Summary
Emerging and re-emerging zoonoses are a significant public health concern and cause considerable socioeconomic problems globally. The emergence of severe acute respiratory syndrome (SARS), highly pathogenic avian influenza (HPAI) H5N1, avian influenza H7N9, and severe fever with thrombocytopenia syndrome (SFTS), and the re-emergence of rabies, brucellosis, and other zoonoses have had a significant effect on the national economy and public health in China, and have affected other countries. Contributing factors that continue to affect emerging and re-emerging zoonoses in China include social and environmental factors and microbial evolution, such as population growth, urbanization, deforestation, livestock production, food safety, climate change, and pathogen mutation. The Chinese government has devised new strategies and has taken measures to deal with the challenges of these diseases, including the issuing of laws and regulations, establishment of disease reporting systems, implementation of special projects for major infectious diseases, interdisciplinary and international cooperation, exotic disease surveillance, and health education. These strategies and measures can serve as models for the surveillance and response to continuing threats from emerging and re-emerging zoonoses in other countries.
Keywords: Emerging zoonoses, Re-emerging zoonoses, Contributing factors, Control strategies and measures, China
1. Introduction
Economic globalization promotes globalization of capital, technology, labor, and commodities, and also contributes to the globalization of emerging infectious diseases. The world is becoming a global community, and a public health event in one geographic region or nation often affects the health and economy beyond that region, sometimes even globally. China has 34 provincial-level administrative regions and 2856 counties, which are covered by urban and rural health systems. The urban health system comprises hospitals of three different levels, disease control and prevention centers, and health authorities, while the rural health system includes county hospitals, health and epidemic prevention stations, and maternal and child health stations. As China has one fourth of the global population and has close ties with other countries through travel and trade, public health events in the country can have an important impact on distant populations in other countries, and the control of infectious diseases in China implies the control of infectious diseases in the world to a large extent.1 China is a major contributor to the spread and control of infectious diseases in the world. For example, the first case of severe acute respiratory syndrome (SARS) arose in 2002 in Guangdong, China, and was transmitted to Hong Kong, and then to other countries.2 The goose/Guangdong/1/1996 H5N1 virus found in a goose farm in southern China in 1996 is considered the precursor of the currently circulating highly pathogenic avian influenza (HPAI) H5N1 viruses in more than 60 countries.3
Most of these emerging infectious diseases are zoonoses, or zoonotic diseases, a group of diseases and infections naturally transmitted from vertebrate animals to humans. These diseases have a negative impact on economic development and human health in both developing and industrial countries.4 Of the 1415 species documented as pathogenic to humans in 2001, 61% originated from animals or animal products, and 70% of these zoonotic infections originated from wildlife.5 In this review, we summarize the major emerging and re-emerging zoonoses and contributing factors, and the strategies and measures being used to deal with the challenges of these diseases in China.
2. Major emerging and re-emerging zoonoses in China
The Chinese government established an infectious diseases reporting system in the 1950s. Since then, the number of notifiable diseases has increased from 15 to 41 (in 2011), with emerging zoonoses accounting for over 50% (Table 1 ); in 2011, 18% of cases and 36% of deaths resulted from emerging zoonoses.6 SARS, HPAI, rabies, Japanese encephalitis, brucellosis, and schistosomiasis japonica are considered major emerging zoonoses in China.7
Table 1.
Notifiable zoonotic diseases in China in 2011
| Disease | Pathogen | Category of diseasea |
|---|---|---|
| Plague | Yersinia pestis | A |
| Cholera | Vibrio cholerae | A |
| Severe acute respiratory syndrome (SARS) | SARS coronavirus | B |
| Acquired immunodeficiency syndrome (AIDS) | Human immunodeficiency virus (HIV) | B |
| Hepatitis E | Hepatitis E virus | B |
| Highly pathogenic avian influenza (HPAI) | Highly pathogenic avian influenza virus (HPAI virus) | B |
| H1N1 influenza | Influenza A virus subtype H1N1 | B |
| Hemorrhagic fever with renal syndrome (HFRS) | Hantaan virus | B |
| Rabies | Rabies virus | B |
| Japanese encephalitis | Japanese encephalitis virus (JEV) | B |
| Dengue fever | Dengue virus | B |
| Anthrax | Bacillus anthracis | B |
| Tuberculosis | Mycobacterium tuberculosis | B |
| Brucellosis | Brucella | B |
| Leptospirosis | Leptospira | B |
| Schistosomiasis | Schistosoma japonicum | B |
| Typhus | Orientia tsutsugamushi | C |
| Kala-azar | Leishmania donovani | C |
| Hydatid disease | Echinococcus granulosus, E. multilocularis | C |
The infectious diseases in China are divided into three categories (A, B, and C) according to their transmission modes, speed, and hazard to humans.
2.1. Severe fever with thrombocytopenia syndrome (SFTS)
In May 2007, a life-threatening disease, characterized by the sudden onset of fever, thrombocytopenia, and leukopenia, designated severe fever with thrombocytopenia syndrome (SFTS), was first described in several provinces in central and northeast China.8 In 2009, the novel bunyavirus, also named SFTS virus (SFTSV), belonging to the family of Bunyaviridae, genus Phlebovirus, was identified as the causative agent of this disease.9
SFTS has been found in the provinces of Henan, Hubei, Shandong, Anhui, Liaoning, and Jiangsu, and has often occurred in spring and summer, especially in hilly regions.9 People are generally susceptible, especially those who live or work in the hills, mountains, and forests. Some SFTS patients have reported that they had seen or been bitten by ticks before the onset of their illness, and Haemaphysalis longicornis has been found infected with SFTSV.10 Specific antibody to SFTSV antigens has been detected in animals in endemic regions.11 SFTSV is therefore proposed to be transmitted by contact with animals and/or vectors.
The virus is also frequently detected in urine, feces, or throat specimens of patients, and thus can be transmitted from person to person through contact with the blood or secretions of patients.12 Several outbreaks of family clusters of SFTS have also provided evidence of person-to-person transmission.8, 13 Recently, a novel bunyavirus was detected in two patients with a history of tick bite in the USA, whose clinical features were similar to those of SFTS, suggesting that SFTS has become an emerging zoonosis worldwide.14
2.2. SARS
SARS is an emerging zoonosis caused by a new type of coronavirus in the world. The earliest cases of SARS occurred in Guangdong Province in November 2002, spread rapidly to Hong Kong and other provinces in China, and then from Hong Kong to other countries. China reported 5327 of 8098 (66%) cases of SARS and 349 of 774 (45%) deaths during the 2003 outbreak.2 The origin of the virus was never definitely confirmed, but coronavirus has been isolated from palm civets, raccoon dogs, ferret badgers, cats, and bats, which might serve as the animal reservoirs or infection sources for humans.15, 16, 17, 18
2.3. HPAI H5N1
Since the first identification of HPAI H5N1 virus in Guangdong in 1996, the disease has been found in poultry, including chickens, ducks, and geese, in 16 provinces of China, and in wild aquatic birds from Qinghai and Tibet.19 There were 36 confirmed human cases infected with H5N1 and 25 deaths during 2005–2011 in China.20 HPAI is spread by direct contact with infected birds or indirect contact with the virus contaminants, and humans are mainly infected by contact with infected chickens. Wild birds play an important role as long-distance animal reservoirs of HPAI virus.21
2.4. Avian influenza H7N9
In March 2013, a novel reassortant avian-origin influenza A (H7N9) virus was isolated from three patients who died following a severe lower respiratory tract disease. One of these patients was from Anhui Province and the other two from Shanghai City.22 Up to October 2013, China had reported a total of 136 cases infected with H7N9 virus in eight provinces and two municipalities, of which 45 were fatal, showing a fatality rate of 33.1%.23
Surveillance in live-poultry markets demonstrated that pigeons, chickens, and ducks were all positive for H7N9 virus, but did not present any clinical symptoms.24 Several H7N9 patients reported that they had been in close contact with birds at wet markets, or had consumed poultry from wet markets, showing the zoonotic introduction of H7N9 virus from avian species to humans.24, 25 Avian species carrying H7N9 virus, including their secretions and excretions, may be the source of infections. The virus may be transmitted through the respiratory tract or by exposure to the secretions or excretions of the infected birds. Direct exposure to the virus can also result in infection.26 Although there is no confirmed evidence of human-to-human transmission, H7N9 virus poses a potentially high risk to humans, as the virus can bind to both human-type and avian-type receptors.27, 28
2.5. Rabies
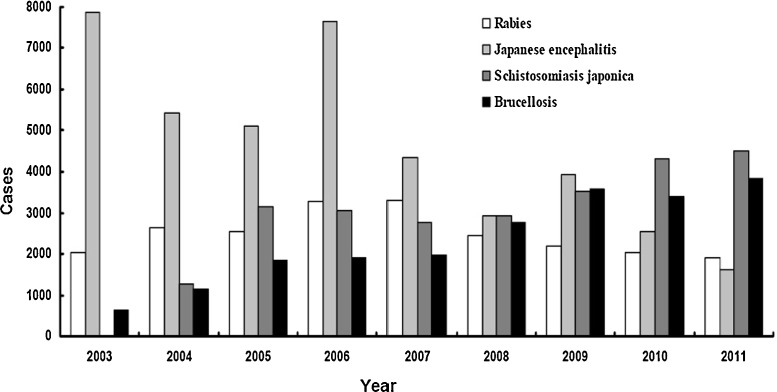
Rabies is a fatal zoonotic disease of almost all mammalian species, with a history of more than 2000 years in China. Since the 1950s, there have been three rabies epidemic peaks in China.29 The first occurred in the mid-1950s resulting in up to 1900 annual deaths; the second peak occurred in the early 1980s with annual deaths of 5537; the third peak occurred in the early 2000s with annual deaths of 2400, and up to 3300 in 2007. In 1996, only 21 provinces including 98 counties reported rabies, but the number reached 23 provinces including 984 counties in 2007.30 During the last 10 years, human infection with rabies virus has continued at a high level of 2000 annual cases, with another peak reached in 2007 of 3300 cases (Figure 1 ). The high incidence of rabies is due to low vaccination coverage and a high population density of cats and dogs. Domestic and stray dogs play an important role in the transmission of rabies in China, with 85–95% of human rabies cases resulting from dog bites.31, 32
Figure 1.
Reported cases of the four major zoonotic diseases – rabies, Japanese encephalitis, schistosomiasis japonica, and brucellosis – in China during 2003–2011. The data were derived from epidemic information of notifiable infectious diseases in China. The brucellosis cases are expressed as 10% of the real incidences, and the cases of the other three diseases are expressed as the actual incidences. The number of cases of schistosomiasis japonica was not available for the year 2003, because the disease was first considered a notifiable disease in 2004.
2.6. Japanese encephalitis
Japanese encephalitis is an emerging vector-borne disease in South and East Asia, including China. It is estimated that three billion people live in the endemic regions, and the annual incidence is approximately 30 000–50 000 cases worldwide.33 In China, Japanese encephalitis has spread to all of the provinces, except Xinjiang and Qinghai, and there were 39 805 cases and 1876 deaths reported during the last 10 years, probably due to population growth, climate change, pig rearing, and the lack of vaccination surveillance (Figure 1). Japanese encephalitis virus (JEV) is transmitted by the mosquito Culex tritaeniorhynchus in northern China, and Culex pipiens quinquefasciatus in southern China.34 Water birds, especially herons and egrets are the main animal reservoirs, and pigs are important amplifier hosts, from which JEV can be transmitted to humans and other vertebrates by the bites of mosquitoes.35
2.7. Brucellosis
Brucellosis is distributed worldwide, particularly in developing countries, with an annual occurrence of more than 500 000 cases.36 In China, human brucellosis was epidemic in the mid-1950s and 1970s, and a substantially decreased incidence was observed in the mid-1990s.37 However, the disease has re-emerged rapidly during the past 10 years, from 6448 new cases in 2003 to 38 151 in 2011, possibly due to frequent market circulation and a lack of disease quarantine (Figure 1). Humans are usually infected via direct contact with infected animals, by eating or drinking contaminated animal products, or by inhaling airborne agents.38
2.8. Schistosomiasis japonica
Schistosomiasis japonica remains a major public health problem in China, although the number of human cases of schistosomiasis reached an historical low of 694 788 by 2000, due to the implementation of integrated control strategies.39 However, the disease re-emerged in 2001, and there were 843 011 infected people, including 1114 acute cases and 24 441 advanced cases by 2003. There are 65 million people at risk of infection, and 74 000 infected cattle and a snail-infested area of 3787 km2.40 A total of 3145 new cases of human schistosomiasis were reported in 2005, and this increased to 4483 in 2011, probably resulting from large population movements and changing ecology. Bovines, particularly water buffaloes (Bubalus bubalis), play an important role in the transmission of the disease in China, while other animals, such as rats, dogs, pigs, sheep, and goats may contribute only minimally to overall transmission of the disease.41
3. Important factors contributing to emerging and re-emerging zoonoses
The factors contributing to emerging and re-emerging zoonoses are complicated and include microbial evolution and social and environmental factors. Microbial evolution is the intrinsic factor that leads to the emergence of new pathogens, and social and environmental factors are external factors, including population growth, mobility, and urbanization, and environmental changes such as agriculture and livestock production, deforestation, and climate change. These have all had important effects on emerging and re-emerging zoonotic diseases in China.
3.1. Population growth
China is the most populous country in the world, with1.3 billion people on land of 9.6 million square kilometers – approximately 20% of the total world population living on 6.7% of the world land. The average population density is 130 people per square kilometer, with an uneven distribution of over 400 in the eastern coastal region, 200 in the central region, and less than 10 in the western region.42 About 16 million babies are born every year; the population is still increasing annually by 0.57% and will reach 1.5 billion by 2033.43 The increased susceptible population will increase the potential for disease transmission, and increased population density can directly contribute to the spread of respiratory diseases, as occurred in the SARS outbreak in 2003.44
The number of older people is also growing in China. The percentage of the population aged over 60 years is now about 10%, and will reach 14.8% in 2015, i.e. up to 200 million. Aging is related to an increased susceptibility to some zoonotic diseases; the incidence of active pulmonary tuberculosis in individuals aged over 60 years is three times the average incidence in China.45 In addition, the incidence of malignant tumors increases with age, and those populations receiving immunosuppressive therapy are susceptible to opportunistic pathogens. For example, Toxoplasma gondii is an important opportunistic pathogen associated with malignant disorders and HIV patients.46, 47
3.2. Urbanization
In addition to rapid population growth, China is rapidly becoming more urbanized. By the end of 2010, China had a total urban population of 665 million, accounting for 50% of the total population; this is expected to reach around 70% by 2035, surpassing the average in the world.48 This massive urbanization has significant public health implications, increasing the occurrence of emerging and re-emerging zoonoses transmitted by water or air, as well as those diseases transmitted through animals or insects, due to the poor healthcare systems and inadequate infrastructure.48
Accumulated garbage provides food and habitats for rodents and other stray animals, and inadequate water supplies make it necessary to store water in containers, which will become an ideal habitat for mosquito larvae. These factors contribute to emerging and re-emerging zoonotic diseases related to arthropods and rodents, such as Japanese encephalitis, dengue fever, and epidemic hemorrhagic fever.49
Urbanization provides opportunities for rural migrants, including higher salaries and improved health conditions. Unfortunately, not all people can find a satisfying job in the city, due to low educational levels and poor professional skills. Because of the commercialization of blood and blood products in China, the sale of plasma by poor people has become a popular way of making a living.50 The reused needles and inadequately sterile equipment facilitate the transmission of HIV through blood and blood products.51
Urbanization also promotes population movement and migration. Most people are from rural areas, but work in urban environments, hence regularly travelling between the two sites. Due to financial constraints and overcrowded and unsanitary living conditions, these people are more vulnerable to infectious diseases, especially HIV and viral hepatitis. Additionally, their mobility promotes the transmission of infectious disease agents.48
3.3. Deforestation
Human population growth and over-exploitation of the land, such as logging, mining, road construction, and agricultural production, has resulted in deforestation. These changes in turn cause a cascade of factors that aggravate the emergence of zoonotic diseases, such as forest fragmentation, population migration, pathogen introduction, and environmental pollution.52
Deforestation forces wildlife to leave their habitat, increasing interactions among humans, wildlife, and livestock, and thus the potential for the transmission of pathogens directly or indirectly to human beings.53, 54 Some zoonotic diseases, including mountain and desert subtypes of leishmaniasis in western China, Lyme disease in northern China, and SFTS in central and northeast China caused by a novel bunyavirus, are related to deforestation, and humans are infected by the bites of wild vectors, Phlebotomus chinensis for leishmaniasis, Ixodes persulcatus for Lyme disease, and Haemaphysalis longicornis for SFTS during agricultural activities on the land changed from a forest.9, 55, 56
3.4. Livestock production
Livestock production in China has expanded at an unprecedented rate over the past three decades. The consumption of pigs increased 3.7-fold from 160 million in 1978 to 610 million in 2008, of cattle increased 1.5-fold from 70 million to 106 million, of sheep and goats increased 1.65-fold from 170 million to 281 million, and of poultry increased 7.3-fold from 170 million to 9700 million.57 Large-scale farms and scattered small-scale individual farms coexist in China, and the latter account for the majority.58 Almost every household in rural areas is engaged in livestock production, including pigs, chickens, bovines, sheep, ducks, etc. Different domestic animals live in the same environment, which provides a dynamic micro-environment that contributes to interspecies transmission, even from animals to humans.59, 60
Another example is human echinococcosis in Tibetan pastoral communities in western China, in which dogs, domestic animals (yaks, sheep, and goats), and wild animals living in the same environment are involved in the transmission cycle of Echinococcus granulosus and Echinococcus multilocularis, contributing to the high prevalence of human echinococcosis.61
Due to the practice of scattered small-scale individual farming and the lack of meat inspection at such small operations, farmers often slaughter their animals for their own consumption. Compared with professionals in modern abattoirs, the local farmers do not wear protective clothing or gloves, resulting in high incidences of brucellosis infection in this population and other livestock-related occupations, such as herdsmen, coat and leatherworkers, and veterinarians.37 On the other hand, the large-scale farms, also termed concentrated animal feeding operations (CAFO), are increasing at an unprecedented rate in China, which will increase the risk of disease transmission.
Chinese livestock production is also challenged with the threat of diseases; the use of some antibiotics in animal feed has been allowed for a long time for the prevention of animal diseases and to promote animal growth.62 However, the overuse of antibiotics speeds up the production of drug resistance of bacteria and the emergence of zoonotic disease. Streptococcus suis is a porcine pathogen and occasional cause of disease in humans. Human S. suis outbreaks were reported in 1998 and 2005; they affected 25 and 204 people, and killed 14 and 38 people, respectively.63 The high mortality rate and high proportion of toxic shock-like syndrome in these outbreaks were found to be associated with drug resistance and the rapid evolution of virulence of S. suis serotype 2.64
3.5. Food safety
Food-borne pathogens are also associated with the emergence and re-emergence of zoonotic diseases. In 2005, 1.8 million people worldwide died of diarrheal diseases due to contaminated food and drinking water.65 Although the burden of diseases caused by food-borne pathogens remains largely unknown in China, there have been many public health emergencies related to food-borne pathogens during the past 20 years. For example, a large outbreak of human angiostrongyliasis occurred in Beijing in 2006, affecting 160 persons.66 From 2004 to 2009, 15 outbreaks of human trichinellosis occurred in China, resulting to 1387 cases and four deaths.67 Other important food-borne zoonotic pathogens include Salmonella spp, Campylobacter spp, Escherichia coli, and hepatitis E virus.68
Many factors are associated with the emergence and re-emergence of food-borne zoonotic diseases, including the global market in meat and farm animals, the habit of eating raw or undercooked food or wildlife, and the increasingly immunocompromised population.65
3.6. Climate change
Global temperatures are rising, and will increase by 1.8–5.8 °C by the end of the 21st century.69 The annual average temperature in China has increased significantly, reaching 0.5 to 0.8 °C, and will increase by 1.3–2.1 °C in 2020, and by 2.3–3.3 °C in 2050.70 The increased temperature can enhance pathogen development, disease transmission, and host susceptibility.71
Animal and vector-borne diseases are strongly affected by climate change. Increased temperatures will accelerate the development of mosquitoes. Since mosquitoes lay their eggs in water-filled containers, rainfall often affects larval habitats and vector populations. In some cases, rainfall may change larval habitats and vector populations. Excessive rain can remove habitats through flooding, while limited rainfall may create new habitats, when water in the rivers is drawn into pools to create novel breeding sites for a number of mosquito species during the dry season.69
A typical example is Schistosomiasis japonicum, whose intermediate host Oncomelania hupensis is usually limited to areas with a mean January temperature of above 0 °C. Due to the increasing temperature, schistosomiasis japonica will be transmitted to non-endemic areas in the north, and the additional risk area is expected to be increased by 783 883 km2 by 2050, equivalent to 8.1% of the surface area of China.72
Waterborne infectious diseases are also strongly affected by climate change. During times of drought, water scarcity results in poor sanitation, therefore, many people can be exposed to potentially contaminated water. In contrast, excess rainfall and flooding can also cause epidemics of waterborne infectious diseases, including bacterial and protozoan diarrheal illnesses.73, 74
3.7. Pathogen mutation
Changes in natural conditions often lead to genetic evolution of microorganisms, such as genetic reassortment and recombination, horizontal transfer of virulence elements, and antimicrobial resistance, possibly resulting in the transition of microbes from non-pathogenic to pathogenic, from low virulence to high virulence, thus causing the emergence of zoonotic diseases.
One example of the effects of genetic reassortment on the emergence and re-emergence of zoonotic diseases is HPAI H5N1.75 Influenza A viruses possess a genome of segmented RNA fragments and genetic reassortment usually happens.76 If a host is co-infected with two different subtypes of avian influenza virus, new viral particles will probably be assembled by genetic reassortment, in which some gene fragments come from one subtype and some come from another. This genetic reassortment contributes to newly emerging pathogens of humans and animals, such as the recent emergence of the swine-origin influenza A (H1N1) virus.77
The SARS coronavirus HKU1 (CoV HKU1), isolated from patients in Hong Kong from 2003 to 2005, was divided into three clusters: genotypes A, B, and C. However, the phylogenetic analysis of different genomic regions showed that the nucleotide sequences of putative proteins (nsp) 4 to nsp6 of the genotype A strains were clustered with the genotype B strains, while nsp7 and nsp8 and from nsp10 to nsp16 from the genotype A strains were clustered with the genotype C strains, suggesting that genetic recombination between genotypes A and B generated genotype C.78 This genetic recombination in coronaviruses could easily generate novel coronavirus genotypes or species that could cross host species barriers and cause the emergence of major zoonotic diseases.
S. suis sequence type (ST) 7, in which the tetracycline resistance gene, tetM, was detected, caused two large outbreaks and sporadic infections in China.63 S. suis ST7 acquired tetM on the conjugative transposon Tn916 via horizontal transfer.79 The abuse of tetracyclines on swine farms could have provided the selective pressure for clone amplification and spreading, thus causing the outbreak of S. suis ST7.
4. Control strategies and measures
As in many countries, responsibilities for health issues in humans, domestic animals, and wild animals in China are separated into several different ministries and levels of government, including the Ministry of Health, Ministry of Agriculture, and Ministry of Forestry, respectively. In order to respond to these emerging and re-emerging zoonoses, the Chinese government has endeavored to enhance the prevention and control of infectious diseases since the 2003 SARS epidemic.
4.1. Formulation of laws and regulations
The Chinese government has developed and enacted a series of laws and regulations for the prevention and control of infectious diseases in humans, domestic animals, and wild animals, such as the Infectious Diseases Prevention Law of the People's Republic of China, Emergency Regulations on Public Health Emergencies, Major Animal Disease Emergency Ordinance, Animal Epidemic Prevention Law of the People's Republic of China, National Sudden Major Animal Disease Contingency Plans, Terrestrial Wild Animal Epidemic Sources and Disease Monitoring Standards.80, 81, 82 These laws and regulations define the responsibilities of governments at all levels, and help them to report, control, treat, and take other emergency measures against infectious diseases in humans, domestic animals, and wild animals.83
4.2. Establishment of the disease reporting systems
The Chinese government has also established disease reporting systems for humans, domestic animals, and wild animals. In fact, a routine reporting system for selected infectious diseases in humans was established in the 1950s.11 Currently the Ministry of Health has improved the disease reporting system, which was switched from paper, or electronic file-based reporting to web-based reporting. In the past, hospitals and clinics submitted case reports to their county or district Center of Disease Control and Prevention (CDC), and subsequently the local CDC submitted the reports to the National CDC.84 Using the present reporting system, hospitals and clinics can immediately and directly report cases through the internet, allowing public health officials to have real-time information on diseases, and decide to enforce any needed control strategies.1
The Ministry of Forestry has constructed a disease monitoring and reporting system for wild animals. Currently, China has established 350 national monitoring stations and 768 provincial, municipal and county stations or points, which cover the key areas of activities of the vast majority of migratory birds and has effectively prevented the spread of avian influenza by migratory birds.85 The Ministry of Agriculture has also established a disease reporting system for domestic animals and cross-border animals.86
The State Council has stressed the need to enforce cooperation and information sharing between ministries and between different levels of government. Therefore, the Chinese government can respond effectively and efficiently when confronted with a disease crisis.
4.3. Implementation of special projects for major infectious diseases
The Chinese government has carried out a special project for the prevention and control of major infectious diseases, whose aims are to reduce the incidence and mortality of AIDS, viral hepatitis, and tuberculosis, and to improve the emergency response and capabilities of infectious disease prevention and control. The project has also focused on the detection of infectious diseases and laboratory monitoring with standardized technology, providing technical support for emergency decisions related to infectious diseases.87
Based on thematic studies, the laboratory network, common technology platform, academic union, demonstration areas, and the effective integration of superior forces, a multi-linked network technology system has been developed, and the prevention and control technology support system for infectious diseases has been established.87 The project has improved the ability to deal with the traditional infectious diseases and has also contributed to the significantly enhanced emergency response abilities against emerging zoonotic diseases. For example, in response to the pandemic caused by a novel swine-origin influenza A (H1N1) virus in 2009, an effective and safe vaccine for various age groups was first developed in China, which played an important role in controlling the influenza epidemic.88 Another major breakthrough in the field of global etiological research since the discovery of the SARS coronavirus, is the discovery in 2009 that a novel bunyavirus had been responsible for a life-threatening illness associated with fever and thrombocytopenia in China since 2007.9
4.4. Surveillance of exotic diseases
A large number of wild animals, including wild boar, geese, gazelles, red deer, wild birds, and captive wild zoo animals (canines, felines, and monkeys) are imported into China from Africa, South America, Oceania, Asia, and other countries every year.89, 90 To prevent certain exotic diseases that are emerging abroad but are not found in China, such as bovine spongiform encephalopathy, Ebola hemorrhagic fever, West Nile fever, and monkeypox, from entering China, the Chinese government has strengthened surveillance and control of cross-border transmission of these exotic diseases and has established the Joint Control Mechanism of Transboundary Animal Diseases in China and neighboring countries. This includes the establishment of direct dialogue mechanisms at the national level, provincial veterinary departments, and animal disease information sharing platforms, and transboundary animal disease prevention and control stations.91, 92
4.5. Emphasis on interdisciplinary and international cooperation
Recent outbreaks of the emerging HPAI H5N1 and SARS, and the re-emerging rabies, brucellosis, and other zoonoses in China, represent examples of interactions among humans, animals, and their environment that contribute to the presence and spread of emerging infectious diseases. The rise in emerging and resurging zoonoses threatens not only human health, but also animal health and socioeconomic development. The Chinese government has established interdisciplinary and cross-sector collaborations and communications among human, animal, and environment health services, which reflect the ‘One Health’ strategy to confront emerging infectious disease. Moreover, the emphasis should be to build international early warning systems by international collaboration and coordination to detect unknown infectious diseases of international public health importance.93
4.6. Health education
Most emerging and re-emerging zoonotic diseases come from wildlife. China has 56 ethnic groups with different ways of life and customs, and some people have a habit of consuming wild animals and raw meat.94, 95 Therefore, healthy eating habits have been recommended and the consumption of wild animals is prohibited by legislation. All cutting boards, knives, and other materials touching uncooked meat should be separated from those used for cooked meat, and also washed thoroughly with soap and water.96
Pets may be another important source of zoonotic diseases in China, including rabies, toxoplasmosis, and salmonellosis.97, 98 Proper care of pets may prevent the transmission of pet-borne zoonotic diseases to humans, including compulsory vaccination of pets, keeping pet living areas clean, and washing hands thoroughly after handling pets.99 The public are educated about the risk factors associated with wildlife and pets.100, 101
5. Conclusions
In the past 10 years, the emergence of SARS, HPAI H5N1, avian influenza H7N9, and SFTS, and the re-emergence of rabies, brucellosis, and other zoonoses, have not only posed a significant national burden on economies and public health in China, but have also affected other countries. These zoonotic diseases represent examples of interactions among humans, animals, and their environment, which continue to have an important impact on zoonotic diseases, facilitating the emergence of new diseases or the re-emergence of old ones. The social and natural conditions that promote the spread of zoonotic diseases include rapid population growth, increasing population migration due to urbanization, changes in the habitats of host animals and vectors by deforestation, backyard animal breeding accompanied by concentrated animal feeding operations, the global market in meat and farm animals, the habit of eating raw or undercooked food and wildlife, and the increasingly immunocompromised individuals.
As no one knows what, when, and where the next novel zoonoses will emerge, we must be prepared for the unexpected.102 Since the 2003 SARS epidemic, the Chinese government has devised new strategies and has taken measures to deal with the emergence and re-emergence of zoonotic diseases, including issuing laws and regulations for the prevention and control of infectious diseases of humans, domestic animals, and wild animals, the establishment of disease reporting systems for humans, domestic animals, and wild animals, the implementation of special projects for major infectious diseases to improve the emergency response and capabilities of infectious diseases prevention and control, the surveillance of exotic diseases to prevent cross-border transmission of zoonotic diseases, an emphasis on interdisciplinary and international cooperation according to the ‘One Health’ concept, and proposing a healthy, active lifestyle. These strategies and measures can serve as models for surveillance and the response to threats from major emerging and re-emerging zoonotic diseases in other countries.
Acknowledgements
Research in the authors’ laboratory was funded, in part, by the National Natural Science Foundation of China (Grant No. 31372430), the Special Fund for Agro-scientific Research in the Public Interest (Grant Nos. 201303037, 201303042), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (Grant No. 2013BAD12B04), the Science Fund for Creative Research Groups of Gansu Province (Grant No. 1210RJIA006), the Open Funds of State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (SKLVEB2013KFKT006), and the National Basic Research Program of China (“973” Program; Grant No. 2012CB722501).
Conflict of interest: We declare that we have no conflicts of interest.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
References
- 1.Zhang L., Wilson D.P. Trends in notifiable infectious diseases in China: implications for surveillance and population health policy. PLoS One. 2012;7:e31076. doi: 10.1371/journal.pone.0031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam W.K., Zhong N.S., Tan W.C. Overview on SARS in Asia and the world. Respirology. 2003;8:S2–S5. doi: 10.1046/j.1440-1843.2003.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan L., Bahl J., Smith G.J., Wang J., Vijaykrishna D., Zhang L.J. The development and genetic diversity of H5N1 influenza virus in China, 1996–2006. Virology. 2008;380:243–254. doi: 10.1016/j.virol.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Zoonoses and veterinary public health 2004. Geneva: WHO; Available at: http://www.who.int/zoonoses/emerging_zoonoses/en (accessed July 25, 2012).
- 5.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health of the People's Republic of China. The epidemic of legal infectious diseases in China in 2011, 2011 (In Chinese). China: MOH; Available at: http://www.moh.gov.cn/publicfiles/business/htmlfiles/wsb/pyqxx/list.htm (accessed July 28, 2012).
- 7.Wang L., Wang Y., Jin S., Wu Z., Chin D.P., Koplan J.P., Wilson M.E. Emergence and control of infectious diseases in China. Lancet. 2008;372:598–605. doi: 10.1016/S0140-6736(08)61365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao C.J., Guo X.L., Qi X., Hu J.L., Zhou M.H., Varma J.K. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis. 2011;53:1208–1214. doi: 10.1093/cid/cir732. [DOI] [PubMed] [Google Scholar]
- 9.Yu X.J., Liang M.F., Zhang S.Y., Liu Y., Li J.D., Sun Y.L. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X.L., Wang X.J., Li J.D. Isolation, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals (In Chinese) Bing Du Xue Bao. 2012;28:252–257. [PubMed] [Google Scholar]
- 11.Cui F., Cao H.X., Wang L., Zhang S.F., Ding S.J., Yu X.J. Clinical and epidemiological study on severe fever with thrombocytopenia syndrome in Yiyuan County, Shandong Province, China. Am J Trop Med Hyg. 2013;88:510–512. doi: 10.4269/ajtmh.11-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y.Z., He Y.W., Dai Y.A., Xiong Y., Zheng H., Zhou D.J. Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin Infect Dis. 2012;54:527–533. doi: 10.1093/cid/cir804. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Li Q., Hu W., Wu J., Wang Y., Mei L. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12:156–160. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- 14.McMullan L.K., Folk S.M., Kelly A.J., MacNeil A., Goldsmith C.S., Metcalfe M.G. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 15.Wang M., Yan M., Xu H., Liang W., Kan B., Zheng B. SARS-CoV infection in a restaurant from palm civet. Emerg Infect Dis. 2005;11:1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Z., Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133:74–87. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Poder S. Feline and canine coronaviruses: common genetic and pathobiological features. Adv Virol. 2011;2011:609465. doi: 10.1155/2011/609465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martina B.E., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin V., Pfeiffer D.U., Zhou X., Xiao X., Prosser D.J., Guo F. Spatial distribution and risk factors of highly pathogenic avian influenza (HPAI) H5N1 in China. PLoS Pathog. 2011;7:e1001308. doi: 10.1371/journal.ppat.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health of the People's Republic of China. The epidemic of the highly pathogenic avian influenza in China during 2005-2011, 2012 (In Chinese). China: MOH; Available at: http://www.moh.gov.cn/publicfiles/business/htmlfiles/wsb/pyqxx/list.htm. (accessed August 11, 2012).
- 21.Prosser D.J., Cui P., Takekawa J.Y., Tang M., Hou Y., Collins B.M. Wild bird migration across the Qinghai-Tibetan plateau: a transmission route for highly pathogenic H5N1. PLoS One. 2011;6:e17622. doi: 10.1371/journal.pone.0017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 23.Dudley J.P., Mackay I.M. Age-specific and sex-specific morbidity and mortality from avian influenza A (H7N9) J Clin Virol. 2013;58:568–570. doi: 10.1016/j.jcv.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H., Wu J.T., Cowling B.J., Liao Q., Fang V.J., Zhou S. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet. 2013 doi: 10.1016/S0140-6736(13)61904-2. pii: S0140-6736(13)61904–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam T.T., Wang J., Shen Y., Zhou B., Duan L., Cheung C.L. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. 2013;502:241–244. doi: 10.1038/nature12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C., Wang J., Su W., Gao S., Luo J., Zhang M. Relationship between domestic and wild birds in live poultry market and a novel human H7N9 virus in China. J Infect Dis. 2014;209:34–37. doi: 10.1093/infdis/jit478. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J., Wang D., Gao R., Zhao B., Song J., Qi X. Biological features of novel avian influenza A (H7N9) virus. Nature. 2013;499:500–503. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q., Shi J., Deng G., Guo J., Zeng X., He X. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science. 2013;341:410–414. doi: 10.1126/science.1240532. [DOI] [PubMed] [Google Scholar]
- 29.Wu X., Hu R., Zhang Y., Dong G., Rupprecht C.E. Reemerging rabies and lack of systemic surveillance in People's Republic of China. Emerg Infect Dis. 2009;15:1159–1164. doi: 10.3201/eid1508.081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong W., Jiang Y., Za Y., Zeng Z., Shao M., Fan J. Temporal and spatial dynamics of rabies viruses in China and Southeast Asia. Virus Res. 2010;150:111–118. doi: 10.1016/j.virusres.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Jin Z., Sun G.Q., Zhou T., Ruan S. Analysis of rabies in China: transmission dynamics and control. PLoS One. 2011;6:e20891. doi: 10.1371/journal.pone.0020891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang X., Luo M., Zhang S., Fooks A.R., Hu R., Tu C. Pivotal role of dogs in rabies transmission. China. Emerg Infect Dis. 2005;11:1970–1972. doi: 10.3201/eid1112.050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erlanger T.E., Weiss S., Keiser J., Utzinger J., Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y., Li M., Wang H., Liang G. Japanese encephalitis and Japanese encephalitis virus in mainland China. Rev Med Virol. 2012;22:301–322. doi: 10.1002/rmv.1710. [DOI] [PubMed] [Google Scholar]
- 35.van den Hurk A.F., Ritchie S.A., Mackenzie J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 36.Seleem M.N., Boyle S.M., Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140:392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Deqiu S., Donglou X., Jiming Y. Epidemiology and control of brucellosis in China. Vet Microbiol. 2002;90:165–182. doi: 10.1016/s0378-1135(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 38.Franco M.P., Mulder M., Gilman R.H., Smits H.L. Human brucellosis. Lancet Infect Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang L.D., Guo J.G., Wu X.H., Chen H.G., Wang T.P., Zhu S.P. China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14:1475–1483. doi: 10.1111/j.1365-3156.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X.N., Wang L.Y., Chen M.G., Wu X.H., Jiang Q.W., Chen X.Y. The public health significance and control of schistosomiasis in China—then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 41.McManus D.P., Gray D.J., Li Y., Feng Z., Williams G.M., Stewart D. Schistosomiasis in the People's Republic of China: the era of the Three Gorges Dam. Clin Microbiol Rev. 2010;23:442–466. doi: 10.1128/CMR.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baidu Encyclopedia. Demographics of China (In Chinese). 2012 Available at: http://baike.baidu.com/view/244361.htm (accessed August 19, 2012).
- 43.Wikipedia, the free encyclopedia. Demographics of China. 2011 Available at: http://en.wikipedia.org/wiki/Demographics_of_China (accessed August 19, 2012).
- 44.Fang L.Q., de Vlas S.J., Feng D., Liang S., Xu Y.F., Zhou J.P. Geographical spread of SARS in mainland China. Trop Med Int Health. 2009;14:S14–S20. doi: 10.1111/j.1365-3156.2008.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., Zhang H., Jiang S., Wang J., Liu X., Li W. Active pulmonary tuberculosis case detection and treatment among floating population in China: an effective pilot. J Immigr Minor Health. 2010;12:811–815. doi: 10.1007/s10903-010-9336-6. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira M.S., Borges A.S. Some aspects of protozoan infections in immunocompromised patients—a review. Mem Inst Oswaldo Cruz. 2002;97:443–457. doi: 10.1590/s0074-02762002000400001. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Z., Gao S., Liu Q., Xia X., Liu X., Liu B. Toxoplasma gondii antibodies in cancer patients. Cancer Lett. 2007;254:71–74. doi: 10.1016/j.canlet.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Gong P., Liang S., Carlton E.J., Jiang Q., Wu J., Wang L. Urbanisation and health in China. Lancet. 2012;379:843–852. doi: 10.1016/S0140-6736(11)61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C., Maurer C., Wang Y., Xue S., Davis D.L. Water pollution and human health in China. Environ Health Perspect. 1999;107:251–256. doi: 10.1289/ehp.99107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Z., Rou K., Detels R. Prevalence of HIV infection among former commercial plasma donors in rural eastern China. Health Policy Plan. 2001;15:41–46. doi: 10.1093/heapol/16.1.41. [DOI] [PubMed] [Google Scholar]
- 51.He N., Detels R. The HIV epidemic in China: history, response, and challenge. Cell Res. 2005;15:825–832. doi: 10.1038/sj.cr.7290354. [DOI] [PubMed] [Google Scholar]
- 52.Patz J.A., Daszak P., Tabor G.M., Aguirre A.A., Pearl M., Epstein J. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qing C.E., Huang F.L., Xie L.Z., Tang Z. The spread and control of wildlife-derived zoonosis (In Chinese) J Public Health Prev Med. 2007;18:94. [Google Scholar]
- 54.Fu X.L., Ma F.L. The relationship between wildlife and zoonoses and prevention and control of the disease (In Chinese) Pract J Med. 2008;28:623–624. [Google Scholar]
- 55.Wang J.Y., Cui G., Chen H.T., Zhou X.N., Gao C.H., Yang Y.T. Current epidemiological profile and features of visceral leishmaniasis in People's Republic of China. Parasit Vectors. 2012;5:31. doi: 10.1186/1756-3305-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao Q., Hou X., Geng Z., Wan K. Distribution of Borrelia burgdorferi sensu lato in China. J Clin Microbiol. 2011;49:647–650. doi: 10.1128/JCM.00725-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y.L. Primary analysis of Chinese animal husbandry development (In Chinese) J Hebei Agric Sci. 2008;166:142–143. [Google Scholar]
- 58.Bedard B.G., Hunt T. The emerging animal health delivery system in the People's Republic of China. Rev Sci Tech. 2004;23:297–304. doi: 10.20506/rst.23.1.1486. [DOI] [PubMed] [Google Scholar]
- 59.Li Z., Chen H., Jiao P., Deng G., Tian G., Li Y. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J Virol. 2005;79:12058–12064. doi: 10.1128/JVI.79.18.12058-12064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen H., Deng G., Li Z., Tian G., Li Y., Jiao P. The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci U S A. 2004;101:10452–10457. doi: 10.1073/pnas.0403212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li T., Chen X., Zhen R., Qiu J., Qiu D., Xiao N. Widespread co-endemicity of human cystic and alveolar echinococcosis on the eastern Tibetan Plateau, northwest Sichuan/southeast Qinghai, China. Acta Trop. 2010;113:248–256. doi: 10.1016/j.actatropica.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y.Q. Veterinary antibiotics and their resistance (In Chinese) Chin J Antibiot. 2009;34:134–140. [Google Scholar]
- 63.Yu H., Jing H., Chen Z., Zheng H., Zhu X., Wang H. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:914–920. doi: 10.3201/eid1206.051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holden M.T., Hauser H., Sanders M., Ngo T.H., Cherevach I., Cronin A. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One. 2009;4:e6072. doi: 10.1371/journal.pone.0006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newell D.G., Koopmans M., Verhoef L., Duizer E., Aidara-Kane A., Sprong H. Food-borne diseases—the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol. 2010;139:S3–S15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J., Qi H., Diao Z., Zheng X., Li X., Ma S. An outbreak of angiostrongyliasis cantonensis in Beijing. J Parasitol. 2010;96:377–381. doi: 10.1645/GE-2214.1. [DOI] [PubMed] [Google Scholar]
- 67.Cui J., Wang Z.Q., Xu B.L. The epidemiology of human trichinellosis in China during 2004–2009. Acta Trop. 2011;118:1–5. doi: 10.1016/j.actatropica.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Shao D., Shi Z., Wei J., Ma Z. A brief review of foodborne zoonoses in China. Epidemiol Infect. 2011;139:1497–1504. doi: 10.1017/S0950268811000872. [DOI] [PubMed] [Google Scholar]
- 69.Shuman E.K. Global climate change and infectious diseases. N Engl J Med. 2010;362:1061–1063. doi: 10.1056/NEJMp0912931. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Bi P., Sun Y., Hiller J.E. Projected years lost due to disabilities (YLDs) for bacillary dysentery related to increased temperature in temperate and subtropical cities of China. J Environ Monit. 2012;14:510–516. doi: 10.1039/c1em10391a. [DOI] [PubMed] [Google Scholar]
- 71.Harvell C.D., Mitchell C.E., Ward J.R., Altizer S., Dobson A.P., Ostfeld R.S. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 72.Zhou X.N., Yang G.J., Yang K., Wang X.H., Hong Q.B., Sun L.P. Potential impact of climate change on schistosomiasis transmission in China. Am J Trop Med Hyg. 2008;78:188–194. [PubMed] [Google Scholar]
- 73.Auld H., MacIver D., Klaassen J. Heavy rainfall and waterborne disease outbreaks: the Walkerton example. J Toxicol Environ Health A. 2004;67:1879–1887. doi: 10.1080/15287390490493475. [DOI] [PubMed] [Google Scholar]
- 74.Charron D., Thomas M., Waltner-Toews D., Aramini J., Edge T., Kent R. Vulnerability of waterborne diseases to climate change in Canada: a review. J Toxicol Environ Health A. 2004;67:1667–1677. doi: 10.1080/15287390490492313. [DOI] [PubMed] [Google Scholar]
- 75.Cardona C.J., Xing Z., Sandrock C.E., Davis C.E. Avian influenza in birds and mammals. Comp Immunol Microbiol Infect Dis. 2009;32:255–273. doi: 10.1016/j.cimid.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Gutierrez R.A., Naughtin M.J., Horm S.V., San S., Buchy P. A (H5N1) virus evolution in South East Asia. Viruses. 2009;1:335–361. doi: 10.3390/v1030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lei F., Shi W. Prospective of genomics in revealing transmission, reassortment and evolution of wildlife-borne avian influenza A (H5N1) viruses. Curr Genomics. 2011;12:466–474. doi: 10.2174/138920211797904052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woo P.C., Lau S.K., Yip C.C., Huang Y., Tsoi H.W., Chan K.H. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J Virol. 2006;80:7136–7145. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye C., Bai X., Zhang J., Jing H., Zheng H., Du H. Spread of Streptococcus suis sequence type 7, China. Emerg Infect Dis. 2008;14:787–791. doi: 10.3201/eid1405.070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang C.J., Xiang H., Liu D.R., He J.J., Nie S.F. Reflection on legal construction of Chinese public health emergencies (In Chinese) Med Soc. 2007;20:38–40. [Google Scholar]
- 81.China Veterinary Chief Network. Policies and regulations, 2010 (In Chinese). Available at: http://www.cadc.gov.cn/sites/vs/List_21018_23750.html (accessed July 14, 2012).
- 82.The State Forestry Administration of China. Laws and regulations, 2012 (In Chinese). Available at: http://www.forestry.gov.cn//portal/slyy/s/3006/content-441181.html (accessed July 14, 2012).
- 83.Choi S.M., Lam P.Y. Enhancing legal preparedness for the prevention and control of infectious diseases: experience from severe acute respiratory syndrome in Hong Kong. Public Health. 2009;123:242–246. doi: 10.1016/j.puhe.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang H., Xue Y. Investigating public health emergency response information system initiatives in China. Int J Med Inform. 2004;73:675–685. doi: 10.1016/j.ijmedinf.2004.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The State Forestry Administration of China. Terrestrial wild animal epidemic sources and disease monitoring, 2012 (In Chinese). Available at: http://www.forestry.gov.cn/portal/main/s/3602/content-552483.html (accessed August 15, 2012).
- 86.Chinese Veterinary Network. Animal disease surveillance, 2010 (In Chinese). Available at: http://www.cadc.gov.cn/Sites/MainSite/List_2_44593.html (accessed August 16, 2012).
- 87.China Highlights. Significant achievements made in special project for major infectious diseases, 2011 (In Chinese). Available at: http://www.gov.cn/jrzg/2011-03/29/content_1833603.htm (accessed September 11, 2012).
- 88.Zhu F.C., Wang H., Fang H.H., Yang J.G., Lin X.J., Liang X.F. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 89.Wang J.W., Zhang N., Wang Z.L., Wang H., Bao J.Y. Hazard risk analysis of introduction of African swine fever in China (In Chinese) Chin Anim Quarantine. 2009;26:63–66. [Google Scholar]
- 90.Burke R.L., Kronmann K.C., Daniels C.C., Meyers M., Byarugaba D.K., Dueger E. A review of zoonotic disease surveillance supported by the Armed Forces Health Surveillance Center. Zoonoses Public Health. 2012;59:164–175. doi: 10.1111/j.1863-2378.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 91.Zhang B.Q., Lu C.P. Afferent pathways of exotic animal diseases and its prevention and control in China (In Chinese) Chin J Anim Quarantine. 2009;26:61–65. [Google Scholar]
- 92.Bai Q.Y., Sun Y.J., Zhang B.Q. The prevention and control system in the United States of America of exotic animal disease and the enlightenment to China (In Chinese) Chin J Inspection Quarantine. 2010;4:24–26. [Google Scholar]
- 93.Jia J. One World One Health (In Chinese) Chin J Comp Med. 2010;20:1–4. [Google Scholar]
- 94.Kruse H., Kirkemo A.M., Handeland K. Wildlife as source of zoonotic infections. Emerg Infect Dis. 2004;10:2067–2072. doi: 10.3201/eid1012.040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li T., He S., Zhao H., Zhao G., Zhu X.Q. Major trends in human parasitic diseases in China. Trends Parasitol. 2010;26:264–270. doi: 10.1016/j.pt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Liu Q., Wei F., Gao S., Jiang L., Lian H., Yuan B. Toxoplasma gondii infection in pregnant women in China. Trans R Soc Trop Med Hyg. 2009;103:162–166. doi: 10.1016/j.trstmh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 97.Enriquez C., Nwachuku N., Gerba C.P. Direct exposure to animal enteric pathogens. Rev Environ Health. 2001;16:117–131. doi: 10.1515/reveh.2001.16.2.117. [DOI] [PubMed] [Google Scholar]
- 98.Ryan C.P. Where do pets fit into human quarantines? J Public Health. 2007;29:70–71. doi: 10.1093/pubmed/fdl035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qi M., Wang R., Ning C., Li X., Zhang L., Jian F. Cryptosporidium spp. in pet birds: genetic diversity and potential public health significance. Exp Parasitol. 2011;128:336–340. doi: 10.1016/j.exppara.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 100.Wang J.W., Zhao Y.J., Lin Y. Causes and control strategies of frequent occurrence of zoonoses in China (In Chinese) Prog Vet Med. 2006;27:104–107. [Google Scholar]
- 101.Dong Y. Relationship between pets and human health (In Chinese) Chin J Comp Med. 2011;20:160–162. [Google Scholar]
- 102.Morse S.S., Mazet J.A., Woolhouse M., Parrish C.R., Carroll D., Karesh W.B. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]