Abstract
Practitioners may be called on to treat rodents with respiratory diseases or to advise clients concerning the care of these rodents. Respiratory diseases of mice, rats, guinea pigs, and Syrian hamsters are well known because of the use of these species in research, whereas few or no reports of respiratory disease in rodents of other species exist. Features of the respiratory diseases of these four commonly encountered species are reviewed, including causes; clinical signs; diagnostic procedures; preventive measures; and, where appropriate, therapies.
Although rodents do not constitute a large percentage of patients in most practices, substantial numbers of rodents are kept as pets, especially in urban areas, as are snakes, for which mice and rats are raised and sold as food. Thus, practitioners could be called on to treat respiratory diseases in rodents or to advise clients regarding prevention of such diseases. Most available information regarding respiratory diseases in rodents concerns animals used in research, however. In that setting, the emphasis is on detection of potential pathogens and on diagnosis of subclinical disease, and the objective is excluding or eliminating infected animals from a research colony to prevent complications to research. Animals that are found to be infected or that become ill rarely are treated; thus, information concerning treatment and immunization is scant. For species not commonly used in laboratory studies, there is little information regarding occurrence of respiratory disease. Reviewed here are the causes, clinical signs, lesions, diagnostic procedures, preventive measures, and, where available, treatments of respiratory diseases of four commonly encountered rodent species: mice, rats, guinea pigs, and Syrian hamsters. Additional information is available in several texts.9., 12., 13., 14., 16., 31.
Proper husbandry is important in preventing respiratory disease in rodents. Many of the causative organisms discussed below are carried in many animals without causing overt disease. Clinical disease can occur as a consequence of stress, poor nutrition, concurrent infections, advanced age, and environmental and other factors. Clinical respiratory disease also can result when acquisition of a new animal results in introduction of a pathogen into a group of rodents that have not been exposed previously. Many of the agents discussed below commonly are found in wild rodents; thus, care should be taken to prevent exposure to vermin. These principles are equally important whether the animals are kept as individual pets or as breeding colonies. Fortunately, the diseases discussed below are not known to present significant risk of zoonotic disease, nor are any known to pose a significant hazard for snakes or other carnivorous animals that feed on rodents.
Because of the need for disease diagnosis and prevention in research settings, many serologic and other tests for rodent respiratory infections are commercially available. Such methods rarely would be employed in private practice, but some of the practices used to maintain disease-free status in laboratory rodents, such as health assessment of potential breeding stock and maintaining a closed colony that is protected from exposure to other rodents, can help clients maintaining breeding colonies to avoid reduced productivity caused by disease. Health assessment procedures for laboratory rodents typically include a combination of serologic testing, bacteriologic culture, parasitologic examination, and histopathologic examination, and can be quite expensive, but basic procedures to detect the most important pathogens can be conducted at modest expense. As a general rule, testing followed by treating or culling is inefficient or ineffective for controlling most chronic infections in rodent breeding colonies; rather, an infected colony should be destroyed and replaced with disease-free stock. For this to be effective, of course, the colony would have to be managed to protect the animals from reintroduction of pathogens.
Viral Diseases
In most circumstances, viral infections of rodents do not result in severe disease but can do so in very young animals or animals carrying potential secondary bacterial pathogens. Viral infections often are enzootic in breeding colonies, in which there is continual introduction of new susceptible hosts. The young are protected from acute disease by maternal antibodies, and, as passive immunity wanes, acquire active immunity without developing clinical disease. Such infections often go unnoticed but can affect breeding performance and can increase susceptibility to bacterial respiratory pathogens. Viral infections in breeding colonies often can be controlled by cessation of breeding for a few weeks, allowing the infection to die out.
Sendai Virus
Sendai virus is a type 1 paramyxovirus, of which mice, rats, and hamsters are the natural hosts.15., 24., 25., 27., 28., 29. Serologic test results suggest that guinea pigs can be infected, but no disease is known in that species. Sendai virus is very contagious, transmission occurring readily by contact and aerosol.
In mice, Sendai virus infection in breeding colonies typically is enzootic and subclinical. Introduction into a nonimmune population can result in epizootic disease, however, the severity of which depends on age, genetic factors, and presence of other potential pathogens. Adults usually have mild respiratory signs (“chattering”) and recover fully in a few days, whereas suckling, weanling, and aged mice can have more severe disease with respiratory distress. Susceptibility also is increased in protein-deprived mice. Infection in pregnant mice can result in prolonged gestation, and survivors of neonatal infection sometimes grow poorly. Acute outbreaks in breeding colonies can cause production to fall, then return to normal in a few weeks, although enzootic subclinical infection can persist.
Gross lesions are often absent, but in severe cases there may be gray-purple patches in the lungs. Microscopic lesions can occur throughout the respiratory tract. Mild disease is characterized by mild necrotizing rhinitis, laryngitis, tracheitis, bronchitis, bronchiolitis, and possibly by mild patchy pneumonitis. Pneumonitis and airway mucosal changes, such as syncytia, hyperplasia, and squamous metaplasia, are more prominent in mice having increased susceptibility owing to genetic factors. In such cases, the terminal bronchioles can be severely damaged, and in survivors can be scarred, distorted, or have polypoid outgrowths of the mucosa.
Sendai virus disease in rats is similar to that in mice, although clinical disease rarely is observed. As in mice, susceptibility varies with age and genetic factors. In both mice and rats, Sendai virus infection can alter antibody responses and other measures of immunity and can increase susceptibility to other respiratory pathogens, such as Mycoplasma pulmonis. In hamsters, acute infection is characterized by mild lesions similar to those described in mice and rats, but clinical disease has not been reported.
Lesions of Sendai virus are not specific; however, in mice, Sendai virus is by far the most likely diagnosis when such lesions are present. In rats, respiratory tract lesions of Sendai virus infection cannot be distinguished from those caused by coronavirus infection, and some rat coronavirus strains cause mild or minimal salivary gland, lacrimal gland, or eye lesions. Specific diagnosis of Sendai virus infection is by serologic testing. The virus can be isolated in eggs or cell culture, and viral antigen is readily demonstrated in tissues by immunohistochemical methods, but such methods are practical only in a research setting.
Coronaviruses of Rats
Sialodacryoadenitis virus (SDAV) and Parker’s rat coronavirus (RCV) are named strains of rat coronavirus.29 Sialoadenitis and dacryoadenitis are prominent features of SDAV infection, but both strains affect the respiratory tract. Other coronavirus strains also have been isolated from rats but are not well characterized. Mice reportedly are experimentally susceptible to disease similar to SDAV, but natural SDAV disease in mice has not been reported.
Coronavirus infection probably is the single most common viral infection of rats. Coronavirus is very contagious; transmission is by contact and aerosol. Natural infection can be epizootic, if newly introduced into a susceptible colony, or enzootic within a breeding colony. Age and genetic factors affect susceptibility. Clinical disease severity varies greatly. When infection is enzootic, there can be minimal signs, such as mild conjunctivitis in sucklings. In epizootics, there may be only mild respiratory signs, or the classic signs of SDAV infection, including cervical swelling, conjunctivitis, keratitis, and porphyrin staining around the eyes. The morbidity rate is high, but there is no mortality in the absence of secondary disease.
Gross findings include corneal erosion, ulceration, and perforation. Ventral cervical edema surrounding the submandibular salivary glands can be prominent in SDAV infection. No gross lesions are evident in the respiratory tract. Microscopic respiratory tract lesions are characterized by mild necrotizing rhinitis, laryngitis, tracheitis, bronchitis, and bronchiolitis. Pneumonitis, if present, usually is mild and patchy, and the inflammatory response is mostly lymphocytic, although alveolar epithelial necrosis also can be evident in sucklings and weanlings. There can be residual lung lesions characterized by tiny scars and lymphoid cell collections, but these are not diagnostically useful because they are similar to residual lesions of various inflammatory causes. In SDAV infection, acute glandular lesions are characterized by diffuse necrosis of the submaxillary salivary gland acini and ducts, with similar changes in the Harderian glands. There also can be multifocal necrotizing inflammation of the parotid gland and infraorbital lacrimal gland, mild multifocal necrotizing cervical lymphadenitis, and mild multifocal thymic necrosis. Resolving lesions in the Harderian gland are characterized by prominent squamous metaplasia. Residual lymphoid collections in the Harderian gland can persist for some time and can be a useful diagnostic clue. In the eye, there is acute keratoconjunctivitis in the early stages, which can resolve or progress to keratitis with ulceration, scarring, and even perforation, in which case there can be secondary bacterial anterior uveitis or panophthalmitis. RCV also causes rhinitis, tracheitis, and other respiratory tract lesions, and can be associated with eye lesions. SDAV infection strongly exacerbates M. pulmonis disease.
In acute SDAV disease, classic lesions of sialoadenitis are diagnostic. Respiratory tract lesions cannot be distinguished from those caused by Sendai virus. Diagnosis of subclinical enzootic infections is by serologic testing. Serologic test results can be negative in acutely affected rats, inasmuch as they may not have had time to develop detectable antibody. Other methods are seldom if ever needed. Viral antigen can be demonstrated readily by indirect immunofluorescence, but antigen disappears rapidly after about 5 to 7 days.
Adenovirus Pneumonia in Guinea Pigs
Several outbreaks of spontaneous adenoviral pneumonia in guinea pigs have been reported.8., 26. The morbidity rate is low, but mortality rates can be high. The major clinical sign in affected guinea pigs is dyspnea. Gross lesions are red or gray-purple patches in the lungs, particularly in the cranial lobes and hilar areas. As is typical of respiratory adenoviral infections, the predominant histologic lesion is necrotizing bronchiolitis, with many bronchioles filled with cellular debris and lacking viable epithelial cells. In other airways, the epithelium can be hyperplastic. Typical basophilic intranuclear inclusions are present in both intact and sloughed cells. The diagnosis is based on histologic and electron microscopic findings. The virus has not been isolated.
Adenoviral infections also occur in mice but do not affect the respiratory tract.28
Miscellaneous Viruses
Pneumonia virus of mice (PVM) is a member of the pneumoviruses, which are paramyxoviruses. Mice and rats are the major hosts, but serologic evidence indicates that several other rodent species can be infected, including hamsters, guinea pigs, gerbils, and cotton rats.27., 28., 29. Transmission is by contact and aerosol, but PVM is considered to be less readily transmitted than Sendai virus. Natural infections are subclinical and rarely, if ever, recognized by means other than serologic testing. Experimental inoculation results in lesions similar to those of Sendai virus infection, although with less tendency to cause proliferative bronchiolar lesions. PVM infection has been little studied and probably is of little significance except in immunodeficient laboratory mice, although it is possible that it could increase susceptibility to bacterial respiratory pathogens.
K virus is a polyomavirus that causes pneumonitis and other lesions in infant mice after experimental inoculation.11., 28. It infects endothelial cells, with prominent basophilic nuclear inclusions. No natural disease is known. Mice are the only known hosts, and it is found naturally in wild mice.
Infection with guinea pig cytomegalovirus (CMV), a herpesvirus, is common among guinea pigs if not ubiquitous.26 It is transmitted by secretions and urine, and through the placenta. In ordinary infections, inclusions can be present in salivary glands, cervical lymph nodes, and kidneys. As in other species, disseminated disease occurs rarely and is characterized by widespread multifocal necrotizing inflammation with syncytia, cytomegaly, and inclusions, especially in the lung and lymphoid tissues, but there are no specific clinical signs. In other species, disseminated CMV disease usually is associated with immunosuppression or immunodeficiency. It has not been established whether this also is the case in guinea pigs, but there is increased susceptibility during pregnancy. A presumptive diagnosis could be based on histopathologic or electron microscopic findings, but definitive diagnosis requires specific identification of the virus by isolation or by molecular techniques. CMVs occur in other rodents but are not known to affect the respiratory system.
Bacterial Diseases
Recommended antibiotic treatments for bacterial diseases in rodents are provided in several texts,9., 12., 13., 14., 16., 31. although few if any antibiotics are approved for use in rodents. In guinea pigs and hamsters, many antibiotics can induce necrotizing cecocolitis caused by Clostridium difficile,26., 27. especially penicillins, lincomycin, clindamycin, and erythromycin,13 and should not be used. Selected recommended treatments are given in Table 1. When giving intramuscular injections in smaller species, one must take particular care to avoid nerve damage. In general, chronic infections are difficult if not impossible to eliminate with antibiotics, although disease severity can be reduced.
Table 1.
Selected Antibiotics.
| Antibiotic | Dosage and Route |
|---|---|
| Chloramphenicol | 20–50 mg/kg PO q 6 to 12 h |
| Ciprofloxacin | 7–20 mg/kg PO q 12 h |
| Doxycy cline | 5 mg/kg PO q 12 h |
| Enrofloxacin | 5–10 mg/kg PO, SC, or IM q 12 h; 50–200 mg/L in drinking water |
| Oxytetracycline | 60 mg/kg IM q 3 days; 100 mg/kg SC daily; 3 g/L in drinking water |
| Trimethoprim-sulfamethoxazole | 30 mg/kg q 12 h PO or IM; 2 mL injectable solution per L of drinking water |
| Tylosin | 10 mg/kg SC daily |
PO, per os; IM, intramuscular injection; SC, subcutaneous injection.
Data from Harkness JE, Wagner JE: The Biology and Medicine of Rabbits and Rodents. Baltimore, Williams & Wilkins, 1995; and from Richardson VCG: Diseases of Small Domestic Rodents. Ames, IA, Iowa State University Press, 1997.
Murine Respiratory Mycoplasmosis
Murine respiratory mycoplasmosis (MRM) is caused by Mycoplasma pulmonis, a member of the Mollicutes. M. pulmonis infection is common in rats and should be considered essentially ubiquitous in rats other than specific pathogenfree laboratory stocks.32 It is by far the most common cause of clinical respiratory disease in pet rats. M. pulmonis infection also occurs in mice, but less commonly than in rats.17., 32. Infection is thought to be transmitted directly or by aerosol from dam to sucklings, and infection of pups in utero or at birth probably also occurs as a result of genital infection. Infection is persistent and probably lifelong, despite local and systemic immune responses. Strains of the organism vary widely in virulence for both rats and mice. M. pulmonis also has been isolated from Syrian hamsters, guinea pigs, and cotton rats, but no disease is known in these species.
M. pulmonis parasitizes the surface of the respiratory epithelium and colonizes the nasal passages and middle ears preferentially. Progression to bronchopulmonary disease can occur and is accelerated by various factors. These factors have been studied most extensively in rats, and include excessive ammonia from soiled cage bedding, concurrent infection with viruses such as SDAV or Sendai virus, deficiency of vitamin A or E, susceptible genotype, and increasing age. In mice, Sendai virus infection and genotype are known to affect disease expression. Thus, proper husbandry is particularly important in management of this disease.
M. pulmonis infection often is subclinical. More severe rhinitis results in nasal discharge and characteristic respiratory sounds (commonly characterized as “snuffling” in rats and “chattering” in mice). Brown-red ocular discharge can be observed. This is a nonspecific sign probably related to obstruction of the lacrimal ducts because of rhinitis, and the pigmentation is due to the porphyrin present in the lacrimal secretions, not to blood. Head tilt occurs occasionally as a result of severe otitis. Progression to severe bronchopulmonary disease can lead to weight loss, dyspnea, and, rarely, death. In rats, the presenting sign in some cases is reduced fertility resulting from endometritis, salpingitis, and perioophoritis.
Gross lesions may not be obvious, although exudate in the middle ears can be observed. In advanced disease, bronchial inflammation, atelectasis, and pneumonia result in patches, or even entire lung lobes, with a cobblestone appearance ( Fig. 1). Abscesses can be present, particularly if there is concurrent infection with cilia-associated respiratory (CAR) bacillus (see later). In genital infections, there may be abscesses of the ovarian bursae and oviducts, and the uterus can contain exudate. Regional lymph nodes are enlarged. Microscopically, the characteristic response to M. pulmonis infection is chronic suppurative mucosal inflammation.
Figure 1.
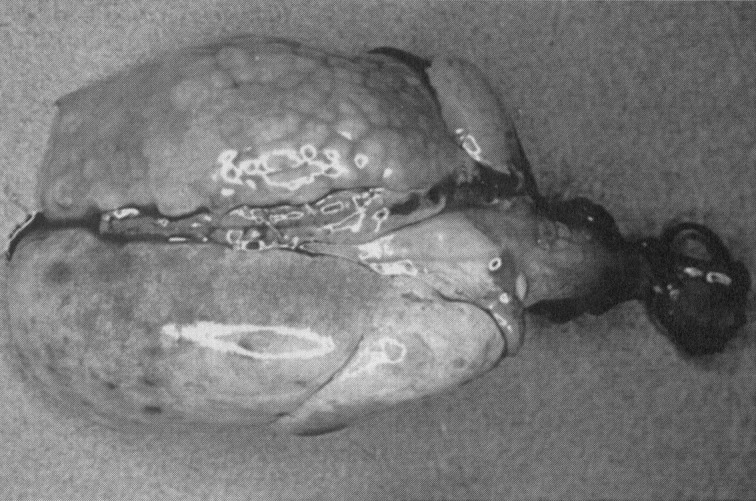
Mycoplasma pulmonis disease in a rat. The left lung is atelectatic and has a cobblestone appearance.
Courtesy of J. Russell Lindsey, DVM
Diagnosis of overt M. pulmonis disease is straightforward. The organism can be safely assumed to be either the primary cause or a major contributory pathogen in most cases of chronic respiratory disease in rats and probably mice as well. In acute or epizootic respiratory disease, one should suspect other agents either as the primary cause or, such as in the case of Sendai virus or rat coronaviruses, as a co-pathogen, predisposing the animals to rapid development of mycoplasmal disease. In most cases, M. pulmonis is readily isolated on appropriate mycoplasma medium. Other bacteria can be isolated in cases of respiratory disease in mice and rats, such as Pasteurella pneumotropica, Klebsiella pneumoniae, Bordetella bronchiseptica, and others. These organisms probably are opportunists or secondary invaders, although it is possible that they could increase disease severity. Serologic testing for M. pulmonis infection is commercially available, although one should be aware that serologic testing sometimes fails to detect subclinical infections, and results can be confounded by antibodies to other rodent mycoplasmas.
Tylosin, tetracycline, and enrofloxacin commonly are recommended for treatment of pet rats with respiratory disease. Few scientific studies of effectiveness have been conducted, but tylosin administered in drinking water has been shown to reach concentrations in serum and lung well above minimal in vitro inhibitory concentrations.6 In contrast, tetracycline administered in drinking water was found to be poorly absorbed and also reduced water consumption.30 In the author’s experience, either tylosin or oxytetracycline given to rats in the drinking water reduces the numbers of M. pulmonis organisms in the respiratory tract, but neither completely eliminates infection, even after prolonged treatment, and both reduce water consumption.
Cilia-associated Respiratory (CAR) Bacillus
CAR bacillus infection was first reported in association with respiratory disease in rats in 1980; however, the organisms are evident in electron micrographs published in the 1960s and have been found in archived tissues collected in the 1950s.33 The organisms are gram-negative, filamentous rods; have no capsule, flagella, pili, or filaments; are motile in cultures by gliding and flexing; and are known only as parasites of the respiratory mucosa. CAR bacillus has not been cultivated on conventional bacteriologic media but can be grown in embryonated chicken eggs, cell culture, and various cell culture media supplemented with serum. Genetic analysis indicates that CAR bacillus isolates from rats are related to flavobacteria.
The epizootiology of CAR bacillus is largely unknown. Most reported cases of CAR bacillus disease have been in conventional (i.e., not specific pathogenfree) laboratory rats, in which it is of worldwide distribution. Disease also has been reported in wild rats, in conventional laboratory mice, and in a South African hamster (Mystromys albicaudatus). Experimental inoculation is reported to induce mild disease in Syrian hamsters, but guinea pigs are resistant.18., 34. Thus, for practitioners, CAR bacillus is most important as a consideration in respiratory disease in rats, but infection also could occur in other rodent species.
CAR bacillus disease is so similar to MRM as to be indistinguishable without histopathology. This usually is of little practical importance because M. pulmonis also is present in most cases, although disease caused by CAR bacillus alone does occur. Subclinical infection probably is more common than overt disease. Disease in rats appears to develop similarly to MRM. The organism parasitizes the respiratory epithelial surface. Chronic inflammation results, and disease develops slowly over a period of weeks or months. Strong serum antibody responses are demonstrable but do not eliminate the infection. Whether disease progression is affected by factors that affect MRM expression, such as concurrent infections, has not been established. Gross and microscopic lesions are closely similar to those of MRM, although in CAR bacillus disease there is a somewhat greater tendency toward occurrence of bronchial abscesses ( Fig. 2) and mucopurulent alveolitis. CAR bacilli can be present anywhere there is ciliated respiratory epithelium, including the Eustachian tubes and middle ears. The organisms are most easily demonstrated histochemically with a silver impregnation method such as Warthin-Starry, but if numerous they can be seen in sections stained with hematoxylin and eosin, where they impart basophilic staining to the ciliary surface.
Figure 2.
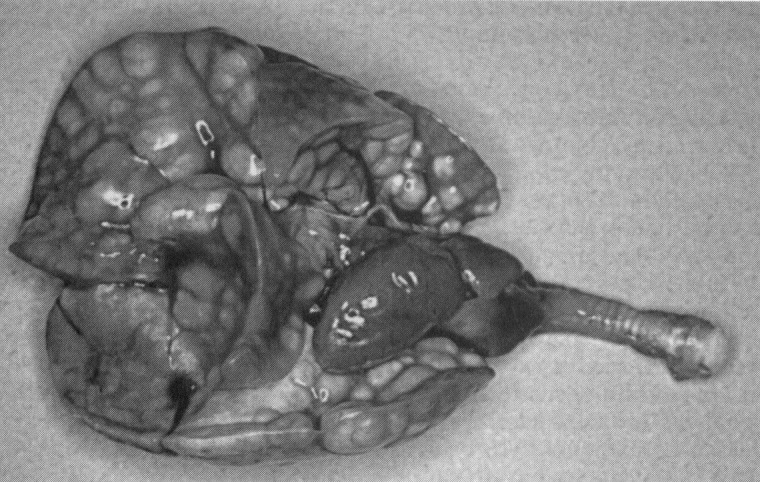
Cilia-associated respiratory bacillus disease in a rat. Numerous bronchial abscesses are present, and the right middle and azygous lobes are atelectatic.
Courtesy of Matthew van Zwieten, DVM, PhD
Diagnosis of CAR bacillus disease is nearly always made by histopathology, although a rapid diagnosis is possible by examination of silver impregnation–stained scrapings of tracheal mucosa.20 The organisms require specialized culture conditions, lengthy cultivation, and experience for isolation. Commercial serologic testing is available, but the sensitivity and specificity of existing tests is not clear.
Little information is available concerning treatment of CAR bacillus infection. Sulfamerazine at 500 mg/L in the drinking water is reported to be effective in experimentally infected laboratory mice.19
Corynebacterium kutscheri
Corynebacterium kutscheri is a gram-positive, metachromatic, diphtheroid bacillus. Mice, rats, and hamsters are the natural hosts, but clinical disease in hamsters has not been reported.17., 27., 28., 29. Most infections are dormant or subclinical; clinical disease usually is associated with immunosuppression, concurrent infection, or dietary deficiency, although apparently unprovoked epizootics have been observed. Sites where the organism resides in natural dormant infection are uncertain, but it has been found in the oropharynx, middle ear, submandibular lymph nodes, respiratory tract, digestive tract, and preputial glands. Modes of transmission are unproved but are probably by the fecal-oral route and by contact or aerosol. Experimentally infected rats can have the organism in the mouth and submandibular nodes for at least 8 weeks. Subclinically infected animals are presumed to act as carriers.
C. kutscheri affects the respiratory tract primarily in rats, although disease in both rats and mice typically results from hematogenous dissemination of the organism. In rats, the classic manifestation is multiple, pinpoint to 1-cm, yellow–tan areas of pneumonia. Fibrinopurulent pleuritis can occur where lesions are adjacent to the pleura. In such cases, clinical signs, if observed, would be respiratory. In acute disease, similar lesions can occur in the liver, kidneys, subcutis, abdominal serosae, and other organs; in chronic disease, abscesses can occur in these locations. C. kutscheri also can cause subcutaneous abscesses and can induce arthritis, with lameness and swollen joints. In mice, polyarthritis and disseminated visceral lesions are more typical than respiratory disease, with visceral lesions occurring most frequently in the liver and kidneys. Acutely affected mice often die suddenly without premonitory signs.
Microscopically, lesions are characteristically necrotizing and suppurative, containing fibrin and numerous bacteria in early stages or, in later stages, dense collections of neutrophils and lesser numbers of macrophages, with necrotic centers and fewer bacteria. Intralesional bacteria usually are readily observed in sections stained with tissue Gram’s stains and have a characteristic morphology, being gram-positive, pleomorphic short rods in jumbled clumps.
In practice settings, diagnosis of C. kutscheri infection usually is made postmortem and is based on necropsy findings and culture results. A finding of microscopic lesions with characteristic organisms in sections is sufficient for a presumptive diagnosis. Detection of dormant infections is difficult, and no commercial serologic test is available. If a breeding colony were to experience repeated or epizootic occurrences, replacement with C. kutscheri–free stock should be considered.
Streptococcus pneumoniae
Streptococcus pneumoniae is a respiratory pathogen for rats and guinea pigs,26., 29. and has been associated with respiratory disease in hamsters, although its role as a primary pathogen for hamsters is unclear.9., 27. It is gram positive and encapsulated, with a classic double-lancet shape, although it also occurs as short chains of cocci. Capsular types 4 and 19 are found in guinea pigs; in rats, types 2, 3, and 19 are most common, and types 8, 16, and 35 also are found. Human carriage of S. pneumoniae is common, but it is not known whether rodent infections are acquired from humans.
Pneumococcal infections in rodents are often subclinical. The organism is found in the nasal passages and middle ears and is transmitted by aerosol and contact. Epizootics of severe disease have occurred in rats and guinea pigs, and young rats can be affected more often than adults. In guinea pigs, the prevalence of carriers in infected colonies can be more than 50%, and outbreaks of disease tend to be associated with poor husbandry and stress. Susceptibility is greater in young or pregnant guinea pigs.
In rats, clinical signs are nasal discharge or respiratory distress, or death occurring acutely. The classic lesion is fibrinopurulent pleuritis, with yellow-tan, opaque, friable or elastic material, more or less adherent to the pleural surface, which is dull and granular. There is pericarditis in some cases. Usually there also is pneumonia, with firm, gray-purple or mottled purple and yellow-tan areas in the lungs, but the pneumonia can be overshadowed by the pleuritis. Mild or subclinical disease is characterized by suppurative rhinitis and otitis media. Suppurative or necrotizing and suppurative arthritis, meningitis, hepatitis, splenitis, peritonitis, and orchitis occur in a few cases. Large numbers of organisms usually are evident in gram-stained exudate or sections.
In guinea pigs, a variety of clinical signs can be observed, including anorexia, ruffled fur, wet nose, and dyspnea; abortion; or deaths without signs. Lesions are similar to those in rats and include suppurative bronchopneumonia, often accompanied by fibrinopurulent pleuritis; fibrinopurulent pericarditis, peritonitis, otitis media, endometritis, meningitis, arthritis, and osteomyelitis. Such lesions indicate that bacteremia or septicemia is common in both species.
Subclinical infection can be detected by nasopharyngeal culture. In overt disease, characteristic lesions and organisms having typical morphology in gram-stained tissue sections or impressions is sufficient for a presumptive diagnosis. Confirmation is by culture.
Streptococcus zooepidemicus
Streptococcus zooepidemicus is a νhemolytic streptococcus of Lancefield Group C. Infection is common in guinea pigs.26 Transmission probably is by aerosol, contact, and fomites. The organism usually causes chronic suppurative lesions, especially lymphadenitis, with the cervical nodes being most commonly affected. Acute disease occasionally occurs, however, including epizootics with high morbidity and mortality rates, especially in young animals. This form is similar to pneumococcal disease, and is commonly characterized by fibrinopurulent pleuritis, pericarditis, and bronchopneumonia. Septicemic dissemination also can occur, leading to myocarditis, peritonitis, meningitis, and other lesions. Diagnosis is based on characteristic lesions, in which myriad gram-positive cocci are seen in gram-stained sections, and on cultural isolation of the organism. Elimination of S. zooepidemicus from a colony probably requires replacing the animals; culling and treatment with antibiotics generally have not been successful.
Bordetella bronchiseptica
Bordetella bronchiseptica, a short, gram-negative rod or coccobacillus, is a significant respiratory pathogen for guinea pigs.26 Infection is maintained by carriers, which harbor the organism in the upper respiratory tract and which can exceed 20% of the animals in a colony. Young animals are more susceptible. Signs are nonspecific or there can be dyspnea or exudate around the external nares. Typical gross lesions are mucopurulent to blood-tinged exudate in the trachea, serous fluid in the pleural cavity, and gray-red patches of consolidation in the lungs. Microscopically, there is suppurative to fibrinopurulent tracheitis, bronchitis, and bronchopneumonia. Pleuritis and pericarditis are present in some cases. B. bronchiseptica also is occasionally recovered from tympanic bullae in cases of otitis media. Diagnosis is based on cultural identification of the organism, but, because carriage is common, care should be taken to differentiate bordetellosis from similar disease caused by such organisms as streptococci. Formalin- or merthiolate-killed bacterins are reported to be useful to eradicate colony infection. Animals of many other species can harbor B. bronchiseptica and are a potential source of infection.
B. bronchiseptica probably is an opportunistic pathogen for rats;29 it has been shown to cause rhinitis and bronchopneumonia after experimental inoculation and has been recovered from cases of spontaneous bronchopneumonia. In many cases of spontaneous respiratory disease in which B. bronchiseptica is isolated, there is concurrent infection with other respiratory pathogens, such as coronaviruses or Mycoplasma pulmonis.
Miscellaneous Bacteria
In most cases, these miscellaneous agents cannot be identified specifically unless cultures are done, either clinically or at necropsy, and some would be identified only by specialized techniques.
Pasteurella pneumotropica is a gram-negative coccobacillus of the family Pasteurellaceae. Natural hosts include mice, rats, hamsters, guinea pigs, and many other species. Infection usually is not apparent. Transmission probably occurs by a variety of routes. In mice, P. pneumotropica is present in a high percentage of apparently healthy animals and is found in the respiratory, digestive, and reproductive tracts; in the urinary bladder; and on the skin and conjunctiva.28 The organism was once regarded as a major respiratory pathogen of mice, but it is now considered to be an opportunist.17., 28. It is reported to complicate Mycoplasma pulmonis disease,4 but this has not been confirmed experimentally. Diagnosis is made by culture and by ruling out other pathogens. In rats, P. pneumotropica is a common commensal that can be isolated from many organs, including the respiratory tract.29 As in mice, it is considered an opportunist. P. pneumotropica also occasionally is associated with pneumonia in hamsters.9., 27.
Proteus mirabilis is an occasional cause of disease in mice, mostly in immuno-deficient laboratory mice but also in immunocompetent mice in a few cases.28 Disease is often septicemic, with suppurative lesions in various organs, including pneumonia, hepatitis, splenitis, pyelonephritis, and peritonitis. Klebsiella pneumoniae commonly occurs in the intestinal flora of rats and mice and is considered an opportunistic pathogen in these species. It has been associated with suppurative rhinitis in rats and with pneumonia in mice.17., 29. Isolation of a previously unrecognized Haemophilus sp from respiratory tracts of rats has been reported to be associated with mild pneumonia.21 Strains of Chlamydia trachomatis and Chlamydia psittaci are capable of causing pneumonitis in mice, but all reported instances have been in laboratory mice in which mouse tissues were serially passaged.17
There is one report of respiratory disease caused by Mycobacterium avium infection in laboratory mice in a commercial breeding facility.36 The source of the infection was thought to be the drinking water, and there were no clinical signs. A few affected mice had raised, tan subpleural nodules up to 5 mm in diameter. Microscopic lesions were characterized by multifocal granulomatous pneumonia with multinucleate giant cells. Lesions were most prevalent near small bronchioles. Organisms were evident in acid fast-stained sections.
Chronic respiratory disease similar to MRM and caused by a novel mycoplasma-like organism has been described in rats and mice.1., 10. The agent, originally termed grey lung virus, has been shown by genetic analysis to be a mycoplasma distinctly different from Mycoplasma pulmonis.22 It has not been cultured on artificial medium, and very little is known of its prevalence or significance as a cause of spontaneous disease.
Cases of pneumonia in guinea pigs have been associated with bacteria in addition to those listed above ( Fig. 3), including Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumoniae, and Streptobacillus moniliformis 26 Citrobacter freundii is an occasional opportunistic pathogen in guinea pigs, and there is one report of an epizootic of fibrinopurulent pleuropneumonia and septic thrombi in the lung, liver, and spleen in one colony, in which 115 of 1300 animals died.23 Weanlings appeared to be more susceptible than adults. Pseudomonas aeruginosa was described in one report as the cause of pulmonary botryomycosis in two guinea pigs.3 Yersinia pseudotuberculosis is an uncommon cause of miliary pneumonia or caseous pulmonary abscesses in guinea pigs. The organism is transmitted by means of fecal contamination by vermin. Acute disease is characterized by enteritis. Subacute and chronic disease results from septicemic dissemination of the organism, resulting in multifocal lesions in the lungs, lymph nodes, liver, and spleen.26 Similar disease can occur in hamsters.9., 27.
Figure 3.
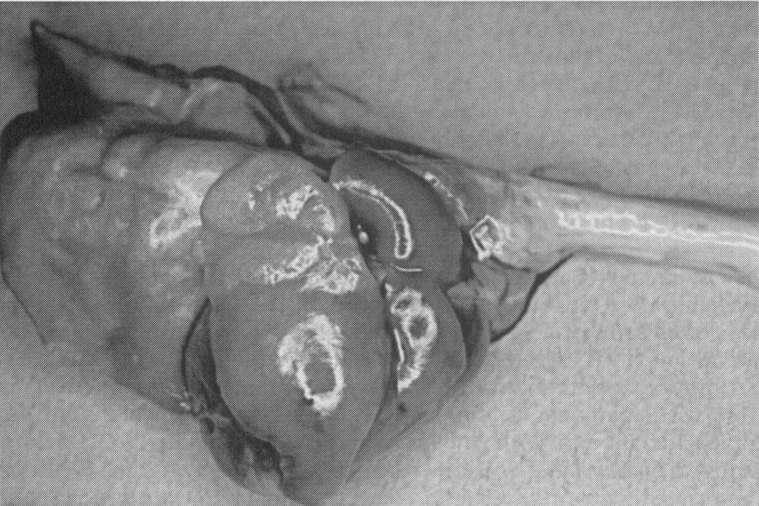
Mixed gram-negative bacterial pneumonia in a guinea pig. The right apical lobe and the ventral portion of the right middle lobe are affected.
Pneumocystis carinii is not a bacterium but is related to fungi.7 It is an important respiratory pathogen in immunodeficient laboratory mice, rats, and guinea pigs but does not cause disease in immunocompetent rodents.
Neoplasms
Pulmonary alveolar tumors arising from Type II epithelial cells are common in older mice of some laboratory strains.28., 35. These can be single or multiple and are pale, rounded, and frequently located beneath the pleura, so that small lesions are prominently visible. They are composed of uniform cuboidal to columnar cells arranged in acinar or papillary patterns, with minimal stroma. Many of these tumors do not behave aggressively and are incidental postmortem findings. Adenocarcinomas do occur, however, and can penetrate the pleura, establish metastases in the pleural cavity, and even invade the thoracic wall. Bronchiolar adenomas and adenocarcinomas originating from Clara cells also occur in mice but less commonly than alveolar tumors. Mammary carcinoma and hepatocellular carcinoma must be considered in the differential diagnosis, inasmuch as these commonly metastasize to the lungs. Neoplasms also must be differentiated from focal alveolar hyperplasia of mucin-containing epithelial cells (pulmonary adenomatosis), a condition of unknown cause that occurs occasionally in aged mice,35 and from multifocal inflammatory lesions.
Pulmonary tumors are reported to be among the commonest neoplasms of guinea pigs.26., 35. The majority are papillary adenomas. They occur most often in animals older than 3 years and can be multiple. Primary lung tumors are rarely reported in other rodent species, and upper respiratory tract neoplasms are rare in all rodents.35
Clinical diagnosis of lung tumors in pet rodents by radiography is possible; however, advanced cases probably would be recognized only after the patient develops respiratory distress. Benign lesions or early malignant ones are nearly always incidental necropsy findings. Definitive diagnosis is by histopathology.
Miscellaneous Conditions
A variety of miscellaneous lesions occur in the respiratory tract of rodents. Most of these conditions are of unknown or poorly understood cause and are not associated with clinical disease, but practitioners using histopathologic services of diagnostic laboratories should be aware of them.
In mice, particularly older animals of some inbred laboratory strains, accumulations of irregular spicule-like eosinophilic crystals in the terminal airways and alveoli occur occasionally.28 The crystals usually are associated with macrophages and are predominantly intracellular. In some cases, the crystals are associated with a mild granulomatous reaction accompanied by accumulation of lymphocytes. The condition, commonly termed pulmonary histiocytosis or crystal pneumonitis, also has been observed in wild mice. Alveolar histiocytosis is rather common in rats.2 The lesions are grossly visible as discrete, pale, slightly elevated, plaquelike areas, chiefly in the dorsal pleura. Microscopically, there is subpleural accumulation of foamy, lipid-containing macrophages. There is no eosinophilic crystal accumulation as in mice. Alveolar histiocytosis similar to that in rats also occurs in hamsters.27
Granulomatous inflammation in response to inhaled particulate matter, such as fragments of bedding material (foreign body pneumonitis) occurs as an incidental finding in mice,28 rats,29 and guinea pigs.26 In guinea pigs, prominent circumscribed nodular accumulations of lymphocytes adjacent to pulmonary vessels are common.26 The stimulus inciting these accumulations is not known. Lesions of soft tissue mineralization or metastatic calcification, a presumably nutritional condition affecting a wide variety of organs and tissues in guinea pigs, also can occur in the lungs.26 Trophoblastic emboli are reported to be common in pregnant hamsters.5
Clinical signs of respiratory distress can occur in heart failure resulting from atrial thrombosis. In white or albino animals, cyanosis can be evident. The condition is clinically silent in many cases. Atrial thrombosis occurs spontaneously in mice, rats, and hamsters, particularly in older animals, and is a common cause of death in older hamsters.27., 28. Thrombi can occur in either atrium but occur more commonly on the left. Septic thrombi are occasionally seen, but most do not appear to result from bacterial infection.
Principles of diagnosis, treatment, and prevention of respiratory diseases of rodents do not differ substantially from those applicable in other species, involving mostly the common sense application of knowledge concerning the diseases occurring in each species. The objective of this review is to provide a concise source of information concerning causes, clinical signs, diagnosis, treatment, and prevention of respiratory diseases of mice, rats, guinea pigs, and hamsters.
Acknowledgments
The author thanks Drs. J. Russell Lindsey and Matthew Van Zwieten for use of their photographs.
References
- 1.Andrewes C.H., Glover R.E. Grey lung virus: An agent pathogenic for mice and other rodents. Br J Exp Pathol. 1946;26:379–386. [PMC free article] [PubMed] [Google Scholar]
- 2.Anver M.R., Cohen B.J. Lesions associated with aging. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. The Laboratory Rat, Vol I. Biology and Diseases. Academic Press; New York: 1979. p. 377. [Google Scholar]
- 3.Bostrom R.E., Huckins J.G., Kroe D.J. Atypical fatal pulmonary botryomycosis in two guinea pigs due to Pseudomonas aeruginosa. J Am Vet Med Assoc. 1969;155:1195. [PubMed] [Google Scholar]
- 4.Brennan P.C., Fritz T.E., Flynn R.J. Role of Pasteurella pneumotropica and Mycoplasma pulmonis in murine pneumonia. J Bacteriol. 1969;97:337. doi: 10.1128/jb.97.1.337-349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burek J.D., Goldberg B., Hutchins G. The pregnant Syrian hamster as a model to study intravascular trophoblasts and associated maternal blood vessel changes. Vet Pathol. 1979;16:553. doi: 10.1177/030098587901600508. [DOI] [PubMed] [Google Scholar]
- 6.Carter K.K., Hietala S., Brooks D.L. Tylosin concentrations in rat serum and lung tissue after administration in drinking water. Lab Anim Sci. 1987;37:468. [PubMed] [Google Scholar]
- 7.Edman J.C., Kovacs J.A., Masur H. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988;334:519. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- 8.Feldman S.H., Richardson J.A., Clubb F.J., Jr. Necrotizing viral bronchopneumonia in guinea pigs. Lab Anim Sci. 1990;40:82. [PubMed] [Google Scholar]
- 9.Frisk C.S. Bacterial and mycotic diseases. In: Van Hoosier G.L. Jr., McPherson C.W., editors. Laboratory Hamsters. Academic Press; Orlando, FL: 1987. p. 111. [Google Scholar]
- 10.Gay F.W. Association of a mycoplasma-like agent with chronic pneumonia and bronchiectasis in the rat. J Bacteriol. 1969;97:441. doi: 10.1128/jb.97.1.441-444.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenlee J.E., Clawson S.H., Phelps R.C. Distribution of K-papovavirus in infected newborn mice. J Comp Pathol. 1994;111:259. doi: 10.1016/s0021-9975(05)80004-0. [DOI] [PubMed] [Google Scholar]
- 12.Hawk C.T., Leary S.L. Iowa State University Press; Ames, IA: 1995. Formulary for Laboratory Animals. [Google Scholar]
- 13.Harkness J.E., Wagner J.E. Williams & Wilkins; Baltimore: 1995. The Biology and Medicine of Rabbits and Rodents. [Google Scholar]
- 14.Hillyer E.V., Quesenberry K.E. WB Saunders; Philadelphia: 1996. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery. [Google Scholar]
- 15.Jacoby R.O., Bhatt P.N., Jonas A.M. Viral diseases. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. The Laboratory Rat, Vol I. Biology and Diseases. Academic Press; New York: 1979. p. 271. [Google Scholar]
- 16.Laber-Laird K., Swindle M.M., Flecknell P.A. Pergamon Press; New York: 1996. Handbook of Rabbit and Rodent Medicine. [Google Scholar]
- 17.Lindsey J.R., Cassell G.H., Davidson M.K. Mycoplasmal and other bacterial diseases of the respiratory system. In: Foster H.L., Small J.D., Fox J.G., editors. The Mouse in Biomedical Research, Vol II. Diseases. Academic Press; New York: 1983. p. 21. [Google Scholar]
- 18.Matsushita S., Joshima H., Matsumoto T. Transmission experiments of cilia-associated respiratory bacillus in mice, rabbits and guinea pigs. Lab Anim. 1989;23:96. doi: 10.1258/002367789780863664. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita S., Suzuki E. Prevention and treatment of cilia-associated respiratory bacillus in mice by use of antibiotics. Lab Anim Sci. 1995;45:503. [PubMed] [Google Scholar]
- 20.Medina L.V., Chladny J., Fortman J.D. Rapid way to identify the cilia-associated respiratory bacillus: Tracheal mucosal scraping with a modified microwave Steiner silver impregnation. Lab Anim Sci. 1996;46:113. [PubMed] [Google Scholar]
- 21.Nicklas W. Haemophilus infection in a colony of laboratory rats. J Clin Microbiol. 1989;27:1636. doi: 10.1128/jcm.27.7.1636-1639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemark H.C., Leach R., Mitchelmore D. Grey lung disease agent, an uncultured wallless prokaryote, appears to be a new mycoplasma species. Abstract 012-5, Tenth international congress of the International Organization for Mycoplasmology. IOM Letters. 1994;3:486. [Google Scholar]
- 23.Ocholi R.A., Chima J.C., Uche E.M. An epizootic infection of Citrobacter freundii in a guineapig colony [short communication] Lab Anim. 1988;22:335. doi: 10.1258/002367788780746278. [DOI] [PubMed] [Google Scholar]
- 24.Parker J.C., Ganaway J.R., Gillett C.S. Viral diseases. In: Van Hoosier G.L. Jr., McPherson C.W., editors. Laboratory Hamsters. Academic Press; Orlando: 1987. p. 95. [Google Scholar]
- 25.Parker J.C., Richter C.B. Viral diseases of the respiratory system. In: Foster H.L., Small J.D., Fox J.G., editors. The Mouse in Biomedical Research, Vol II. Diseases. Academic Press; New York: 1983. p. 109. [Google Scholar]
- 26.Percy D.H., Barthold S.W. Pathology of Laboratory Rodents and Rabbits. Iowa State University Press; Ames, IA: 1993. Guinea pig; p. 146. [Google Scholar]
- 27.Percy D.H., Barthold S.W. Pathology of Laboratory Rodents and Rabbits. Iowa State University Press; Ames: 1993. Hamster; p. 115. [Google Scholar]
- 28.Percy D.H., Barthold S.W. Pathology of Laboratory Rodents and Rabbits. Iowa State University Press; Ames, IA: 1993. Mouse; p. 3. [Google Scholar]
- 29.Percy D.H., Barthold S.W. Pathology of Laboratory Rodents and Rabbits. Iowa State University Press; Ames, IA: 1993. Rat; p. 71. [Google Scholar]
- 30.Porter W.P., Bitar Y.S., Strandberg J.D. Absence of therapeutic blood concentrations of tetracycline in rats after administration in drinking water. Lab Anim Sci. 1985;35:71. [PubMed] [Google Scholar]
- 31.Richardson V.C.G. Iowa State University Press; Ames, IA: 1997. Diseases of Small Domestic Rodents. [Google Scholar]
- 32.Schoeb T.R., Davis J.K., Lindsey J.R. Murine respiratory mycoplasmosis, rat and mouse. In: Jones T.C., Mohr U., Hunt R.D., editors. International Life Sciences Institute Monographs on Pathology of Laboratory Animals: Respiratory System. Springer-Verlag; Berlin: 1996. p. 117. [Google Scholar]
- 33.Schoeb T.R., Lindsey J.R. Cilia-associated respiratory bacillus infection, rat, mouse, and rabbit. In: Jones T.C., Mohr U., Hunt R.D., editors. International Life Sciences Institute Monographs on Pathology of Laboratory Animals: Respiratory System. Springer-Verlag; Berlin: 1996. p. 325. [Google Scholar]
- 34.Shoji-Darkye Y., Itoh T., Kagiyama N. Pathogenesis of CAR bacillus in rabbits, guinea pigs, Syrian hamsters, and mice. Lab Anim Sci. 1991;41:567. [PubMed] [Google Scholar]
- 35.Squire R.A., Goodman D.G., Valerio M.G. Tumors. In: Benirschke K., Garner F.M., Jones T.C., editors. Pathology of Laboratory Animals. Springer-Verlag; New York: 1978. p. 1051. [Google Scholar]
- 36.Waggie K.S., Wagner J.E., Lentsch R.H. A naturally occurring outbreak of Mycobacterium avium-intracellulare infections in C57BL/6N mice. Lab Anim Sci. 1983;33:249. [PubMed] [Google Scholar]


