Highlights
-
•
Hospitalizations for respiratory viruses are seasonal in Vietnam
-
•
Respiratory syncytial virus peaks in the late summer months, and inuenza A in April to June
-
•
No clear seasonality is seen for human rhinovirus
-
•
Human parainuenza 3 and human rhinovirus are positively associated with dew point
-
•
This work can inform the timing of inuenza and RSV vaccination and the judicious use of antibiotics in Vietnam
Keywords: Respiratory viruses, Seasonality of transmission, weather effects on transmission
Abstract
Background
Acute respiratory infections (ARIs) are the most common causes of death in children under 5 years of age. While the etiology of most pneumonia and ARI episodes is undiagnosed, a broad range of ARI-causing viruses circulate widely in South East Asia. However, the patterns and drivers of the seasonal transmission dynamics are largely unknown. Here we identify the seasonal patterns of multiple circulating viruses associated with hospitalizations for ARIs in Nha Trang, Vietnam.
Methods
Hospital based enhanced surveillance of childhood ARI is ongoing at Khanh Hoa General Hospital in Nha Trang. RT-PCR was performed to detect 13 respiratory viruses in nasopharyngeal samples from enrolled patients. Seasonal patterns of childhood ARI hospital admissions of various viruses were assessed, as well as their association with rainfall, temperature, and dew point.
Results
Respiratory syncytial virus peaks in the late summer months, and influenza A in April to June. We find significant associations between detection of human parainfluenza 3 and human rhinovirus with the month's mean dew point. Using a cross-wavelet transform we find a significant out-of-phase relationship between human parainfluenza 3 and temperature and dew point.
Conclusions
Our results are important for understanding the temporal risk associated with circulating pathogens in Southern Central Vietnam. Specifically, our results can inform timing of routing seasonal influenza vaccination and for when observed respiratory illness is likely viral, leading to judicious use of antibiotics in the region.
Introduction
Acute respiratory infections (ARIs) in South East Asia cause substantial morbidity and mortality, especially in children under 5 years of age (Walker et al., 2013). Pneumonia continues to be the number one cause of under 5 death despite effective treatments (Wardlaw et al., 2006). A substantial contributor to this is the largely unknown etiology of most pneumoniae, with both viral and bacterial origin. The patterns of pneumonia and other ARI hospitalizations serves as a proxy for determining the transmission dynamics of viruses and bacteria contributing to these hospitalizations, and is of key importance in understanding and limiting burden of this childhood killer.
In addition to informing on the relative likelihood of the potential viral etiology of a pneumonia case based on seasonally circulating pathogens, knowledge of seasonal influenza epidemiological dynamics can aid in informing optimal timing for vaccination efforts (Saha et al., 2014, Lambach et al., 2015) and judicious use of antivirals (Althouse et al., 2013, Tanaka et al., 2014). Identification of low incidence seasons would provide a target window for vaccination, with the hope of maximizing population immunity before the onset of the influenza season. Similarly, when respiratory syncytial virus (RSV) vaccination becomes available, knowledge of its seasonality will be useful to maximize benefit (Drysdale et al., 2016, Anderson et al., 2013), and in the potential administration of passive immunoprophylaxis with palivizumab to reduce the number of severe outcomes associated with RSV infection among high-risk infants (Committee on Infectious Diseases, 2009). Finally, knowledge of seasonal patterns of virus circulation can inform the clinical use of antibiotics, again limiting use when viral circulation is traditionally high to minimize antibiotic resistance (Van Nguyen et al., 2013, Laxminarayan et al., 2013, Laxminarayan et al., 2016, Van Boeckel et al., 2014).
Previous work has identified the potential etiology of pneumonia and other ARIs in Southern Central Vietnam (Do et al., 2011, Yoshida et al., 2010). Adenovirus (AoV), bocavirus (HBoV), coronavirus (CoV), human metapneumovirus (HMPV), human parainfluenza 1–4 viruses (HPIV1–4), human rhinovirus (HRV), Influenza A and B, and RSV all contribute to the disease burden and circulate widely. Information on viral transmission dynamics across seasons in Vietnam is relatively under-explored. Do et al. found seasonality of RSV infections and slight seasonality of HMPV infections in Ho Chi Minh City, but no seasonality of influenza (Do et al., 2011). Several studies have similarly found high variability in Influenza A & B incidence over the year (Nguyen et al., 2007, Le et al., 2015, Thai et al., 2015). Yoshida et al. found RSV occurring in the hot months, influenza A in the cool months, and year-round detection of HRV in Nha Trang (Yoshida et al., 2010). Few other studies have examined seasonality of these common viruses across South East Asia.
Here we examine the seasonal trends of hospitalizations and circulation of multiple viruses in Nha Trang, Vietnam. Using enhanced hospital based surveillance of childhood ARI we identify seasonal patterns in hospitalizations as a proxy for transmission and explore the relationship of hospitalizations associated with virus detection with rainfall, temperature, and dew point, to try and identify contributing factors to observed seasonality.
Methods
Study site
The study site is Nha Trang, central Vietnam, where the study population has been described previously (Yoshida et al., 2014, Yoshida et al., 2010, Flasche et al., 2014). The hospital based enhanced surveillance of childhood ARI is ongoing. We analyze data from January 29, 2007 to April 26, 2012 at Khanh Hoa General Hospital (KHGH) which is the only tertiary care facility located in Khanh Hoa Province. According to the field site census survey in July 2006, the study catchment area encompassing the 16 non-touristic of the 27 communes in Nha Trang city, had 198,729 residents including 13,952 children less than 5 years of age. An ARI case was defined as any child presenting to KHGH with cough or/and difficulty in breathing. Before study enrollment, informed consent was obtained from parents of children who presented with ARI and lived in the study catchment area. Clinical and demographic information, chest radiographs (CXR), laboratory data, and nasopharyngeal (NP) samples were collected from all enrolled patients. KHGH is the only hospital in Nha Trang, Khanh Hoa province and the only one accessible for residents of the catchment area. Hence for incidence calculations we assume that all children with ARI are eligible to be hospitalized and enrolled into the study and use the population of the catchment area as denominator. Acute respiratory infection patients with normal CXR were categorized as upper respiratory tract infection (URTI). Patients with abnormal CXR were categorized as lower respiratory tract infection (LRTI).
NP samples were collected at the time of admission and viral nucleic acid was extracted using QIA viral RNA minikit (QIAGEN Inc., Valencia, CA). Four multiplex-PCR assays (1: influenza A, influenza B, RSV, hMPV; 2: PIV-1, -2, -3, and -4; 3: rhinovirus, coronavirus 229E, coronavirus OC43; 4: adenovirus and bocavirus) were performed to detect 13 respiratory viruses in each NP sample. A second confirmatory-PCR was performed for samples positive on the initial PCR test. Samples positive for both PCR assays were defined as positive. Reverse transcription-PCR (RT-PCR) assays were performed using one-step RT-PCR kit from QIAGEN. For the multiplex PCR and hemi-nested PCR assays, TaqDNA polymerase (Promega, San Luis Obispo, CA) was used as previously described (Yoshida et al., 2010). Positive templates were used in each assay for quality control.
Weather data
Three weather variables – rainfall (inches), temperature (F∘), and dew point (the temperature to which air must be cooled in order to reach saturation with water) – were collected from the Nha Trang Station (ID: 488770) reported by the US National Oceanic and Atmospheric Administration (National Oceanic and Atmospheric Administration, 2017). We considered monthly averages of all weather variables as well as both the weather variable on the day of admission (t 0) as well as averaged over the previous 7 days (t −7 to t −1) assuming up to a week incubation period for the viral infections (see supplementary material) (Lessler et al., 2009). The weather in Nha Trang central Vietnam is warm throughout the year (between 20 and 30 C∘). In terms of temperature, December to February months are cooler (referred to here as the winter months) while June to August months are hottest months (referred to here as the summer months). September, October, November are the wettest months.
Statistical analysis
For each PCR+ for virus a series of log-link Poisson models were fit to assess respective seasonality with calendar month as the main predictor, log-commune population size as an offset term, monthly averaged rainfall, temperature, and dew point, and calendar year as adjusting variables. The outcome was monthly aggregate cases, with resulting coefficients as incidence rate ratios as compared to January. We excluded hospitalizations with more than one virus detected in the nasopharynx to adjust for a potential bias through inclusion of cases who by virtue of being co-infected may otherwise have been asymptomatic (Althouse and Scarpino, 2015). This approach underestimates the true incidence of NP carriage among ARI cases but allows estimation of seasonal patterns that are not biased by other circulating viruses; although co-circulation of bacteria was not accounted for. We assessed the numbers and variety of viruses in ARI hospitalizations using binomial proportion tests for each virus.
Cross-wavelet transform
To examine the relationship between monthly average rain, temperature, and dew point and incidence hospitalized childhood ARI infections, we estimated the cross-wavelet transform between the z-standardized time series (we subtracted the mean of the time series and divided by the standard deviation) of weather and viral detections (Cazelles et al., 2008). The cross-wavelet transform identifies regions of high power in phase-space and identifies the relative phases of each time series, i.e., in-phase or out-of-phase (Grinsted et al., 2004). The wavelet transform can be thought of a Fourier transformation over time that can identify what is the dominant frequency composing a time series as the signal changes in time. The cross-wavelet transform allows us to compare how two time signals co-vary: we can identify if the presence of a particular frequency at a given time in the time series of hospitalizations corresponds to the presence of that same frequency at the same time in a weather covariate (Chaves and Pascual, 2006). Additionally, we can identify the magnitude by which weather precedes or follows hospitalizations through the phase angle of the two time series. Finally, we can identify the statistical significance of the identified constituent frequencies over time by comparing the observed frequencies to a red-noise process.
Sensitivity analyses
Sensitivity analyses, presented in the supplementary materials, were performed as follows: 1) case counts of less than 70 per virus over the whole study period were deemed too low for robust statistical inference; 2) alternative Poisson regression models where the reference category is July; 3) logistic regression models were formulated as an alternative to the Poisson regressions above with detection of a virus by PCR (yes/no) as the outcome, with month as the main predictor, adjusted for weather, commune of residence, age, sex, smoking indoors, socioeconomic status (SES), and calendar year, with weather variables on the day of admission (t 0) as well as averaged over the previous 7 days (t −7 to t −1); and 4) additional wavelet analyses of viral isolations not presented in the main text.
Results
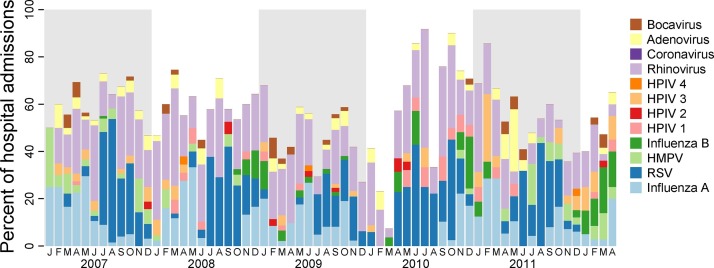
The study enrolled 3431 children between 2007 to 2013. Among those, 374 (11%) had multiple viruses detected in their NP swabs, for 59 presence of viruses in the NP was not determined, and were excluded from the analyses, thus the total study population was 2998. Among all cases with a virus detected, HRV, RSV, and Influenza A were the most frequently detected viruses, with 569 (33.5% of all viral detections), 455 (26.8%), and 282 (16.6%) detections, respectively (Table 1 and Figure 1 ). Counts of bocavirus (HBoV), coronavirus (CoV), and human parainfluenza 1, 2, and 4 viruses (HPIV1–4) were less than 70 and are reported in the supplementary material.
Table 1.
Annual hospitalizations by virus. Table shows counts of hospitalizations for adenovirus (ADV), human metapneumovirus (HMPV), human parainfluenza virus 3 (HPIV3), influenza A, influenza B, rhinovirus (HRV), and respiratory syncytial virus (RSV). Viruses with less than 70 cases were excluded from this table and are presented in the supplement. Counts of all virus detections (including those presented in the supplement are in the column ‘any virus’).
| ADV | HMPV | HPIV3 | HRV | Influenza A | Influenza B | RSV | Any virus | |
|---|---|---|---|---|---|---|---|---|
| 2007 | 21 | 28 | 19 | 137 | 84 | 1 | 149 | 451 |
| 2008 | 15 | 10 | 10 | 134 | 51 | 8 | 85 | 330 |
| 2009 | 20 | 1 | 9 | 129 | 74 | 10 | 68 | 333 |
| 2010 | 12 | 2 | 4 | 95 | 25 | 24 | 84 | 271 |
| 2011 | 13 | 24 | 17 | 61 | 37 | 8 | 69 | 235 |
| 2012 | 6 | 7 | 12 | 13 | 11 | 19 | 0 | 76 |
| Total (%) | 87 (5.1%) | 72 (4.2%) | 71 (4.2%) | 569 (33.5%) | 282 (16.6%) | 70 (4.1%) | 455 (26.8%) | 1696 (100%) |
Figure 1.
Monthly hospitalizations by virus in Nha Trang, Vietnam. Figure shows monthly detections of virus as percentages of all enrolled ARI hospitalizations.
Seasonality
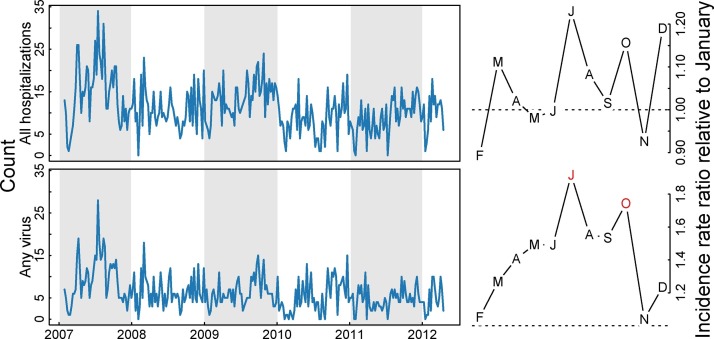
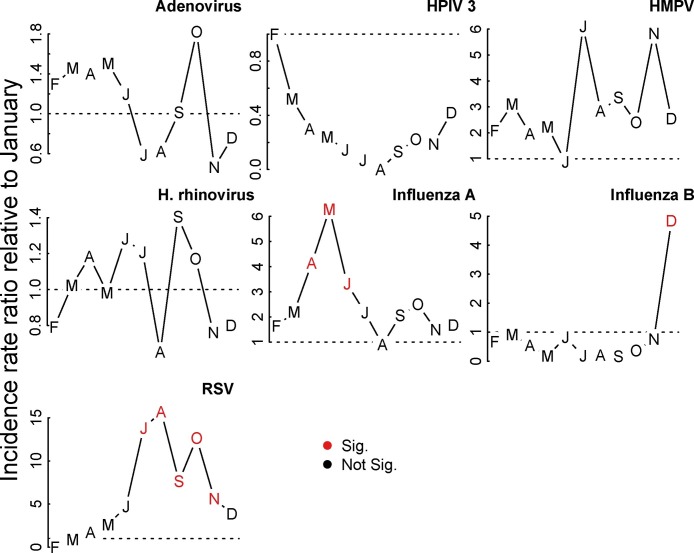
Strong seasonality, as defined by at least three consecutive months with a consistently higher or lower incidence than expected (IRR or OR greater or less than 1, respectively) and at least one of those statistically significantly different from the baseline, was observed for Influenza A, RSV, and the presence of any virus (Figure 2, Figure 3 ). RSV peaked in July through November, with August seeing a 15.69 (95% confidence interval [CI]: 3.05, 80.56) times higher risk of identifying RSV as the sole viral agent from the nasopharynx of a childhood case as compared to January. Influenza A peaked in May with an IRR of 6.28 (95% CI: 2.2, 17.89) as compared to January. Estimates of odds ratios from the supplemental logistic regression are qualitatively similar to the Poisson regression, save for HPIV3, which exhibits a consistent yet non-significant peak in the cool months in the primary analysis which becomes significant in the supplemental analysis (see supplemental material).
Figure 2.
Seasonality of hospitalizations and any viral detection in Nha Trang, Vietnam. Figure shows weekly counts of all hospitalizations (top row) and any virus detection (bottom row). Right-hand column shows model-adjusted incidence rate ratios for month of year as compared to January. Red months indicates statistically significant deviations from January.
Figure 3.
Seasonality of common respiratory viruses in Nha Trang, Vietnam. Figure shows model-adjusted incidence rate ratios for month of year as compared to January. Red months indicates statistically significant deviations from January.
Association with weather
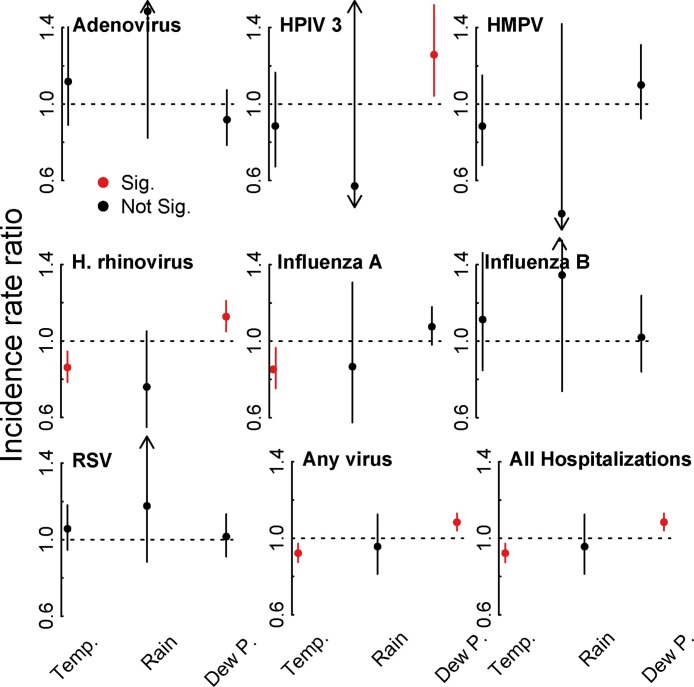
Weather patterns over the study period were similar to patterns before and after the study period (supplementary material, Figure S3). Monthly average rainfall (in inches), temperature (1∘ F), and dew point (1∘ F) correlated with the seasonality of some of our endpoints. Figure 4 shows the incidence rate ratios for the three weather effects from the seasonally-adjusted models. Overall hospitalizations for ARI were negatively associated with temperature (IRR 0.92 per 1 degree increase, 95% CI: 0.87, 0.97, p = 0.003) and positively associated with dew point (IRR 1.08 per 1 degree increase, 95% CI: 1.04, 1.13, p < 0.001). Of the few other significant effects, Influenza A and HRV had a negative associations with temperature (IRR 0.85, 95% CI: 0.75, 0.9, p = 0.0116, and IRR 0.86, 95% CI: 0.79, 0.94, p < 0.001, respectively), and HPIV3 and HRV were positively associated with dew point, with IRRs 1.26 (95% CI: 1.04, 1.52, p = 0.0164) and 1.13 (95% CI: 1.05, 1.21, p = 0.0009), respectively. Logistic regression found that RSV was positively associated with the previous week's rain, with an odds ratio of of 1.90 (95% CI: 1.21, 2.99, p = 0.0053). Previous week's temperature was marginally associated with RSV (OR: 1.14 (95% CI: 0.98, 1.33, p = 0.08) (see supplementary material).
Figure 4.
Weather effects. Figure shows model-adjusted incidence rate ratios for the main three weather effects: monthly mean rainfall (in inches), temperature (1∘ F), and dew point (1∘ F).
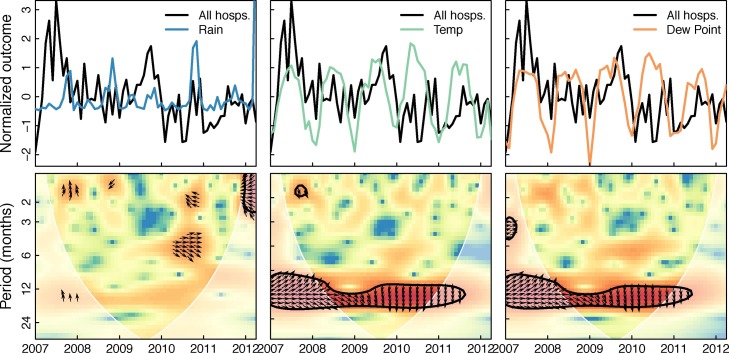
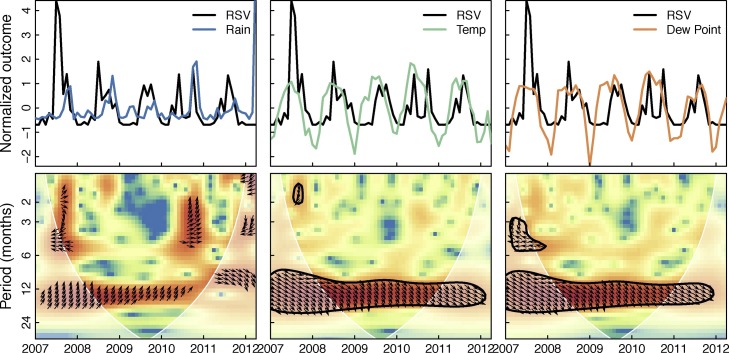
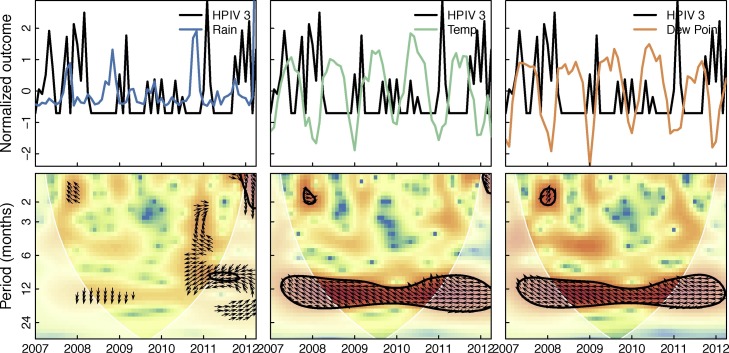
Figure 5 shows the cross-wavelet transform of all hospitalizations and the three weather variables in the month of admission. Significant bands of high power around 1 year can be seen for temperature and dew point. This indicates that hospitalizations and temperature and dew point share variability at yearly frequencies over the study period, and the phase relationship indicates that changes in weather slightly precede changes in hospitalizations (arrows point about 45∘ down). Figure 6 shows the cross-wavelet transform of RSV and the three weather variables with similar significance bands around 1 year for temperature and dew point. The phase indicates temperature and dew point lead RSV incidence. While not significant, RSV was found to be leading rainfall by 90∘ (3 months) at the one year period band (see supplementary material). Significant bands of power were seen between temperature and dew point with HPIV3 with an indicated phase relationship of nearly completely out-of-phase (Figure 7 ). Similar patterns were seen (1 year significant bands between temperature and dew point and virus) for ADV, HBoV, CoV, HPIV1,2&4, HRV, and influenza A, though the phase differences varied across these viruses; HMPV and influenza B had bands lying outside the cone of influence (ie, not statistically significant; see supplementary material). These results in general indicate strong associations between weather covariates and viral hospitalizations at a yearly timescale.
Figure 5.
Cross-wavelet transform of the z-standardized all hospitalization counts and weather time series. Figure shows the cross-wavelet of all hospitalization counts and rainfall, temperature, and dew point. Colors indicate increasing cross-wavelet power (strength of coherence between the time series) blue to red. The 5% significance level against red noise is shown as a thick contour and the cone of influence (within which the wavelets are not influenced by the edges of the time series) is shown in white shading. The relative phase relationship is shown as arrows (with in-phase pointing right, out-of-phase pointing left, and weather leading hospitalizations by 90∘ pointing straight down).
Figure 6.
Cross-wavelet transform of the z-standardized RSV and weather time series. Figure shows the cross-wavelet of RSV and rainfall, temperature, and dew point. Colors indicate increasing cross-wavelet power (strength of coherence between the time series) blue to red. The 5% significance level against red noise is shown as a thick contour and the cone of influence (within which the wavelets are not influenced by the edges of the time series) is shown in white shading. The relative phase relationship is shown as arrows (with in-phase pointing right, out-of-phase pointing left, and weather leading RSV by 90∘ pointing straight down).
Figure 7.
Cross-wavelet transform of the z-standardized HPIV3 and weather time series. Figure shows the cross-wavelet of HPIV3 and rainfall, temperature, and dew point. Colors indicate increasing cross-wavelet power (strength of coherence between the time series) blue to red. The 5% significance level against red noise is shown as a thick contour and the cone of influence (within which the wavelets are not influenced by the edges of the time series) is shown in white shading. The relative phase relationship is shown as arrows (with in-phase pointing right, out-of-phase pointing left, and weather leading HPIV3 by 90∘ pointing straight down).
Discussion
Here we have identified seasonal trends of several common respiratory viruses in hospitalized children in Nha Trang, Vietnam. By fitting a series of statistical models to the observed data, we allow the data to identify salient features contributing to the seasonality of these viruses. We evaluated seasonal patterns and associations with weather of hospitalizations for several respiratory viruses using three lines of evidence: 1) Poisson regression examining the relative incidence across months of virus detections adjusted for weather covariates, 2) cross-wavelet transforms of hospitalizations with viral detections, and 3) a sensitivity analysis with a logistic regression model finding odds ratio of hospitalizations with viral detections and weather variables.
Any viral detection showed distinct seasonality with peaks in May through September, a negative association with temperature, positive association with dew point, and cross-wavelets indicating temperature and dew point leading viral detection. Of commonly detected viruses, RSV, Influenza A, and HPIV3 had significant seasonality. RSV peaked in July through December, was positively associated with the week's previous average rainfall. Cross-wavelets showed temperature and dew point to lead RSV, rain was found to non-significantly fall behind RSV at 1 year frequencies, and precede RSV at shorter (<100 day) frequencies. Finally, HPIV3 while not significant, had peaks in January and February, was positively associated with dew point, and was completely out of phase with temperature and dew points in cross-wavelet analyses. These results contribute to the growing body of knowledge on the epidemiology of respiratory pathogens in South East Asia and Southern Central Vietnam.
Using a cross-wavelet transform we evaluated the time-dependence of virus hospitalizations with the weather covariates. We found strong yearly associations with RSV over the study period with temperature and dew point in phase with hospitalizations. This seasonality is opposite to observed seasonality in temperate climates, where RSV typically peaks in winter (Tang and Loh, 2014, Shek and Lee, 2003). Recent reviews of RSV seasonality in tropical regions highlights the uncertainty in the effects of weather on RSV transmission. Studies in Brazil (Straliotto et al., 2002), Hawaii (Reese and Marchette, 1991), India (Agrawal et al., 2009), Kenya (Hazlett et al., 1988), and Malaysia (Chew et al., 1998, Chan et al., 2002, Khor et al., 2012), have shown negative associations between temperature and RSV, while studies in Hong Kong (Chan et al., 1999), Mexico (Yusuf et al., 2007), Singapore (Chew et al., 1998), and Taiwan (Huang et al., 2001) have shown positive associations. We find strongly positive associations between temperature and RSV hospitalizations with a slight lead of temperature on RSV. This is evident both from the cross-wavelet transform and the regression results indicating a positive effect of the previous week's temperature on RSV.
There is less uncertainty in the role of rainfall on RSV transmission in tropical areas, where the majority of work indicates that RSV generally occurs during rainy seasons (Shek and Lee, 2003). Colombia (Bedoya et al., 1996), The Gambia (Weber et al., 1998), Hong Kong (Chan et al., 1999), Kenya (Hazlett et al., 1988), Malaysia (Chan et al., 2002), and Papua New Guinea (Hierholzer et al., 1994) all show positive RSV associations with rainfall. Omer et al. (Omer et al., 2008) showed significant positive associations between rainfall and temperature in the previous 8 days and RSV incidence in Lombok, Indonesia. We find similar associations, with the mean rainfall and temperature over the previous 7 days having odds ratios for RSV of 1.90 and 1.14, respectively. This association is plausible as the incubation period of RSV is estimated to be between 4 and 5 days (Lessler et al., 2009). Detailed contact tracing studies, coupled with climatological data could refine this association.
Somewhat surprisingly, the cross-wavelet transform of RSV and rain showed no significant association, though areas of high power were observed in the 1-year and 6-month bands, with phase indicating RSV leading weather. However, none of the viruses studied here showed appreciable associations with rain in the cross-wavelet transform, possibly indicating the dominance of other weather effects (temperature and dew point) on virus hospitalizations.
As with previous studies examining HPIV incidence, we found a predominance of HPIV3 (72 detections) compared to 41, 13, and 3 for HPIV1, 2, and 4, respectively. Typical seasonality for HPIV3 is the spring and early summer months in the temperate regions (Fry et al., 2006, Hall, 2001), and has little to no observed seasonality in the tropics and subtropics (Chew et al., 1998, Branche and Falsey, 2016). We find evidence for winter peaks in HPIV3 hospitalizations when employing both the Poisson regression model as well as the logistic regression model to estimate odds ratios, though the peaks were not statistically significant when controlling for weather.
The cross-wavelet transforms reveals HPIV3 to vary significantly at 1 year periodicity with temperature and dew point across the study. It also shows that HPIV3 hospitalizations are nearly completely out of phase with temperature and dew point throughout the study period. HPIV3 has been associated with low temperature and low relative humidity (Miller and Artenstein, 1967) though there is in general a paucity of data on the transmission routes of HPIV3 (Pica and Bouvier, 2012, Meissner et al., 1984). Future work could explore in more depth the epidemiological relationship between HPIV and weather variables.
This study is not without limitations. First, while we have nearly 6 years of data, this is still a relatively short period to assess long-term seasonal trends, or to increase confidence in the estimates of seasonal patterns. However, the length of the analyzed time series is similar to other studies examining seasonal trends in viral respiratory pathogens in the tropics (Chew et al., 1998, Hall, 2001), and gives indications for areas of future study. Second, we excluded individuals with more than one virus detected. Examination of changes in the seasonality of other viruses and coinfection over this period is worthy of study and is outside the scope of this paper. Third, this study used hospital-based surveillance, necessarily presenting the most ill children. We take as an assumption that hospitalizations are a fraction of all transmission and severity of illness is not related to weather. Finally, this study examines the influence of weather on viral hospitalizations and does not address other drivers of seasonal patterns of transmission, such as school closures (Fine and Clarkson, 1982), differences in other social behavior such as contact rates (Cook et al., 1990, Dushoff et al., 2004), susceptible recruitment through births (Metcalf et al., 2012), or possible seasonal changes in host immune responses (Dowell et al., 2003). Future work examining these drivers in this setting is necessary.
Limitations aside, our study adds to the body of literature on seasonality of common respiratory patterns in tropical regions and will be of use when consideration of the epidemiology of these pathogens is necessary. For example the timing of influenza peaks in the mid-spring months (April, May, June) would indicate routine vaccination in the winter months would be of biggest impact. Similarly, knowing RSV peaks in the late summer/early fall when RCP is at its lowest may help in limiting unnecessary antibiotic (Laxminarayan and Malani, 2007) or antiviral use (Tanaka et al., 2014). In Vietnam and most of Asia, most antibiotics are acquired from a pharmacist without a formal prescription (Van Nguyen et al., 2013). This fact makes results like those presented here of high importance to public health decision-makers to inform pharmacists of seasonality of respiratory infection etiologies and urge judicious prescribing practices.
Additional future work could include examination of the effects of contact clustering (Hébert-Dufresne and Althouse, 2015), co-infection (Althouse et al., 2013, Hébert-Dufresne et al., 2013), and asymptomatic carriers (Althouse and Scarpino, 2015) on transmission of the examined viruses all of which may be influenced by weather.
Contributions
Study design: BMA, SF, HH, LMY; Data collection: LMY, LNM, VDT; Data analysis: BMA, SF; Writing first draft: BMA, Writing subsequent drafts: all authors; Contributed intellectually: all authors
Ethical approval
The study was approved by institutional review boards in the National Institute of Hygiene and Epidemiology, Vietnam and the Institute of Tropical Medicine, Nagasaki University, Japan.
Acknowledgments
BMA and HH were supported by Bill & Melinda Gates through the Global Good Fund. SF and LMY were partially supported by Bill & Melinda Gates Foundation (BMGF OPP1139859). The pediatric ARI surveillance study in Nha Trang, Vietnam was supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from Ministry of Education, Culture, Sport, Science & Technology in Japan, and Japan Agency for Medical Research and Development (AMED). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2018.08.001.
Appendix B. Supplementary Data
The following is the supplementary data to this article:
References
- Agrawal A.S., Sarkar M., Chakrabarti S., Rajendran K., Kaur H., Mishra A.C. Comparative evaluation of real-time pcr and conventional rt-pcr during a 2 year surveillance for influenza and respiratory syncytial virus among children with acute respiratory infections in kolkata, india, reveals a distinct seasonality of infection. J Med Microbiol. 2009;58(12):1616–1622. doi: 10.1099/jmm.0.011304-0. [DOI] [PubMed] [Google Scholar]
- Althouse B.M., Scarpino S.V. Asymptomatic transmission and the resurgence of bordetella pertussis. BMC Med. 2015;13(1):146. doi: 10.1186/s12916-015-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse B.M., Patterson-Lomba O., Goerg G.M., Hébert-Dufresne L. The timing and targeting of treatment in influenza pandemics influences the emergence of resistance in structured populations. PLoS Comput Biol. 2013;9(2):e1002912. doi: 10.1371/journal.pcbi.1002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L., Dormitzer P., Nokes D., Rappuoli R., Roca A., Graham B. Strategic priorities for respiratory syncytial virus (rsv) vaccine development. Vaccine. 2013;31:B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya V.I., Abad V., Trujillo H. Frequency of respiratory syncytial virus in hospitalized infants with lower acute respiratory tract infection in colombia. Pediatr Infect Dis J. 1996;15(12):1123–1124. doi: 10.1097/00006454-199612000-00014. [DOI] [PubMed] [Google Scholar]
- Branche A.R., Falsey A.R. Parainfluenza virus infection. Seminars in respiratory and critical care medicine, 37. 2016:538–554. doi: 10.1055/s-0036-1584798. Thieme Medical Publishers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazelles B., Chavez M., Berteaux D., Ménard F., Vik J.O., Jenouvrier S. Wavelet analysis of ecological time series. Oecologia. 2008;156(2):287–304. doi: 10.1007/s00442-008-0993-2. [DOI] [PubMed] [Google Scholar]
- Chan P., Sung R., Fung K., Hui M., Chik K., Adeyemi-Doro F. Epidemiology of respiratory syncytial virus infection among paediatric patients in hong kong: seasonality and disease impact. Epidemiol Infect. 1999;123(02):257–262. doi: 10.1017/s0950268899002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P., Chew F., Tan T., Chua K., Hooi P. Seasonal variation in respiratory syncytial virus chest infection in the tropics. Pediatr Pulmonol. 2002;34(1):47–51. doi: 10.1002/ppul.10095. [DOI] [PubMed] [Google Scholar]
- Chaves L.F., Pascual M. Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med. 2006;3(8):e295. doi: 10.1371/journal.pmed.0030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew F., Doraisingham S., Ling A., Kumarasinghe G., Lee B. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol Infect. 1998;121(01):121–128. doi: 10.1017/s0950268898008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. on Infectious Diseases et al., Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections, Pediatrics 124(6)(2009)1694–1701. [DOI] [PubMed]
- Cook S., Glass R., LeBaron C., Ho M.-S. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68(2):171. [PMC free article] [PubMed] [Google Scholar]
- Do A.H.L., van Doorn H.R., Nghiem M.N., Bryant J.E., thi Hoang T.H., Do Q.H. Viral etiologies of acute respiratory infections among hospitalized vietnamese children in ho chi minh city, 2004-2008. PloS One. 2011;6(3) doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell S.F., Whitney C.G., Wright C., Rose C.E., Jr., Schuchat A. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9(5):574. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale S.B., Sande C.J., Green C.A., Pollard A.J. 2016. Rsv vaccine use-the missing data. [DOI] [PubMed] [Google Scholar]
- Dushoff J., Plotkin J.B., Levin S.A., Earn D.J. Dynamical resonance can account for seasonality of influenza epidemics. Proc Natl Acad Sci. 2004;101(48):16915–16916. doi: 10.1073/pnas.0407293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P.E., Clarkson J.A. Measles in england and wales-i: an analysis of factors underlying seasonal patterns. Int J Epidemiol. 1982;11(1):5–14. doi: 10.1093/ije/11.1.5. [DOI] [PubMed] [Google Scholar]
- Flasche S., Takahashi K., Vu D.T., Suzuki M., Nguyen T.H.-A., Le H. Early indication for a reduced burden of radiologically confirmed pneumonia in children following the introduction of routine vaccination against haemophilus influenzae type b in nha trang, vietnam. Vaccine. 2014;32(51):6963–6970. doi: 10.1016/j.vaccine.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A.M., Curns A.T., Harbour K., Hutwagner L., Holman R.C., Anderson L.J. Seasonal trends of human parainfluenza viral infections: United states, 1990-2004. Clin Infect Dis. 2006;43(8):1016–1022. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- Grinsted A., Moore J.C., Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes Geophys. 2004;11(5/6):561–566. [Google Scholar]
- Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Hazlett D., Bell T., Tukei P., Ademba G., Ochieng W., Magana J. Viral etiology and epidemiology of acute respiratory infections in children in nairobi, kenya. Am J Trop Med Hyg. 1988;39(6):632–640. doi: 10.4269/ajtmh.1988.39.632. [DOI] [PubMed] [Google Scholar]
- Hébert-Dufresne L., Althouse B.M. Complex dynamics of synergistic coinfections on realistically clustered networks. Proc Natl Acad Sci. 2015;112(33):10551–10556. doi: 10.1073/pnas.1507820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert-Dufresne L., Patterson-Lomba O., Goerg G.M., Althouse B.M. Pathogen mutation modeled by competition between site and bond percolation. Phys Rev Lett. 2013;110(10):108103. doi: 10.1103/PhysRevLett.110.108103. [DOI] [PubMed] [Google Scholar]
- Hierholzer J., Tannock G., Hierholzer C.M., Coombs R., Kennett M.L., Phillips P. Subgrouping of respiratory syncytial virus strains from australia and papua new guinea by biological and antigenic characteristics. Arch Virol. 1994;136(1–2):133–147. doi: 10.1007/BF01538823. [DOI] [PubMed] [Google Scholar]
- Huang Y.-C., Lin T.-Y., Chang L.-Y., Wong K.-S., Ning S.-C. Epidemiology of respiratory syncytial virus infection among paediatric inpatients in northern taiwan. Eur J Pediatr. 2001;160(9):581–582. doi: 10.1007/s004310100803. [DOI] [PubMed] [Google Scholar]
- Khor C.-S., Sam I.-C., Hooi P.-S., Quek K.-F., Chan Y.-F. Epidemiology and seasonality of respiratory viral infections in hospitalized children in kuala lumpur, malaysia: a retrospective study of 27 years. BMC Pediatr. 2012;12(1):32. doi: 10.1186/1471-2431-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambach P., Alvarez A.M.R., Hirve S., Ortiz J.R., Hombach J., Verweij M. Considerations of strategies to provide influenza vaccine year round. Vaccine. 2015;33(47):6493–6498. doi: 10.1016/j.vaccine.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R., Malani A. Earthscan; 2007. Extending the cure: policy responses to the growing threat of antibiotic resistance. [Google Scholar]
- Laxminarayan R., Duse A., Wattal C., Zaidi A.K., Wertheim H.F., Sumpradit N. Antibiotic resistance–the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R., Matsoso P., Pant S., Brower C., Røttingen J.-A., Klugman K. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- Le T.T., Pham T.H., Pham T.H., Nguyen L.K.H., Nguyen C.T., Hoang V.M.P. Circulation of influenza b lineages in northern viet nam, 2007-2014. West Pac Surveill Response J. 2015;6(4):17. doi: 10.5365/WPSAR.2015.6.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9(5):291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H.C., Murray S.A., Kiernan M.A., Snydman D.R., McIntosh K. A simultaneous outbreak of respiratory syncytial virus and parainfluenza virus type 3 in a newborn nursery. J Pediatr. 1984;104(5):680–684. doi: 10.1016/s0022-3476(84)80943-9. [DOI] [PubMed] [Google Scholar]
- Metcalf C., Lessler J., Klepac P., Cutts F., Grenfell B. Impact of birth rate, seasonality and transmission rate on minimum levels of coverage needed for rubella vaccination. Epidemiol Infect. 2012;140(12):2290–2301. doi: 10.1017/S0950268812000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.S., Artenstein M.S. Aerosol stability of three acute respiratory disease viruses. Proc Soc Exp Biol Med. 1967;125(1):222–227. doi: 10.3181/00379727-125-32054. [DOI] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration, Global summary of the day (GSOD) global summary of the day (GSOD), https://www7.ncdc.noaa.gov/cdo/cdoselect.cmd?datasetabbv=gsod&countryabbv=&georegionabbv, accessed feb. 20, 2017.
- Nguyen H.L., Saito R., Ngiem H.K., Nishikawa M., Shobugawa Y., Nguyen D.C. Epidemiology of influenza in hanoi, vietnam, from 2001 to 2003. J Infect. 2007;55(1):58–63. doi: 10.1016/j.jinf.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Omer S., Sutanto A., Sarwo H., Linehan M., Djelantik I., Mercer D. Climatic, temporal, and geographic characteristics of respiratory syncytial virus disease in a tropical island population. Epidemiol Infect. 2008;136(10):1319–1327. doi: 10.1017/S0950268807000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Curr Opin Virol. 2012;2(1):90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese P.E., Marchette N.J. Respiratory syncytial virus infection and prevalence of subgroups a and b in hawaii. J Clin Microbiol. 1991;29(11):2614–2615. doi: 10.1128/jcm.29.11.2614-2615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Chadha M., Al Mamun A., Rahman M., Sturm-Ramirez K., Chittaganpitch M. Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and south-eastern asia. Bull World Health Organ. 2014;92(5):318–330. doi: 10.2471/BLT.13.124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek L.P.-C., Lee B.-W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev. 2003;4(2):105–111. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Straliotto S.M., Siqueira M.M., Muller R.L., Fischer G.B., Cunha M.L., Nestor S.M. Viral etiology of acute respiratory infections among children in porto alegre, rs, brazil. Rev Soc Bras Med Trop. 2002;35(4):283–291. doi: 10.1590/s0037-86822002000400002. [DOI] [PubMed] [Google Scholar]
- Tanaka M.M., Althouse B.M., Bergstrom C.T. Timing of antimicrobial use influences the evolution of antimicrobial resistance during disease epidemics. Evol Med Public Health. 2014;2014(1):150–161. doi: 10.1093/emph/eou027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Loh T.P. Correlations between climate factors and incidence–a contributor to rsv seasonality. Rev Med Virol. 2014;24(1):15–34. doi: 10.1002/rmv.1771. [DOI] [PubMed] [Google Scholar]
- Thai P.Q., Choisy M., Duong T.N., Thiem V.D., Yen N.T., Hien N.T. Seasonality of absolute humidity explains seasonality of influenza-like illness in vietnam. Epidemics. 2015;13:65–73. doi: 10.1016/j.epidem.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S.A. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- Van Nguyen K., Do N.T.T., Chandna A., Nguyen T.V., Van Pham C., Doan P.M. Antibiotic use and resistance in emerging economies: a situation analysis for viet nam. BMC Public Health. 2013;13(1):1158. doi: 10.1186/1471-2458-13-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C.L.F., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw T., Salama P., Johansson E.W., Mason E. Pneumonia: the leading killer of children. Lancet. 2006;368(9541):1048. doi: 10.1016/S0140-6736(06)69334-3. [DOI] [PubMed] [Google Scholar]
- Weber M.W., Dackour R., Usen S., Schneider G., Adegbola R.A., Cane P. The clinical spectrum of respiratory syncytial virus disease in the gambia. Pediatr Infect Dis J. 1998;17(3):224–230. doi: 10.1097/00006454-199803000-00010. [DOI] [PubMed] [Google Scholar]
- Yoshida L.M., Suzuki M., Yamamoto T., Nguyen H.A., Nguyen C.D., Nguyen A.T. Viral pathogens associated with acute respiratory infections in central vietnamese children. Pediatr Infect Dis J. 2010;29(1):75–77. doi: 10.1097/INF.0b013e3181af61e9. [DOI] [PubMed] [Google Scholar]
- Yoshida L.-M., Suzuki M., Thiem V.D., Smith W.P., Tsuzuki A., Huong V.T.T. Population based cohort study for pediatric infectious diseases research in vietnam. Trop Med Health. 2014;42(Suppl. 2):S47–S58. doi: 10.2149/tmh.2014-S07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S., Piedimonte G., Auais A., Demmler G., Krishnan S., Van Caeseele P. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135(07):1077–1090. doi: 10.1017/S095026880600776X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.