Summary
Adrenal infections are an important but under-recognized clinical entity. The adrenal gland can be infected by a myriad of pathogens including fungi, viruses, parasites, and bacteria. Infection can directly or indirectly cause tissue damage and alteration in endocrine function. Direct damage occurs via microbial replication and local production of toxic compounds, such as endotoxins. Indirect damage results from alterations in the regulation of a host's immunologic and endocrine mediators in response to damage by a microbe at a distant site. Variations in pathogen tropism, adrenal anatomy, and host immune integrity contribute to the progression of active disease and discernable adrenal dysfunction. Early recognition and intervention in the case of adrenal infection can significantly improve outcome, demonstrating the need for increased clinical suspicion in the appropriate clinical setting.
Keywords: Adrenal gland, Adrenal insufficiency, Adrenal infection, Adrenalitis
Introduction
Infections of the adrenal gland are an important yet relatively uncommonly recognized clinical entity. Although autoimmune destruction represents the primary cause of adrenal dysfunction in developed countries (80–90%), infectious etiologies represent the major cause of Addison's disease in the developing world, with Mycobacterium tuberculosis being the most common causative agent.1 The adrenal gland can be directly infected by various microbial pathogens, including a diverse array of viruses, fungi, and bacteria.1
Immunocompromised individuals are at greatest risk for either primary adrenal infection or disseminated microbial disease involving the adrenal gland. However, numerous case reports describe individuals with objectively normal immune function who nonetheless have clinical or pathological evidence of primary adrenal involvement in the setting of an indolent infection.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Due to the non-specific nature of the symptomatology and laboratory manifestations (e.g., fatigue, anorexia, low blood pressure, hyperkalemia, hypernatremia, hypoglycemia1) and the fact that 80–90% of the gland must be destroyed before the patient will demonstrate overt Addisonian symptoms,17 antemortem diagnosis is rare. However, prompt recognition combined with appropriate therapy early in the disease course may prove beneficial,13, 15, 16, 18, 19, 20 thereby warranting a high clinical suspicion for adrenal involvement in the appropriate clinical scenario.
Adrenal dysfunction can also occur in response to infections at distant sites or in disseminated disease due to alterations in the host's physiochemical milieu.14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 It is generally understood that individuals who are responding to the stress of an illness (e.g., a systemic infection) will manifest an increase in the functioning of the hypothalamo-pituitary–adrenal axis (HPA-axis) leading to an overall increase in systemic corticosteroid levels. Increased cortisol secretion has been shown to shift the balance in the Th1/Th2 cell ratio towards a Th2 response.21, 33 This shift, along with age-related changes in dehydroepiandrosterone (DHEA), is thought to enhance the infectivity of M. tuberculosis (where a Th1 dominated response is considered to be protective) and a cycle of positive immunological alterations is induced to the benefit of the bacteria. In addition to the up-regulation of the HPA-axis to secondary infection, endotoxin and exotoxins can induce both functional and pathological changes to the adrenal gland.32, 34
In addition to M. tuberculosis, hypercortisolism, both endogenous or due to the administration of high dose corticosteroids, is associated with a significant increased risk for infection with Nocardia asteroides, Aspergillus spp, Cryptococcus neoformans, and Pneumocystis (carinii) jirovecii.35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 Additional organisms associated with disease in the setting of high systemic cortisol levels include Strongyloides stercoralis,49, 50 Staphylococcus aureus,41, 51 Histoplasma capsulatum,52 and mycobacterial disease, such as M. tuberculosis 53, 54 or Mycobacterium chelonae.55 Importantly, the signs and symptoms of disease can be obscured by the hypercortisolism. The increased incidence of serious infections in the setting of hypercortisolism is due to the complex deregulation of the normal inflammatory response, such as alterations in cytokine production, decreased migration of effector cells to sites of inflammation, and impaired cytotoxic functions (reviewed in56), resulting in an inability of the host to effectively combat invading pathogens.
In glandular infection, variations of the particular tropism of the implicated agent determine the differential rates of adrenal involvement from organism to organism regardless of host immune integrity (this is however to be distinguished from glandular destruction). For example, autopsy studies have shown that adrenal involvement occurs in 85–90%16, 57 of infections due to Paracoccidioides brasiliensis, a dimorphic fungus, regardless of host immune status. However, clinical symptomatology and overt adrenal dysfunction were found in only 14–20%16, 58 of individuals with P. brasiliensis adrenal infection, suggesting a possible variation in either the virulence and destructivity of the organism or, potentially, in host immune responses. The interaction between an endogenous host response and microbial growth and/or antigen production influences the destructive capabilities and disease manifestations of a particular microorganism.59
Pathological effects of infection
Microbes affect the adrenal glands either by direct invasion of tissue substrate or via secondary mediators through downstream pathways and biochemical modulators. The interplay of endotoxin produced locally or from a distant source and adrenocorticotrophin (ACTH) set up conditions that predispose the adrenal gland to hemorrhage and, potentially, the Waterhouse–Friderichsen syndrome. The Waterhouse–Friderichsen syndrome represents a paradigm for the interrelationship of host and microbe within the adrenal gland with the disease being characterized by acute adrenal gland insufficiency and profound shock. The syndrome is typically attributed to infection with Neisseria meningitidis,18 but it may occur in fulminant infections with other microbes, including smallpox.60 In his pioneering clinical and laboratory work, Friderichsen described the Waterhouse–Friderichsen syndrome in cases due to N. meningitidis, Streptococcus spp, Haemophilus influenzae, Corynebacterium diphtheriae, and Staphylococcus aureus, and he was able to experimentally induce adrenal hemorrhage in guinea pigs by injections of Bacillus anthracis, Clostridium tetani, Streptococcus spp and several Gram-negative bacilli.61
The anatomical distribution of vessels to the adrenal makes the gland particularly prone to hemorrhage in the setting of increased venous pressure.14 In a study conducted upon rabbits, Levin and Cluff62 discovered that the local or systemic administration of endotoxin resulted in adrenal hemorrhage, but only after priming with ACTH. ACTH causes increased blood flow to the adrenal,14 which suggests that a hyperactive gland is a necessary predisposing condition for hemorrhage. Other studies of Waterhouse–Friderichsen cases have demonstrated changes in the cortices, such as compact cells, pseudotubules, and degenerate cortical cells, as well as extensive deposition of fibrin thrombi in many organs including the adrenal, indicating a role of disseminated intravascular coagulation (DIC) in the syndrome.63 However, case reports exist with no overt clinical evidence of DIC,30 suggesting that the effects of ACTH and endotoxin are sufficient to induce the hemorrhagic syndrome. In addition to endotoxin, exotoxin has been studied and shown to have distinct pathological effects upon the adrenal gland. Kuwajima32 injected sensitized rats with the histamine sensitizing factor (HSF) of Bordetella pertussis which resulted in cytolysis, vacuolation, cellular necrosis, and lipid depletion in the adrenal zona fasciculata.
The predilections of certain fungi for the adrenals and their pathological effects on the gland have been studied. P. brasiliensis primarily causes destruction by embolic infection of small vessels by large fungal cells, leading to endovasculitis and granuloma formation. Caseation necrosis is responsible for the largest loss of glandular tissue and it is most likely a consequence of local tissue ischemia secondary to the fungal emboli.16, 57, 64 The high local concentrations of corticosteroids have been postulated to account for the tropism of H. capsulatum for the adrenals.65 H. capsulatum adrenal lesions are found most commonly in the zona reticularis where there are elevated levels of corticoids downstream from the area of secretion, zona fasciculata to the medullary venous system.15, 65 The development of adrenal vasculitis can cause extensive glandular destruction resulting in frank adrenal insufficiency.66 As in P. brasiliensis, the affected vessels are unable to adequately perfuse adrenal tissue, leading to caseation necrosis and massive glandular infarction.
Physiochemical effects of infection
Microbial infection alters the biochemical and endocrinologic milieu of the host. Alterations in the functioning of upstream endocrine regulators and/or downstream hormonal mediators can feedback upon the adrenals and cause an upregulation in HPA-axis function that increases the amounts of circulating corticosteroids.20, 27, 34, 67 It has been demonstrated that administration of endotoxin causes a prolonged activation of the HPA-axis, mainly due to a release of the cytokines IL-1, IL-6, and TNF-α from stimulated peripheral immune cells initially acting directly on corticotropin releasing factor (CRF).34 In human sepsis, the HPA-axis is upregulated resulting in elevated cortisol levels and, in the acute phase, is associated with increased ACTH release.34 When sepsis is protracted the patient enters a ‘stress-conditioned’ stage of HPA-axis adaptation wherein ACTH is low and overall plasma cortisol is elevated suggesting a direct role of immunological mediators upon the adrenal gland. Along with the increase in glucocorticoids, there is a concomitant decrease in the level of androgens and mineralocorticoids with dissociation between the reduced aldosterone secretion and elevated renin level. However, numerous studies have questioned whether or not persistently elevated plasma cortisol represents an adequate response to the protracted illness or if the HPA-axis is inadequately responding to the prolonged stress.68, 69 There are indications that during protracted critical illness there is a relative adrenocortical insufficiency despite normal or elevated total serum cortisol levels,34 and the incidence of adrenal insufficiency in septic patients is high.70
The HPA-axis is frequently disturbed in tuberculosis. Administration of the M. tuberculosis cell-wall component lipoarabinomannan (LAM), mycobacterial heat shock protein-65 KD, or M. tuberculosis culture filtrate produces a similar response in normal hosts to that elicited by lipopolysaccharide (LPS).21 During active pulmonary tuberculosis measurements of basal cortisol levels and responses to ACTH stimulation have shown that cortisol reserve is normal or increased in patients with active disease. Patients with more extensive pulmonary disease have more significant changes in cortisol diurnal variation than those with limited disease suggesting that the adrenals are more responsive to active disease.21, 71, 72 The cytokine that appears to be produced in greatest amounts is TNF-α, which stimulates macrophage aggregation and granuloma formation to contain the bacteria73 while simultaneously activating the HPA-axis. The upregulation in the HPA-axis and subsequent rise in cortisol levels promotes a predominantly Th2 response. In addition to the protracted stress response inducing a decrease in androgen production, increasing age causes declining DHEA(S) levels so that by the time an individual is 80 years old concentrations are about 25% of those at age 25.74 Low levels of DHEA(S) often occur in patients with tuberculosis, suggesting a role for both increased cortisol and decreased androgens in the pathogenesis of the disease.21, 75 The interplay between infection, endocrine function, and immunomodulatory cytokines dictates the overall response both systemically and locally at the adrenal level thereby delineating the role of the HPA-axis and adrenal hormones in general in causing disease both in the adrenal gland and at distant sites.
Viruses
Infection with the human immunodeficiency virus (HIV) predisposes individuals to numerous other infections, including viral diseases such as cytomegalovirus (CMV), that result in adrenal infection and dysfunction.1, 24, 25, 29, 76, 77, 78 However, direct destruction of the adrenal by HIV is unusual.79 In autopsy studies, the adrenal gland is the most commonly involved endocrine organ in patients with HIV.80, 81 One autopsy study of 128 patients with AIDS demonstrated that the adrenal gland was pathologically compromised in 99.2% of the subjects.78 It is estimated that adrenal insufficiency occurs in 5–8% of HIV-infected individuals, which is substantially higher than the incidence in the general population.29 In addition to direct infection by HIV,82 proposed etiologies for adrenal malfunction include opportunistic infections (i.e., CMV), AIDS-associated neoplasms (i.e., Kaposi sarcoma, non-Hodgkin's lymphoma), hemorrhage, viral-induced autoimmune destruction, and adverse effects of chemotherapeutics.29
From autopsy studies, CMV appears to play the most significant role in adrenal damage during HIV infection. One study of adrenal pathology in 41 patients with AIDS showed that CMV was the most common infection seen in the adrenal gland, occurring in 21 cases (51%).25 Similarly, additional postmortem studies focusing on adrenal pathology in 2577 and 12878 AIDS patients revealed that CMV adrenalitis occurred in 56% and 48.4%, respectively. In individuals with AIDS, CMV is usually disseminated, but CMV adrenalitis may occur without clinical evidence of dissemination.83 CMV appears to cause a mixed inflammatory infiltrate with the cortex–medulla junction being the area of greatest injury,24, 78 and the amount of necrosis in the region is directly correlated with the degree of direct CMV involvement.25 In spite of the extensive involvement of CMV in the adrenal gland of HIV-infected patients, antemortem diagnosis remains a rare occurrence.77 The discordance is most likely due to the stimulation of the HPA-axis and the need for near total glandular destruction before clinical symptoms become obvious. Studies have shown that as few as 26% of patients have correctly diagnosed antemortem CMV adrenalitis.77
Disturbance of adrenal function can occur at all stages of HIV infection. Though clinically silent, elevation of ACTH and cortisol levels occurs early in infection.20 As HIV infection progresses, overt and clinically apparent insufficiency can occur with normal to low ACTH levels.20 As in endotoxemia, the early rise in basal cortisol appears to be an adaptive response to a stressor. In addition, advancing HIV infection increases the cortisol-binding globulin (CBG) in the serum in response to stimulation of the adrenal cortex by IL-1β and IL-6.26 In advanced HIV disease, adrenal ‘burnout’, co-infection by opportunistic microbes, anti-adrenal cell antibodies (unique to HIV infection),84 and increased peripheral cortisol resistance are all posited as mechanisms to explain the progression to overt adrenal failure.20, 26, 27 Interestingly, there is a case report of an individual with AIDS undergoing treatment for Pneumocystis pneumonia who developed adrenal failure due to CMV in the setting of the administration of high doses of exogenous steroids.85 As in tuberculosis, the shift towards cortisol production and away from androgen may enhance the induction of Th2 type responses while inhibiting protective Th1 responses thereby advancing HIV infection86 and disease due to co-infecting viruses.
The actual incidence of adrenal insufficiency in HIV-infected patients varies from study to study and depends upon the parameters used to assess adrenal function and the HPA-axis, Center for Disease Control (CDC) staging of HIV infection, and clinical status of the patient. Estimates place the overall incidence of adrenal insufficiency between 5% and 8%, with CMV remaining the most likely exacerbating agent.87 A prospective study of 60 patients with advanced AIDS (CD4 <50), found that 25% had adrenal dysfunction at baseline and these patients all had detectable CMV antigenemia.19 Follow-up of 34 of the patients revealed that 16 (47%) progressed to overt adrenal insufficiency.19 A separate study of 30 AIDS patients found a significant correlation between adrenal insufficiency and the presence of CMV retinitis and CMV antigenemia.23
Controversy exists as to the appropriate diagnostic parameters for a screening test to delineate possible adrenal insufficiency, which likely accounts for some of the disparities in numbers of cases reported by different authors.20 Screening in HIV-infected individuals has proven to be complex.88 This is evident in a study of 28 critically ill HIV patients screened with both a low-dose ACTH (LD-ACTH) and high-dose ACTH (HD-ACTH) using both <18 μg/dl and <25 μg/dl of cortisol as a cutoff to define an abnormal response to the test.27 With a stress cortisol of <18 μg/dl, 50% of patients screened positive for adrenal insufficiency, whereas changing the diagnostic threshold to <25 μg/dl resulted in a positive test for adrenal insufficiency in 75% of patients. The results varied from 7% to 46% with the addition of the LD-ACTH and HD-ACTH to the assessed parameters, highlighting the deceptive nature of adrenal diagnostics and inter-test variability.
Certain viruses have mutagenic potential. In particular, Epstein–Barr virus-associated lymphoma of the adrenal gland has also been reported.89, 90, 91, 92, 93 The majority of these cases have been in individuals infected with HIV. Interestingly, the adrenal deregulation of glucocorticoid levels that often occurs in HIV infected patients may significantly increase reactivation of latent Epstein–Barr virus.94 Adrenalitis can also occur during acute Epstein–Barr virus infection.95
Additional relatively common viruses can also cause significant adrenal disease in certain clinical settings. For example, neonatal infections with echoviruses, particularly serotypes 6 and 11, are associated with lethal disseminated intravascular coagulation resulting in severe damage to multiple organs, including adrenal hemorrhagic necrosis.96, 97, 98, 99, 100, 101 Herpes simplex virus (HSV) (Figure 1 ) similarly can cause damage to the adrenals in fulminant disease in neonates.102 However, apparently immunologically intact individuals can have adrenal infection due to HSV.103 Interestingly, experimental data from mice suggests that the rapid infection of the central nervous system during primary HSV type 1 or 2 occurs via the adrenal gland.104, 105, 106 Early in HSV type 1 and 2 infection, the adrenal glands frequently contain the highest number of viral particles of any organ.107
Figure 1.
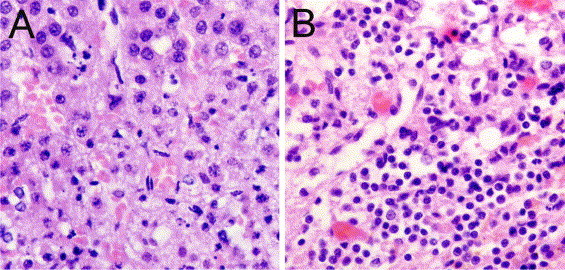
Representative images of (A) acute and (B) chronic adrenalitis. (A) Acute adrenalitis: normal adrenal cortex (top) and focal necrosis. (B) Chronic adrenalitis with lymphocytic infiltration. Examples are from two cases of systemic herpes virus infection. Tissue was stained with hemotoxylin and eosin (magnification: ×40).
Other less common hemorrhagic viruses also have a high potential for catastrophic adrenal disease. Lethal infections with filoviruses, such as Ebola virus, are characterized by massive apoptotic lysis of cells in multiple organs including liquefaction of the adrenals.108 Although the Marburg viruses have a lower case fatality rate, they too can cause damage to the adrenal, particularly the cortical cells.108, 109 Similarly, Lassa virus, an arenavirus, has been shown to infect the adrenals.110
Adrenal involvement can also occur during infection with the relatively recently identified severe acute respiratory syndrome associated coronavirus (SARS-CoV).111 However, the cellular abnormalities seen in the adrenal could be due to direct cytopathic effects by the virus or due to systemic inflammatory responses. Interestingly, SARS-CoV deregulates the host's corticosteroid stress response by producing peptides that are molecular mimics of ACTH.112 Antibodies to the viral peptides bind both viral protein and host ACTH, which reduces the host's ability to secrete corticosteroids, resulting in a state of adrenal insufficiency. It has been postulated that the administration of glucocorticoids in SARS-CoV infection may abrogate or otherwise modify infection.112 It is also noteworthy that influenza virus type A infection can affect the production or release of ACTH.113 Prior to the availability of cortisone, influenza virus (particularly Asian influenza) was typically fatal in patients with adrenal insufficiency.114 There is recent experimental evidence to suggest that the highly pathogenic H5N1 avian influenza A viruses currently circulating in Southeast Asia have the capacity to cause severe adrenal damage, since they cause multifocal necrosis of adrenal cells in some fowl species.115 However, an autopsy study of a single patient who died of H5N1 influenza only detected viral RNA by reverse transcription-polymerase chain reaction in lung, intestine, and spleen, with viral replication being localized only to the lung.116
Fungi
Many pathogenic fungi are known to affect the adrenal glands in both immunocompetent and immunocompromised individuals, with the highest incidence occurring in individuals with defects in cell-mediated immunity. Adrenal infection most frequently occurs in the setting of disseminated infection. In endemic areas, pathogenic fungi can cause higher rates of adrenal insufficiency than any other infectious etiology. For example, up to half of patients with severe, disseminated H. capsulatum infection have been found to have an infected adrenal gland.9, 15 It is postulated that the reason for the tropism of H. capsulatum for the gland is the local production and release of glucocorticoids and a relative lack of reticuloendothelial cells within the adrenal.117 Destruction of the gland itself is thought to occur via direct infection by H. capsulatum leading to an extracapsular and intracapsular vasculitis causing local ischemia and caseation.15 Typical adrenal involvement occurs during disseminated infection and is frequently bilateral with radiographic evidence of suprarenal masses, however, unilateral involvement has also been described.8, 9, 15 Though histoplasmosis typically occurs in endemic areas, cases occur worldwide.9 Autopsy data from several studies of disseminated H. capsulatum have described adrenal involvement in 30–50%9, 15 of the cases, however there remains a discordance between postmortem pathological diagnosis and antemortem clinical suspicion.15 The rate of clinical adrenal insufficiency in patients with adrenal histoplasmosis is estimated to be between 5% and 50%.9, 118 There is evidence that early antifungal therapy can lead to at least partial recovery of adrenal function,119 though most cases require long-term corticosteroid maintenance.15
P. brasiliensis is a dimorphic fungus endemic to Brazil and other South American countries. Paracoccidioidomycosis (PCM) is a chronic, progressive, suppurative and granulomatous disease that often leads to adrenal involvement.7 Autopsy studies have demonstrated adrenal involvement in 44–80% of the studied cases,120, 121 with frank adrenal dysfunction noted in 14–44% of affected patients.16 Although calcifications may be detected by computed tomography,2 near complete destruction of the gland can occur without calcification.6 Importantly, patients with disseminated PCM, adrenal dysfunction, and Addisonian symptoms who receive antifungals over a 1–2 year period may fully recover adrenal function.16 Antifungal therapy, if prescribed in the early stages of the disease is thought to prevent fungal embolism thereby reestablishing blood flow and reducing adrenal necrosis and progression to adrenal dysfunction.64 In spite of the substantial rates of adrenal involvement in PCM, the clinical suspicion for adrenal insufficiency is often low as the symptomatology may be attributed to the systemic mycosis.6 The efficacy of early intervention in preventing the progression to adrenal dysfunction warrants an increased clinical suspicion of adrenal involvement in patients presenting with signs and symptoms of PCM.
Like the other dimorphic fungi described above, Blastomyces dermatitidis has a high affinity for the adrenal gland, however, it does not appear to cause a similar rate of overt adrenal failure. Radiographs demonstrate bilaterally enlarged adrenal glands in most cases of adrenal involvement.122 Autopsy studies have demonstrated a 10% involvement of the adrenal gland in cases of disseminated blastomycosis.123 However, the patients did not have an antemortem or postmortem diagnosis of adrenal failure. In a review of 90 patients with blastomycosis, only one patient with symptoms consistent with adrenal insufficiency was discovered.124 However, there are case reports describing patients with Addison's disease caused by blastomycosis.122, 125
C. neoformans is an encapsulated yeast-like fungus that typically infects immunodeficient patients, particularly individuals with advanced AIDS. Despite the propensity of this fungus to cause disseminated disease, adrenal dysfunction is uncommon.13 However, as with blastomycosis, case reports of primary adrenal cryptococcosis causing adrenal failure exist.5, 12 Autopsy studies on AIDS patients have demonstrated adrenal cryptococcosis in up to 5.5% of cases.78 It appears that near total replacement of the adrenal gland with C. neoformans and caseating granulomas are necessary for adrenal dysfunction to become clinically apparent.5 Apparently immunologically competent individuals may also develop cryptococcosis involving the adrenal gland.4, 12 Infection may consist of an isolated lesion with no obvious adrenal dysfunction4 or can result in disseminated cryptococcosis and adrenal failure due to direct infection of the fungus.12 There is even a report of cryptococcosis in a patient with apparently intact cell mediated immunity whose adrenal glands served as a source for persistent fungemia despite appropriate antifungal therapy that only resolved with bilateral adrenalectomy.126
Pulmonary infections with P. (carinii) jirovecii occur in individuals with defects in Th1 cell immunity, as in the case of advanced HIV. Additionally, visceral dissemination can occur, including to the adrenal glands. For example, two of nine patients with AIDS and visceral calcifications secondary to P. jirovecii were shown to have adrenal disease.127 Visceral sites may shield P. jirovecii from antifungal therapy setting up a source for recurrent pulmonary infections.128 The rate of adrenal dysfunction due to direct involvement of the fungus appears to be low, but even an apparently immunologically intact individual with isolated adrenal involvement can develop a fatal Addisonian crisis.129 Additional mycoses associated with adrenal failure include coccidioidomycosis130, 131 and candidiasis.132
It is noteworthy that a major category of antifungal medications, the azoles, can adversely affect adrenal function presumably via liver cytochrome P450-mediated interactions. In particular, the inhibition of CYP3A4 (the most abundant cytochrome P450 in humans) significantly impacts steroid catabolism.133 The most potent antifungal that inhibits the P450 system is ketoconazole (an azole that currently is infrequently used in the USA and Europe), but adrenal insufficiency can occur during the administration of other azoles.134, 135, 136, 137 Additional important inhibitors of the P450 system include antibacterials (such as macrolides and isoniazid) and antiviral agents (such as ritonavir and delavirdine). Hence, clinicians should carefully consider the potential risks associated with the use of an azole or other medications that affect glandular function in patients at risk for, or with suspected or proven, adrenal dysfunction.
Bacteria
Bacteria can affect the adrenal gland by direct infection and tissue damage, by the production of exotoxins or endotoxins, and by deregulation of the physiological host response. M. tuberculosis is the most common bacterial agent associated with adrenal destruction. The mycobacterium disseminates to the adrenal gland hematogenously, where it can reside without clinical symptomatology for up to 10 years.21 In a large retrospective study of 13 762 patients, active tuberculosis was found in 6.5% of all cases with 6% of the patients with active M. tuberculosis disease demonstrating adrenal insufficiency.138 In 25% of the patients with adrenal involvement the infection was restricted to the adrenal gland. Interestingly, M. tuberculosis appears to cause direct adrenal dysfunction by inducing degeneration of cells within the adrenal cortex.71 The radiographic appearance of the adrenal gland correlates with the length and activity of the tuberculosis within the gland. Large glands occur in recent active infection whereas small, atrophied, or calcified glands appear to represent inactive or remote infection.71 Studies have attempted to discern whether treatment at any of the radiographic stages of disease improves adrenal function. A study that looked at tuberculosis patients with bilaterally enlarged adrenal glands and frank Addisonian crisis found that treatment with anti-tuberculosis drugs does not improve or help recover adrenal functionality.139 In addition, treatment with rifampin, itself an inducer of hepatic enzymes that metabolize glucocorticoids, can cause an individual with a stressed HPA-axis and minimal cortisol reserve to enter into Addisonian crisis, warranting careful monitoring of critically ill tuberculosis patients. The atypical mycobacterium Mycobacterium avium can involve the adrenals of AIDS patients, with one study finding the organism in five out of 41 cases.25 Although adrenal failure may occur, infiltrative disease has been reported without clinically apparent dysfunction.140 The role of M. avium in adrenal disease is uncertain and most adrenal destruction is thought to be due to concomitant infection with CMV and not by direct effects of the mycobacterium.
As mentioned above, Waterhouse–Friderichsen syndrome is a rapidly progressing entity in which bacterial sepsis appears to induce bilateral adrenal hemorrhage. The most common etiological agent associated with the syndrome is the bacteria N. meningitidis,18 however it can also occur during systemic disease due to group A streptococcus, pneumococci, Haemophilus influenzae, Klebsiella oxytoca, Capnocytophaga canimorsus, Pasteurella multocida, and Ewingella americana.11, 14, 30, 31, 141, 142, 143, 144, 145, 146, 147, 148 Though the bacteria associated with the syndrome are rarely found in the pathological adrenal specimens of the patients who die during this syndrome, it is felt that the bacteria must also reside in the adrenal tissue and that it is most likely not demonstrated secondary to the heavy use of antibiotics antemortem. The disorder is thought to be brought about by the stressed adrenals response to endotoxin precipitating a local DIC in a physiologically altered gland.
Parasites
Parasitic infections of the adrenal gland are rare occurrences with frequency rates dependant upon the organism, residence in endemic areas, and host immune integrity. Case reports have demonstrated adrenal involvement with such diverse pathogens as Microsporidia spp,149, 150 amebic species,151 Trypanosoma spp,152, 153 Leishmania spp,154, 155 and Echinococcus spp.156, 157 The nature of involvement of the adrenal gland varies significantly depending on the microbe. Echinococcus spp cause hydatid disease, which presents with diffuse cystic involvement of visceral organs. The most commonly involved organs are the lungs and liver, with adrenal involvement representing 0.5% of studied cases,156, 157 usually as part of a generalized infection and, more rarely, as primary cysts. It is estimated that hydatid disease accounts for 6–7% of all diagnosed cases of adrenal cysts.156, 157 Visceral leishmaniasis can also cause cystic adrenal disease, both in immunocompetent155 and immunocompromised individuals.154 Rarely, amebic species have been found in cystic lesions of the adrenal gland. For example, there is a case report of a previously healthy Mexican patient presenting with Balamuthia mandrillaris encephalitis, in which amebic trophozoites were incidentally observed in the left adrenal gland during postmortem examination.151 In immunocompromised patients (particularly in individuals with AIDS) various species of microsporidia cause a wide array of pathological features ranging from localized infection of the gastrointestinal tract to a diffuse visceral infection mimicking other more commonly diagnosed opportunistic infections. Adrenal involvement in disseminated microsporidia induces large necrotic lesions with histiocytic and fibrotic reactions within the gland substrate.149, 150 In infections due to Trypanosoma cruzi, the adrenal may serve as a reservoir for the organism.152, 153 In fact, investigators have correlated infection within the central vein of the adrenal gland and the development of chagasic myocarditis.152, 153
Conclusions
The adrenal gland can be infected by a multitude of pathogenic microorganisms that not only exert detrimental effects on the organ by local tissue destruction but also through disturbances in the homeostasis of the host's HPA-axis. Recent advances in clinical studies have demonstrated a role for low dose corticosteroid replacement in individuals with vasopressor-dependant septic shock regardless of overall adrenal function.158, 159, 160 Several recent comprehensive meta-analyses and reviews have concluded that low dose steroids should be used in this setting.158, 159, 160 Clinical trials published prior to 1989 demonstrated a distinct survival disadvantage in enrolled patients given corticosteroid replacement.158 However, most of these trials used an ineffective high dose replacement regimen (defined as 30 mg/kg methylprednisolone or equivalent steroid preparations administered up to four times during a short course of 1 or 2 days) as opposed to a low dose regimen (defined as a daily dose of 200–300 mg of hydrocortisone or equivalent administered for 5–7 days or longer).159 Recent studies using the revised ‘low-dose’ replacement dosing of corticosteroids, with the greatest benefit demonstrated with hydrocortisone, have shown a significant survival advantage in individuals suffering from vasopressor-dependant septic shock but not pure sepsis in the absence of shock (relative benefit range 1.13–3.24, 95% CI 0.86–7.01).158, 159 Low dose treatment should be begun as early as possible, but positive effects may be seen in patients with late septic shock. Similar to assessment in individuals with HIV infection, adrenal function testing in critically ill patients has not been shown to be a reliable predictive tool to determine the potential efficacy of replacement steroids regardless of overt adrenal insufficiency or a defined relative adrenal insufficiency.159
By continuing to advance studies that delineate the nature of adrenal infections and the interplay between host response and disease progression, the myriad disparate infectious entities that culminate in adrenal pathology can better be clinically addressed. Innovations in laboratory diagnostics, such as PCR,161 and improvements in imaging techniques,162, 163 including computed tomography–fine-needle aspiration biopsy,164 increase the ability of clinicians to diagnose indolent infection processes earlier. Improved methods for diagnosing adrenal function in diverse populations are urgently needed. Though infections of the adrenal gland continue to be under-recognized, the possible benefits of early recognition and intervention, particularly for fungal pathogens, warrant increased clinical suspicions especially during systemic infections.
Acknowledgements
JDN is supported in part by NIH AI056070-01A2, an Albert Einstein College of Medicine Center for AIDS Research grant, and an Infectious Disease Society of America Wyeth Vaccine Young Investigator Research Award. Adrenal images kindly provided by Dr J.S. Nosanchuk and Dr B.A. Summers, Cornell University, Ithaca, NY.
Conflict of interest: No conflict of interest to declare.
Corresponding Editor: Raymond A. Smego, Sohar, Oman
References
- 1.Arlt W., Allolio B. Adrenal insufficiency. Lancet. 2003;361:1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 2.Faical S., Borri M., Hauache O., Ajzen S. Addison's disease caused by Paracoccidioides brasiliensis: diagnosis by needle aspiration. AJR Am J Roentgenol. 1996;166:461–462. doi: 10.2214/ajr.166.2.8553971. [DOI] [PubMed] [Google Scholar]
- 3.Powers C.N., Rupp G.M., Maygarden S.J., Frable W.J. Fine-needle aspiration cytology of adrenal cryptococcosis: a case report. Diagn Cytopathol. 1991;7:88–91. doi: 10.1002/dc.2840070123. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y.C., Cheng D.L., Liu C.Y., Yen M.Y., Wang R.S. Isolated cryptococcosis of the adrenal gland. J Intern Med. 1991;230:285–287. doi: 10.1111/j.1365-2796.1991.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 5.Shah B., Taylor H.C., Pillay I., Chung-Park M., Dobrinich R. Adrenal insufficiency due to cryptococcosis. JAMA. 1986;256:3247–3249. [PubMed] [Google Scholar]
- 6.Leal A.M., Bellucci A.D., Muglia V.F., Lucchesi F.R. Unique adrenal gland imaging features in Addison's disease caused by paracoccidioidomycosis. AJR Am J Roentgenol. 2003;181:1433–1434. doi: 10.2214/ajr.181.5.1811433. [DOI] [PubMed] [Google Scholar]
- 7.Murray H.W., Littman M.L., Roberts R.B. Disseminated paracoccidioidomycosis (South American blastomycosis) in the United States. Am J Med. 1974;56:209–220. doi: 10.1016/0002-9343(74)90599-3. [DOI] [PubMed] [Google Scholar]
- 8.Singh S.K., Bhadada S.K., Singh S.K., Sharma O.P., Arya N.C., Shukla V.K. Histoplasmosis: an unusual presentation. J Assoc Physicians India. 2000;48:923–925. [PubMed] [Google Scholar]
- 9.Kumar N., Singh S., Govil S. Adrenal histoplasmosis: clinical presentation and imaging features in nine cases. Abdom Imaging. 2003;28:703–708. doi: 10.1007/s00261-003-0010-5. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan R., Sharma U., Trivedi N., Parsad M., Kansra U., Bhandari S. Histoplasma capsulatum in adrenal gland aspirate—a case report. Indian J Pathol Microbiol. 2000;43:165–168. [PubMed] [Google Scholar]
- 11.Doherty S. Fatal pneumococcal Waterhouse–Friderichsen syndrome. Emerg Med. 2001;13:237–239. doi: 10.1046/j.1442-2026.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- 12.Walker B.F., Gunthel C.J., Bryan J.A., Watts N.B., Clark R.V. Disseminated cryptococcosis in an apparently normal host presenting as primary adrenal insufficiency: diagnosis by fine needle aspiration. Am J Med. 1989;86:715–717. doi: 10.1016/0002-9343(89)90453-1. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita A., Nakazawa H., Akiyama H., Takeuchi K., Kawai R., Oohashi K. Disseminated cryptococcosis presenting with adrenal insufficiency and meningitis: resistant to prolonged antifungal therapy but responding to bilateral adrenalectomy. Intern Med. 1992;31:1401–1405. doi: 10.2169/internalmedicine.31.1401. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton D., Harris M.D., Foweraker J., Gresham G.A. Waterhouse–Friderichsen syndrome as a result of non-meningococcal infection. J Clin Pathol. 2004;57:208–209. doi: 10.1136/jcp.2003.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roubsanthisuk W., Sriussadaporn S., Vawesorn N., Parichatikanond P., Phoojaroenchanachai M., Homsanit M. Primary adrenal insufficiency caused by disseminated histoplasmosis: report of two cases. Endocr Pract. 2002;8:237–241. doi: 10.4158/EP.8.3.237. [DOI] [PubMed] [Google Scholar]
- 16.Do Valle A., Guimaraes M., Cuba J., Wanke B., Tendrich M. Recovery of adrenal function after treatment of paracoccidioidomycosis. Am J Trop Med Hyg. 1993;48:626–629. doi: 10.4269/ajtmh.1993.48.626. [DOI] [PubMed] [Google Scholar]
- 17.Huebener K., Treugut H. Adrenal cortex dysfunction: CT findings. Radiology. 1984;150:195–199. doi: 10.1148/radiology.150.1.6689760. [DOI] [PubMed] [Google Scholar]
- 18.Bosworth D. Reversible adrenocorticol insufficiency in fulminant meningococcemia. Arch Intern Med. 1979;139:823–824. [PubMed] [Google Scholar]
- 19.Hoshino Y., Yamashita N., Nakamura T., Iwamoto A. Prospective examination of adrenocortical function in advanced AIDS patients. Endocr J. 2002;49:641–647. doi: 10.1507/endocrj.49.641. [DOI] [PubMed] [Google Scholar]
- 20.Eledrisi M., Verghese A. Adrenal insufficiency in HIV infection; a review and recommendations. Am J Med Sci. 2001;321:137–144. doi: 10.1097/00000441-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kelestimur F. The endocrinology of adrenal tuberculosis: the effect of tuberculosis on the hypothalamic-pituitary–adrenal axis and adrenocortical function. J Endocrinol Invest. 2004;27:380–386. doi: 10.1007/BF03351067. [DOI] [PubMed] [Google Scholar]
- 22.Geusau A., Stingl G. Primary adrenal insufficiency in two patients with the acquired immunodeficiency syndrome associated with disseminated cytomegaloviral infection. Wien Klin Wochenschr. 1997;109:845–849. [PubMed] [Google Scholar]
- 23.Hoshino Y., Nagata Y., Gatanaga H., Hosono O., Morimoto C., Tachikawa N. Cytomegalovirus (CMV) retinitis and CMV antigenemia as a clue to impaired adrenocortical function in patients with AIDS. AIDS. 1997;11:1719–1724. doi: 10.1097/00002030-199714000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Nakamine H., Shimizu E., Nishino E., Takenaka T., Maeda J., Hanaoka M. Autopsy findings in a Japanese patient with acquired immunodeficiency syndrome. Acta Pathol Jpn. 1987;37:1797–1809. doi: 10.1111/j.1440-1827.1987.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 25.Glasgow B., Steinsapie K., Anders K., Layfield L. Adrenal pathology in the acquired immune deficiency syndrome. Am J Clin Pathol. 1985;84:594–597. doi: 10.1093/ajcp/84.5.594. [DOI] [PubMed] [Google Scholar]
- 26.Mayo J., Collazos J., Martinez E., Ibarra S. Adrenal function in the human immunodeficiency virus-infected patient. Arch Intern Med. 2002;162:1095–1098. doi: 10.1001/archinte.162.10.1095. [DOI] [PubMed] [Google Scholar]
- 27.Marik P.E., Kiminyo K., Zaloga G.P. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30:1267–1273. doi: 10.1097/00003246-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Hinz S., McCormack D., van der Spuy Z.M. Endocrine function in HIV-infected women. Gynecol Endocrinol. 2002;16:33–38. [PubMed] [Google Scholar]
- 29.Huang Y.-W., Chang C.-C., Sun H.-Y., Chen M.-Y., Hung C.-C., Chang S.-C. Primary adrenal insufficiency in patients with acquired immunodeficiency syndrome: report of four cases. J Microbiol Immunol Infect. 2004;37:250–253. [PubMed] [Google Scholar]
- 30.Karakousis P.C., Page K.R., Varello M.A., Howlett P.J., Stieritz D.D. Waterhouse–Friderichsen syndrome after infection with group A streptococcus. Mayo Clin Proc. 2001;76:1167–1170. doi: 10.4065/76.11.1167. [DOI] [PubMed] [Google Scholar]
- 31.Tsokos M. Fatal Waterhouse–Friderichsen syndrome due to Ewingella americana infection. Am J Forensic Med Pathol. 2003;24:41–44. doi: 10.1097/01.PAF.0000051704.91568.A6. [DOI] [PubMed] [Google Scholar]
- 32.Kuwajima Y. Morphological alterations of the adrenal gland following administration of histamine-sensitizing-factor of Bordetella pertussis. Osaka City Med J. 1980;26:61–66. [PubMed] [Google Scholar]
- 33.Rook G.A., Hernandez-Pando R., Lightman S.L. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994;15:301–303. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 34.Beishuizen A., Thijs L. Endotoxin and the hypothalamo-pituitary–adrenal (HPA) axis. J Endotoxin Res. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 35.Graham B.S., Tucker W.S., Jr. Opportunistic infections in endogenous Cushing's syndrome. Ann Intern Med. 1984;101:334–338. doi: 10.7326/0003-4819-101-3-334. [DOI] [PubMed] [Google Scholar]
- 36.Boscaro M., Fallo F., Sonino N. Disseminated nocardiosis in a patient with Cushing's syndrome. J Endocrinol Invest. 1994;17:443–445. doi: 10.1007/BF03347735. [DOI] [PubMed] [Google Scholar]
- 37.Kang C.I., Kim S.H., Kim H.B., Oh M.D., Kim S.Y., Choe K.W. Disseminated cryptococcosis in a patient with pituitary Cushing's disease. Korean J Intern Med. 2003;18:199–201. doi: 10.3904/kjim.2003.18.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew P.A., Takezawa K. Pulmonary cryptococcosis and pituitary Cushing's disease. Diagn Cytopathol. 1998;18:365–367. doi: 10.1002/(sici)1097-0339(199805)18:5<365::aid-dc13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Kramer M., Corrado M.L., Bacci V., Carter A.C., Landesman S.H. Pulmonary cryptococcosis and Cushing's syndrome. Arch Intern Med. 1983;143:2179–2180. [PubMed] [Google Scholar]
- 40.Ferguson R.P., Cryptococcosis Cushing's syndrome. Ann Intern Med. 1977;87:65–66. doi: 10.7326/0003-4819-87-1-65. [DOI] [PubMed] [Google Scholar]
- 41.Bakker R.C., Gallas P.R., Romijn J.A., Wiersinga W.M. Cushing's syndrome complicated by multiple opportunistic infections. J Endocrinol Invest. 1998;21:329–333. doi: 10.1007/BF03350337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McQuillen D.P., Schroy P.C., Hesketh P.J., Sugar A.M. Pneumocystis carinii pneumonia complicating somatostatin therapy of Cushing's syndrome in a patient with metastatic pancreatic islet cell carcinoma and Zollinger–Ellison syndrome. Am J Gastroenterol. 1991;86:512–514. [PubMed] [Google Scholar]
- 43.Findling J.W., Buggy B.P., Segerson T.P., Raff H. Pneumocystis carinii pneumonia complicating intermittent Cushing's syndrome. Wis Med J. 1986;85:23–25. [PubMed] [Google Scholar]
- 44.Fulkerson W.J., Newman J.H. Endogenous Cushing's syndrome complicated by Pneumocystis carinii pneumonia. Am Rev Respir Dis. 1984;129:188–189. doi: 10.1164/arrd.1984.129.1.188. [DOI] [PubMed] [Google Scholar]
- 45.Anthony L.B., Greco F.A. Pneumocystis carinii pneumonia: a complication of Cushing's syndrome. Ann Intern Med. 1981;94:488–489. doi: 10.7326/0003-4819-94-4-488. [DOI] [PubMed] [Google Scholar]
- 46.Huang T.P., Wang P.W., Liu R.T., Tung S.C., Jean W.Y., Lu Y.C. Ectopic ACTH syndrome with nocardiosis—a case report. Changgeng Yi Xue Za Zhi. 1994;17:371–377. [PubMed] [Google Scholar]
- 47.Pesce C.M., Quaglia A.C. Nocardia lung infection with hematogenous spread in a woman with adrenal cortical hyperfunction. Eur J Respir Dis. 1984;65:613–615. [PubMed] [Google Scholar]
- 48.Higgins T.L., Calabrese L.H., Sheeler L.R. Opportunistic infections in patients with ectopic ACTH-secreting tumors. Cleve Clin Q. 1982;49:43–49. doi: 10.3949/ccjm.49.1.43. [DOI] [PubMed] [Google Scholar]
- 49.Goh S.K., Chow P.K., Chung A.Y., Tan B.H., Tan P.H. Strongyloides colitis in a patient with Cushing's syndrome. Gastrointest Endosc. 2004;59:738–741. doi: 10.1016/s0016-5107(04)00289-5. [DOI] [PubMed] [Google Scholar]
- 50.Namisato S., Motomura K., Haranaga S., Hirata T., Toyama M., Shinzato T. Pulmonary strongyloidiasis in a patient receiving prednisolone therapy. Intern Med. 2004;43:731–736. doi: 10.2169/internalmedicine.43.731. [DOI] [PubMed] [Google Scholar]
- 51.Dunlap N.E., Grizzle W.E., Heck L.W., Jr. Unsuspected Cushing's disease in a patient with fatal staphylococcal bacteremia and multiple pituitary adenomas. Am J Med. 1989;86:217–221. doi: 10.1016/0002-9343(89)90273-8. [DOI] [PubMed] [Google Scholar]
- 52.Tan T.T., Choy Y.W., Norizan M.A., Meah F., Khalid B.A. Adrenal histoplasmosis in Cushing's syndrome with bilateral adrenocortical nodular hyperplasia. Med J Malaysia. 1990;45:154–158. [PubMed] [Google Scholar]
- 53.Tsubota A., Shishiba Y., Shimizu T., Ozawa Y., Sawano S., Yamada S. Masked Cushing's disease in an aged man associated with intraventricular hemorrhage and tuberculous peritonitis. Jpn J Med. 1991;30:233–237. doi: 10.2169/internalmedicine1962.30.233. [DOI] [PubMed] [Google Scholar]
- 54.Hill A.T., Stewart P.M., Hughes E.A., McLeod D.T. Cushing's disease and tuberculosis. Respir Med. 1998;92:604–606. doi: 10.1016/s0954-6111(98)90320-1. [DOI] [PubMed] [Google Scholar]
- 55.Haas S.R., Hodge M.B., Duncan R.A. Cushing's syndrome presenting as disseminated cutaneous Mycobacterium chelonae infection. Clin Infect Dis. 2001;33:e51–e53. doi: 10.1086/322629. Epub 2001 Aug 6. [DOI] [PubMed] [Google Scholar]
- 56.Lionakis M.S., Kontoyiannis D.P. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 57.Torres C., Duarte E., Guimares J.P., Moreira L.F. Destructive lesion of the adrenal gland in South American blastomycosis (Letz’ disease) Am J Pathol. 1952;28:145–155. [PMC free article] [PubMed] [Google Scholar]
- 58.Del Negro G., Melo E.H., Rodbard D., Melo M.R., Layton J., Wachslicht-Rodbard H. Limited adrenal reserve in paracoccidioidomycosis: cortisol and aldosterone responses to 1-24 ACTH. Clin Endocrinol. 1980;13:553–559. doi: 10.1111/j.1365-2265.1980.tb03423.x. [DOI] [PubMed] [Google Scholar]
- 59.Casadevall A., Pirofski L. The damage–response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voelcker A. Pathologic report. Middlesex Hosp J. 1894;12:279. [Google Scholar]
- 61.Friderichsen C. Waterhouse–Friderichsen syndrome (W.-F. S.) Acta Endocrinol. 1955;18:489–492. doi: 10.1530/acta.0.0180482. [DOI] [PubMed] [Google Scholar]
- 62.Levin J., Cluff L. Endotoxemia and adrenal hemorrhage. A mechanism for the Waterhouse–Friderichsen syndrome. J Exp Med. 1965;121:247–260. doi: 10.1084/jem.121.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox B. Venous infarction of the adrenal glands. J Pathol. 1976;119:65–89. doi: 10.1002/path.1711190202. [DOI] [PubMed] [Google Scholar]
- 64.Osa S.R., Peterson R.E., Roberts R.B. Recovery of adrenal reserve following treatment of disseminated South American blastomycosis. Am J Med. 1981;71:298–301. doi: 10.1016/0002-9343(81)90131-5. [DOI] [PubMed] [Google Scholar]
- 65.Frenkel J.K. Role of corticosteroids as predisposing factors in fungal diseases. Lab Invest. 1962;11:1192–1208. [PubMed] [Google Scholar]
- 66.Goodwin R.A., Shapiro J.L., Thurman G.H., Thurman S.S., Des Prez R. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980;59:1–33. [PubMed] [Google Scholar]
- 67.Silverman M.N., Pearce B.D., Biron C.A., Miller A.H. Immune modulation of the hypothalamic-pituitary–adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamberts S.W., Bruining H.A., de Jong F.H. Corticosteroid therapy in severe illness. N Engl J Med. 1997;337:1285–1292. doi: 10.1056/NEJM199710303371807. [DOI] [PubMed] [Google Scholar]
- 69.Beishuizen A., Thijs L.G. Relative adrenal failure in intensive care: an identifiable problem requiring treatment? Best Pract Res Clin Endocrinol Metab. 2001;15:513–531. doi: 10.1053/beem.2001.0167. [DOI] [PubMed] [Google Scholar]
- 70.Briegel J., Forst H., Haller M., Schelling G., Kilger E., Kuprat G. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single center study. Crit Care Med. 1999;27:723–732. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 71.Kelestimur F., Unlu Y., Ozesmi M., Tolu I. A hormonal and radiological evaluation of adrenal gland in patients with acute or chronic pulmonary tuberculosis. Clin Endocrinol. 1994;41:53–56. doi: 10.1111/j.1365-2265.1994.tb03784.x. [DOI] [PubMed] [Google Scholar]
- 72.York E.L., Enarson D.A., Nobert E.J., Fanning F.A., Sproule B.J. Adrenocortical function in patients investigated for active tuberculosis. Chest. 1992;101:1338–1341. doi: 10.1378/chest.101.5.1338. [DOI] [PubMed] [Google Scholar]
- 73.Barnes P.F., Abrams J.S., Lu S., Sieling P.A., Rea T.H., Modlin R.L. Patterns of cytokine production by mycobacterium reactive human T-cell clones. Infect Immun. 1993;61:197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orentreich N., Brind J.L., Rizer R.L., Vogelman J.H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 75.Keven K., Uysal A.R., Erdogan G. Adrenal function during tuberculous infection and effects of antituberculosis treatment on endogenous and exogenous steroids. Int J Tuberc Lung Dis. 1998;2:419–424. [PubMed] [Google Scholar]
- 76.Angulo J.C., Lopez J.I., Flores N. Lethal cytomegalovirus adrenalitis in a case of AIDS. Scand J Urol Nephrol. 1994;28:105–106. doi: 10.3109/00365599409180481. [DOI] [PubMed] [Google Scholar]
- 77.Dore G., Marriott D., Duflou J. Clinico-pathological study of cytomegalovirus (CMV) in AIDS autopsies: under-recognition of CMV pneumonitis and CMV adrenalitis. Aust N Z J Med. 1995;25:503–506. doi: 10.1111/j.1445-5994.1995.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 78.Rodrigues D., Reis M., Teixeira V., Silva-Vergara M., Filho D.C., Adad S. Pathologic findings in the adrenal glands of autopsied patients with acquired immunodeficiency syndrome. Pathol Res Pract. 2002;198:25–30. doi: 10.1078/0344-0338-00180. [DOI] [PubMed] [Google Scholar]
- 79.Sellmeyer D.E., Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev. 1996;17:518–532. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- 80.Welch K., Finkbeiner W., Alpers C.E., Blumenfeld W., Davis R.L., Smuckler E.A. Autopsy findings in the acquired immune deficiency syndrome. JAMA. 1984;252:1152–1159. [PubMed] [Google Scholar]
- 81.Hofbauer L.C., Heufelder A.E. Endocrine implications of human immunodeficiency virus infection. Medicine (Baltimore) 1996;75:262–278. doi: 10.1097/00005792-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Dluhy R. The growing spectrum of HIV related endocrine abnormalities. J Clin Endocrinol Metab. 1990;70:563–565. doi: 10.1210/jcem-70-3-563. [DOI] [PubMed] [Google Scholar]
- 83.Pinching A.J. Cytomegalovirus infection in the acquired immune deficiency syndrome. J Antimicrob Chemother. 1989;23(Suppl. E):31–36. doi: 10.1093/jac/23.suppl_e.31. [DOI] [PubMed] [Google Scholar]
- 84.Salim Y.S., Faber V., Wiik A., Andersen P.L., Hoier-Madsen M., Mouritsen S. Anti-corticosteroid antibodies in AIDS patients. APMIS. 1988;96:889–894. doi: 10.1111/j.1699-0463.1988.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 85.Razzaq F., Dunbar E.M., Bonington A. The development of cytomegalovirus-induced adrenal failure in a patient with AIDS while receiving corticosteroid therapy. HIV Med. 2002;3:212–214. doi: 10.1046/j.1468-1293.2002.00114.x. [DOI] [PubMed] [Google Scholar]
- 86.Clerici M., Bevilacqua M., Vago T., Villa M.L., Shearer G.M., Norbiato G. An immunoendocrinological hypothesis of HIV infection. Lancet. 1994;343:1552–1553. doi: 10.1016/s0140-6736(94)92944-0. [DOI] [PubMed] [Google Scholar]
- 87.Masharani U., Schambelan M. The endocrine complications of acquired immunodeficiency syndrome. Adv Intern Med. 1993;38:323–336. [PubMed] [Google Scholar]
- 88.Eledrisi M.S., Verghese A.C. Adrenal insufficiency in HIV infection: a review and recommendations. Am J Med Sci. 2001;321:137–144. doi: 10.1097/00000441-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Ohsawa M., Tomita Y., Hashimoto M., Yasunaga Y., Kanno H., Aozasa K. Malignant lymphoma of the adrenal gland: its possible correlation with the Epstein–Barr virus. Mod Pathol. 1996;9:534–543. [PubMed] [Google Scholar]
- 90.Suankratay C., Shuangshoti S., Mutirangura A., Prasanthai V., Lerdlum S., Shuangshoti S. Epstein–Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis. 2005;40:1521–1528. doi: 10.1086/429830. Epub 2005 Apr 12. [DOI] [PubMed] [Google Scholar]
- 91.Ohshima K., Kobari S., Kuroiwa S., Nakanishi K., Shibuya T., Nagafuchi S. Heterogeneity of systemic extra-nodal Epstein–Barr virus-associated lympho-histiocytic tumor—ten autopsy cases of human immunodeficiency virus-negative Japanese. Pathol Res Pract. 1997;193:257–265. doi: 10.1016/s0344-0338(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 92.Jimenez-Heffernan J.A., Hardisson D., Palacios J., Garcia-Viera M., Gamallo C., Nistal M. Adrenal gland leiomyoma in a child with acquired immunodeficiency syndrome. Pediatr Pathol Lab Med. 1995;15:923–929. doi: 10.3109/15513819509027028. [DOI] [PubMed] [Google Scholar]
- 93.Prevot S., Neris J., de Saint Maur P.P. Detection of Epstein–Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Arch. 1994;425:321–325. doi: 10.1007/BF00196156. [DOI] [PubMed] [Google Scholar]
- 94.Cacioppo J.T., Kiecolt-Glaser J.K., Malarkey W.B., Laskowski B.F., Rozlog L.A., Poehlmann K.M. Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein–Barr virus. Horm Behav. 2002;42:32–41. doi: 10.1006/hbeh.2002.1801. [DOI] [PubMed] [Google Scholar]
- 95.Hertel N.T., Jacobsen B.B., Pedersen F.K., Heilmann C. Adrenocortical insufficiency associated with Epstein–Barr virus infection in a patient with the Wiskott–Aldrich syndrome. Eur J Pediatr. 1987;146:603–604. doi: 10.1007/BF02467365. [DOI] [PubMed] [Google Scholar]
- 96.Ventura K.C., Hawkins H., Smith M.B., Walker D.H. Fatal neonatal echovirus 6 infection: autopsy case report and review of the literature. Mod Pathol. 2001;14:85–90. doi: 10.1038/modpathol.3880260. [DOI] [PubMed] [Google Scholar]
- 97.Speer M.E., Yawn D.H. Fatal hepatoadrenal necrosis in the neonate associated with echovirus types 11 and 12 presenting as a surgical emergency. J Pediatr Surg. 1984;19:591–593. doi: 10.1016/s0022-3468(84)80111-6. [DOI] [PubMed] [Google Scholar]
- 98.Mostoufizadeh M., Lack E.E., Gang D.L., Perez-Atayde A.R., Driscoll S.G. Postmortem manifestations of echovirus 11 sepsis in five newborn infants. Hum Pathol. 1983;14:818–823. doi: 10.1016/s0046-8177(83)80304-9. [DOI] [PubMed] [Google Scholar]
- 99.Reyes M.P., Ostrea E.M., Jr., Roskamp J., Lerner A.M. Disseminated neonatal echovirus 11 disease following antenatal maternal infection with a virus-positive cervix and virus-negative gastrointestinal tract. J Med Virol. 1983;12:155–159. doi: 10.1002/jmv.1890120210. [DOI] [PubMed] [Google Scholar]
- 100.Berry P.J., Nagington J. Fatal infection with echovirus 11. Arch Dis Child. 1982;57:22–29. [PMC free article] [PubMed] [Google Scholar]
- 101.Wreghitt T.G., Sutehall G.M., King A., Gandy G.M. Fatal echovirus 7 infection during an outbreak in a special care baby unit. J Infect. 1989;19:229–236. doi: 10.1016/s0163-4453(89)90709-3. [DOI] [PubMed] [Google Scholar]
- 102.Nakamura Y., Yamamoto S., Tanaka S., Yano H., Nishimura G., Saito Y. Herpes simplex viral infection in human neonates: an immunohistochemical and electron microscopic study. Hum Pathol. 1985;16:1091–1097. doi: 10.1016/s0046-8177(85)80176-3. [DOI] [PubMed] [Google Scholar]
- 103.Miyazaki Y., Akizuki S., Sakaoka H., Yamamoto S., Terao H. Disseminated infection of herpes simplex virus with fulminant hepatitis in a healthy adult. A case report. APMIS. 1991;99:1001–1007. doi: 10.1111/j.1699-0463.1991.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 104.Irie H., Harada Y., Kurokawa E., Saito M., Sugawara Y., Ohami H. Early adrenal infection by herpes simplex virus type-1 (Miyama + GC strain): special reference to inoculation dose and spread from the adrenal to the central nervous system. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53:325–331. doi: 10.1007/BF02890259. [DOI] [PubMed] [Google Scholar]
- 105.Hill T.J., Yirrell D.L., Blyth W.A. Infection of the adrenal gland as a route to the central nervous system after viraemia with herpes simplex virus in the mouse. J Gen Virol. 1986;67(Pt 2):309–320. doi: 10.1099/0022-1317-67-2-309. [DOI] [PubMed] [Google Scholar]
- 106.Aita K., Irie H., Koyama A.H., Fukuda A., Yoshida T., Shiga J. Acute adrenal infection by HSV-1: role of apoptosis in viral replication. Arch Virol. 2001;146:2009–2020. doi: 10.1007/s007050170048. [DOI] [PubMed] [Google Scholar]
- 107.Potratz D., Brake B., Dienes H.P., Schulz T.F., Hosp M., Dierich M.P. Herpes simplex virus type 1 and 2 in the adrenal glands: replication and histopathology. Arch Virol. 1986;90:207–222. doi: 10.1007/BF01317371. [DOI] [PubMed] [Google Scholar]
- 108.Mahanty S., Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4:487–498. doi: 10.1016/S1473-3099(04)01103-X. [DOI] [PubMed] [Google Scholar]
- 109.Geisbert T.W., Jaax N.K. Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct Pathol. 1998;22:3–17. doi: 10.3109/01913129809032253. [DOI] [PubMed] [Google Scholar]
- 110.Edington G.M., White H.A. The pathology of Lassa fever. Trans R Soc Trop Med Hyg. 1972;66:381–389. doi: 10.1016/0035-9203(72)90268-4. [DOI] [PubMed] [Google Scholar]
- 111.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wheatland R. Molecular mimicry of ACTH in SARS – implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 2004;63:855–862. doi: 10.1016/j.mehy.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jefferies W.M., Turner J.C., Lobo M., Gwaltney J.M., Jr. Low plasma levels of adrenocorticotropic hormone in patients with acute influenza. Clin Infect Dis. 1998;26:708–710. doi: 10.1086/514594. [DOI] [PubMed] [Google Scholar]
- 114.Skanse B., Miorner G. Asian influenza with adrenocortical insufficiency. Lancet. 1959;1:1121–1122. doi: 10.1016/s0140-6736(59)90708-1. [DOI] [PubMed] [Google Scholar]
- 115.Lee C.W., Suarez D.L., Tumpey T.M., Sung H.W., Kwon Y.K., Lee Y.J. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol. 2005;79:3692–3702. doi: 10.1128/JVI.79.6.3692-3702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uiprasertkul M., Puthavathana P., Sangsiriwut K., Pooruk P., Srisook K., Peiris M. Influenza A H5N1 replication sites in humans. Emerg Infect Dis. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Swartz M.A., Scofield R.H., Dickey W.D., Kirk JLJ, Wilson D.A., Pitha J.V. Unilateral adrenal enlargement due to Histoplasma capsulatum. Clin Infect Dis. 1996;23:813–815. doi: 10.1093/clinids/23.4.813. [DOI] [PubMed] [Google Scholar]
- 118.Wong P., Houston S., Power B., Lalor E., Bain V.G. A case of Histoplasma capsulatum causing granulomatous liver disease and Addisonian crisis. Can J Gastroenterol. 2001;15:687–691. doi: 10.1155/2001/813784. [DOI] [PubMed] [Google Scholar]
- 119.Alevritis E.M., Sarubbi F.A., Jordan R.M., Peiris A.N. Infectious causes of adrenal insufficiency. South Med J. 2003;96:888–890. doi: 10.1097/01.SMJ.0000073269.49575.DF. [DOI] [PubMed] [Google Scholar]
- 120.Franco M., Montenegro M. Sarvier-Edusp; São Paulo, Brazil: 1982. Anatomia patologica. [Google Scholar]
- 121.Colombo A.L., Faical S., Kater C.E. Systematic evaluation of the adrenocortical function in patients with paracoccidioidomycosis. Mycopathologia. 1994;127:89–93. doi: 10.1007/BF01103064. [DOI] [PubMed] [Google Scholar]
- 122.Rimondi A.P., Bianchini E., Barucchello G., Panzavolta R. Addison's disease caused by adrenal blastomycosis: a case report with fine needle aspiration (FNA) cytology. Cytopathology. 1995;6:277–279. doi: 10.1111/j.1365-2303.1995.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 123.Chandler P. Addison's disease secondary to North American blastomycosis. South Med J. 1977;70:863–864. doi: 10.1097/00007611-197707000-00028. [DOI] [PubMed] [Google Scholar]
- 124.Kunkel W.M., Weed L.A., McDonald J.R., Clagett O.T. North American blastomycosis-Gilchrist's disease; a clinicopathologic study of ninety cases. Surg Gyn Obstet. 1954;99:1–26. [PubMed] [Google Scholar]
- 125.Eberle D., Evans R., Johnson R. Disseminated North American blastomycosis. Occurrence with clinical manifestations of adrenal insufficiency. JAMA. 1977;238:2629–2630. doi: 10.1001/jama.238.24.2629. [DOI] [PubMed] [Google Scholar]
- 126.Kawamura M., Miyazaki S., Mashiko S., Sumi M., Ashidate K., Tohda H. Disseminated cryptococcosis associated with adrenal masses and insufficiency. Am J Med Sci. 1998;316:60–64. doi: 10.1097/00000441-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 127.Radin D.R., Baker E.L., Klatt E.C. Visceral and nodal calcification in patients with AIDS-related Pneumocystis carinii infection. AJR Am J Roentgenol. 1990;154:27–31. doi: 10.2214/ajr.154.1.2104720. [DOI] [PubMed] [Google Scholar]
- 128.Pilon V.A., Echols R.M., Celo J.S., Elmendorf S.L. Disseminated Pneumocystis carinii infection in AIDS. N Engl J Med. 1987;316:1410–1411. doi: 10.1056/NEJM198705283162213. [DOI] [PubMed] [Google Scholar]
- 129.Agarwal J., Agarwal G., Ayyagari A., Kar D., Mishra S., Bhatia E. Isolated Pneumocystis carinii infection of adrenal glands causing Addison's disease in a non-immunocompromised adult. Endocr Pathol. 2001;12:87–91. doi: 10.1385/ep:12:1:87. [DOI] [PubMed] [Google Scholar]
- 130.Chowfin A., Tight R. Female genital coccidioidomycosis (FGC), Addison's disease and sigmoid loop abscess due to Coccidioides immites; case report and review of literature on FGC. Mycopathologia. 1999;145:121–126. doi: 10.1023/a:1007058106662. [DOI] [PubMed] [Google Scholar]
- 131.Blair J.E., Smilack J.D., Caples S.M. Coccidioidomycosis in patients with hematologic malignancies. Arch Intern Med. 2005;165:113–117. doi: 10.1001/archinte.165.1.113. [DOI] [PubMed] [Google Scholar]
- 132.Alteras I., Cojocaru I., Balanescu A. Generalized candidiasis associated with Addison's disease. Mykosen. 1969;12:575–577. doi: 10.1111/j.1439-0507.1969.tb04479.x. [DOI] [PubMed] [Google Scholar]
- 133.Plant N.J., Gibson G.G. Evaluation of the toxicological relevance of CYP3A4 induction. Curr Opin Drug Discov Devel. 2003;6:50–56. [PubMed] [Google Scholar]
- 134.Skov M., Main K.M., Sillesen I.B., Muller J., Koch C., Lanng S. Iatrogenic adrenal insufficiency as a side-effect of combined treatment of itraconazole and budesonide. Eur Respir J. 2002;20:127–133. doi: 10.1183/09031936.02.00248002. [DOI] [PubMed] [Google Scholar]
- 135.Albert S.G., DeLeon M.J., Silverberg A.B. Possible association between high-dose fluconazole and adrenal insufficiency in critically ill patients. Crit Care Med. 2001;29:668–670. doi: 10.1097/00003246-200103000-00039. [DOI] [PubMed] [Google Scholar]
- 136.Shibata S., Kami M., Kanda Y., Machida U., Iwata H., Kishi Y. Acute adrenal failure associated with fluconazole after administration of high-dose cyclophosphamide. Am J Hematol. 2001;66:303–305. doi: 10.1002/ajh.1063. [DOI] [PubMed] [Google Scholar]
- 137.Sharkey P.K., Rinaldi M.G., Dunn J.F., Hardin T.C., Fetchick R.J., Graybill J.R. High-dose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother. 1991;35:707–713. doi: 10.1128/aac.35.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lam K., Lo C. A critical examination of adrenal tuberculosis and a 28-year autopsy experience of active tuberculosis. Clin Endocrinol. 2001;54:633–639. doi: 10.1046/j.1365-2265.2001.01266.x. [DOI] [PubMed] [Google Scholar]
- 139.Bhatia E.A., Jain S.K., Gupta R.K., Pandey R. Tuberculous Addison's disease: lack of normalization of adrenocortical function after anti-tuberculous chemotherapy. Clin Endocrinol. 1998;48:355–359. doi: 10.1046/j.1365-2265.1998.00409.x. [DOI] [PubMed] [Google Scholar]
- 140.Guenthner E.E., Rabinowe S.L., Van Niel A., Naftilan A., Dluhy R.G. Primary Addison's disease in a patient with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;100:847–848. doi: 10.7326/0003-4819-100-6-847. [DOI] [PubMed] [Google Scholar]
- 141.Givner L.B. Invasive disease due to group A beta-hemolytic streptococci: continued occurrence in children in North Carolina. South Med J. 1998;91:333–337. doi: 10.1097/00007611-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 142.Hori K., Yasoshima H., Yamada A., Sakurai K., Ohkubo E., Kubota A. Adrenal hemorrhage associated with Klebsiella oxytoca bacteremia. Intern Med. 1998;37:990–994. doi: 10.2169/internalmedicine.37.990. [DOI] [PubMed] [Google Scholar]
- 143.Ip M., Teo J.G., Cheng A.F. Waterhouse–Friderichsen syndrome complicating primary biliary sepsis due to Pasteurella multocida in a patient with cirrhosis. J Clin Pathol. 1995;48:775–777. doi: 10.1136/jcp.48.8.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gertner M., Rodriguez L., Barnett S.H., Shah K. Group A beta-hemolytic Streptococcus and Waterhouse–Friderichsen syndrome. Pediatr Infect Dis J. 1992;11:595–596. doi: 10.1097/00006454-199207000-00019. [DOI] [PubMed] [Google Scholar]
- 145.McKinney W.P., Agner R.C. Waterhouse–Friderichsen syndrome caused by Haemophilus influenzae type b in an immunocompetent young adult. South Med J. 1989;82:1571–1573. doi: 10.1097/00007611-198912000-00029. [DOI] [PubMed] [Google Scholar]
- 146.Mirza I., Wolk J., Toth L., Rostenberg P., Kranwinkel R., Sieber S.C. Waterhouse–Friderichsen syndrome secondary to Capnocytophaga canimorsus septicemia and demonstration of bacteremia by peripheral blood smear. Arch Pathol Lab Med. 2000;124:859–863. doi: 10.5858/2000-124-0859-WFSSTC. [DOI] [PubMed] [Google Scholar]
- 147.Morrison U., Taylor M., Sheahan D.G., Keane C.T. Waterhouse–Friderichsen syndrome without purpura due to Haemophilus influenzae group B. Postgrad Med J. 1985;61:67–68. doi: 10.1136/pgmj.61.711.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piccioli A., Chini G., Mannelli M., Serio M. Bilateral massive adrenal hemorrhage due to sepsis: report of two cases. J Endocrinol Invest. 1994;17:821–824. doi: 10.1007/BF03347786. [DOI] [PubMed] [Google Scholar]
- 149.Tosoni A., Nebuloni M., Ferri A., Bonetto S., Antinori S., Scaglia M. Disseminated microsporidiosis caused by Encephalitozoon cuniculi III (dog type) in an Italian AIDS patient: a retrospective study. Mod Pathol. 2002;15:577–583. doi: 10.1038/modpathol.3880566. [DOI] [PubMed] [Google Scholar]
- 150.Mertens R.B., Didier E.S., Fishbein M.C., Bertucci D.C., Rogers L.B., Orenstein J.M. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod Pathol. 1997;10:68–77. [PubMed] [Google Scholar]
- 151.Riestra-Castaneda J.M., Riestra-Castaneda R., Gonzalez-Garrido A.A., Pena Moreno P., Martinez A.J., Visvesvara G.S. Granulomatous amebic encephalitis due to Balamuthia mandrillaris (Leptomyxiidae): report of four cases from Mexico. Am J Trop Med Hyg. 1997;56:603–607. doi: 10.4269/ajtmh.1997.56.603. [DOI] [PubMed] [Google Scholar]
- 152.Teixeira V. de P., Araujo M.B., dos Reis M.A., dos Reis L., Silveira S.A., Rodrigues M.L. Possible role of an adrenal parasite reservoir in the pathogenesis of chronic Trypanosoma cruzi myocarditis. Trans R Soc Trop Med Hyg. 1993;87:552–554. doi: 10.1016/0035-9203(93)90085-5. [DOI] [PubMed] [Google Scholar]
- 153.Teixeira V. de P., Hial V., Gomes R.A., Castro E.C., Reis M., das G., Rodrigues M.L. Correlation between adrenal central vein parasitism and heart fibrosis in chronic chagasic myocarditis. Am J Trop Med Hyg. 1997;56:177–180. doi: 10.4269/ajtmh.1997.56.177. [DOI] [PubMed] [Google Scholar]
- 154.Mondain-Miton V., Toussaint-Gari M., Hofman P., Marty P., Carles M., De Salvador F. Atypical leishmaniasis in a patient infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:663–665. doi: 10.1093/clinids/21.3.663. [DOI] [PubMed] [Google Scholar]
- 155.Brenner D.S., Jacobs S.C., Drachenberg C.B., Papadimitriou J.C. Isolated visceral leishmaniasis presenting as an adrenal cystic mass. Arch Pathol Lab Med. 2000;124:1553–1556. doi: 10.5858/2000-124-1553-IVLPAA. [DOI] [PubMed] [Google Scholar]
- 156.Akcay M., Akcay G., Balik A., Boyuk A. Hydatid cysts of the adrenal gland: review of nine patients. World J Surg. 2004;28:97–99. doi: 10.1007/s00268-003-6901-3. [DOI] [PubMed] [Google Scholar]
- 157.Bastounis E., Pikoulis E., Leppaniemi A., Cyrochristos D. Hydatid disease: a rare cause of adrenal cyst. Am J Surg. 1996;62:383–385. [PubMed] [Google Scholar]
- 158.Minneci P.C., Deans K.J., Banks S.M., Eichacker P.Q., Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004;141:47–56. doi: 10.7326/0003-4819-141-1-200407060-00014. [DOI] [PubMed] [Google Scholar]
- 159.Keh D., Sprung C.L. Use of corticosteroid therapy in patients with sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32(11 Suppl.):S527–S533. doi: 10.1097/01.ccm.0000142983.15421.11. [DOI] [PubMed] [Google Scholar]
- 160.Annane D., Bellissant E., Bollaert P.E., Briegel J., Keh D., Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004;329:480. doi: 10.1136/bmj.38181.482222.55. Epub 2004 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang S., Rothman R.E. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4:337–348. doi: 10.1016/S1473-3099(04)01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mansmann G., Lau J., Balk E., Rothberg M., Miyachi Y., Bornstein S.R. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–340. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 163.Lerttumnongtum P., Muttarak M., Visrutaratna P., Ya-In C. Imaging features of unusual adrenal masses. Australas Radiol. 2004;48:107–113. doi: 10.1111/j.1440-1673.2004.01268.x. [DOI] [PubMed] [Google Scholar]
- 164.Grover S., Midha N., Gupta M., Sharma U., Talib V. Imaging spectrum in disseminated histoplasmosis: case report and brief review. Australas Radiol. 2005;49:175–178. doi: 10.1111/j.1440-1673.2005.01369.x. [DOI] [PubMed] [Google Scholar]


