Abstract
The objective of this study was to examine the dynamics of bovine corona virus (BCV) and bovine respiratory syncytial virus (BRSV) infections in dairy herds over a 3-year period. The status of 79 dairy herds located in two Northern and two Southern Regions of Sweden were surveyed by measuring antibody concentrations to BCV and BRSV in pooled milk samples from primiparous cows, and in bulk-tank milk twice annually.
In the Southern Regions the percentage of antibody-positive herds remained persistently high (75–100%), whereas in herds based in the Northern Region, the percentage of positive herds for BCV and BRSV was 38–80% and 0–80%, respectively, with antibody levels to BRSV decreasing over time. Pooled milk samples of ‘home-bred’ primiparous animals were found to be most useful in terms of monitoring herd status but could gradually be replaced by bulk-tank sampling once freedom from infection was established.
Keywords: Cattle, Bovine corona virus, Bovine respiratory syncytial virus, Serology, Herd monitoring, Longitudinal study
Introduction
Bovine corona virus (BCV) and bovine respiratory syncytial virus (BRSV) are significant pathogens of both beef and dairy cattle worldwide (Clark, 1993, Valarcher and Taylor, 2007). A survey in England and Wales based on virus antibody concentrations in bulk-tank milk (BTM) samples, found prevalences of 100% (Paton et al., 1998). BCV can cause diarrhoea in calves and winter dysentery in adult cattle as well as respiratory disease of variable severity (Stair et al., 1972, Saif et al., 1986, Saif, 1990, Alenius et al., 1991). Infection with BRSV can lead to outbreaks of severe respiratory disease (Verhoeff et al., 1984, Elvander, 1996, Viuff et al., 1996).
Outbreaks of both viral infections typically occur during the housing (winter) period (Stott et al., 1980), and it remains unclear how the virus survives during the intervening seasons. It is possible that factors such as latency/reactivation, low level circulation of virus within and/or between herds or reservoir hosts may play a role. Sequencing indicates that virus within herds during an outbreak is identical, but varies both temporally and spatially between outbreaks, suggesting that outbreaks are caused by ‘new’ virus rather than through latency or the existence of carrier animals (Larsen et al., 2000, Liu et al., 2006, Bidokhti et al., 2012).
Both infections have significant negative effects on both herd health and animal performance (Tråvén et al., 1999, Beaudeau et al., 2010, Ohlson et al., 2010a) and there is a clear association between higher levels of herd biosecurity levels and lower prevalence of herd infection (Ohlson et al., 2010b). In order to control these infections better, it is important to clarify further some aspects of their epidemiology and to evaluate the accuracy of diagnostic procedures. To the authors’ knowledge, very few longitudinal studies charting the epidemiology of BCV and BRSV infections in cattle herds have been performed. Van der Poel et al. (1993) investigated BRSV infection in six dairy herds over a 1-year period and found a rise in titres but not seroconversion in antibody-positive animals during the summer period. Hägglund et al. (2006) conducted a 1-year serological study of calves in a region of southern Sweden where both BCV and BRSV are endemic and found seroconversion to occur in animals mainly during the winter.
The within-herd spread of both BCV and BRSV is highly effective with the result that all susceptible animals typically become infected (Alenius et al., 1991, Hägglund et al., 2006, Bidokhti et al., 2009). Sampling a subset of young animals has previously been used in testing for bovine viral diarrhoea virus (BVBV) (Houe, 1992). Bulk tank milk (BTM) is also convenient way of monitoring infection status in dairy herds, and the relationship between the BTM antibody titre and the serological status of young animals has previously been evaluated for BVDV (Houe, 1994). However, such an approach has not been used to investigate if there is an association between BTM antibody concentrations and the serological status of young animals to BCV or BRSV.
The main objective of this study was to investigate the dynamics of BCV and BRSV infection in dairy herds and to assess if these dynamics varied between geographical areas. The antibody status of 79 herds located in four regions of Sweden was recorded over a 3-year period. A secondary aim was to evaluate if there was an association between the percentage IgG positivity in BTM samples, and the antibody status of younger cows in order to determine if the former could be used as an estimate of the latter.
Materials and methods
Study population
Four study areas within the counties of Halland, Gotland, Jämtland and Västerbotten were selected (Fig. 1 ). The selection was based on previously estimated differences in herd prevalence of antibodies in BTM samples, and on regional differences in herd density. The prevalence of antibodies in BTM was estimated to be 90–94% and 84–89% in Halland, 95–100% and 84–89% in Gotland, 75–79% and 41–51% in Jämtland, and 70–74% and 41–51% in Västerbotten to BCV and BRSV, respectively (Elvander, 1996, Tråvén et al., 1999). The corresponding herd densities in 2006 were 0.09, 0.11, 0.005 and 0.007 herds/km2, respectively (Swedish Board of Agriculture, 2007).
Fig. 1.
Map of Sweden illustrating the boundaries of the four areas surveyed. H, Halland; G, Gotland; J, Jämtland; and V, Västerbotten. Map created using R statistical package (TEAM, 2008).
A sample of 20 herds was selected from each area: 10 herds with 30–80 cows and 10 with >80 animals. These represented ‘average’ and ‘large’ sized herds under current dairy farming conditions in Sweden (the mean herd size in Sweden was 48 in 2006).
The vast majority of Swedish dairy herds have a non-seasonal calving pattern, and vaccines against BCV and BRSV are rarely used. Herds were eligible for inclusion in the study if their owners were members of the local livestock association and had enrolled in the National Animal Disease Recording System (Emanuelson, 1988), and Swedish Official Milk Recording Scheme (Olsson et al., 2001). Currently >90% of Swedish dairy cows are enrolled in these programs (Mörk et al., 2010). Selected herds were geographically spread over the selected areas, were included once farmer consent was obtained, and were routinely visited by personnel from the local livestock association. Vaccination against BRSV and BCV was not used in participating herds and all were free of BVDV infection as defined by the Swedish eradication program (Lindberg and Alenius, 1999). The herds were also included (in the spring sampling of 2008) as a part of a risk factor study (Ohlson et al., 2010b).
Using farm location details provided by the Swedish Board of Agriculture in 2005, kernel smoothing techniques were applied to illustrate the spatial distribution of dairy farms throughout Sweden, expressed as the number of farms/100 km2. These analyses were based on a regular grid of 2578 × 2578 cells calculated using the Spatial Analyst extension in ArcGIS (version 9.3, ESRI). The bandwidth parameter for the kernel function (used to control the amount of smoothing of the estimated density surface) was fixed at 30 km, and was calculated using the normal optimal method (Bowman and Azzalini, 1997). The locations of the 79 herds were superimposed on this plot to demonstrate the spatial distribution of the study herds relative to the overall national distribution of at risk herds (Fig. 2 ).
Fig. 2.
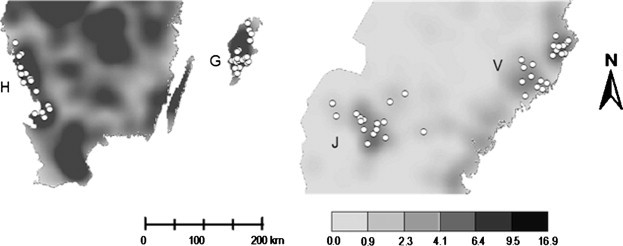
Image plot maps showing density of dairy farms (number of farms/100km2) recorded by the Swedish Board of Agriculture in 2005. Superimposed on each plot are the point locations of the 79 study herds located in the south (Halland [H] and Gotland [G]), and north (Jämtland [J] and Västerbotten [V]).
Sampling procedures
Herds were sampled before (September–October) and after (April–May) the housing season for three consecutive years (from September 2006 to May 2009). For each herd, on each sampling occasion, a pooled milk sample was collected from the five youngest home-bred primiparous cows, and a BTM sample was also obtained, which included milk from the sampled primiparous animals. Sampling was performed by personnel from the local livestock association. Ten millilitre test tubes containing 1.5 mg of the preservative bronopol (2-bromo-2-nitropropane-1.3-diol) were used and samples were not diluted or centrifuged and were stored at −20 °C until analysed. There were 432 pooled samples in total with corresponding BTM samples. Farmers were informed of the antibody status of their herd by mail after each sampling, based on the results obtained from the pooled primiparous cow sample. Herd owners also received basic information regarding the clinical signs and transmission routes of BCV and BRSV infection, as well as advice on biosecurity.
Milk analysis
Samples were analysed for IgG to BCV (Alenius et al., 1991) and BRSV (Elvander et al., 1995) using commercially available indirect ELISAs (Svanovir BCV-Ab and BRSV-Ab, Svanova Biotech). Test sensitivity was estimated at 84.6% (BCV) and 94.6% (BRSV), respectively, and specificity at 100% for both (individual samples, Svanova Manual). There were no data relating to herd sensitivity/specificity for BTM samples. The optical density (OD) at 450 nm was corrected by subtraction of the negative control antigen OD. To adjust for possible day-to-day variation in analysis, the percentage positivity (PP) was calculated as (corrected OD/positive control corrected OD) × 100. For the BTM samples, a PP value of <5 was considered negative. This corresponded to the corrected OD of 0.05, with the positive control having an OD of ‘1’. This cut-off had previously been used to detect antibodies to BCV and BRSV in BTM (Elvander, 1996, Paton et al., 1998, Hägglund et al., 2006). For the pooled milk samples a PP < 20 was deemed negative, closely corresponding to the corrected OD of 0.20, the cut-off for negative individual samples for both BCV and BRSV, as recommended by the manufacturer.
Assessment of infection dynamics
Changes in test results from <20 to >20 for primiparous animal sample pools, and from PP < 5 to PP ⩾ 5 for BTM samples, were classified as a change in herd status from antibody negative to positive and the reverse situation resulted in herds changing from positive to negative status.
Statistical analysis
A Kruskal–Wallis equality-of-populations rank test was used to compare median herd size between the areas for each of the two herd-size groups, and also to compare median PPs in BTM between negative and positive herds based on the primiparous pooled sample. The proportion of positive herds in each region on each sampling occasion was compared using a Fisher’s exact test. The association between herd antibody status to BCV and BRSV was evaluated using the χ 2 test, repeated for each sampling occasion.
Results
Of the 79 herds initially sampled in the autumn of 2006, 73 remained in the study for the duration of the survey period and six dropped out as the farms ceased milk production. Two additional herds entered the study: ‘Halland’ in spring 2007 and ‘Jämtland’ in autumn 2007. For the Gotland region, samples during the autumn 2008 period were lost during transport. Fig. 2 illustrates the distribution of the selected herds and the herd densities in these areas. Median herd size at the start of the study was 45 for the 30–80 group and 110 for the >80 sized herds: there were no significant differences between the four regions as regards median herd size of these two categories of herd.
Primiparous pooled samples
A summary of the results of analysis of the primiparous pooled samples at each sampling occasion is given in Table 1 . At study commencement, 12 and 13 herds were antibody negative to BCV and BRSV, respectively. Changes in herd status from antibody negative to positive occurred 26 times in 25 herds for BCV and 14 times in 13 herds for BRSV, respectively. Changes in antibody status from negative to positive were present at all five follow-up sampling points, and also occurred during the two summer seasons (five herds for BCV and two herds for BRSV, respectively). A change from antibody positive to negative status occurred 29 times in 28 herds for BCV and 32 times in 32 herds for BRSV.
Table 1.
Summary of six sampling occasions from autumn (a) 2006 to spring (s) 2009 regarding antibody status to bovine coronavirus (BCV) and bovine respiratory syncytial virus (BRSV), measured in pooled milk samples of five home-bred primiparous cows in 79 dairy herds in four areas in Sweden.
| BCV |
BRSV |
|||||||
|---|---|---|---|---|---|---|---|---|
| Positive/totala | Neg-posb | Pos-negc | Incd | Positive/totala | Neg-posb | Pos-negc | Incd | |
| Halland | ||||||||
| a 2006 | 18/19 | NA | NA | NA | 16/19 | NA | NA | NA |
| s 2007 | 18/18 | 1 | 0 | 100 | 17/18 | 2 | 0 | 67 |
| a 2007 | 17/18 | NA | 1 | NA | 15/18 | 0 | 1 | 50 |
| s 2008 | 16/18 | 0 | 1 | 0 | 17/18 | 2 | 0 | 33 |
| a 2008 | 16/17 | 1 | 0 | 50 | 16/17 | 0 | 0 | 0 |
| s 2009 | 16/17 | 0 | 0 | 0 | 16/17 | 0 | 0 | 0 |
| Gotland | ||||||||
| a 2006 | 19/20 | NA | NA | NA | 19/20 | NA | NA | NA |
| s 2007 | 18/20 | 1 | 2 | 100 | 19/20 | 1 | 1 | 67 |
| a 2007 | 18/20 | 1 | 0 | 50 | 18/20 | 1 | 2 | 100 |
| s 2008 | 17/20 | 1 | 3 | 100 | 15/20 | 0 | 3 | 67 |
| a 2008 | missing | - | - | - | missing | - | - | - |
| s 2009 | 19/19 | 3 | 0 | 100 | 18/19 | 3 | 0 | 100 |
| Jämtland | ||||||||
| a 2006 | 16/20 | NA | NA | NA | 16/20 | NA | NA | NA |
| s 2007 | 14/20 | 1 | 3 | 25 | 11/20 | 0 | 5 | 0 |
| a 2007 | 8/21 | 1 | 7 | 17 | 2/21 | 0 | 9 | 0 |
| s 2008 | 10/16 | 4 | 0 | 40 | 1/16 | 0 | 1 | 0 |
| a 2008 | 11/17 | 2 | 0 | 17 | 0/17 | 0 | 1 | 0 |
| s 2009 | 11/18 | 0 | 1 | 0 | 1/18 | 1 | 0 | 6 |
| Västerbotten | ||||||||
| a 2006 | 14/20 | NA | NA | NA | 15/20 | NA | NA | NA |
| s 2007 | 11/20 | 1 | 4 | 17 | 15/20 | 1 | 1 | 20 |
| a 2007 | 8/20 | 1 | 4 | 11 | 14/20 | 0 | 1 | 0 |
| s 2008 | 10/19 | 4 | 1 | 33 | 12/19 | 0 | 1 | 0 |
| a 2008 | 8/19 | 0 | 2 | 0 | 10/19 | 1 | 3 | 14 |
| s 2009 | 13/19 | 4 | 0 | 45 | 9/19 | 2 | 3 | 25 |
NA, not applicable.
Number of positive herds/number of sampled herds.
Number of herds converting from antibody negative (neg) to positive (pos).
Number of herds converting from antibody positive to negative.
Incidence of antibody conversion from negative to positive for herds at risk (antibody negative on previous sampling).
The infection dynamics of BRSV and BCV are detailed in Table 2 . The majority of herds that remained negative throughout the study were located in Jämtland and Västerbotten: 6/6 and 8/9 herds for BCV and BRSV, respectively. There was a positive association between BCV and BRSV primiparous pool status (P < 0.005; odds ratio [OR] 4.2–8.4) on all sampling occasions, except for autumn 2008 where the association was not significant likely due to the missing data. The proportion of positive herds did not differ significantly between the four study areas in autumn 2006, for either BCV or BRSV. On almost all follow-up samplings, however, there were a significantly higher proportion of herds positive to BCV and BRSV in Halland and Gotland, compared with those in Jämtland and Västerbotten.
Table 2.
Dynamics of antibody status to bovine coronavirus (BCV) and bovine respiratory syncytial virus (BRSV), in pooled milk samples from five primiparous cows from 79 dairy herds in four areas in Sweden, stratified by the results of the first sampling in primiparous pools and in bulk tank milk (BTM). The results relate to six sampling points between October 2006 and April 2009.
| Result at first test BTM/primiparous pool | Negative throughout study | New exposure - remained positive | New exposure - returned to negative | Multiple new exposures (fluctuated) | |
|---|---|---|---|---|---|
| BRSV (n = 4) | - / - | 100% | 0% | 0% | 0% |
| BRSV (n = 12) | + / - | 41.7% | 33.3% | 8.3% | 16.7% |
| BCV (n = 2) | - / - | 100% | 0% | 0% | 0% |
| BCV (n = 9) | + / - | 22.2% | 66.7% | 11.1% | 0% |
| Positive throughout study | Turned negative - remained negative | Turned negative then new exposure | Multiple new exposures (fluctuated) | ||
| BRSV (n = 64) | + / + | 50.0% | 33.3% | 16.7% | 0% |
| BCV (n = 69) | + / + | 59.4% | 13.0% | 27.5% | 0% |
Bulk tank milk samples
At study commencement, two and four herds were antibody negative to BCV and BRSV in their BTM, respectively, and remained seronegative throughout the study period. One herd in Jämtland, comprising 147 milking cows, remained antibody negative to both viruses throughout the survey. In addition, three herds in each case became seronegative for BCV and BRSV, respectively. These herds were all located in Jämtland and Västerbotten, except for one herd, antibody negative to BCV, located in Halland. One of these herds, located in Västerbotten, became positive to BCV whereas the rest remained seronegative.
Association between pooled and BTM samples
A total of 435 primiparous pooled milk samples were analysed during the study period, of which 432 had a corresponding BTM sample. Scatterplots of the PP value in BTM against the corresponding pooled sample are shown in Fig. 3 . There was a considerable variation in the PP values for the BTM samples corresponding to the seronegative primiparous pools. All antibody negative BTM samples (PP < 5) however, had a corresponding negative primiparous pool sample (PP ⩽ 20). Furthermore, all BTM samples with a PP ⩽ 30 had a corresponding antibody negative primiparous pooled milk sample for both viruses.
Fig. 3.
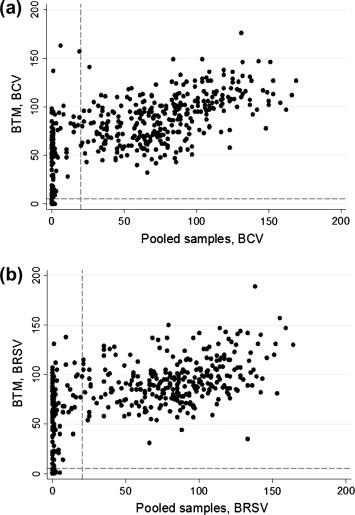
Scatterplots of serological percentage positivity (PP) to: (a) bovine coronavirus (BCV) and (b) bovine respiratory syncytial virus (BRSV). The results of pooled milk samples of five home-bred primiparous cows and of the corresponding bulk tank milk sample (BTM) are given on the horizontal and vertical axes, respectively. Dashed horizontal and vertical lines represent the cut-offs for negative BTM (PP < 5) and pooled (PP < 20) samples, respectively.
The median PP in the BTM was lower in samples that had a negative corresponding primiparous pooled sample to both BCV and BRSV (P < 0.001), which remained significant when the BTM-negative samples were excluded. The majority of the negative primiparous pooled samples had a corresponding positive BTM sample (Table 3 ). Median and mean PP values, stratified by the results of the pooled milk samples, are detailed in Table 4 .
Table 3.
Relationship between antibody status to bovine coronavirus (BCV) and bovine respiratory syncytial virus (BRSV), in pooled milk samples from five primiparous cows (pool), and corresponding antibody status in bulk tank milk (BTM) samples, expressed as number of samples. Cut-off set at percentage positivity of <20 for pooled and <5 for BTM, samples, respectively.
| Positive BTM | Negative BTM | Total | |
|---|---|---|---|
| BCV | |||
| positive pool | 323 | 0 | 323 |
| negative pool | 92 | 17 | 109 |
| BRSV | |||
| positive pool | 287 | 0 | 287 |
| negative pool | 116 | 30 | 146 |
Table 4.
Median, mean and standard deviation (STD) for the percentage positivity (PP) of immunoglobulin (Ig) G to bovine coronavirus (BCV) and bovine respiratory syncytial virus (BRSV) measured in pooled milk samples from five primiparous cows (pool) and corresponding bulk tank milk (BTM) samples, stratified by the antibody status of the primiparous pool. Results of negative and positive BTM samples also detailed. Cut-off for negative samples set at PP<20 for pooled, <5 for BTM samples, respectively.
| BCV |
BRSV |
|||||||
|---|---|---|---|---|---|---|---|---|
| number | median | mean | STD | number | median | mean | STD | |
| Negative pool | ||||||||
| pool | 109 | 1 | 2.6 | 4.8 | 147 | 1 | 2.1 | 4.1 |
| BTM | 109 | 53 | 49.2 | 35.1 | 146 | 59.5 | 51.4 | 35.9 |
| Positive pool | ||||||||
| pool | 323 | 78 | 80.4 | 32.3 | 288 | 86 | 85.7 | 31.4 |
| BTM | 323 | 91 | 89.7 | 24.1 | 286 | 92 | 89.7 | 24.1 |
| BTM | ||||||||
| negative | 17 | 0 | 0.7 | 1.1 | 30 | 1 | 1 | 1.2 |
| positive | 415 | 83 | 82.2 | 28.9 | 403 | 86 | 85.8 | 27.7 |
Discussion
Our results indicate that the dynamics of BCV and BRSV infection differ with geographical location: in Halland and Gotland in the South, the percentage of antibody-positive herds as reflected in the primiparous contrast to herds in Jämtland and Västerbotten in the North where there was greater fluctuation pooled samples was persistently 75–100%, in in seropositivity (38–80% and 0–80% for BCV and BRSV, respectively). Herds that remained seronegative throughout the study period (except for one herd for BRSV) were located in the Southern Regions surveyed. The incidence of new infections was higher in Halland and Gotland compared to in Jämtland and Västerbotten.
It could be speculated that the information provided to the farmers twice yearly had an impact in terms of improved biosecurity on study farms. Also, the greater herd densities in Halland and Gotland compared to Jämtland and Västerbotten could possibly result in a larger infection pressure in these Southern Regions. The missing samples from the Gotland herds from the autumn of 2008 were unlikely to have significantly altered our overall findings as the sampling from spring 2009 gave similar results to those taken during the same season in 2008.
The fact that there were a few herd conversions from antibody negative to positive (six and two for BCV and BRSV, respectively), between April/May and September/October, suggests viral circulation, albeit at a low level, during the summer when animals are kept outdoors. It is possible that these herds had become newly infected at the previous sampling time but antibody concentrations had not yet reached detectable levels. However, it is likely that either the farmers and/or veterinarians involved in the sampling would have become aware of such new infections. Given how readily both BCV and BRSV transmit within a group of cattle, herds negative in primiparous pooled samples were likely to have been free from the active spread of infection for at least 2 years (corresponding to the age of the cows included). Such a time-span would strongly suggest that a herd converting from antibody negative to positive had recently been introduced to virus rather than being continuously exposed to virus circulating within the herd or being excreted by a carrier animal.
The herds in Jämtland showed no evidence of circulating BRSV by the end of the study, suggesting the possibility of ‘self-clearance’, i.e. clearance without the use of vaccination and/or increased biosecurity. The one herd in this region that did become seropositive in spring 2009 had a PP value of 21, which was just above the cut-off for negative samples (PP < 20). Such a low value suggests this change was not due to an outbreak of BRSV: it is possible that an animal was included in the sample ‘pool’ that was not home-bred or had been excluded from the herd for some reason. No other samples where the antibody status changed from negative to positive had such a marginal PP value. When the herds from Jämtland were resampled in 2010, all were antibody negative to BRSV in pooled milk samples from primiparous cows.
The negative primiparous pooled samples exhibited considerable variation in PP values in the corresponding BTM sample. In many cases it was not possible to distinguish primiparous pool-negative from pool-positive herds using the PP value of the BTM sample, but all antibody negative BTM samples had corresponding negative primiparous pool samples for both BCV and BRSV. We concluded that the measurement of antibodies to BCV and BRSV in BTM is an effective method of monitoring herds in areas where virus circulation has been very low or absent for a long time period. However, our findings also suggest that the use of primiparous pooled milk samples is more effective in identifying susceptible herds in the early phases of control programs in endemic areas and for the purposes of animal trading/movement. However, it is crucial that the cows included in the pooled sample are home-bred in order to truly reflect herd infection status.
The results of our study suggest it may be possible to establish regions free of BRSV infection, as reflected in the initiation of voluntary control programs that include the majority of the herds and that involve local veterinarians and livestock associations. Animal trading between low- and high-prevalence regions and between herds of differing antibody status involves the risk of introducing these viruses to potentially highly-susceptible, immunologically-naive cattle. One interesting option is to decrease the incidence of BRSV infection by establishing regional populations of herds certified free of this infection.
Conclusions
We found that the dynamics of BCV and BRSV infection varied between our two Southern and two Northern study areas. All of the herds in one of the Northern Regions remained free of BRSV transmission during the 3-year study period. Our findings also identified that individual herds in less cattle-dense areas can stay free of BRSV and BCV infection. Between-herd transmission of BRSV and BCV occurred when animals were kept outdoors between late spring and early autumn, which suggests ongoing circulation of the viruses, albeit at a low level, over this time-period. Pooled milk samples from primiparous cows are a convenient way to monitor herd infection status, and can gradually, if freedom from infection is established, be replaced by monitoring of BTM samples.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors wish to acknowledge Associate Professor M. Stevenson for valuable assistance and Ms. M. Hjort for her skilful laboratory work. This study was funded by the Swedish Farmers’ Foundation for Agricultural Research.
References
- Alenius S., Niskanen R., Juntti N., Larsson B. Bovine coronavirus as the causative agent of winter dysentery: Serological evidence. Acta Veterinaria Scandinavica. 1991;32:163–170. doi: 10.1186/BF03546976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudeau F., Ohlson A., Emanuelson U. Associations between bovine coronavirus and bovine respiratory syncytial virus infections and animal performance in Swedish dairy herds. Journal of Dairy Science. 2010;93:1523–1533. doi: 10.3168/jds.2009-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidokhti M.R., Tråvén M., Fall N., Emanuelson U., Alenius S. Reduced likelihood of bovine coronavirus and bovine respiratory syncytial virus infection on organic compared to conventional dairy farms. The Veterinary Journal. 2009;182:436–440. doi: 10.1016/j.tvjl.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidokhti M.R., Tråvén M., Ohlson A., Zarnegar B., Baule C., Belák S., Alenius S., Liu L. Phylogenetic analysis of bovine respiratory syncytial viruses from recent outbreaks in feedlot and dairy cattle herds. Archives of Virology. 2012;157:601–607. doi: 10.1007/s00705-011-1209-3. [DOI] [PubMed] [Google Scholar]
- Bowman A.W., Azzalini A. Oxford University Press; Oxford: 1997. Applied Smoothing Techniques for Data Analysis: The Kernel Approach with S-Plus Illustrations. [Google Scholar]
- Clark M.A. Bovine coronavirus. The British Veterinary Journal. 1993;149:51–70. doi: 10.1016/S0007-1935(05)80210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvander M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. Veterinary Record. 1996;138:101–105. doi: 10.1136/vr.138.5.101. [DOI] [PubMed] [Google Scholar]
- Elvander M., Edwards S., Naslund K., Linde N. Evaluation and application of an indirect ELISA for the detection of antibodies to bovine respiratory syncytial virus in milk, bulk milk, and serum. Journal of Veterinary Diagnostic Investigation. 1995;7:177–182. doi: 10.1177/104063879500700202. [DOI] [PubMed] [Google Scholar]
- Emanuelson U. The national Swedish animal disease recording system. Acta Veterinaria Scandinavica, Suppl. 1988;84:262–264. [PubMed] [Google Scholar]
- Houe H. Serological analysis of a small herd sample to predict presence or absence of animals persistently infected with bovine viral diarrhoea virus (BVDV) in dairy herds. Research in Veterinary Science. 1992;53:320–323. [PubMed] [Google Scholar]
- Houe H. Bovine virus diarrhoea virus: Detection of Danish dairy herds with persistently infected animals by means of a screening test of ten young stock. Preventive Veterinary Medicine. 1994;19:241–248. [Google Scholar]
- Hägglund S., Svensson C., Emanuelson U., Valarcher J.F., Alenius S. Dynamics of virus infections involved in the bovine respiratory disease complex in Swedish dairy herds. The Veterinary Journal. 2006;172:320–328. doi: 10.1016/j.tvjl.2005.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L.E., Tjornehoj K., Viuff B. Extensive sequence divergence among bovine respiratory syncytial viruses isolated during recurrent outbreaks in closed herds. Journal of Clinical Microbiology. 2000;38:4222–4227. doi: 10.1128/jcm.38.11.4222-4227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A.L., Alenius S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Veterinary Microiology. 1999;64:197–222. doi: 10.1016/s0378-1135(98)00270-3. [DOI] [PubMed] [Google Scholar]
- Liu L., Hägglund S., Hakhverdyan M., Alenius S., Larsen L.E., Belak S. Molecular epidemiology of bovine coronavirus on the basis of comparative analyses of the S gene. Journal of Clinical Microbiology. 2006;44:957–960. doi: 10.1128/JCM.44.3.957-960.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörk M.J., Wolff C., Lindberg A., Vågsholm I., Egenvall A. Validation of a national disease recording system for dairy cattle against veterinary practice records. Preventive Veterinary Medicine. 2010;93:183–192. doi: 10.1016/j.prevetmed.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Ohlson A., Emanuelson U., Tråvén M., Alenius S. The relationship between antibody status to bovine corona virus and bovine respiratory syncytial virus and disease incidence, reproduction and herd characteristics in dairy herds. Acta Veterinaria Scandinavica. 2010;52:37. doi: 10.1186/1751-0147-52-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson A., Heuer C., Lockhart C., Tråvén M., Emanuelson U., Alenius S. Risk factors for transmission of bovine coronavirus and bovine respiratory syncytial virus between dairy herds. Veterinary Record. 2010;167:201–206. doi: 10.1136/vr.c4119. [DOI] [PubMed] [Google Scholar]
- Olsson S.O., Baekbo P., Hansson S.O., Rautala H., Osteras O. Disease recording systems and herd health schemes for production diseases. Veterinaria Scandinavica, Suppl. 2001;94:51–60. doi: 10.1186/1751-0147-42-S1-S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton D.J., Christiansen K.H., Alenius S., Cranwell M.P., Pritchard G.C., Drew T.W. Prevalence of antibodies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. Veterinary Record. 1998;142:385–391. doi: 10.1136/vr.142.15.385. [DOI] [PubMed] [Google Scholar]
- Saif L.J. A review of evidence implicating bovine coronavirus in the etiology of winter dysentery in cows: An enigma resolved? Cornell Veterinarian. 1990;80:303–311. [PubMed] [Google Scholar]
- Saif L.J., Redman D.R., Moorhead P.D., Theil K.W. Experimentally induced coronavirus infections in calves: Viral replication in the respiratory and intestinal tracts. American Journal of Veterinary Research. 1986;47:1426–1432. [PubMed] [Google Scholar]
- Stair E.L., Rhodes M.B., White R.G., Mebus C.A. Neonatal calf diarrhea: Purification and electron microscopy of a coronavirus-like agent. American Journal of Veterinary Research. 1972;33:1147–1156. [PubMed] [Google Scholar]
- Stott E.J., Thomas L.H., Collins A.P., Crouch S., Jebbett J., Smith G.S., Luther P.D., Caswell R. A survey of virus infections of the respiratory tract of cattle and their association with disease. Journal of Hygiene. 1980;85:257–270. doi: 10.1017/s0022172400063294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish Board of Agriculture, 2007. Official Statistics of Sweden. In: Yearbook of Agricultural Statistics 2007 including Food Statistics, Jönköping, Sweden, p. 100.
- TEAM, R.D.C., 2008. Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. <http://www.R-project.org>.
- Tråvén M., Björnerot L., Larsson B. Nationwide survey of antibodies to bovine coronavirus in bulk milk from Swedish dairy herds. Veterinary Record. 1999;144:527–529. doi: 10.1136/vr.144.19.527. [DOI] [PubMed] [Google Scholar]
- Valarcher J.F., Taylor G. Bovine respiratory syncytial virus infection. Veterinary Research. 2007;38:153–180. doi: 10.1051/vetres:2006053. [DOI] [PubMed] [Google Scholar]
- Van der Poel W.H., Kramps J.A., Middel W.G., Van Oirschot J.T., Brand A. Dynamics of bovine respiratory syncytial virus infections: A longitudinal epidemiological study in dairy herds. Archives of Virology. 1993;133:309–321. doi: 10.1007/BF01313771. [DOI] [PubMed] [Google Scholar]
- Verhoeff J., Van der Ban M., van Nieuwstadt A.P. Bovine respiratory syncytial virus infections in young dairy cattle: Clinical and haematological findings. Veterinary Record. 1984;114:9–12. doi: 10.1136/vr.114.1.9. [DOI] [PubMed] [Google Scholar]
- Viuff B., Uttenthal A., Tegtmeier C., Alexandersen S. Sites of replication of bovine respiratory syncytial virus in naturally infected calves as determined by in situ hybridization. Veterinary Pathology. 1996;33:383–390. doi: 10.1177/030098589603300403. [DOI] [PubMed] [Google Scholar]


