Abstract
Serum Protein Electrophoresis (SPE) is a useful diagnostic and prognostic tool in human and companion animals medicine: several experiences show that it can be useful in exotic practice as well. The fundamentals of SPE interpretation as well as some normal and pathological patterns for the species most commonly seen in practice are provided.
Keywords: Electrophoresis, Exotic pets, Protein
Key points
-
•
With exotic species, progress in the interpretation of the alteration of plasma proteins has been discontinuous and irregular.
-
•
In several exotic species, especially those of small size, the need to work with small volumes of blood requires the clinician to collect the blood with an anticoagulant.
-
•
In clinical practice, the normal plasma protein concentration is measured very simply by a refractometer.
Hundreds of proteins, having several different functions, circulate in the plasma that are, with the exception of immunoglobulins, largely synthesized in the liver. Plasma proteins in the complex are responsible for the colloidal osmotic effect necessary to maintain blood volume. Other important functions of plasma proteins are the buffer function of the blood pH (15%–20% of the total buffering capacity), transport of hormones, transport of drugs, and blood clotting. Different types of proteins are indispensable for the inflammatory reaction, the immune reaction, and for the process of healing and tissue repair. So, it is easily understood that the measurement of plasma proteins and the determination of their alterations may constitute an important method for assessing the health condition of a patient. Human medicine, such as the dog and cat’s, has taken advantage for decades of this valuable diagnostic tool. With exotic species, progress in the interpretation of the alteration of plasma proteins has been more discontinuous and irregular. Despite this, the usefulness of this test is indisputable. This article analyzes the different applications of plasma protein electrophoresis in small mammals, birds, and reptiles.
Method of assessing proteins
Traditionally in the medicine of dogs and cats, the measurement of protein is performed on the serum, the fluid that is obtained by centrifugation of a blood sample allowed to clot in a test tube for 15 to 20 minutes, the time needed for fibrinogen to be used in the coagulation cascade. In several exotic species, especially of small size, the need to work with small volumes of blood requires the clinician to collect the blood with an anticoagulant (frequently natrium heparin or lithium heparin, sometimes heparinizing the syringe to avoid the coagulation of the sample during collection); therefore, centrifugation is achieved in plasma. The main difference between the 2 products is the absence in the serum of fribrinogen, the protein involved in the processes of coagulation; the concentration of total solids of the plasma is thus slightly higher than that of serum (about 5%) and the electrophoretic pattern from it will result in a higher incidence of β-globulin fraction where the fibrinogen normally migrates. In clinical practice, the normal plasma protein concentration is measured very simply by using a refractometer: a few drops of blood are collected in a heparinized capillary whose centrifugation gives the value of the microhematocrit (percentage of blood occupied by cells compared with plasma) and a small amount of plasma. Refractometry of that plasma allows easy estimation of the concentration of total solids. This method is simple and intuitive; however, it is not reliable if the sample quality is poor. Among the factors that interfere with the measurement of total solids by refractometer include hemolysis, lipemia, hyperbilirubinemia, azotemia, severe hyperglycemia, hypernatremia, hyperchloremia, and administration of colloids, including synthetic hemoglobin–based oxygen carriers. In the absence of these factors, the amount of nonprotein solids present in plasma is relatively constant, therefore by subtracting the nonprotein component (1.5 g/dL) the value of total plasma proteins is obtained.
An increase in the concentration of total solids (hyperproteinemia) must always be evaluated in correlation with the microhematocrit value and reflects mainly dehydration or increased synthesis of globulins for various pathologic conditions. The decrease in total solids (hypoproteinemia), vice versa, may reflect overhydration, decreased synthesis of albumin or immunoglobulin, or protein loss associated with hemorrhage, vasculitis, nephropathy, or enteropathy. With the exception of bleeding, which causes a loss of balanced albumins and globulins, in all pathologies involving protein loss albumin concentration falls to a greater extent than that of the globulins because of lower dimensions of the molecule, which allow easier migration through vascular endothelia. The measurement of total protein is certainly very useful, but for the purposes of a diagnosis that is as accurate as possible, it becomes essential to know the alterations of each protein fraction. For this purpose is used the characteristic of plasma proteins to be separated based on their charge and the consequent mobility in an electric field specially created. This method defines electrophoresis of proteins. The distribution of protein migrants on strips of cellulose acetate or agarose gel is represented graphically by a curve in which the extension and the height of each peak is corresponding to the breadth and the density of each protein fraction on the substrate migration.
Normally, 5 protein fractions are identified in the mammalian plasma, ordered according to the electric charge: albumin (high negative charge and low molecular weight, migrates to the anode and conventionally to the left of the track), α1-globulin, α2-globulin, and β-globulins and γ-globulins that present the greatest molecular weight and the lowest negative charge and then migrate to the right end of the curve. A different protein fraction is present in most birds and reptiles, with an even more negative charge than albumin that is positioned at the left in the same electrophoretic pattern and is therefore referred to as prealbumin. Conventionally, you find a point halfway between the beginning and end of the curve as the limit between the α2-globulin and β-globulins, and a point halfway to the start of β-globulins and the end of the track as the start of γ-globulin. With the exception of albumin, each peak represents a large number of different proteins that can be further separated by more complex examinations.
The different fractions in circulating plasma protein
Prealbumin is the protein fraction that migrates more rapidly and is positioned on the track before albumin. In species that have it, this is calculated with albumin in the ratio A/G. It is not normally detectable in mammals, except occasionally in cats, and it is normally found in human patients where it has the function of direct transport of thyroid hormones and indirectly of vitamin A by acting as a carrier protein that binds retinol. In the various avian species, it is present in variable amounts. For example, in the budgerigar (Melopsittacus undulatus) or in the cockatiel (Nymphicus hollandicus) it can constitute 75% of the total prealbumin + albumin, whereas in African gray parrots (Psittacus erythacus), it represents no more than 10% of total albumin and can also be completely absent.1 In reptiles, the study of plasma proteins is still sporadic and fragmentary data exist, but migrating prealbumin has been reported in some species of chelonians: Caretta caretta,2 Geochelone radiata,3 Chelonia mydas, 4 and sauria (Gallotia spp),5 as well as crocodiles (Caiman spp), but only with subsequent migration on polyacrylamide gels6 apparently have similar transport functions, as in other orders.
Albumin constitutes the main fraction of the plasma proteins (in general between 35% and 50% of total protein and up to 60% to 65% in primates, including humans) and represents the main and more homogeneous peak in the electropherograms of all species. The main functions of albumin are to carry many molecules and maintain the oncotic pressure of the blood.7
The group of globulins include the acute phase proteins (APPs; α-globulins and β-globulins) and immunoglobulins (γ-globulins).
APPs are the body's response to trauma, inflammation, or infection. The local activation of granulocytes and macrophages causes the release of cytokines (mainly interleukin-6) that stimulate the liver to produce a series of glycoproteins. Alpha-1-antitrypsin, α-1-acid glycoprotein, α2-macroglobulin, C-reactive protein, haptoglobin, and fibrinogen are positive APPs, because their concentration increases in response to inflammation; albumin is considered a negative APP, as its synthesis decreases during inflammation, diverting liver protein synthesis to the proteins mentioned previously. The electrophoretic pattern of the APPs migrates to the peak of the α (α-1-acid glycoprotein, α2-macroglobulin, haptoglobin, protein C) and β (C-reactive protein, fibrinogen, complement, ferritin)-globulins, but with huge specific differences.
The group of γ-globulins is mainly composed of immunoglobulins, although some APPs, such as degradation products of the complement, can migrate into this area as well, especially in avian species. Immunoglobulins are quite impressive in size, consisting of 4 amino acid chains, 2 so-called “heavy” and 2 “light.” Depending on the composition of the heavy chains, immunoglobulins are divided into classes named by letters of the Greek alphabet: gamma (IgG), mu (IgM), alpha (IgA), and epsilon (IgE). The IgG antibodies are produced in response to various bacterial, viral, or toxic stimuli; IgE (not present in all species) is mainly involved in allergic reactions; IgA is primarily responsible for the defense processes located at the mucosal level; and IgM has the characteristic of being active in pentameric form (ie, joining in groups of 5), thereby forming a protein complex of imposing size. IgM is secreted by B-lymphocytes and early plasma cells and its main function is to activate complement, whereas IgG is produced later and then plays a predominant action of opsonization, binding to the pathogen and allowing phagocytosis by macrophages. It is normally not possible to differentiate IgM from IgG through normal electrophoresis, but distinguishing the ones by the others is very important because it allows us to date the pathologic process in acute (IgM only) or chronic (IgG with or without IgM) and to differentiate an active process (IgM) by a seropositivity alone (IgG).
Electrophoresis of proteins in the rabbit
Electrophoresis serum is used as a “screening” of interpretation of the humoral response and during not specific processes. Recent studies in rabbits have tried to bring to the surface the true diagnostic value of this report, in particular we tried to find a link between hypergammaglobulinemia and one of the diseases most commonly encountered in clinical practice veterinary medicine in lagomorphs: Encephalitozoon cuniculi.
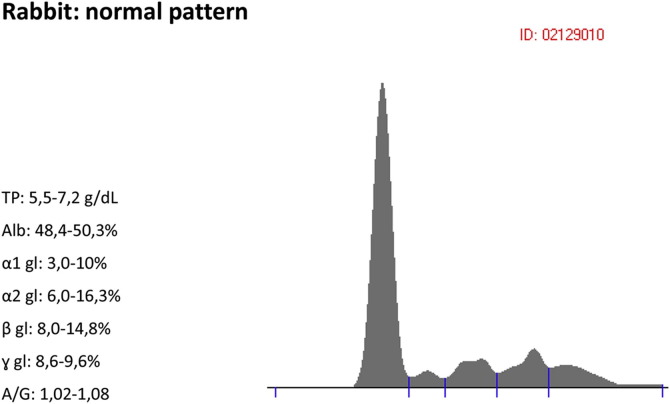
The blood for sampling is collected in plain tubes (the use of tubes with accelerant is not possible because of the reduced volume of collectable blood in this species) and left to rest for about 30 minutes. The serum obtained is isolated and frozen at a temperature of –18°C to be examined in the shortest possible time. Laboratory data have shown that a single cycle of freezing does not alter the electrophoretic pattern, whereas prolonged freezing determines a variation in the albumin/globulin ratio in favor of the latter. The serum of the rabbit contains, as in other mammals, various protein fractions: albumin, α-globulins, β-globulins, and γ-globulins. The α-globulins and β-globulins are generally divided into subunits of α1 and α2, and β1 and β2 (Fig. 1 ). Albumin constitutes the main fraction responsible for the oncotic pressure of body fluids, whereas the α1-globulin, α2-globulin, β-globulins, and γ-globulins are a smaller fraction responsible for the humoral response of the organism. Unlike the guinea pig, however, the area occupied by β-globulins is wider than that of the α-globulins. In rabbits, the normal Serum Protein Electrophoresis (SPE) pattern lacks a clear distinction between β1-globulins and β2-globulins, as present in dogs and cats, but when gammopathies in the β region occur, usually an extension of the electrophoretic band is seen, with consequent demarcation of the 2 peaks. The α1-globulins, together with the α2-globulins and β-globulins, increase in case of acute inflammation, expressing a more rapid response compared with that cell mediated. The γ fraction includes circulating immunoglobulins, complement, the degradation products of complement, and to a lesser extent some proteins that characterize the acute phase.8 C-reactive protein is one of the first proteins produced by the body following an insult to any tissue. Despite being defined as an APP, it is also found in the course of chronic processes.9 Placed inside a magnetic field (principle of electrophoresis of proteins), because of its shape, weight, and molecular electrostatic charge, such protein migrates to the right (toward the anode or negative electrode) in the area of β-globulins and γ-globulins to influence the path. Other APPs, such as aptoglobulina, α1 acid glycoprotein, and ceruloplasmin are normally found in the α and β fractions. In course of encephalitozoonosis we have seen a decrease of albumin, primarily because of the increased synthesis by hepatocytes of numerous immunoproteins (especially Ig) with consequent widening of the electrophoretic band in the region of β and γ. The β-globulin and γ-globulin can be found in course of chronic inflammatory processes (like bacterial, viral, or fungal infections), parasitism (E cuniculi), cancer, or immune-mediated diseases. In the specific case of protozoal infection by E cuniculi, healthy animals and seropositive animal SPE patterns only differ significantly in the γ fraction.10 Results are very similar to those obtained by Didier in 1995, in a study of dogs showing clinical signs of encephalitozoon infection.11 The hypergammaglobulinemia during encephalitozoonosis is usually mild to moderate; this value may be altered when rabbits with impaired immune response come in contact with the parasite, which may react with a decrease of circulating IgG and a possible increase in IgM.12 The titration of IgM and IgG allows us to clarify the response against the parasite carried by the body at the time of collection. IgM increases usually in the early stages when the parasite exerts its action, decreasing up to be absent on the 38th day, whereas IgG increases more slowly in the course of infection but remains detectable for years.13 The changes of γ-globulin have been shown to not be significantly different in animals with IgM titratable compared with animals with IgG increased. This shows that the hypergammaglobulinemia is an artifact resulting in a chronic inflammatory process. In support of this theory, it is believed that the organism remains latent in the body until it occurs in a suppression of the immune system, a phase in which the body eliminates spores in the urine.14
Fig. 1.
Normal pattern for a rabbit.
Contrary to what occurs in avian species, and in accordance with what occurs in dogs, hemolysis increases the β fraction, not the γ fraction.
Electrophoresis of proteins in the ferret
In the otherwise rich literature on diseases of the ferret, electrophoresis is quite neglected, mainly because the main source of information on ferret diseases is US research, where electrophoresis is relatively unknown: the only application is described as an aid in diagnosis of Aleutian disease. Actually, it is a useful diagnostic tool in ferret species and its interpretation is not very different from that of the dog and cat. Being that the ferret is an animal of sufficient size, allowing access to the veins of good gauge as the cranial cava, it is recommended to draw blood without anticoagulant (heparin needle) to obtain serum instead of plasma and thus avoid the deposition of fibrinogen, which will inevitably alter the peak of β-globulins.
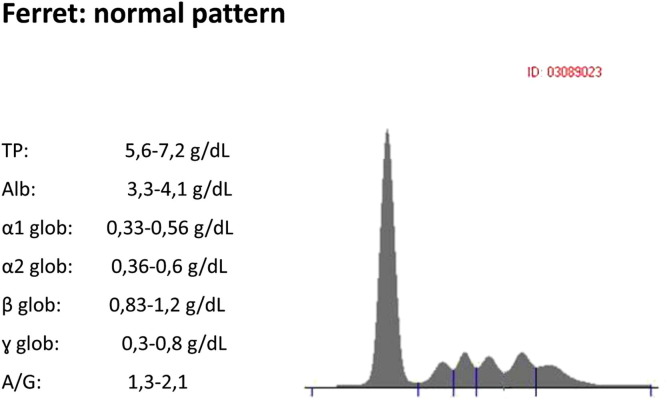
In a healthy ferret, as in dogs, the proteins migrate in the electric field in dividing fractions: albumin, α1-globulin, α2-globulin, β1-globulin, β2-globulin, and γ-globulin. Albumins represent approximately half of circulating proteins, for which the A/G ratio is about 1. IgA migrates in the field of α2 and β1 globulins, IgM in the field of β2 globulin or “fast” γ globulin, whereas the IgG in the field of slow γ-globulin (the far end of the track) (Fig. 2 ).
Fig. 2.
Normal pattern for a ferret.
In ferrets, dehydration progresses very quickly in case of insufficient intake or increased losses, so hyperproteinemia is a frequently encountered clinical sign (for example in diarrhea, intestinal obstruction, acute gastritis, or renal failure) but in the case of noninfectious diseases, proteins raise in their entirety for hemoconcentration, so the pattern will result basically normal; in the case of inflammatory diseases, globulins increase, altering the electrophoretic curve. The most characteristic alteration is described in patients suffering from Aleutian disease. This disease, fortunately is infrequent (but certainly underdiagnosed) in Italy, is caused by a parvovirus (ADV) that affects the Mustelidae family, including mink and ferret. It is transmitted by feces and all body secretions and is particularly insidious because it evolves as a chronic disease, debilitating the ferret to the invariably fatal outcome. Aleutian disease causes lymphoplasmacytic infiltration and precipitation of immune complexes in various tissues from which a variety of symptoms is shown by sick animals. Blood tests are often vague, showing nonregenerative anemia, abnormal liver enzymes, and uremia; however, electrophoresis is revealing. Patients with ADV in fact have a marked hyperproteinemia with values up to 10 to 12 g/dL or more, but at the same time an important hypoalbuminemia: γ-globulins rise instead to constitute 20% to 60% of total protein, drawing a characteristic “horned” path and seriously altering the A/G ratio. Other diseases can cause hypergammaglobulinemia, although not as marked as ADV: other viruses such as influenza or distemper, pyoderma, kidney diseases with no protein loss, certain tumors, and systemic mycoses (Blastomycosis, Coccidioides spp). Another, more frequent, viral disease is catarrhal enteritis (ECE) caused by a coronavirus: kits are often asymptomatic carriers for adults who become instead gravely ill. In case of ECE, electrophoresis is characterized at the beginning by moderate hypoalbuminemia, which becomes more and more severe during the following weeks. Following the evolution of the disease, an increase in α2 or β1 may be noticed, meaning the production of IgA in the acute phase, then a peak of gammaglobulines even if significantly below that of ADV. After the second or third week, there is often hypogammaglobulinemia, evidence of the failure of the immune response.
It is also interesting to note the changes in the electrophoretic pattern in the case of liver disease. In the case of hepatic neoplasia, often the protein curve does not differ much from that of a healthy animal, except for a modest elevation of α2-globulins. More marked is the alteration in case of hepatic lipidosis and acute cholangitis-cholangiohepatitis, when the increase of α2-globulins is greater and reflects an intense inflammation. Chronic hepatitis and cholestasis phenomena, intrahepatic or extrahepatic, are reflected instead in a β-globulin peak, where transferrin, C-reactive protein, and generally the IgM migrate.
The decrease in the percentage of globulin is uncommon and is usually associated with renal failure or lymphoma. The hypoproteinemia usually indicates a defect in protein synthesis and has a negative prognostic value.15
Electrophoresis of proteins in hystricomorph rodents
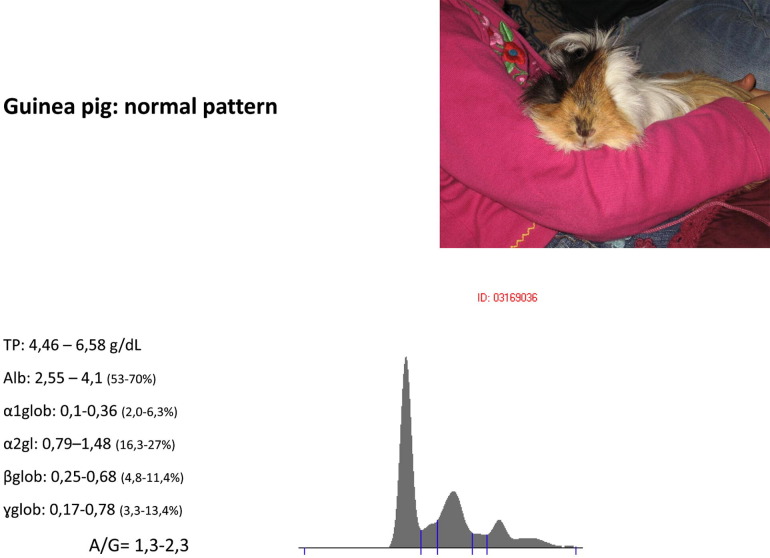
The electrophoretic pattern of the guinea pig (Cavia porcellus) has been thoroughly studied in the experimental for which we know either paths in different normal farming conditions and adjustment of the normal bacterial flora (Specific pathogen free subjects and the like) and the reaction to various pathologies experimentally induced; however, very little is described in the field of clinical practice about the response of animals living in uncontrolled conditions that get sick spontaneously. Clinically healthy guinea pig serum proteins are separated into 7 sections: albumin, α1-globulin, α2-globulin, β1-globulin, β2-globulin (often divided into 2 peaks), and γ-globulins, and in some subjects prealbumins have been occasionally signaled (Fig. 3 ). The area occupied by β-globulins and γ-globulins in the complex is restricted in comparison with the well-developed area of the α-globulins and A/G ratio is high. By immunoelectrophoresis, up to 30 different protein migrants in those areas can be individuated, although not all were necessarily present in the same sample. An interesting study describes the changes in the electrophoretic pattern during the evolution of experimentally induced tuberculosis infection in a group of guinea pigs.16 At the end of the first week, the electrophoretic pattern was still virtually unchanged, whereas at the fulfillment of the second, a large majority of individuals showed a marked increase in α2-globulins and the appearance of a new peak in the same region, characteristic of sick individuals and never found in healthy ones, named α2-T. At the third week, the α1 started to decrease whereas α2 increased further with increasing importance of α2-T peak, which persisted until the death of the subjects. More detailed studies have identified this protein as a glycoprotein, not macroglobulin. It is interesting to note that the response to tubercular infection occurs in the region of α2 in several species, including humans. Guinea pig lipoproteins tend instead to migrate in the β-globulin peak. The same study also analyzed the serum of guinea pigs inoculated with killed mycobacteria: the electrophoresis of these subjects showed no increase of α2-globulins nor the appearance of α2-T, which can then be considered the sign of an organic reaction to the pathogen, but all the samples at the third week after inoculation showed a marked hypergammaglobulinemia. In another study, reaction to infection by Entamoeba histolytica was investigated, which consisted of hypoalbuminemia and increase of α-globulins and β-globulins.17
Fig. 3.
Normal pattern for a guinea pig.
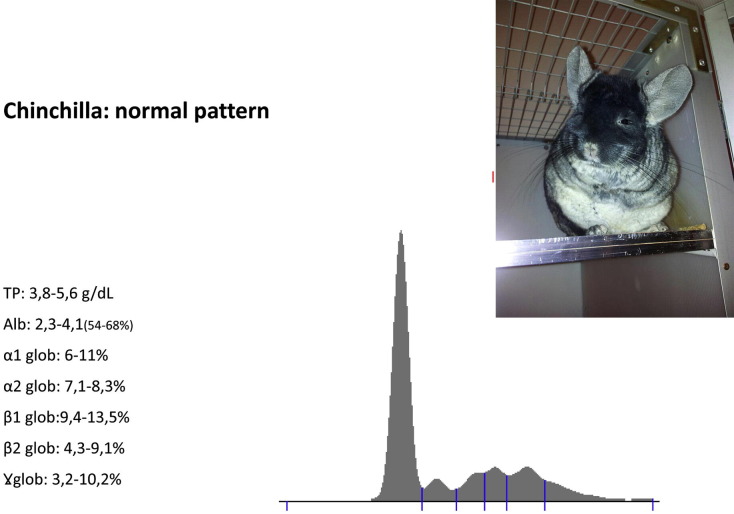
The importance of electrophoresis in the diagnosis of spontaneous disease in the guinea pig and chinchilla is currently studied: patterns from clinically healthy individuals are shown as a starting point for further observations (Fig. 4 ).
Fig. 4.
Normal pattern for a chinchilla.
Electrophoresis of proteins in Psittaciformes
The first studies of serum proteins in birds were performed on domestic chickens, showing many similarities with the layout of mammals (eg, the production of APPs18, 19), but also several differences: the widespread presence of prealbumin, for example, the lowest concentration of γ-globulin, and conversely the more marked response to inflammatory stimuli in the β-globulin field. Unfortunately for the needs of clinical practice, however, not only may the electrophoretic patterns of parrots differ from those of chickens,20 but significant differences are found also among the different species of Psittaciformes (Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10 ). This variability has led some investigators to doubt the possibility of obtaining reliable results by electrophoresis in avian species, especially with regard to the differentiation between the various globulin fractions.21 The answer to this question is probably a better standardization of the techniques of electrophoresis and graphical representation, as well as a better understanding of the normal values of the different species. Normally, electrophoresis of birds is run on plasma samples obtained from heparinized blood: the percentages are derived from densitometric analysis of each protein fraction deposited on the gel and absolute values (g/dL) calculated by multiplying the percentage of each fraction for the value of total protein obtained from the refractometer.
Fig. 5.
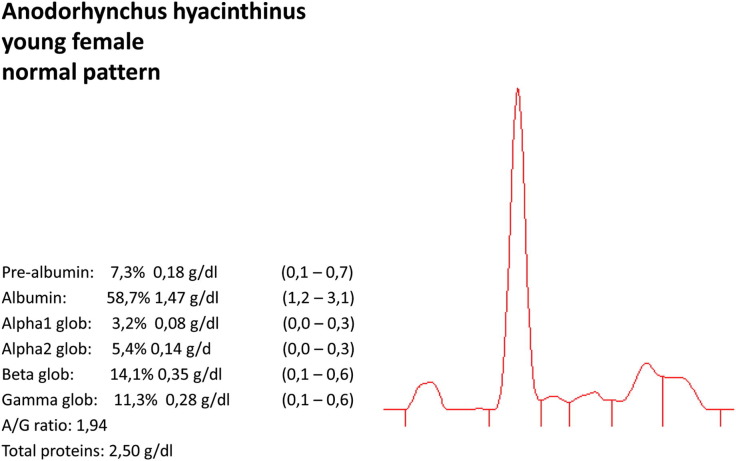
Normal pattern for Anodorhynchus hyacinthinus, young female.
Fig. 6.
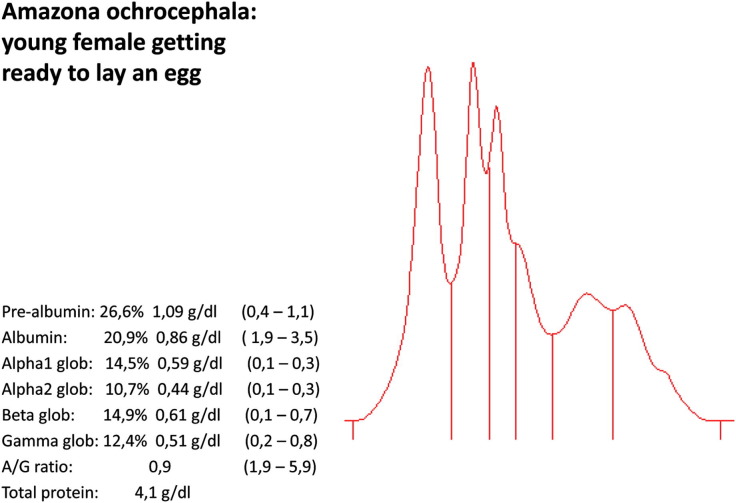
An Amazona ochrocephala (young female) getting ready to lay an egg.
Fig. 7.
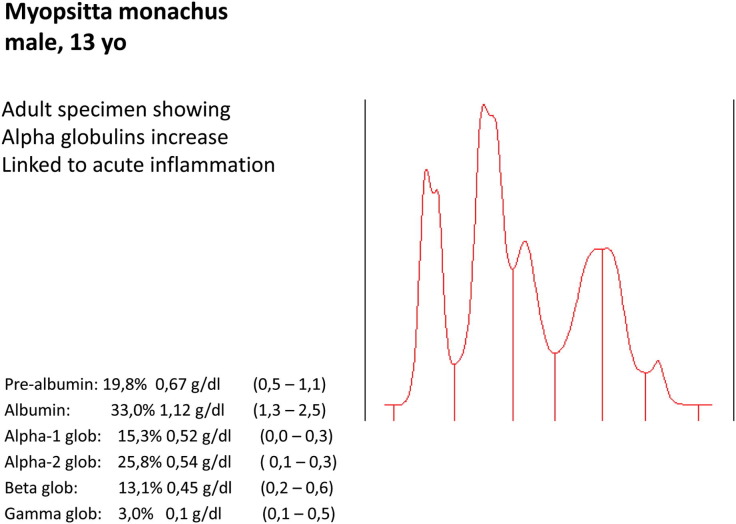
Myopsitta monachus (male, 13 years old). Adult specimen showing α-globulin increase. It is linked to acute inflammation.
Fig. 8.
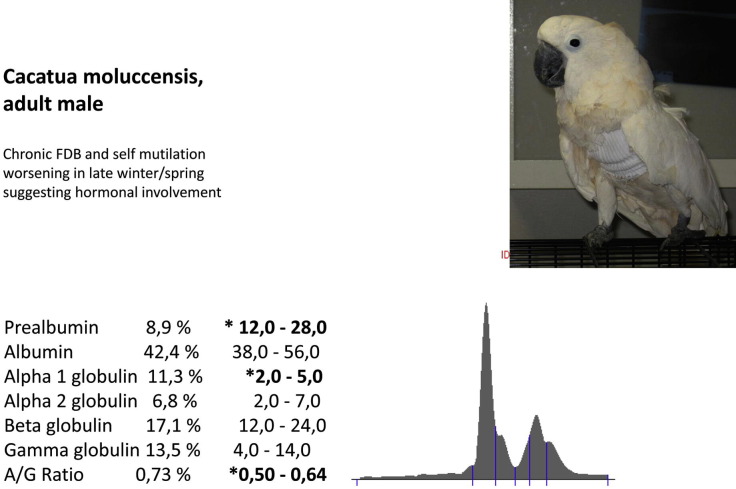
Cacatua moluccensis (adult male). Chronic FDB and self-mutilation worsening in late winter/spring suggesting hormonal involvement.
Fig. 9.
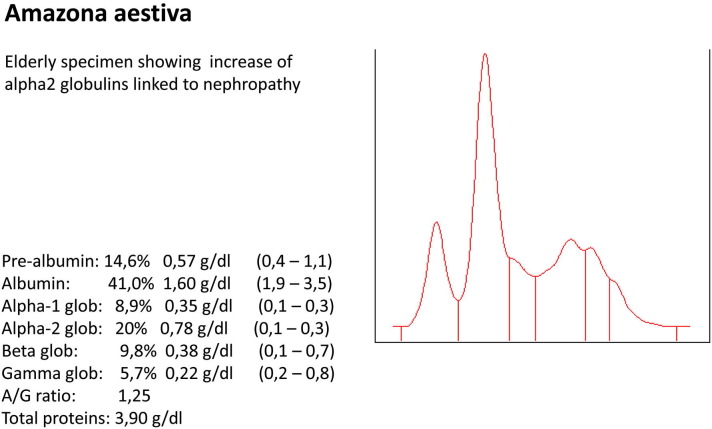
Amazona aestiva. Elderly specimen showing increase of α2-globulins linked to nephropathy.
Fig. 10.
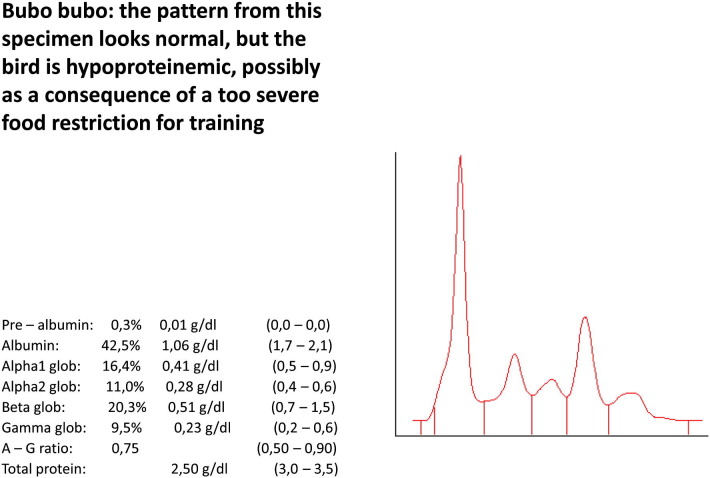
Bubo bubo. The pattern from this specimen looks normal, but the bird is hypoproteinemic, possibly as a consequence of a too severe food restriction for training.
The pattern of the normal chicken shows a marked peak of albumin, and globulins are divided between the β-globulin (IgM, IgA) and γ-globulin (IgG): transferrin, APP, migrates mainly in the β-globulins. In birds, the albumin protein fraction is undoubtedly preeminent; an interspecific important difference is noted in the group of prealbumin. Whereas in some species, such as the African gray, prealbumin is nearly or totally absent, similar to chickens, other species, such as the budgerigar or the monk parakeet (Myopsitta monachus), may have even more prealbumin than albumin. Conventionally, the A/G ratio is calculated by dividing the sum of prealbumin + albumin for the sum of the globulins. According to some investigators, however, it may be more correct to subtract the prealbumin, because it is not properly albumin and even a globulin. The opinion of the author is that this precaution not only is of little practical value but also potentially counterproductive, being that prealbumin, as albumin, has a negative APP and therefore its quantification, when present, has validity in the diagnosis of acute inflammation.
The β-globulins are the predominant group of globulins in all Psittaciformes: some species, such as the African gray, appear regularly divided into 2 subfractions, but the clinical value of this feature is still unclear.22 The β-globulins increase from 15% to more than 35% in the Psittaciformes with chlamydiosis, aspergillosis, or sarcocystosis.23, 24 An increase of β-globulins has also been described in chickens experimentally inoculated with turpentine and other irritants,18, 19 demonstrating that most APPs (transferrin, fibrinogen, β lipoprotein, complement) in avian species migrate in this sector.25 The transferrin appears to be the major APP in avian species (in contrast to what happens in mammals) and, although it is not specific to a particular disease, has an important prognostic value: its short half-life (24–48 hours) makes the monitoring of β-globulins an excellent indicator of the effectiveness of a therapy.
The α-globulins instead represent the group less defined and the whose measurement determines the highest margin of error.22 Altogether they represent only 4% to 8% of total protein, and already this modest quantity (in comparison with that of mammals and also of other avian orders, such as Falconiformes) makes it difficult to precisely separate it from the 2 contiguous predominant peaks, albumins and β-globulins. They are divided into 2 fractions: the area of α1-globulins hosts mainly the α1 antitrypsin and in normal paths is not detectable as a well-defined peak; even in sick parrots an increase of this fraction has almost never been described, unlike what was observed in mammals. In some cases has been noted a selective increase dell'alfa1 antitrypsin, with or without moderate hypoalbuminemia, in Psittaciformes with heavy parasitism.25 The main protein in the α2 area is instead α2 macroglobulin. The characteristic of this protein is its large size (molecular weight > 400,000) for which it is still retained by the filter even in the presence of serious renal damage. One of the few cases when significant elevations of α2 are described is precisely acute nephritis, in which most of the proteins are lost through the kidneys and the percentage of macroglobulin increases accordingly: in response to other phlogistic stimuli, instead, increments much more moderate and inconstant are noticed. Modest alterations of α-globulins have also been described in relation to the activation of the ovary, similarly as described in the human species. The increase of transferrin that occurs during this stage, however, also induces a concomitant peak of β-globulins.
The γ-globulins are a minor fraction in the electrophoretic pattern of the Psittaciformes, on average 10% to 15% of the total protein. In the area of γ-globulin migrate mainly IgG so we see increments in this area only at a later stage of the pathology. Diseases in which we expect massive increases in γ-globulin are chronic diseases such as chlamydiosis or mycobacteriosis. Aspergillosis also belongs to the group of chronic diseases but, because of its immunosuppressing activity, often patients suffering from chronic fungal forms have apparently normal electrophoresis, as the humoral response is inhibited. Approximately 30% of patients suffering from aspergillosis show cyclical peaks in the β-globulins area, and only a small percentage show hypergammaglobulinemia. The normal response to a chronic inflammatory process is the production of a wide variety of antibodies that are expressed in a series of γ-globulin peaks or, more commonly, in a single peak with a large base: this phenomenon is named polyclonal gammopathy. In some cases, however, a single or very few immunoglobulins are massively produced, a phenomenon that is expressed in a peak-to-narrow base similar to that of albumin and is defined oligoclonal or monoclonal gammopathy. Normally behind this phenomenon there is a neoplastic proliferation of one or a few lines of B lymphocytes, even if in some cases, monoclonal gammopathies may occur in response to non-neoplastic stimuli as well, but anyway very serious.
It is worth considering that the low reliability reported by some investigators about the avian electrophoresis is to be traced back to improper collection, retention, and selection of the sample. Cray and colleagues22 showed that serum refrigeration causes a discrete change in the albumin/globulin ratio, causing a progressive difficulty in defining and quantifying the α-globulins. Conversely, a freezing of the sample did not show adverse effects. Hemolysis is a phenomenon unfortunately present when sampling blood from small patients and difficult to control. In the dog, the products of hemolysis tend to migrate in the β-globulin region; conversely, those of birds seem to migrate primarily in the region of γ-globulin. This may be attributable to a structural difference of the protein or by a different technique analysis.22 Lipemia is another frequent alteration especially in the serum of some South American species (Amazona spp, Miopsitta, Pionus spp): the electropherogram of a lipemic sample shows an artificial elevation in the region of β-globulins. In contrast, the β-globulins would be below the normal values related in the literature, in which serum would be used in place of plasma because the fibrinogen (absent in serum) migrates essentially in the β region.26 This is, however, the only significant difference between the tracks from the 2 different spin cycles.
Electrophoresis of Falconiformes
Electrophoresis of birds of prey basically follows the same general considerations of Psittaciformes: common use of plasma instead of serum, need to standardize techniques, and to study the parameters of normality among the different species (Figs. 11 and 12 ). Compared with Psittaciformes, most Falconiformes do not present prealbumin or present it in a minimal amount: vice versa, apparently healthy Falconiformes have levels of α1-globulins and α2-globulins, markedly higher than the parrots. Major differences regarding the β-globulins and γ-globulins are not described. Electrophoresis is an important tool for the diagnosis of chlamydiosis and aspergillosis in Falconiformes. A text antibody positive for Chlamydia spp acquires a very different meaning in the presence of electrophoretic curve indicative of acute or chronic inflammation than in absence of any alteration; a marked alteration of electropherogram with prevailing in the region of the β-globulins can support a presumptive diagnosis of aspergillosis also in case of a negative antibody test.27 The electrophoresis has proved useful as a diagnostic and prognostic tool in Falconiformes even in the case of diseases such as mycobacteriosis, abscesses, and osteomyelitis.
Fig. 11.
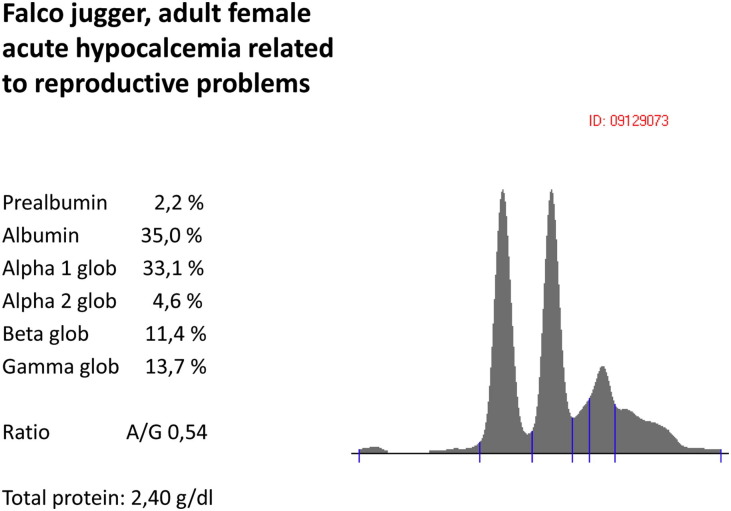
Falco jugger (adult female). Acute hypocalcemia related to reproductive problems.
Fig. 12.
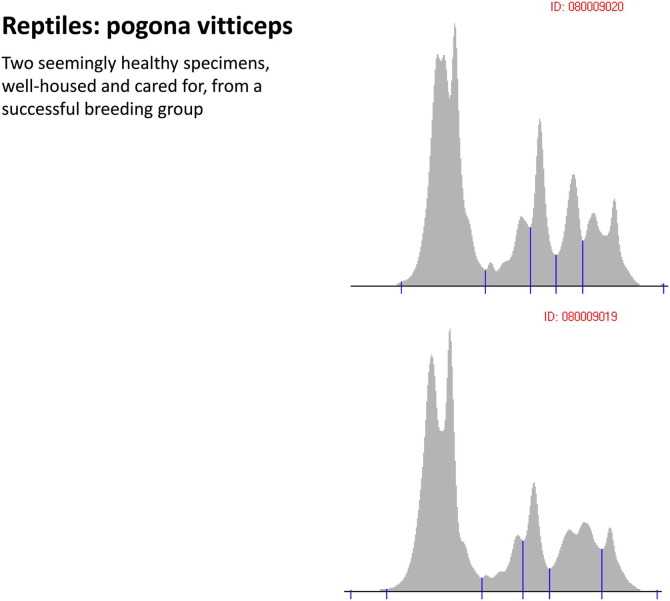
Pogona vitticeps. Two seemingly healthy specimens, well housed and cared for, from a successful breeding group.
Electrophoresis of reptiles
The use of electrophoresis in reptiles is still sporadic and most of the available data are anecdotal; nevertheless, they are interesting as a first element for future study. Serum proteins of reptiles migrate in a manner quite similar to those of birds and are divided on the track in the fractions of albumin, α1-globulin, α2-globulin, β-globulins, and γ-globulins. Prealbumin appears in some species (eg, Geochelone radiata and some turtles), in which it presumably has the same function of transport as in other animals. Albumins are also the preponderant portion of the curve in reptiles, whereas the globulins as a complex, like in birds, are in minimal concentration in healthy subjects.28 In the fraction of α-globulins and β-globulins migrate mainly APPs and complement, whereas the γ-globulins are circulating antibodies; both β-globulin and γ-globulin can configure a single peak or two. Even in the absence of standardization, it can be seen how the electrophoretic pattern changes in subjects markedly ill compared with healthy subjects in reptiles as well. In an iguana (Iguana iguana) with evidence of osteometabolic disease, for example, is frequently observed the decrease of albumins and the marked increase in β globulins (APPs) that indicates suffering hepatic and renal function as a cause or consequence of the metabolic problem.28 The alteration of the ratio A/G is one of the most interesting parameters to observe and evaluate, as it can reveal a dysproteinemia even in the presence of a normal value of total protein, thus inducing the clinician to further investigation. An important obstacle to the use of electrophoresis, as well as the biochemistry as an assessment of the health status of a reptile, is the marked variability linked not only to the species but also to temperature, ambient humidity, photoperiod, and in general to the season, that for heterothermic animals has much more influence on homeostasis than in homeothermic animals. Only the collection of an adequate volume of data will clarify the actual diagnostic value of this test in reptiles.
References
- 1.Cray C., Tatum L.M. Applications of protein electrophoresis in avian diagnostics. J Avian Med Surg. 1998;12(1):4–10. [Google Scholar]
- 2.Gicking J.C., Allen M.F., Kendal E.H. Plasma protein electrophoresis of the Atlantic Loggerhead Sea Turtle Caretta caretta. J Herpetol Med Surg. 2004;14(3):13–18. [Google Scholar]
- 3.Zaias J., Norton T., Fickel A. Biochemical and hematologic values for 18 clinically healthy radiated tortoises (Geochelone radiata) on St Catherines Island, Georgia. Vet Clin Pathol. 2006;35(3):321–332. doi: 10.1111/j.1939-165x.2006.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 4.Work T.M., Rameyer R.A., Balazs G.H. Immune status of free-ranging green turtles with fibropapillomatosis from Hawaii. J Wildl Dis. 2001;37(3):574–581. doi: 10.7589/0090-3558-37.3.574. [DOI] [PubMed] [Google Scholar]
- 5.Martinez S., Rodriguez Dominguez M.A., Mateo J.A. Comparative haematology and blood chemistry of endangered lizards (Gallotia species) in the Canary Islands. Vet Rec. 2004;155(9):266–269. doi: 10.1136/vr.155.9.266. [DOI] [PubMed] [Google Scholar]
- 6.Coppo J.A., Mussart N.B., Barboza N.N. Electrophoretic proteinogram reference interval from Argentina Northeastern captive caimans (crocodylia: Alligatoridae) InVet. 2006;8(1):129–137. [Google Scholar]
- 7.Kaneko J.J., Harvey J.W., Bruss M.L. Academic Press Elsevier; 2008. Clinical biochemistry of domestic animals. [Google Scholar]
- 8.Ceron J.J., Eckersall P.D., Martinez-Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol. 2005;34(2):85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 9.Martinez Subiela S., Tecles F., Eckersall P.D. 2002 Serum concentration of acute phase proteins in dogs with leishmaniasis. Vet Rec. 2002;150(8):241–244. doi: 10.1136/vr.150.8.241. [DOI] [PubMed] [Google Scholar]
- 10.Cray C. New testing options for the diagnosis of Encephalitozoon cuniculi in rabbits. Exotic DVM. 2009;11(2):27–28. [Google Scholar]
- 11.Didier E.S., Vossbrinck C.R., Baker M. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–422. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 12.Cox J.C. Altered immune responsiveness associated with Encephalitozoon cuniculi infection in Rabbits. Infect Immun. 1977;15(2):392–395. doi: 10.1128/iai.15.2.392-395.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobottka I., Iglauer F., Schuler T. Acute and long term humoral immunity following active immunization of rabbits with inactivated spores of various Encephalitozoon species. Parasitol Res. 2001;87(1):1–6. doi: 10.1007/s004360000297. [DOI] [PubMed] [Google Scholar]
- 14.Harcourt-Brown F.M., Holloway H.K. Encephalitozoon cuniculi in pet rabbits. Vet Rec. 2003;152:427–431. doi: 10.1136/vr.152.14.427. [DOI] [PubMed] [Google Scholar]
- 15.Boussarie P.D. L’electrophorese des proteins seriques en pathologie du furet (Mustela putorius furo) Bull Acad Vet Fr. 2007;160 n4. [Google Scholar]
- 16.Dardas T.J., Mallmann V.H. Electrophoretic and immunoelectrophoretic studies of sera from normal, tuberculous and non infected tuberculin sensitive guinea pigs. J Bacteriol. 1966;92(1) doi: 10.1128/jb.92.1.76-81.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atchley F.O., Auernheimer A.H., Wasley M.A. Electrophoretic studies of blood serum proteins in Guinea pigs inoculated with Entoameba histolytica. Parasitology. 1961;47(2) [PubMed] [Google Scholar]
- 18.Xie H., Huff G.R., Huff W.E. Identification of ovotransferrin as an acute phase protein in chickens. Poult Sci. 2002;81(1):112–120. doi: 10.1093/ps/81.1.112. [DOI] [PubMed] [Google Scholar]
- 19.Tohjo H., Miyoshi F., Uchida E. Polyacrylamide gel electrophoretic patterns of chicken serum in acute inflammation induced by intramuscular injection of turpentine. Poult Sci. 1995;74(4):648–655. doi: 10.3382/ps.0740648. [DOI] [PubMed] [Google Scholar]
- 20.Archer F.J., Battison A.L. Differences in electrophoresis patterns between plasma albumins of the cockatiel (Nymphicus hollandicus) and the chicken (Gallus gallus domesticus) Avian Pathol. 1997;26:865–870. doi: 10.1080/03079459708419260. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal K.L., Johnston M.S., Shofer F.S. Assessment of the reliability of plasma electrophoresis in birds. Am J Vet Res. 2005;66(3):375–378. doi: 10.2460/ajvr.2005.66.375. [DOI] [PubMed] [Google Scholar]
- 22.Cray C., Rodriguez M., Zajas J. Protein electrophoresis of psittacine plasma. Vet Clin Pathol. 2007;36(1):64–72. doi: 10.1111/j.1939-165x.2007.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 23.Ivey E.S. Serologic and plasma protein eletrophoretic findings in 7 psittacine birds with aspergillosis. J Avian Med Surg. 2000;14(2):103–106. [Google Scholar]
- 24.Cray C., Zielezienski-Roberts K., Bonda M. Serologic diagnosis of Sarcocystosis in Psittacine birds: 16 cases. J Avian Med Surg. 2005;19(3):208–215. [Google Scholar]
- 25.Cray C. Plasma protein electrophoresis: an update. Proc Annu Conf Assoc Avian Vet. 1997:209–212. [Google Scholar]
- 26.Lumeij J.T., Maclean B. Total protein determination in pigeon plasma and serum: comparison of refractometric methods with the Biuret method. J Avian Med Surg. 1996;10(3):150–152. [Google Scholar]
- 27.Tatum L.M., Zajas J., Mealey B.K. Protein electrophoresis as a diagnostic and prognostic tool in raptor medicine. J Zoo Wildl Med. 2000;31(4):497–502. doi: 10.1638/1042-7260(2000)031[0497:PEAADA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Zaias J., Cray C. Protein electrophoresis: a tool for the reptilian and amphibian practitioner. J Hum Mov Stud. 2002;12:30–32. [Google Scholar]