palliative procedures
The goal of palliative procedures is restoration of sufficient air flow creating an alternative nasal passage via a stoma on the dorsal aspect of the nasal cavities. Even if this procedure can be combined with extraction procedures, the standard palliative approach is aimed at alleviating the obstruction without extraction of affected tooth/teeth. Reported variations of this permanent rhinostomy technique via a dorsal approach may or may not include the use of a stent.31, 34 The patient is placed in sternal recumbency with the head slightly elevated. The skin is incised over the midline with a linear incision or with a biopsy punch, as the rhinostomy site and the stent opening will be round. Rhinotomy of the nasal bones is performed with a 2.5-mm or 3-mm intramedullary pin, and the nasal cavity is exposed. A small, plastic catheter is cut to proper length and inserted into the nasal cavity, just dorsal to the mass if the goal is to keep the rhinostomy site open,37 or beyond (caudally) the pseudo-odontoma to allow passage of air into the rhinopharynx31, 34 (Fig. 17 ). The use of a metal stent has been reported.46 The stent can be removed or exchanged for a smaller one a few weeks postoperatively to keep the skin from closing over the bony rhinostomy opening. Advantages include short anesthesia and surgery time, simplicity, rapid recovery, and quick improvement of respiratory symptoms. However, it should be noted that this surgical option does not stop the dysplastic process; therefore, the rhinostomy site and/or the stent may eventually no longer be sufficient. Another disadvantage is that postoperative management of the rhinostomy site includes removal of mucus and debris. This may or may not be easy depending on the patient’s temperament.
Fig. 17.
Rhinostomy for palliative treatment of pseudo-odontoma in a prairie dog. (A) The anesthetized prairie dog is placed in sternal recumbency. The area over the nasal bones is surgically prepared and draped with a transparent adhesive drape. A circular portion of the skin is removed using a biopsy punch, and the nasal bone is exposed. A 2.5-mm intramedullary pin is used to drill a small rhinotomy access. (B) The rhinotomy site is then enlarged using a small bur. (C) A plastic stent, consisting a of a 1-mL syringe, is placed within the rhinotomy access and sutured to the skin, to keep the rhinostomy site patent. (D) The plastic stent is removed 10 days after the surgical procedure.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Surgical debulking of the mass also has been reported in conjunction with more invasive surgical approaches.35, 37 Temporary tracheostomy is a viable alternative, although often not successful for long-term management of these cases.23
Causative procedures
Causative dental and surgical techniques instead pursue extraction of the diseased tooth/teeth compressing the upper airways.3, 5, 7, 23, 33, 37, 47, 69, 72, 73
The goal of a primary treatment is extraction of the affected maxillary incisor tooth or teeth. Mandibular incisors can be extracted during the same surgery, or at a later time as a second procedure to decrease initial anesthesia.5
Standard intraoral extraction
The standard technique for extraction is technically similar to that described for rabbits, but it is much more challenging in prairie dogs because of apical deformities and dental ankylosis that are always present to some extent.3, 33, 69 Also, affected teeth may have a very short clinical crown, making the use of extraction forceps difficult. The most frequent complication is fracture of the tooth while attempting removal, which represents a treatment failure.69 Contoured 19-gauge needles are recommended to luxate the incisor teeth. However, if severe deformity and/or ankylosis are present, this technique is not practical and not effective because the reserve crown would invariably fracture.
Transpalatal intraoral approach
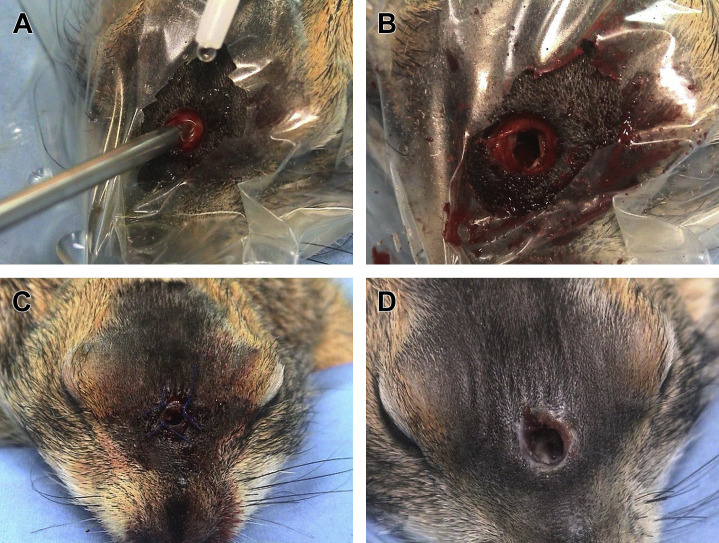
Indications for the transpalatal approach7, 35, 67, 69 are represented by complete fracture of the clinical crown below the gingival level, and in cases in which the apex is deformed with a ventral prominence, causing an evident deformity of the hard palate. With the patient placed in dorsal recumbency and a mouth gag positioned to keep the mouth wide open, an incision is performed over the mucosa of the hard palate. The mucosa is then bluntly dissected free from the underlying hard tissue to expose the ventral aspect of the deformed apex, which is ankylotic with the incisive bone (Fig. 18 ). The apex and the proximal aspect of the reserve crown are then meticulously separated from the ankylotic attachment. Complete extraction or debulking is performed depending on the case. The mucosa is then sutured over the defect.
Fig. 18.
Transpalatal approach for extraction of pseudo-odontoma in a prairie dog. (A) The patient is placed in dorsal recumbency with a mouth gag and a cheek dilator. Direct tracheal intubation via tracheotomy was performed in this case. Note deformity of the hard palate due to the pseudo-odontoma affecting the left maxillary incisor (white arrow). (B) The mucosa has been incised, dissected free, and retracted by a small hemostat to expose the deformed apex of the pseudo-odontoma ankylotic with the incisive bone.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
Rhinotomy extraoral technique
The rhinotomy technique with a dorsal approach has been reported.35, 47, 73
The reserve crowns do not actually perforate the incisive bones penetrating the nasal cavities. However, this approach is effective to access the dorsal aspect of the reserve crown of the maxillary incisor teeth affected by pseudo-odontoma, which protrude making the nasal meatuses much narrower. This technique is also effective to create a temporary or permanent rhinostomy site, usually larger than the palliative rhinostomy. The procedure is performed with the patient in sternal recumbency and the head slightly elevated. After a skin incision on the midline and proper retraction of soft tissues, the rhinotomy is performed by burring the nasal bones. The reserve crowns and the apex of diseased teeth are then manipulated for complete extraction or debulking.
Lateral approach through the incisive bone
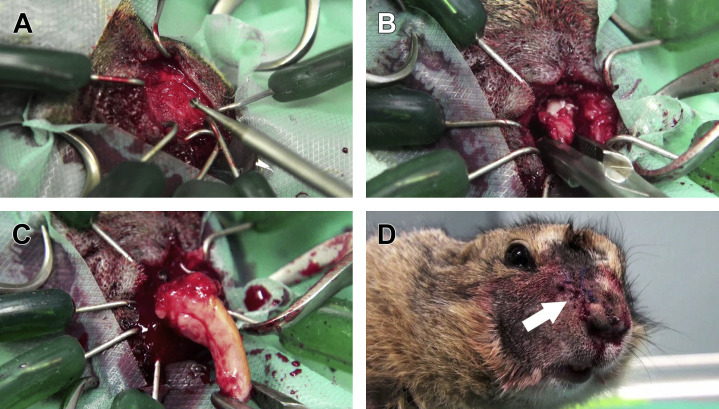
Originally described by one of the authors (V.C),49, 72 this technique is completely extraoral and extranasal, and has the advantage to approach the reserve crown and the apex on their lateral aspect, therefore approaching the “shell” of the pseudo-odontoma, which is the incisive bone (Fig. 19 ). Because the ipsilateral nasal cavity is not entered, excessive bleeding is usually not a concern. The patient is placed in lateral recumbency, and the lateral aspect of the upper lip is shaved, aseptically prepared, and surgically dressed. A skin incision is performed over the incisive bone, and hemostasis is controlled with cotton-tip applicators. Blunt dissection of the underlying soft tissues allows exposure of the lateral aspect of the incisive bone, and a specialized retractor (eg, the Lone Star Retractor; Lone Star Medical Products, Inc. Stafford, TX) is positioned. Osteotomy of the incisive bone is performed with a small 2-mm ball-tipped bur, until the reserve crown of the pseudo-odontoma becomes visible with the typical folds of dentine. The reserve crown is then meticulously worked with small dental elevators or contoured needles, to resolve ankylosis. However, the bone fenestration may not be sufficient to extract the pseudo-odontoma in one whole piece. Therefore, the reserve crown may be intentionally cut to allow retrograde extraction of the rostral portion of the reserve crown and the clinical crown (when present), and the anterograde extraction of the proximal portion of the reserve crown and the deformed apex. The skin is sutured over the site as routine.
Fig. 19.
Extraction of the right maxillary incisor tooth affected by pseudo-odontoma via the lateral approach to the incisive bone. (A) With the patient anesthetized and placed in lateral recumbency, the lateral aspect of the incisive bone is exposed. Osteotomy is performed with a small 2-mm ball-tipped bur to expose the lateral aspect of the reserve crown of the diseased incisive tooth. (B) Extraction of the rostral portion of the reserve crown. (C) Complete extraction of the diseased tooth, including the deformed apex. (D) Suture of the skin (arrow) and postoperative cosmetic appearance of the surgical access.
(Courtesy of Vittorio Capello, DVM, Milano, Italy; with permission.)
The lateral approach also has been reported with the patient in sternal recumbency, creating a bone window between the nasal and the incisive bone.74 However, it reports debulking via extraction only of the apical portion of the pseudo-odontoma, whereas the rostral portion of the reserve crown and the clinical crown remain in place.74
Summary
The order Rodentia is divided into 3 groups based on anatomic and functional differences of the masseter muscle. Myomorph and sciuromorph species have elodont incisors and anelodont cheek teeth, unlike hystrichomorph species, which have full anelodont dentition. Diseases of incisors and cheek teeth of rat-like and squirrel-like rodents result in a wide variety of symptoms and clinical signs. Appropriate diagnostic procedures are essential to a definitive diagnosis, prognosis, and treatment plan. Pseudo-odontomas of prairie dogs are challenging and require surgical expertise for effective treatment.
Footnotes
The authors have nothing to disclose.
References
- 1.Crossley D.A. Clinical aspects of rodent dental anatomy. J Vet Dent. 1991;8:131–134. [PubMed] [Google Scholar]
- 2.Nowak R., Paradiso J. 4th edition. The Johns Hopkins University Press; Baltimore (MD): 1983. Walker’s mammals of the world. [Google Scholar]
- 3.Capello V., Gracis M., Lennox A.M. Zoological Education Network; Lake Worth (FL): 2005. Rabbits and rodents dentistry handbook; pp. 1–274. [Google Scholar]
- 4.Lennox A.M., Bauck L. Sections four: small rodents. Basic anatomy, physiology, husbandry and clinical techniques. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits and rodents clinical medicine and surgery. 3rd edition. Elsevier Saunders; St Louis (MO): 2012. pp. 339–353. [Google Scholar]
- 5.Capello V., Lennox A.M. Section VI: general topics. Small mammal dentistry. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits and rodents clinical medicine and surgery. 3rd edition. Elsevier Saunders; St Louis (MO): 2012. pp. 452–471. [Google Scholar]
- 6.Legendre L.F.J. Oral disorders of exotic rodents. Vet Clin North Am Exot Anim Pract. 2003;6:601–628. doi: 10.1016/S1094-9194(03)00041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capello V. Diagnosis and treatment of dental disease in pet rodents. J Exotic Pet Med. 2008;17(2):114–123. [Google Scholar]
- 8.Chiasson R.B. The phylogenetic significance of rodent cheek pouches. J Mammal. 1954;35:425–427. [Google Scholar]
- 9.Goshal N.G., Bal H.S. Histomorphology of the hamster cheek pouch. Lab Anim. 1990;24:228–233. doi: 10.1258/002367790780866083. [DOI] [PubMed] [Google Scholar]
- 10.Cox P.G., Jeffery N. Reviewing the morphology of the jaw-closing musculature in squirrels, rats, and guinea pigs with contrast-enhanced microCT. Anat Rec. 2011;294:915–928. doi: 10.1002/ar.21381. [DOI] [PubMed] [Google Scholar]
- 11.Cox P.G., Rayfiel E.J., Fagan M.J. Functional evolution of the feeding system in rodents. PLoS One. 2012;7(4):1–11. doi: 10.1371/journal.pone.0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druzinsky R.E. Functional anatomy of incisal biting in Aplodontia rufa and sciuromorph rodents—Part 1: masticatory muscles, skull shape and digging. Cells Tissues Organs. 2010;191:510–522. doi: 10.1159/000284931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jekl V. Rodents: dentistry. In: Keeble E., Meredith A., editors. BSAVA manual of rodents and ferrets. British Small Animal Veterinary Association; Gloucester (United Kingdom): 2009. pp. 86–95. [Google Scholar]
- 14.Yarto-Jaramillo E. Respiratory system anatomy, physiology and disease: guinea pigs and chinchillas. Vet Clin Exot Anim. 2011;14:339–355. doi: 10.1016/j.cvex.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Druzinsky R.E. Functional anatomy of incisal biting in Aplodontia rufa and sciuromorph rodents—Part 2: sciuromorphy is efficacious for production of force at the incisors. Cells Tissues Organs. 2010;192:50–63. doi: 10.1159/000284930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becht G. Comparative biologic-anatomical researches on mastication in some mammals. Proc K Ned Akad Wet C. 1953;56:508–527. [Google Scholar]
- 17.Hiiemae K., Ardran G.M. A cinefluorographic study of mandibular movement during feeding in the rat (Rattus norvegicus) J Zool. 1968;154:139–154. [Google Scholar]
- 18.Reese S., Fehr M. Small mammals. In: Krautwald-Junghanns M.E., Pees M., Reese S., editors. Diagnostic imaging of exotic pets. Schlütersche Verlagsgesellschaft mbH & Co; Hannover (Germany): 2011. pp. 143–307. [Google Scholar]
- 19.Zuri I., Terkel J. Reversed palatal perforation by upper incisors in ageing blind mole-rats (Spalax ehrenbergi) J Anat. 2001;199:591–598. doi: 10.1046/j.1469-7580.2001.19950591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson M., Hart D., Pigott G.H. The effects of diet on the incidence of periodontitis in rats. Lab Anim. 1991;25:247–253. doi: 10.1258/002367791780808374. [DOI] [PubMed] [Google Scholar]
- 21.Vincent A.L., Rodrick G.E., Sodeman W.A., Jr. The pathology of the Mongolian gerbil (Meriones unguiculatus): a review. Lab Anim Sci. 1979;29:645–651. [PubMed] [Google Scholar]
- 22.Yankell S.L. Oral disease in laboratory animals: animal models of human dental disease. In: Harvey C.E., editor. Veterinary dentistry. WB Saunders Co; Philadelphia: 1985. p. 281. [Google Scholar]
- 23.Bennet RA. Odontomas in prairie dogs. In: Proceedings of the North Am Vet Conference. Orlando (FL): 2007. p. 1707–10.
- 24.Losco P.E. Dental dysplasia in rats and mice. Toxicol Pathol. 1995;23(6):677–688. doi: 10.1177/019262339502300605. [DOI] [PubMed] [Google Scholar]
- 25.Head K.W., Cullen J.M., Dubielzig R.R. WHO histological classification of tumors of domestic animals, second series. Armed Forces Institute of Pathology; Washington, DC: 2003. Histological classification of tumors of the alimentary system of domestic animals; pp. 46–57. [Google Scholar]
- 26.Thoma K.H., Goldman H.M. Odontogenic tumors: a classification based on observations of the epithelial, mesemchymal, and mixed varieties. Am J Pathol. 1946;22:443–449. [PubMed] [Google Scholar]
- 27.Walsh K.M., Denholm L.J., Cooper B.J. Epithelial odontogenic tumours in domestic animals. J Comp Pathol. 1987;97:503–521. doi: 10.1016/0021-9975(87)90002-8. [DOI] [PubMed] [Google Scholar]
- 28.Boy S.C., Steenkamp G. Odontoma like tumours of squirrel elodont incisors-elodontomas. J Comp Pathol. 2006;135:56–61. doi: 10.1016/j.jcpa.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Jang D.D., Kim C.K. Spontaneous complex odontoma in Sprague-Dawley rat. J Vet Med Sci. 2002;64(3):289–291. doi: 10.1292/jvms.64.289. [DOI] [PubMed] [Google Scholar]
- 30.Ida-Yonemochi H., Noda T., Shimokawa H. Disturbed tooth eruption in osteopetrotic (op/op) mice: histopathogenesis of tooth malformation and odontomas. J Oral Pathol Med. 2002;31(6):361–373. doi: 10.1034/j.1600-0714.2002.00087.x. [DOI] [PubMed] [Google Scholar]
- 31.Wagner R.A., Garman R.H., Collins B.M. Diagnosing odontomas in prairie dogs. Exotic DVM Magazine. 1999;1(1):7–10. [Google Scholar]
- 32.Phalen D.N., Antinoff N., Fricke M.E. Obstructive respiratory disease in prairie dogs with odontomas. Vet Clin Exot Anim Pract. 2000;3:513–517. doi: 10.1016/s1094-9194(17)30085-3. [DOI] [PubMed] [Google Scholar]
- 33.Capello V. Incisor extraction to resolve clinical signs of odontoma in a prairie dog. Exotic DVM. 2002;4(1):9. [Google Scholar]
- 34.Wagner R., Johnson D. Rhinotomy for treatment of odontoma in prairie dogs. Exotic DVM. 2001;3:29–34. [Google Scholar]
- 35.Crossley D.A. Small mammal dentistry (part I) In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits and rodents clinical medicine and surgery. 2nd edition. Elsevier Saunders; St Louis (MO): 2004. pp. 370–379. [Google Scholar]
- 36.Greenacre C.B. Spontaneous tumours of small mammals. Vet Clin Exot Anim. 2004;7:627–651. doi: 10.1016/j.cvex.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Capello V. Dentistry of prairie dogs and other squirrels. In: American board of veterinary practitioners symposium 2014 proceedings. Nashville (TN): 2014. p. 1–4.
- 38.Capello V., Lennox A.M., Ghisleni G. Elodontoma in two guinea pigs. J Vet Dent. 2015;32(2):111–119. doi: 10.1177/089875641503200205. [DOI] [PubMed] [Google Scholar]
- 39.Jekl V., Hauptman K., Skoric M. Elodontoma in a degus (Octodon degus) J Exotic Pet Med. 2008;17:216–220. [Google Scholar]
- 40.Barbolt T.A., Bhandari J.C. Ameloblastic odontoma in a rat. Lab Anim Sci. 1983;33(6):583–584. [PubMed] [Google Scholar]
- 41.Ernst H., Rittinghausen S., Mohr U. Ameloblastic odontoma in a Sprague-Dawley rat. Dtsch Tierarztl Wochenschr. 1989;96(4):207–209. [PubMed] [Google Scholar]
- 42.Philippart C., Arys A., Dourov N. Experimental odontomas in osteopetrotic op/op rats. J Oral Pathol Med. 1994;23(5):200–204. doi: 10.1111/j.1600-0714.1994.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 43.Dayan D., Waner T., Harmelin A. Bilateral complex odontoma in a Swiss (CD-1) male mouse. Lab Anim. 1994;28(1):90–92. doi: 10.1258/002367794781065898. [DOI] [PubMed] [Google Scholar]
- 44.Nyska A., Waner T., Tal H. Spontaneous ameloblastic fibro-odontoma in a female mouse. Oral Pathol Med. 1991;20(5):250–252. doi: 10.1111/j.1600-0714.1991.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 45.Lu X., Rios H.F., Jiang B. A new osteopetrosis mutant mouse strain (ntl) with odontoma-like proliferations and lack of tooth roots. Eur J Oral Sci. 2009;117(6):625–635. doi: 10.1111/j.1600-0722.2009.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulliot C., Mentré V. Original rhinostomy technique for the treatment of pseudoodontoma in a prairie dog (Cynomys ludovicianus) J Exotic Pet Med. 2013;22:76–81. [Google Scholar]
- 47.Smith M., Dodd J.R., Hobson P. Clinical techniques: Surgical removal of elodontomas in the black tailed prairie dog (Cynomys ludovicianus) and eastern fox squirrel. J Exotic Pet Med. 2013;22:258–264. [Google Scholar]
- 48.Mitchell SC. Rhinotomy for treatment of odontoma in an Eastern gray squirrel. In: AEMV Proceedings. Oakland (CA): 2012. p. 71.
- 49.Capello V. Odontomas in rodents: surgical treatment of pseudo-odontomas in prairie dogs. In: AEMV Proceedings. Oakland (CA): 2012. p. 105–9.
- 50.Slootweg P.J., Kuijpers M.H.M., van de Kooij A.J. Rat odontogenic tumors associated with disturbed tooth eruption. J Oral Pathol Med. 1996;25:481–483. doi: 10.1111/j.1600-0714.1996.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 51.Berman J.J., Rice J.M. Odontogenic tumours produced in Fischer rats by a single intraportal injection of methylnitrosourea. Arch Oral Biol. 1980;25:213–220. doi: 10.1016/0003-9969(80)90025-4. [DOI] [PubMed] [Google Scholar]
- 52.Percy D.H., Bathold S.W. Blackwell Publishing; Oxford (United Kingdom): 2007. Pathology of laboratory rodents and rabbits; pp. 3–309. [Google Scholar]
- 53.Harness J.E., Wagner J.E. Specific diseases and conditions. In: Harness J.E., Wagner J.E., editors. The biology and medicine of rabbits and rodents. 4th edition. Williams ad Wilkins; Philadelphia: 1995. pp. 171–322. [Google Scholar]
- 54.Capello V. Surgical techniques in pet hamsters. Exotic DVM. 2003;5(3):32–37. [Google Scholar]
- 55.West W.L., Gaillard E.T., O'Connor S.A. Fibroma (myxoma) molle in a hamster (Mesocricetus auratus) Contemp Top Lab Anim Sci. 2001;40(6):32–34. [PubMed] [Google Scholar]
- 56.Mouser P., Cole A., Lin T.L. Maxillary osteosarcoma in a prairie dog (Cynomys ludovicianus) J Vet Diagn Invest. 2006;18:310–312. doi: 10.1177/104063870601800317. [DOI] [PubMed] [Google Scholar]
- 57.Yamate J., Yamamoto E., Nabe M. Spontaneous adenocarcinoma immunoreactive to cyclooxygenase-2 and transforming growth factor-beta1 in the buccal salivary gland of a Richardson's ground squirrel (Spermophilus richardsonii) Exp Anim. 2007;56(5):379–384. doi: 10.1538/expanim.56.379. [DOI] [PubMed] [Google Scholar]
- 58.Sainsbury A.W., Kountouri A., DuBoulay G. Oral disease in free-living red squirrels (Sciurus vulgaris) in the United Kingdom. J Wildl Dis. 2004;40(2):185–196. doi: 10.7589/0090-3558-40.2.185. [DOI] [PubMed] [Google Scholar]
- 59.Miles A.E.W., Grigson C. Cambridge University Press; Cambridge (United Kingdom): 1990. Colyer’s variation and diseases of teeth of animals; p. 672. [Google Scholar]
- 60.Wiggs R.B., Lobprise H.B. Dental and oral disease in rodents and lagomorphs. In: Wiggs R.B., Lobprise H.B., editors. Veterinary dentistry principles and practice. Lippincott-Raven; Philadelphia: 1997. pp. 518–537. [Google Scholar]
- 61.Jekl V., Knotek Z. Evaluation of a laryngoscope and a rigid endoscope for the examination of the oral cavity of small mammals. Vet Rec. 2007;160:9–13. doi: 10.1136/vr.160.1.9. [DOI] [PubMed] [Google Scholar]
- 62.Taylor M. Endoscopy as an aid to the examination and treatment of the oropharyngeal disease of small herbivorous mammals. Semin Avian Exot Pet. 1999;8:139–141. [Google Scholar]
- 63.Hernandez-Divers S.J. Exotic mammal diagnostic endoscopy and endosurgery. Vet Clin Exot Anim. 2010;13:255–272. doi: 10.1016/j.cvex.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez-Divers S.J. Clinical technique: dental endoscopy of rabbits and rodents. J Exotic Pet Med. 2008;17:87–92. [Google Scholar]
- 65.Silverman S., Tell L.A. Elsevier Saunders; St. Louis (MO): 2005. Radiology of rodents, rabbits and ferrets. An atlas of oral anatomy and positioning. [Google Scholar]
- 66.Capello V., Lennox A.M. Wiley Blackwell; Ames (IA): 2008. Clinical radiology of exotic companion mammals; pp. 1–486. [Google Scholar]
- 67.Capello V., Lennox A.M., Widmer W.R. The basic of radiology. In: Capello V., Lennox A.M., editors. Clinical radiology of exotic companion mammals. Wiley-Blackwell; Ames (IA): 2008. pp. 2–51. [Google Scholar]
- 68.Bennet R.A. Soft tissue surgery. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits and rodents clinical medicine and surgery. 3rd edition. Elsevier Saunders; St Louis (MO): 2012. pp. 373–391. [Google Scholar]
- 69.Capello V. Common surgical procedures in pet rodents. J Exotic Pet Med. 2011;20(4):294–307. [Google Scholar]
- 70.Johnson D.H. Over-the-endoscope endotracheal intubation of small exotic mammals. Exotic DVM. 2005;7(2):18–23. [Google Scholar]
- 71.Capello V. Intubation techniques for exotic companion mammals. In: Proceedings of the North Am Vet Conference. Orlando (FL): 2009. p. 1829–31.
- 72.Capello V. We are not smaller rabbits, either. Dentistry of other rodent species and ferrets. In: Proceedings of the North Am Vet Conference. Orlando (FL): 2011. p. 1681–4.
- 73.Johnson DH. Rhinotomy approach for the removal of pseudo-odontoma in sciuromorphs. In: Proceedings of the AEMV Conference. Indianapolis (IN): 2013. p. 42.
- 74.Pelizzone I., Vitolo G.D., D’Acierno M. Lateral approach for excision of maxillary incisor pseudo-odontoma in prairie dogs (Cynomys ludovicianus) In Vivo. 2016;30:61–68. [PubMed] [Google Scholar]