Abstract
The immune spectrum of severe acute respiratory syndrome (SARS) is poorly understood.
To define the dynamics of the immune spectrum in SARS, serum levels of cytokines, chemokines, immunoglobulins, complement and specific antibodies against SARS-associated coronavirus (SARS-CoV) were assayed by enzyme-linked immunosorbent assay (ELISA), and phenotypes of peripheral lymphocytes were analyzed by flow cytometry in 95 SARS-infected patients. Results showed that interleukin (IL)-10 and transforming growth factor β (TGF-β) were continuously up-regulated during the entirety of SARS. Regulated on activation normally T cell-expressed and secreted (RANTES) levels were decreased, while monocyte chemoattractant protein-1 (MCP-1) was elevated in acute patients. Immunoglobulins and complement were elevated during the first month of SARS. Both serum-positive rates and titers of specific IgM and IgG antibodies responding to SARS-CoV peaked at days 41–60 from the onset of SARS. CD4+ and CD8+ T lymphocytes decreased significantly in acute-phase. CD3+CD8+CD45RO+ T lymphocytes were decreased by 36.78% in the convalescent patients. Conclusion: SARS-CoV seemed to elicit effective humoral immunity but inhibited cellular immunity, especially CD8+ memory T lymphocytes over time. Prolonged overproduction of IL-10 and TGF-β may play an important role in the disease.
Keywords: Severe acute respiratory syndrome, Immune monitoring, Immune system
Abbreviations: ALT, aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; C, complement; CoV, coronavirus; ctr, control; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; IFN-γ, interferon γ; Ig, immunoglobulin; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor κB; PBMC, peripheral blood mononuclear cell; RANTES, regulated on activation normally T cell-expressed and secreted; SARS, severe acute respiratory syndrome; SARS-CoV, SARS-associated coronavirus; S.D., standard deviation; TGF, transforming growth factor; TNF, tumor necrosis factor
1. Introduction
Severe acute respiratory syndrome (SARS) is caused by SARS-associated coronavirus (SARS-CoV) [1], [2]. It is known that human coronaviruses usually infect the upper respiratory tract and cause the common cold [3], whereas SARS-CoV infects the lower respiratory tract, leading to pulmonary destruction [4]. Although antibody induction and lymphopenic responses to SARS-CoV have been briefly described elsewhere [5], the precise immune and inflammatory responses following SARS-CoV infection remain unclear. Moreover, the rapidly reported results by other authors deal merely with one or two aspects of anti-viral immunity, i.e. either antibody induction, changes in T lymphocytes or alteration of cytokines. The initial studies showed that not only lung but also immune cells were targets of SARS-CoV [4]. What then, is the overall immune spectrum of SARS: the profile of humoral and cellular immunity and their importance in SARS; whether cytokines and chemokines play a role in pathogenesis of SARS; the status of immune memory function of lymphocytes in SARS? It is particularly important to explore a full, informative description of the immune response and pathogenesis in SARS. This will greatly help us in understanding the pathogenic mechanisms, as well as improving patient management and developing a vaccine to completely control SARS epidemics.
The panel of cytokines (Th1 cytokines interferon γ (IFN-γ), tumor necrosis factor (TNF)-a, interleukin (IL)-2 and IL-12; Th2 cytokines IL-4, IL-6 and IL-10) reflects the overall balance within the immune system; chemokines function briefly in inflammatory processes, acting as regulatory bridge molecules between innate and acquired immunity. The complement system is an important component of innate immunity and major anti-viral effectors. Besides the soluble mediators mentioned above, lymphocytes, especially T and B lymphocytes, play a central role in specific anti-viral immunity for clearing the virus. We thus decided to define the immune response profile, focusing mainly on cytokine/chemokine balance and lymphocyte subtypes to give an overview of the immune spectrum against SARS-CoV.
Therefore, we characterized systemically the spectrum of immune and inflammatory responses in 95 SARS-infected healthcare workers. Our results indicate that SARS-CoV seem to elicit effective humoral immunity but inhibit cellular immunity. An imbalance of Th2 over Th1 immunity, i.e. prolonged overproduction of IL-10 and transforming growth factor β (TGF-β), may play an important role in the disease. These observations are hypothesized to create an imbalance in immune function that could be associated with SARS pathogenesis, through direct destruction of lymphocytes by SARS or indirectly through impairment of cellular immunity by SARS-induced humoral mediators.
2. Materials and methods
2.1. Patients and clinical features
From February 1 to March 9, 2003, we identified 95 hospital-contact-exposed healthcare workers (nurses, physicians, radiologists, clerical staff, trainees and paramedics) who participated in caring for other SARS patients in our region. whose disease met the case definition of SARS (revised by the Chinese Ministry of Health on April 14, 2003) at the Second Affiliated Hospital of Sun Yat-sen University, in Guangzhou. The 95 patients were enrolled in the study. All had a definite close-contact history with a person who was a suspect, and alanine aminotransferase (ALT), aspartate aminotransferase (AST) and serum creatinine levels were elevated significantly within 10–14 days from onset of SARS, as described previously [6]. Approximately 10% of the patients suffered from hypoxemia. There was a 3–7 days (3.6 ± 1.5) latency period prior to the onset of symptoms. All 95 patients developed fever (temperature > 38 for more than 24 h), and 25 of them had rigor (Table 1 ). About 30% of patients presented with dyspnea, pleurisy and myalgia. Over 80% of the patients reported a productive cough and malaise. Mild leukopenia and thrombocytopenia were observed at initial presentation in about 30% of the patients. Within 1 week of onset of SARS, significant leukopenia (decreased to 3.4 × 109 per l) and thrombocytopenia (decreased to 159.0 × 109 per l) were observed. ALT was present in most cases, which is similar to previous reports [7], [8]. Three patients were treated with mechanical ventilation. After empirical treatment with anti-viral regimens, gamma globulin and/or corticosteroids, all of these patients survived to recover from all symptoms. Most of these patients have good prognoses, and no sequelae have been observed to date.
Table 1.
Characteristics of the patients on presentation*
| Characteristic | No. (%) of individuals |
|---|---|
| Sex | |
| Male | 19 (20) |
| Female | 76 (80) |
| Age | 28.7 ± 9.5 |
| Major symptoms | |
| Fever (temperature > 38 C for 24 h) | 95 (100) |
| Rigor | 25 (26.3) |
| Cough | 95 (100) |
| Sputum production | 84.21 (80) |
| Dyspnea | 30 (31.6) |
| Pleurisy | 28 (29.5) |
| Malaise | 95 (100) |
| Myalgia | 32 (33.7) |
| Physical signs | |
| Crackles | 34 (35.8) |
| Percussion dullness | 88 (92.7) |
| Red blood cell count (×1012 per l) | 4.38 ± 0.5 |
| Hemoglobin (g/dl) | 129.3 ± 16.8 |
| White blood cell count (×109 per l) | 5.6 ± 2.1 |
| Lymphocyte (%) | 13.9 ± 13.5 |
| Platelet count (×109 per l) | 176.6 ± 72.6 |
| ALT (U/l) | 14.6 ± 6.0 |
| AST (U/l) | 22.7 ± 5.8 |
| Creatinine (μmol/l) | 79.2 ± 13.2 |
| Oxygen saturation on room air (%) | 97 ± 2.3 |
* Plus-minus values are mean ± S.D. Normal ranges are as follows: for red blood cell count, 3.5 × 1012 to 5.5 × 1012 per l; for hemoglobin, 110–160 g/dl; for white blood cell count, 4 × 109 to 10 × 109 per l; for lymphocyte percentage, 20–40%; for platelet, 100 × 109 to 300 × 109 per l; for ALT, 5–40 U/l; for AST, 5–40 U/l; for creatinine, 44–133 μmol/l.
Control subjects were 60 healthy adult volunteers (male = 13, 21.6%; female = 47, 78.4%) with mean age 29.5 years old, sampled in a time sequence similar to that of the study subjects. Informed consent was obtained from the patients. The institutional review board and research ethics board of each participating institution approved this study.
2.2. Samples
Blood samples from the patients were consecutively collected on the day of admission, during hospitalization and during the convalescent phase of the disease. The acute-phase was defined as the period between the onset of the disease and the time when the patient became clinically stable, and the convalescent phase was defined as the period from release from the hospital and soon after. Peripheral blood mononuclear cells (PBMCs) from convalescent patients were isolated using Hypaque–Ficoll (Promega) and frozen in liquid nitrogen in 5% (v/v) plus 95% (v/v) autologous serum.
2.3. Cytokine and chemokine determination
Serum concentrations of cytokines, including interleukins (IL-2, IL-4, IL-10), IFN-γ, TNF-β and TGF-β, and chemokines, including regulated on activation normally T cell-expressed and secreted (RANTES) and monocyte chemoattractant protein-1 (MCP-1), were measured using the quantitative sandwich enzyme immunoassay technique (ELISA kit, R&D system, Minneapolis, MN). The 96-well polystyrene microplate was pre-coated with recombinant human TGF-β soluble receptor type II or murine monoclonal antibody against IL-2, IL-4, IL-10, IFN-γ, TNF-β, RANTES or MCP-1. Briefly, 50 or 100 μl of assay diluent was added to each well, as specified. Then, 50, 100 or 200 μl of the serum sample and standard or control buffer were added. After 2 h incubation at room temperature, the plate was aspirated and washed three or four times. Two hundred micoliters of conjugate was added, and the incubation/wash was repeated. Then, 200 μl of substrate solution was added, and the plate was incubated at room temperature for 20 or 30 min. Finally, 50 μl of stop solution was added and the optical density (OD) was read within 30 min on a microplate reader (CliniBio 128c, Austria) at 450 nm with reference wavelengths set to 550 and 620 nm.
2.4. Serological assays
General humoral immune parameters (IgA, IgG, IgM, C3, C4) were evaluated from serum samples using a Beckman ARRAY 360 System (Beckman Coulter, Galway, Ireland).
Specific IgM and IgG antibodies responding to SARS-CoV were measured by enzyme-linked immunosorbent assay (ELISA) using the SARS antibody assay kit developed by the Chinese Academy of Military Sciences and Chinese Academy of Medicine (Huada Co. Ltd., Beijing). The 96-well polystyrene microplate was pre-coated with lysates of SARS-CoVs and SARS-CoV-infected Vero-E6 cells. Briefly, 100 μl of assay diluent was added to each well. Then, 10 μl of serum sample and standard or control buffer were added. After incubation for 30 min at 37 °C, the plate was aspirated and washed five times. One hundred microliters of conjugated murine anti-human IgG was added, and the plate was then incubated for 20 min at 37 °C and washed five times. Then, 50 μl of substrate solution A and 50 μl of substrate solution B were sequentially added and the plate was incubated at room temperature for 10 min. Finally, 50 μl of stop solution was added and OD was read within 30 min on a microplate reader (CliniBio 128c, Austria) at 450 nm with reference wavelengths set to 630 nm.
2.5. Flow cytometry analysis
Acute-phase flow assay was performed on a real-time sample. Heparinized peripheral blood collected from the acute-phase patients underwent red blood cell lysis using Tris–HCl–NH4Cl (pH 7.56), washing with PBS and labeling with FITC-, APC- and/or PE-conjugated antibodies (BD & Pharmingen). Convalescent-phase flow assay was performed in frozen samples. The recovered PBMCs from patients and control subjects were cultured overnight at 37 °C in RPMI-1640 (Gibco BRL) supplemented with 5% human AB type serum and 40 U/ml gentamicin (Gibco BRL) and labeled with FITC-, APC- and/or PE-conjugated murine anti-human monoclonal antibodies. The CD3, CD3CD4, CD3CD8, CD3CD4CD45RACD45RO, CD3CD8CD45RACD45RO and CD19CD45RACD45RO phenotypes of lymphocytes were sequentially analyzed by flow cytometry (FACScalibur, 4 color system, BD Biosciences, CA, US).
2.6. Data management and statistics
Data are given as mean ± standard deviation (S.D.) unless otherwise specified. The results were compared using Student's t-test, Spearman's correlation test or analysis of variance (ANOVA) using SPSS11.0 software. P < 0.05 (bilateral) was considered statistically significant.
3. Results
3.1. Cytokine and chemokine determination
Overall, IL-10 and TGF-βwere continuously overproduced for the entire course of SARS infection, indicating a Th2 cytokine response.
3.1.1. Th1 cytokines
Compared to the normal controls, IL-2 levels of the SARS patients did not change significantly within the first 2 months, but decreased during months 3–5 (Fig. 1 A, P = 0.02, correlation coefficient = 0.176, one-way ANOVA; minimum detectable dose < 7 pg/ml). Notably, during months 4–5, IL-2 levels decreased to their lowest point of 11.71 pg/ml, less than 1/3 that of the normal control levels (P = 0.001, one-way ANOVA). IFN-γ was not altered at either acute or convalescent phases compared with the normal control group (Fig. 1B, minimum detectable dose < 8 pg/ml). TNF-β significantly decreased within the first 2 weeks and again from months 1–2 (Fig. 1E, P = 0.032, and 0.031, respectively; one-way ANOVA; minimum detectable dose < 16 pg/ml), then gradually returned to normal levels over the next 2 months.
Fig. 1.
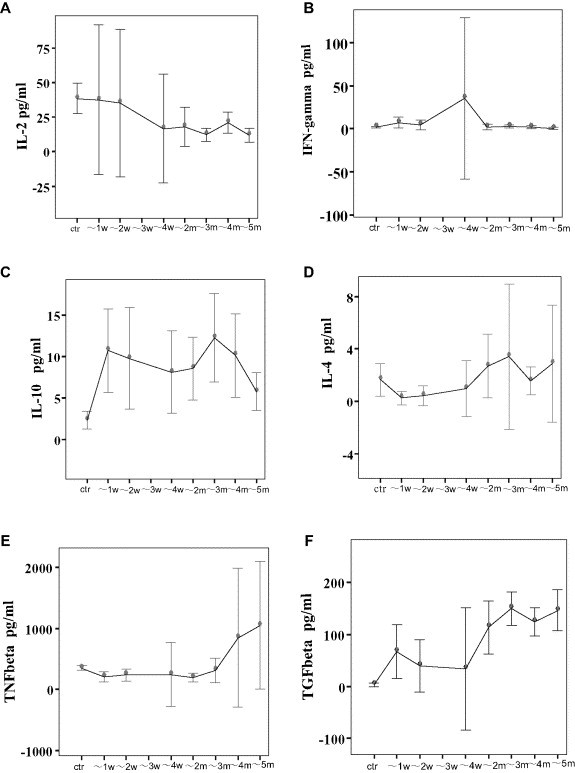
Profiles of Th1/Th2 cytokines and pro-inflammatory /immunosuppressive cytokines in 95 healthcare workers with SARS, as assayed by ELISA. Serum levels of IFN-γ showed no significant change. IL-2 levels did not change significantly within the first 2 months but decreased during months 3–5. Expression of TNF-β was downregulated within week 1 and again during month 2. IL-10 and TGF-β were continuously overproduced for the entire course of SARS infection.
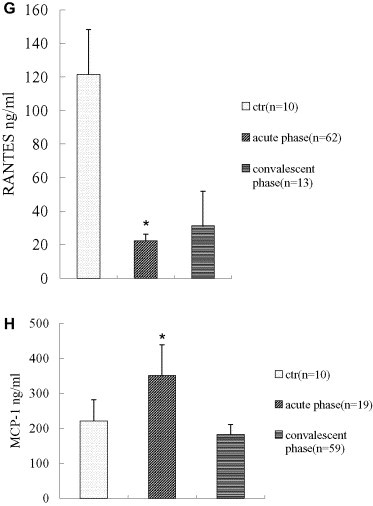
A–F, cytokine expression profiles in patients with SARS: A, IL-2; B, IFN-γ; C, IL-10; D, IL-4; E, TNF-β; F, TGF-β. G, RANTES levels in patients at acute and convalescent phases; H, MCP-1 levels in patients at acute and convalescent phases. “w” = week, “ctr” = control, and “m” = month. *: P < 0.05, one-way ANOVA).
3.1.2. Th2 cytokines
IL-10 levels in the serum of SARS patients were continuously elevated for the 5 months observed in this study (2.47–4.57 times that of normal control levels; Fig. 1C, P < 0.001, correlation coefficient = 0.514, one-way ANOVA; minimum detectable dose < 3.9 pg/ml). Serum IL-4 levels were undetectable in both patients and healthy volunteers (Fig. 1D, minimum detectable dose < 10 pg/ml). TGF-β increased in SARS patients throughout the test period, particularly during month 2 (Fig. 1F, P < 0.001, correlation coefficient = 0.610, one-way ANOVA, minimum detectable dose < 7 pg/ml).
The observed changes in cytokine levels were not correlated to administration of glucocorticoids (P all > 0.05; Spearmen's correlation analysis).
3.1.3. RANTES and MCP-1 expression in acute patients
The two β chemotactic peptides were assayed by ELISA in the patients and the concurrent controls, which showed that the levels of RANTES were decreased in both acute and convalescent-phase patients (Fig. 1G; minimum detectable dose < 2 pg/ml), whereas those of MCP-1 were elevated by around 50% in acute patients but returned to normal levels in the convalescent patients (Fig. 1H; minimum detectable dose < 10 pg/ml).
3.2. Flow cytometry analysis
There was no significant difference in percentages of T lymphocytes in acute-phase SARS patients as compared to healthy controls (P all > 0.30, one-way ANOVA), which was different from other reports [7], [8], [9], [10]. However, percentages of CD4+ and CD8+ T lymphocytes decreased significantly (P all < 0.001, one-way ANOVA), paralleling the results reported elsewhere [7], [8], [9], [10]. The observed decrease in CD4+ T cell percentages in SARS patients was statistically correlated with steroid use (correlation coefficient = 0.372, P < 0.001, Spearman's correlation analysis), but change in CD8+ T lymphocyte percentage was not (correlation coefficient = 0.125, P = 0.401, Spearman's correlation analysis). There was no significant change in ratios of CD4/CD8 in acute-phase patients as compared to healthy controls (P all > 0.90, one-way ANOVA) (Fig. 2 ).
Fig. 2.
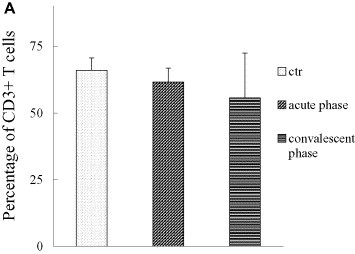
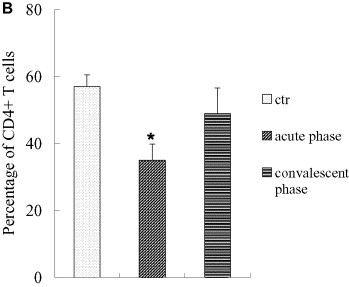
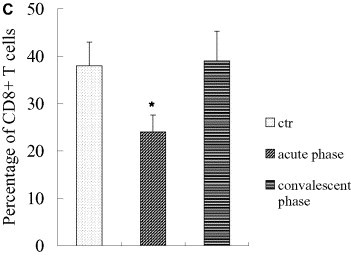
Percentages of CD3+ of total numbers of PBMCs, percentages of CD4+, CD8+ lymphocytes of total numbers of CD3+ T cells, and CD4/CD8 ratios in acute-phase SARS patients. Percentages of T lymphocytes did not change significantly in acute-phase (P all > 0.30, one-way ANOVA), whereas percentages of CD4+ and CD8+ T lymphocytes decreased significantly (*: vs. the control and convalescent groups, P all < 0.001, one-way ANOVA). Alteration in CD4+ T cell percentage correlated statistically with steroid use (correlation coefficient =–0.372, P < 0.001, Spearman's correlation analysis), whereas CD8+ T lymphocytes did not (correlation coefficient =–0.125, P = 0.401, Spearman's correlation analysis). (A, percentages of T lymphocytes in acute patients; B, percentages of CD4+ T lymphocytes in acute-phase patients; C, percentages of CD8+ T lymphocytes in acute-phase patients. *: P < 0.05, one-way ANOVA). The data were collected under the conditions indicated in Section 2.5, Flow cytometry analisis.
There were also no significant changes in amounts of total T lymphocytes, CD4+ or CD8+ T lymphocytes in PBMCs isolated from the convalescent patients compared with healthy controls (P all > 0.05, Fig. 3 , Table 2 ). Similarly, expression of CD45RA on CD19+ B lymphocytes was not substantially altered in convalescent patients compared with healthy controls. CD8+ naïve T (CD3+CD8+CD45RA+) lymphocytes were increased by 72.40% in convalescent patients (P < 0.001) and CD4+ naïve T (CD3+CD8+CD45RA+) lymphocytes by 26.70% compared to that of the normal controls, but the latter was not statistically significant (P = 0.051). CD3+CD8+ memory T (CD3+CD8+CD45RO+) lymphocytes were decreased by 36.78% (P = 0.040) and CD3+CD4+ memory T (CD3+CD4+CD45RO+) lymphocytes by 19.65% in convalescent patients, compared with healthy controls, but the latter was not significant (P = 0.062; Table 2). The observed alterations in CD3+CD8+ naïve T cell and CD3+CD8+ memory T cell percentages were not correlated with use of corticosteroids (correlation coefficient =–0.448 and 0.253, P = 0.055 and 0.296, respectively; Spearman's correlation analysis).
Fig. 3.
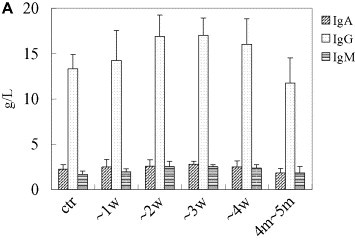
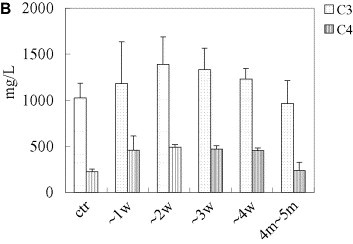
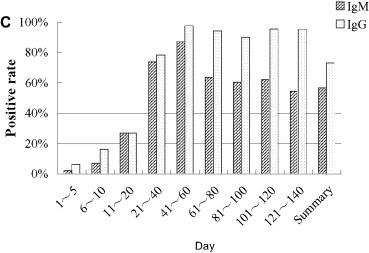
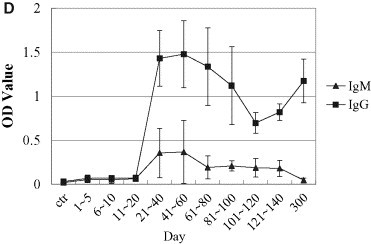
Spectrum of nonspecific and specific humoral immunity in patients with SARS. General humoral immune parameters (IgA, IgG, IgM, C3, C4) were evaluated automatically from serum samples using Beckman ARRAY 360 System. In specific-antibody ELISA assay, the 96-well polystyrene microplate was pre-coated with lysates of SARS-CoVs and SARS-CoV infected Vero-E6 cells, sera were 10× diluted, and conjugated murine anti-human IgG was used as the second antibody. Levels of IgA, IgG, IgM, C3, and C4, κ light chain were notably elevated during the first month of SARS infection and then decreased to normal levels within 2 months thereafter. Both the seropositive rate and the titer of specific IgM and IgG antibodies responding to SARS-CoV increased, peaking 3 weeks after onset of SARS. (A, IgA, IgG and IgM; B, C3 and C4; C, positive rates of specific IgG and IgM responding to SARS-CoV in patients with SARS; D, optic density of specific IgG and IgM responding to SARS-CoV in patients with SARS. “w” = week, “ctr” = control and “m” = month, “OD” = optical density).
Table 2.
Percentages of cell phenotypes of PBMCs collected from convalescent patients with SARS
| CD markers | Controls |
Patients |
Rates of change (%) | F | P | ||
|---|---|---|---|---|---|---|---|
| Percentage | n | Percentage | n | ||||
| CD3+ | 51.35 ± 15.15 | 13 | 55.55 ± 15.80 | 20 | 8.10 | 0.575 | 0.454 |
| CD3+CD4+ | 24.02 ± 7.30 | 13 | 26.46 ± 11.33 | 19 | 10.10 | 0.469 | 0.499 |
| CD3+CD8+ | 17.06 ± 11.59 | 13 | 18.35 ± 8.80 | 19 | 7.50 | 0.128 | 0.723 |
| CD3+CD4+CD45RA+ | 32.40 ± 10.78 | 13 | 41.07 ± 12.65 | 20 | 26.70 | 4.136 | 0.051 |
| CD3+CD4+CD45RO+ | 48.10 ± 11.67 | 13 | 38.65 ± 14.85 | 20 | –19.60 | 3.751 | 0.062 |
| CD3+CD8+CD45RA+ | 37.48 ± 18.62 | 13 | 64.62 ± 15.02 | 20 | 72.40 | 21.297 | <0.001 |
| CD3+CD8+CD45RO+ | 33.03 ± 20.35 | 13 | 20.88 ± 12.22 | 20 | –36.78 | 4.619 | 0.040 |
| CD19+CD45RA+ | 93.70 ± 5.70 | 9 | 94.68 ± 3.00 | 9 | 1.00 | 0.208 | 0.654 |
Note: CD3+CD8+CD45RA+ T lymphocytes increased, while CD3+CD8+CD45RO+ T lymphocytes decreased in patients with SARS, compared with those of normal controls (P all < 0.05, one-way ANOVA). However, there was no considerable change in CD3+, CD3+CD4+, CD3+CD8+, CD3+CD4+CD45RA+, CD3+CD4+CD45RO+, and CD19+CD45RA+ cell numbers in the PBMCs from the convalescent patients with SARS by flow cytometry (P all > 0.05, one-way ANOVA). Rates of change (%) in column 6 stand for alteration of percentage in the patients compared with that of normal controls.
3.3. Humoral immune item assay (serological assay)
All five tested general humoral immune parameters (IgA, IgG, IgM, C3 and C4) notably increased within one month of disease onset, peaked at week 3, and then decreased gradually to normal levels within 2 months. The 1-month increases were statistically significant (P all < 0.05, Fig. 3A, B).
Seropositive rates of specific IgM and IgG antibodies responding to SARS-CoV increased in the 95 SARS patients. From days 1 to 5, the rates increased from 2.04% to 6.12%, then continued to increase to the highest levels of 87.5% and 97.7% at days 41 and 60 (Fig. 3C). By days 121–140, the specific IgM-positive rate had dropped to 54.5%, whereas the IgG-positive rate remained stable at around 96.5%. Similarly to the dynamics of the positive rates, the titers of specific IgM and IgG were elevated along with the disease development, as demonstrated by elevated optical densities of 0.055 and 0.069 at days 1 and 5, and peaks of 0.366 and 1.477 at days 41 and 60', respectively, with control OD value of 0.030 in healthy controls. From day 60 on, the titers of these two specific antibodies decreased, eventually dropping to approximately half of the peak values (Fig. 3D).
4. Discussion
In this study, we found that infection with SARS-CoV triggered vigorous immune disturbances characterized by the following: (1) typical anti-viral nonspecific and specific humoral responses; (2) perturbation of cytokine and chemokine levels; and (3) severe impairment of cellular immunity, including lymphopenia in acute phase and loss of CD8+ memory T cells in convalescent phase.
In the acute-phase SARS patients, the serum levels of IL-2 and IFN-γ did not change significantly, but levels of TNF-β decreased, and IL-10 and TGF-β levels increased significantly, as shown in Fig. 1, suggesting that a complex, predominantly Th2-associated pattern was triggered by SARS-CoV infection during the acute-phase. This differs from the results of previous reports, which found a Th1 pattern response [10], [11], [12]. The reason for this difference is unknown. Interestingly, this Th2 pattern was not statistically correlated with administration of steroids, although steroids can inhibit cytokine production. Also, it could not be due to use of ribavirin, because ribavirin switch cytokine response from a Th2 pattern to a Th1 one [13] and there was no evidence of this observed in this study. Thus, the difference may be due to variations in the study population, genetic heterogeneity of the SARS-CoV strains and discrepancies in the applied analytic methods. Previous work has shown that cytoplasmic parasites can directly activate nuclear factor-kappa B (NF-κB) pathway [14]. And another recent report showed that the SARS E2-spike protein contains a superantigen domain (residues 690–1050) that mimics the T-cell receptor α–βV chain protein [15]. Moreover, the entry of SARS-CoV into lymphocytes has been well established [16]. Therefore, we postulate that cytoplasmic SARS-CoVs could possibly activate Th2 cytokine release. It is well-known that Th2 immune response inhibits cellular immunity but facilitates induction of humoral immunity to help clear persistent viral infections [17]. So, this Th2 immune response may benefit the elimination of persistent extracellular coronavirus particles.
The combination of ribavirin and corticosteroid is empirical therapy in SARS. Administration of steroids in SARS is believed to be based on the following: (1) viruses (including coronaviruses) can induce IFN-γ production and elicit an immune response mainly through the Th1 pathway [18], [19]; (2) massive release of IFN-γ and TNF-α contribute to the pathogenesis of SARS; (3) steroids are able to antagonize the action of these pro-inflammatory cytokines [10], [11], [20]. However, steroid therapy is ineffective in acute respiratory distress syndrome [21] and doubtful in SARS, but has considerable side effects, including avascular necrosis of bone, opportunistic infection, hepatic damage and metabolism disturbance [22], [23], [24], [25], [26], [27]. Unlike other coronavirus [19], SARS presented a Th2 dominance instead of exacerbated immune responses in this study. This might be one of the reasons explaining the ineffectiveness of steroid therapy in SARS. Th2 dominance also suggests that steroid therapy may be unnecessary or should be modulated at acute-phase so as not to adversely affect the clearance of intracellular SARS-CoV. Future studies directed at improving immune function of the patients to restore the Th1/Th2 balance and developing more effective anti-viral therapy to control replication of SARS-CoV are needed.
Chemokines play an essential role in the development of inflammation in response to viral infection [26], and acute respiratory viruses commonly induce inflammatory chemokines, which can amplify inflammatory responses, leading to immunopathology [28]. Since β-chemokines MCP-1 and RANTES predominantly attract monocytes [29], elevation of MCP-1 observed in acute-phase patients most likely reflects the potential role of monocytes in the pathogenesis of SARS. As CD8+ and CD4+ T cells as well as monocytes and macrophages produce RANTES [30], and acute-phase patients experienced a severe decrease in CD4+ and CD8+ T cells, impairment of these two subtypes of T cells may account for the observed downregulation of RANTES in acute-phase. But the reason for and significance of downregulation of RANTES in convalescent patients is unclear, because CD4+ and CD8+ T cells were not decreased in this phase.
Differentiation of naïve T cells into virus-specific CD8+ T cells is critical for the induction of an effective anti-viral immune response [31]. However, the SARS patients in this study showed remarkable decreases in CD4+ and CD8+ T cells at acute-phase, comparable with those found in previous reports [7], [8], [9]. It is supposed that this phenomenon is related to either (1) viral infection of lymphocytes [7], [8], [9], [10], [11]; (2) apoptosis of lymphocytes [4]; (3) humoral mediators, such as cytokines [17]; (4) downregulation of lymphocyte differentiation [11]; or use of cortisol [25]. In this study, the decrease in CD4+ but not CD8+ T cells was definitely related to the administration of steroids. For this reason, the changes in these two subsets of T cells could not fully be explained by the use of cortisol. In addition, we also found significant elevation of IL-10 and TGF-β in patients at acute-phase. These two cytokines are potent downregulators of the cell-mediated immune response [32], [33], and TGF-βwas shown to be involved in pulmonary fibrosis, which is common in SARS [33]. Thus, the overexpression of these two cytokines in this study strongly suggests that they are involved in mediating lymphocyte damage in SARS.
Interpreted in conjunction with the impairment of CD4+ and CD8+ T cells, Th2 predominance and changes in RANTES and MCP-1 indicate that (1) the patients suffered severe immune damage, especially T cell immunity; (2) imbalanced chemokine and cytokine levels may be involved in the pathogenesis of SARS at acute-phase.
Viral infections usually stimulate T lymphocytes, leading to rapid increases in CD8+ T lymphocytes [34]. But in SARS, CD45RO-positive T lymphocytes in circulation were decreased to 6–7% in acute-phase, as demonstrated by immunoelectron microscopy [35]. This raised the concern about the effect of SARS-CoV infection on immune memory of the patients. To observe immune memory function in convalescent patients, we measured peripheral memory T cells. To our surprise, SARS patients also experienced selective damage of peripheral CD8+ memory lymphocytes, and this phenomenon was not statistically correlated with induction of glucocorticoid therapy, even though steroids can induce inhibition of memory T cell development [36], [37]. Moreover, not all of the lymphopenia can be ascribed to exogenous steroids [38]. Taken together, it may suggest that the dramatic reduction in CD8+ memory T cells is caused by other mechanisms. Considerable data have shown that maintenance of memory T cells is fundamentally regulated by cytokines, and Th2 dominance is associated with increased apoptosis of CD45RO+ memory T cells [20], [37]. Thus, we hypothesize that selective damage of cellular immunity, especially the unusual loss of CD8+ memory lymphocytes, is much more likely to result from the prolonged overexpression of IL-10 and TGF-β.
Protective memory CD8+ T cells are crucial for anti-viral immunity [39]. Our study implies that in the case of another SARS-CoV attack, the immune system in these patients may be unable to effectively respond to the pathogen. Therefore, these patients could be re-infected by SARS-CoV. For this reason, immune enhancement measures may be beneficial for the patients. Also, further studies defining the differentiation and functioning of SARS-CoV-infected lymphocytes are needed to clarify whether the changes in T cell subsets at follow-up indicate enhanced SARS-CoV progression after initial infection.
Recent research has demonstrated that the signals triggered in infection/vaccination determine the extent and nature of memory cell development [40]. Taking the above into consideration, it is possible that entry of SARS-CoV into lymphocytes is able to stimulate cytokine imbalances and consequently cause damage of cellular immunity, presumably in an apoptotic manner [20]. Admittedly, SARS-specific T cells would be the most representative parameter of the precise T cell immunity against SARS-CoV. It is necessary to follow up the SARS patients for a longer time to investigate their immune function and to characterize the specific CD8+ T cells of these patients.
Fortunately, SARS-CoV infection did not induce depletion of B lymphocytes and was able to efficiently trigger substantial non-specific and specific humoral immunity in this cohort study, indicative of preservation of B cell function. Recently, neutralizing antibodies against SARS-CoV generated from immunization of mice with either subunit DNA vaccine or SARS-CoVs was shown to induce protective immunity in animals [41], suggesting the possibility and rationale of development of SARS-CoV vaccines that are able to induce neutralizing antibodies.
SARS-CoV is a novel coronavirus and its biology is not fully understood to date. Our findings suggest that SARS-CoV infection can elicit general and specific humoral immunity but may deplete cellular immunity, and overexpression of IL-10 and TGF-β may be responsible for the immunopathy of SARS. This study will provide the basis for a better understanding of the biological features of SARS-CoV, which will be valuable for patient management and design of effective vaccines against SARS-CoV.
Acknowledgements
Financial support: funding from Chinese Ministry of Education (No. 200364), National Basic Research Program of China (2003CB 514107) and Guangdong Provincial Fund of SARS (2003Z3-E0451). We thank Xu Ben-Ling and Xiao Lin for their assistance in data collection and management.
Dr. Jia-Ling Huang, Jian Huang and Zhao-Hui Duan contributed equally to this article
Footnotes
Financial support: Fund from Chinese Ministry of Education, National Basic Research Program of China (2003CB 514107) and Guangdong Provincial Fund of SARS.
References
- 1.Summary table of SARS cases by country, 1 November 2002–7 August 2003. (http//www.who.int/csr/sars/country/2003_08_15/en/).
- 2.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., De Risi J., Yang J.Y., Cox N., Hughes J.M., Le Duc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. New Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. New Engl. J. Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 6.Ooi C.G., Khong P.L., Ho J.C., Lam B., Wong W.M., Yiu W.C., Wong P.C., Wong C.F., Lai K.N., Tsang K.W. Severe acute respiratory syndrome: relationship between radiologic and clinical parameters. Radiology. 2003;229:492–499. doi: 10.1148/radiol.2292030736. [DOI] [PubMed] [Google Scholar]
- 7.Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K., Chan P.K., Ng M.H., Yu L.M., Hui D.S., Tam J.S., Cheng G., Sung J.J. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Br. Med. J. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui W., Fan Y., Wu W., Zhang F., Wang J.Y., Ni A.P. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2003;37:857–859. doi: 10.1086/378587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Jia W.D., Xu A. Measurement of subgroups of peripheral blood T lymphocytes in patients with severe acute respiratory syndrome and its clinical significance. Chin. Med. J. (Engl.) 2003;116:827–830. [PubMed] [Google Scholar]
- 10.Li Z., Guo X., Hao W., Wu Y., Ji Y., Zhao Y., Liu F., Xie X. The relationship between serum interleukins and T-lymphocyte subsets in patients with severe acute respiratory syndrome. Chin. Med. J. (Engl.) 2003;116:981–984. [PubMed] [Google Scholar]
- 11.Nicholls J.M., Poon L.L., Lee K C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:9370–9376. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H., Lit L.C., Hui D.S., Chan M.H., Chung S.S., Sung J.J. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ning Q., Brown D., Parodo J., Cattral M., Gorczynski R., Cole E., Fung L., Ding J.W., Liu M.F., Rotstein O., Phillips M.J., Levy G. Ribavirin inhibits viral-induced macrophage production of TNF, IL1, the procoagulant fgl2 prothrombinase and preserves Th1 but inhibits Th2 cytokine response. J. Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 14.Palmer G.H., Machado T., Jr., Fernandez P., Heussler V., Perinat T., Dobbelaere D.A. Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection. Proc. Natl. Acad. Sci. USA. 1997;94:12527–12532. doi: 10.1073/pnas.94.23.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee R.E. SARS E2-spike protein contains a superantigen between residues 690 through 1050. J. O. I. M. R. 2003;1:3–8. [Google Scholar]
- 16.National Research Project for SARS. Beijing Group Changes in monocyte counts and expression of mCD14 and HLA-DR in the peripheral blood of patients with severe acute respiratory syndrome. Chin. Med. J. (Engl.) 2004;117:624–626. [PubMed] [Google Scholar]
- 17.Karupiah G. Type 1 and type 2 cytokines in antiviral defense. Vet. Immunol. Immunopathol. 1998;63:105–109. doi: 10.1016/s0165-2427(98)00086-5. [DOI] [PubMed] [Google Scholar]
- 18.Perlman S. Pathogenesis of coronavirus-induced infections, Review of pathological and immunological aspects. Adv. Exp. Med. Biol. 1998;440:503–513. [PubMed] [Google Scholar]
- 19.Glass W.G., Rosenberg H.F., Murphy P.M. Chemokine regulation of inflammation during acute viral infection. Curr. Opin. Allergy Clin. Immunol. 2003;3:467–473. doi: 10.1097/00130832-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Annunziato F., Galli G., Nappi F., Cosmi L., Manetti R., Maggi E., Ensoli B., Romagnani S. Limited expression of R5-tropic HIV-1 in CCR5-positive type 1-polarized T cells explained by their ability to produce RANTES, MIP-1alpha, and MIP-1beta. Blood. 2000;95:1167–1174. [PubMed] [Google Scholar]
- 21.Thompson B.T. Glucocorticoids and acute lung injury. Crit. Care Med. 2003;31:S253–S257. doi: 10.1097/01.CCM.0000057900.19201.55. [DOI] [PubMed] [Google Scholar]
- 22.Hong N., Du X.K. A vascular necrosis of bone in severe acute respiratory syndrome. Clin. Radiol. 2004;59:602–608. doi: 10.1016/j.crad.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cyranoski D. Critics slam treatment for SARS as ineffective and perhaps dangerous. Nature. 2003;423:4. doi: 10.1038/423004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G.W. Be alert of the risk of glucocorticoid-induced diabetes in the management of severe acute respiratory syndrome. Chin. J. Endocronol. Metab. 2004;20:1–2. [Google Scholar]
- 25.Panesar N.S., Lam C.W.K., Chan M.H.M., Wong C.K., Sung J.J.Y. Lymphopenia and neutrophilia in SARS are related to the prevailing serem cortisol. Eur. J. Clin. Invest. 2004;34:382–384. doi: 10.1111/j.1365-2362.2004.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oba Y. The use of corticosteroids in SARS. New Engl. J. Med. 2003;348:2034–2035. doi: 10.1056/NEJM200305153482017. [DOI] [PubMed] [Google Scholar]
- 27.Panesar N.S. Lymphopenia in SARS. Lancet. 2003;361:1985. doi: 10.1016/S0140-6736(03)13557-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Multz A.S., Cohen R. Systemic response to pneumonia in the critically ill patient. Semin. Respir. Infect. 2003;18:68–71. [PubMed] [Google Scholar]
- 29.Wu X.M., Wang X.Y., Chen P.H. Potential role of cytokines in idiopathic pulmonary fibrosis. Chin. Crit. Med. 2003;15:362–364. [PubMed] [Google Scholar]
- 30.Kottilil S., Chun T.W., Moir S., Liu S., McLaughlin M., Hallahan C.W., Maldarelli F., Corey L., Fauci A.S. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 2003;187:1038–1045. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 31.Hackney K., Cavanagh D., Kaiser P., Britton P. In vitro and in vivo expression of chicken gamma interferon by a defective RNA of avian coronavirus infectious bronchitis virus. J. Virol. 2003;77:5694–5702. doi: 10.1128/JVI.77.10.5694-5702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunne P.J., Faint J.M., Gudgeon N.H., Fletcher J.M., Plunkett F.J., Soares M.V., Hislop A.D., Annels N.E., Rickinson A.B., Salmon M., Akbar A.N. Epstein–Barr virus-specific CD8+ T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–940. doi: 10.1182/blood-2002-01-0160. 9. [DOI] [PubMed] [Google Scholar]
- 33.Nijhuis E.W., Hinloopen B., van Lier R.A., Nagelkerken L. Differential sensitivity of human naive and memory CD4+ T cells for dexamethasone. Int. Immunol. 1995;7:591–595. doi: 10.1093/intimm/7.4.591. [DOI] [PubMed] [Google Scholar]
- 34.Mor F., Cohen I.R. IL-2 rescues antigen-specific T cells from radiation or dexamethasone-induced apoptosis. Correlation with induction of Bcl-2. J. Immunol. 1996;156:515–522. [PubMed] [Google Scholar]
- 35.Zhong Y.F., Gao X.M., Wang S.L., Xie Z.G., Ma Y., Fang W.G., Zou W.Z. Pathologic study of circulating blood leukocytes in severe acute respiratory syndrome. Chin. J. Med. 2003;83:2137–2141. [PubMed] [Google Scholar]
- 36.Bellinghausen I., Brand U., Steinbrink K., Enk A.H., Knop J., Saloga J. Inhibition of human allergic T-cell responses by IL-10-treated dendritic cells: differences from hydrocortisone-treated dendritic cells. J. Allergy Clin. Immunol. 2001;108:242–249. doi: 10.1067/mai.2001.117177. [DOI] [PubMed] [Google Scholar]
- 37.Tripp R.A. Role of cytokines in the development and maintenance of memory T cells during respiratory viral infection. Curr. Pharm. Des. 2003;9:51–59. doi: 10.2174/1381612033392521. [DOI] [PubMed] [Google Scholar]
- 38.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. J. Am Med. Assoc. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 39.Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed alphabeta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swain S.L. Regulation of the generation and maintenance of T-cell memory: a direct, default pathway from effectors to memory cells. Microbes Infect. 2003;5:213–219. doi: 10.1016/s1286-4579(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]


